Comparison of Different Dietary Fatty Acids Supplement on the Immune Response of Hybrid Grouper (Epinephelus fuscoguttatus × Epinephelus lanceolatus) Challenged with Vibrio vulnificus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diet Preparation
2.2. Bacteria Culture
2.3. Fish and Rearing Condition
2.4. Feeding Experimental Design
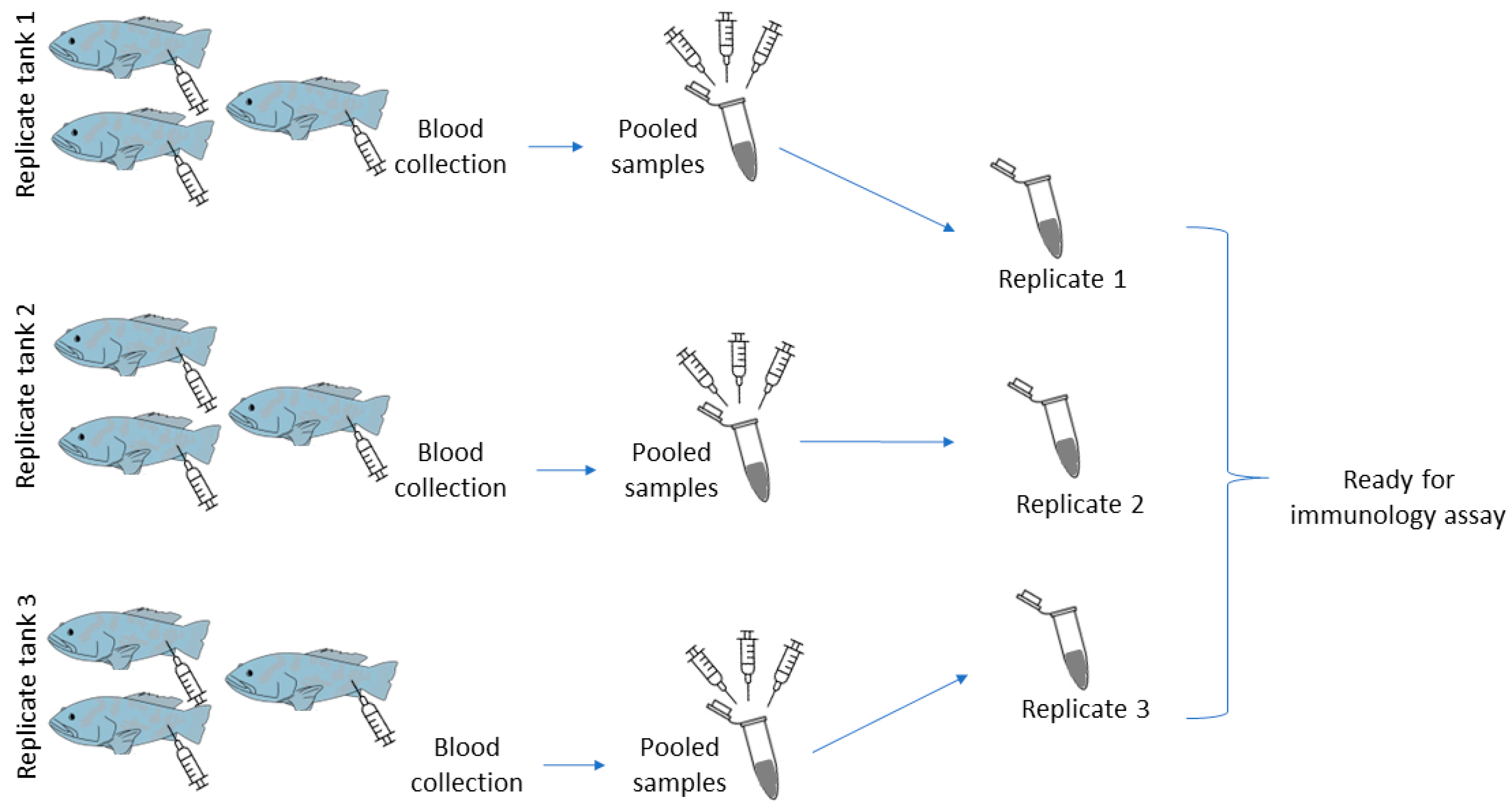
2.5. Sample Collection before the Bacterial Challenge
2.6. Fingerlings Challenged with LD50 of V. vulnificus
2.7. Sample Collection for Post-Bacterial Challenge
2.8. Immunology Assay
2.8.1. White Blood Cell (WBC) and Red Blood Cell (RBC) Counts
2.8.2. Lysozyme Activity Assay
2.8.3. Respiratory Burst Activity Assay
2.8.4. Phagocytic Activity Assay
2.9. Statistical Analysis
3. Results
3.1. Growth Performance and Feeding Efficiency
3.2. Survival and Hematological Parameters in Post-Challenge
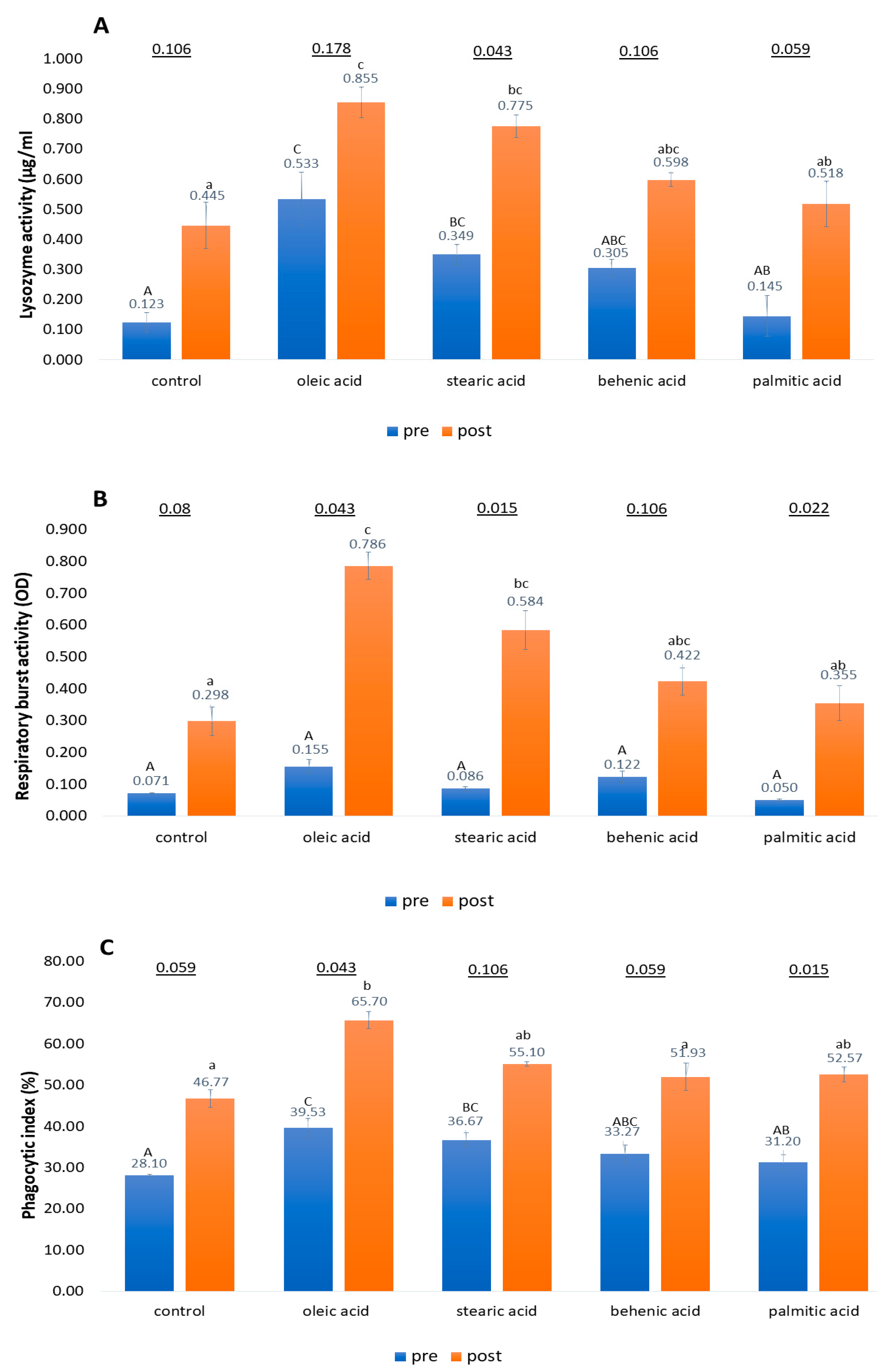
3.3. Lysozyme Activity, Respiratory Burst Activity, and Phagocytic Activity Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO Statistic, Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/fishery/statistics/en (accessed on 24 January 2021).
- Low, C.-F.; Nor Shamsudin, M.; Abdullah, M.; Chee, H.-Y.; Aliyu-Paiko, M. Experimental infection of brown-marbled grouper, Epinephelus fuscoguttatus (Forskal), with Vibrio parahaemolyticus identifies parvalbumin beta-2 subunit I, alpha-2-macroglobulin, nattectin and immunoglobulin light chain, differentially expressed in resist. J. Fish Dis. 2015, 38, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ina-Salwany, M.Y.; Al-saari, N.; Mohamad, A.; Mursidi, F.; Mohd-Aris, A.; Amal, M.N.A.; Kasai, H.; Mino, S.; Sawabe, T.; Zamri-Saad, M. Vibriosis in Fish: A review on disease development and prevention. J. Aquat. Anim. Health 2019, 31, 3–22. [Google Scholar] [CrossRef]
- Austin, B.; Austin, D.A. Bacterial Fish Pathogens: Disease of Farmed and Wild Fish; Springer: Dordrecht, The Netherlands, 2012; ISBN 978-94-007-4883-5. [Google Scholar]
- Vincent, A.T.; Gauthier, J.; Derome, N.; Charette, S.J. The rise and fall of antibiotics in aquaculture. In Microbial Communities in Aquaculture Ecosystems; Derome, N., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–19. [Google Scholar]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef]
- Zargar, A.; Taheri Mirghaed, A.; Mirzargar, S.S.; Ghelichpour, M.; Yousefi, M.; Hoseini, S.M. Dietary ginger administration attenuates oxidative stress and immunosuppression caused by oxytetracycline in rainbow trout (Oncorhynchus mykiss). Aquac. Res. 2020, 51, 4215–4224. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Yousefi, M. Beneficial effects of thyme (Thymus vulgaris) extract on oxytetracycline-induced stress response, immunosuppression, oxidative stress and enzymatic changes in rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2019, 25, 298–309. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Zhang, X.; Rabbi, M.H.; Guo, R.; Shi, S.; Ma, Z.; Liu, Y. Effects of dietary florfenicol contained feeds on growth and immunity of European seabass (Dicentrarchus labrax) in flow-through and recirculating aquaculture system. Aquac. Reports 2021, 19, 100602. [Google Scholar] [CrossRef]
- Mehana, E.; Rahmani, A.; Aly, S. Immunostimulants and Fish Culture: An Overview. Annu. Res. Rev. Biol. 2015, 5, 477–489. [Google Scholar] [CrossRef]
- Firdaus-Nawi, M.; Zamri-Saad, M. Major components of fish immunity: A review. Pertanika J. Trop. Agric. Sci. 2016, 39, 393–420. [Google Scholar]
- Akhter, N.; Wu, B.; Memon, A.M.; Mohsin, M. Probiotics and prebiotics associated with aquaculture: A review. Fish Shellfish Immunol. 2015, 45, 733–741. [Google Scholar] [CrossRef]
- Hixson, S.M. Fish Nutrition and Current Issues in Aquaculture: The Balance in Providing Safe and Nutritious Seafood, in an Environmentally Sustainable Manner. J. Aquac. Res. Dev. 2014, 5. [Google Scholar] [CrossRef]
- Mejri, S.C.; Tremblay, R.; Audet, C.; Wills, P.S.; Riche, M. Essential fatty acid requirements in tropical and cold-water marine fish larvae and juveniles. Front. Mar. Sci. 2021, 8, 557. [Google Scholar] [CrossRef]
- Puertollano, M.A.; Puertollano, E.; Álvarez de Cienfuegos, G.; de Pablo, M.A. Significance of olive oil in the host immune resistance to infection. Br. J. Nutr. 2007, 98, S54–S58. [Google Scholar] [CrossRef]
- Hidalgo, M.A.; Carretta, M.D.; Burgos, R.A. Long Chain Fatty Acids as Modulators of Immune Cells Function: Contribution of FFA1 and FFA4 Receptors. Front. Physiol. 2021, 12, 668330. [Google Scholar] [CrossRef]
- Ishak, W.M.W.; Katas, H.; Yuen, N.P.; Abdullah, M.A.; Zulfakar, M.H. Topical application of omega-3-, omega-6-, and omega-9-rich oil emulsions for cutaneous wound healing in rats. Drug Deliv. Transl. Res. 2019, 9, 418–433. [Google Scholar] [CrossRef]
- Masner, M.; Lujea, N.; Bisbal, M.; Acosta, C.; Kunda, P. Linoleic and oleic acids enhance cell migration by altering the dynamics of microtubules and the remodeling of the actin cytoskeleton at the leading edge. Sci. Rep. 2021, 11, 14984. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Jia, R.; Cao, L.; Ding, W.; Xu, P.; Yin, G. Effects of Rhizoma Alismatis extract on biochemical indices and adipose gene expression in oleic acid-induced hepatocyte injury in Jian carp (Cyprinus carpio var. Jian). Fish Physiol. Biochem. 2018, 44, 747–768. [Google Scholar] [CrossRef]
- Gonçalves-de-Albuquerque, C.F.; Medeiros-de-Moraes, I.M.; Oliveira, F.M.D.J.; Burth, P.; Bozza, P.T.; Castro Faria, M.V.; Silva, A.R.; Castro-Faria-Neto, H.C.D. Omega-9 Oleic Acid Induces Fatty Acid Oxidation and Decreases Organ Dysfunction and Mortality in Experimental Sepsis. PLoS ONE 2016, 11, e0153607. [Google Scholar] [CrossRef] [PubMed]
- Majdoubi, F.-Z.; Benhima, R.; Ouizgane, A.; Farid, S.; Droussi, M.; Guerriero, G.; Hasnaoui, M. Ova Fatty Acids Composition and Spawning Performances of Silver Carp, Hypophthalmichthys molitrix (Morocco). Turkish J. Fish. Aquat. Sci. 2020, 20, 879–888. [Google Scholar] [CrossRef]
- Nurdalila, A.A.; Mayalvanan, Y.; Baharum, S.N. Metabolite profiling of Epinephelus fuscoguttatus infected with vibriosis reveals Omega 9 as potential metabolite biomarker. Fish Physiol. Biochem. 2019, 45, 1203–1215. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Daeman, N.H.; Chong, C.M.; Karami, A.; Kumar, V.; Hoseinifar, S.H.; Romano, N. Comparing the effects of different dietary organic acids on the growth, intestinal short-chain fatty acids, and liver histopathology of red hybrid tilapia (Oreochromis sp.) and potential use of these as preservatives. Fish Physiol. Biochem. 2017, 43, 1195–1207. [Google Scholar] [CrossRef]
- Mohd Faudzi, N.; Yong, A.S.K.; Shapawi, R.; Senoo, S.; Biswas, A.; Takii, K. Soy protein concentrate as an alternative in replacement of fish meal in the feeds of hybrid grouper, brown-marbled grouper (Epinephelus fuscoguttatus) × giant grouper (E. lanceolatus) juvenile. Aquac. Res. 2018, 49, 431–441. [Google Scholar] [CrossRef]
- Firdaus, R.F.; Lim, L.S.; Kawamura, G.; Shapawi, R. Assessment on the acceptability of hybrid grouper, Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂ to soybeanmeal-based diets. Aquac. Aquarium, Conserv. Legis. J. Bioflux Soc. 2016, 9, 284–290. [Google Scholar]
- Adekoya, A.; Porcadilla, M.; Varga, D.; Kucska, B. Replacing fish meal with alternative protein sources in common carp’s feed. Acta Agrar. Kaposváriensis 2018, 22, 18–24. [Google Scholar] [CrossRef]
- Ashraf, M.; Abbas, S.; Hafeez; Rasul, F.; Khan, N.; Zafar, A.; Ehsan, M.; Muhammad Naeem, M. Effect of different levels of cellulose on growth and survival of rohu (Labeo rohita) fingerlings. Glob. J. Anim. Sci. Res. 2014, 2, 321–326. [Google Scholar]
- Nurdalila, A.A.; Natnan, M.E.; Baharum, S.N. The effects of amino acids and fatty acids on the disease resistance of Epinephelus fuscoguttatus in response to Vibrio vulnificus infection. 3 Biotech 2020, 10, 544. [Google Scholar] [CrossRef]
- Chaklader, M.R.; Howieson, J.; Fotedar, R.; Siddik, M.A.B. Supplementation of Hermetia illucens Larvae in Poultry By-Product Meal-Based Barramundi, Lates calcarifer Diets Improves Adipocyte Cell Size, Skin Barrier Functions, and Immune Responses. Front. Nutr. 2021, 7, 613158. [Google Scholar] [CrossRef]
- Henry, M.A.; Gasco, L.; Chatzifotis, S.; Piccolo, G. Does dietary insect meal affect the fish immune system? The case of mealworm, Tenebrio molitor on European sea bass, Dicentrarchus labrax. Dev. Comp. Immunol. 2018, 81, 204–209. [Google Scholar] [CrossRef]
- Bakhshi, F.; Najdegerami, E.H.; Manaffar, R.; Tokmechi, A.; Rahmani Farah, K.; Shalizar Jalali, A. Growth performance, haematology, antioxidant status, immune response and histology of common carp (Cyprinus carpio L.) fed biofloc grown on different carbon sources. Aquac. Res. 2018, 49, 393–403. [Google Scholar] [CrossRef]
- Yeh, S.-P.; Chang, C.-A.; Chang, C.-Y.; Liu, C.-H.; Cheng, W. Dietary sodium alginate administration affects fingerling growth and resistance to Streptococcus sp. and iridovirus, and juvenile non-specific immune responses of the orange-spotted grouper, Epinephelus coioides. Fish Shellfish Immunol. 2008, 25, 19–27. [Google Scholar] [CrossRef]
- Biller-Takahashi, J.; Takahashi, L.; Saita, M.; Gimbo, R.; Urbinati, E. Leukocytes respiratory burst activity as indicator of innate immunity of pacu Piaractus mesopotamicus. Brazilian J. Biol. 2013, 73, 425–429. [Google Scholar] [CrossRef]
- Romano, N.; Kanmani, N.; Ebrahimi, M.; Chong, C.M.; Teh, J.C.; Hoseinifar, S.H.; Nurul Amin, S.M.; Kamarudin, M.S.; Kumar, V. Combination of dietary pre-gelatinized starch and isomaltooligosaccharides improved pellet characteristics, subsequent feeding efficiencies and physiological status in African catfish, Clarias gariepinus, juveniles. Aquaculture 2018, 484, 293–302. [Google Scholar] [CrossRef]
- Cheng, A.; Tu, C.; Chen, Y.; Nan, F.; Chen, J. The immunostimulatory effects of sodium alginate and iota-carrageenan on orange-spotted grouper Epinephelus coicoides and its resistance against Vibrio alginolyticus. Fish Shellfish Immunol. 2007, 22, 197–205. [Google Scholar] [CrossRef] [PubMed]
- ATCC. ATCC Animal Cell Culture Guide. Available online: https://www.atcc.org/resources/culture-guides/animal-cell-culture-guide (accessed on 27 May 2021).
- Matsuyama, H.; Yano, T.; Yamakawa, T.; Nakao, M. Opsonic effect of the third complement component (C3) of carp (Cyprinus carpio) on phagocytosis by neutrophils. Fish Shellfish Immunol. 1992, 2, 69–78. [Google Scholar] [CrossRef]
- Craig, S.; Helfrich, L.A.; Kuhn, D.; Schwarz, M.H. Understanding fish nutrition, feeds, and feeding. Virginia Coop. Ext. 2017, 420–256 (FST-269P). Available online: https://www.pubs.ext.vt.edu/FST/FST-269/FST-269.html (accessed on 27 March 2019).
- Natnan, M.E.; Low, C.-F.; Chong, C.-M.; Bunawan, H.; Baharum, S.N. Integration of Omics Tools for Understanding the Fish Immune Response Due to Microbial Challenge. Front. Mar. Sci. 2021, 8, 668771. [Google Scholar] [CrossRef]
- Al-Khalaifah, H. Modulatory Effect of Dietary Polyunsaturated Fatty Acids on Immunity, Represented by Phagocytic Activity. Front. Vet. Sci. 2020, 7, 569939. [Google Scholar] [CrossRef]
- Calder, P.C.; Waitzberg, D.L.; Klek, S.; Martindale, R.G. Lipids in Parenteral Nutrition: Biological Aspects. J. Parenter. Enter. Nutr. 2020, 44, S21–S27. [Google Scholar] [CrossRef]
- Tang, S.; Guo, S.; Wang, J.; Wang, Y.; Fu, S.; Shen, Z. Relationship Between Polyunsaturated Fatty Acids and Animal Production: A Review. Kafkas Univ. Vet. Fak. Derg. 2019, 25. [Google Scholar] [CrossRef]
- Li, X.; Cui, K.; Fang, W.; Chen, Q.; Xu, D.; Mai, K.; Zhang, Y.; Ai, Q. High level of dietary olive oil decreased growth, increased liver lipid deposition and induced inflammation by activating the p38 MAPK and JNK pathways in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2019, 94, 157–165. [Google Scholar] [CrossRef]
- Fadjar, M.; Andajani, S.; Zaelani, K. Squid (Loligo edulis) ink raw extract as an anti– vibriosis substance in grouper (Epinephelus fuscoguttatus) juvenile culture infected by Vibrio alginolyticus. AACL Bioflux 2016, 9, 422–428. [Google Scholar]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Satheeshkumar, P.; Ananthan, G.; Senthilkumar, D.; Khan, A.B.; Jeevanantham, K. Comparative investigation on haematological and biochemical studies on wild marine teleost fishes from Vellar estuary, southeast coast of India. Comp. Clin. Path. 2012, 21, 275–281. [Google Scholar] [CrossRef]
- Fazio, F. Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture 2019, 500, 237–242. [Google Scholar] [CrossRef]
- Ishimine, N.; Honda, T.; Yoshizawa, A.; Kawasaki, K.; Sugano, M.; Kobayashi, Y.; Matsumoto, T. Combination of White Blood Cell Count and Left Shift Level Real-Timely Reflects a Course of Bacterial Infection. J. Clin. Lab. Anal. 2013, 27, 407–411. [Google Scholar] [CrossRef]
- Taşbozan, O.; Gökçe, M.A. Fatty Acids in Fish. In Fatty Acids; InTech: Rang-Du-Fliers, France, 2017. [Google Scholar]
- Wu, F.-C.; Chen, H.-Y. Effects of dietary linolenic acid to linoleic acid ratio on growth, tissue fatty acid profile and immune response of the juvenile grouper Epinephelus malabaricus. Aquaculture 2012, 324–325, 111–117. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, C.; You, C.; Chen, B.; Wang, S.; Li, Y. Effects of different dietary ratios of docosahexaenoic to eicosapentaenoic acid (DHA/EPA) on the growth, non-specific immune indices, tissue fatty acid compositions and expression of genes related to LC-PUFA biosynthesis in juvenile golden pompano Trachin. Aquaculture 2019, 505, 488–495. [Google Scholar] [CrossRef]
- Selvam, C.; Powell, M.D.; Liland, N.S.; Rosenlund, G.; Sissener, N.H. Impact of dietary level and ratio of n-6 and n-3 fatty acids on disease progression and mRNA expression of immune and inflammatory markers in Atlantic salmon (Salmo salar) challenged with Paramoeba perurans. PeerJ 2021, 9, e12028. [Google Scholar] [CrossRef]
- Nayak, S.; Khozin-Goldberg, I.; Cohen, G.; Zilberg, D. Dietary supplementation with ω6 LC-PUFA-rich algae modulates zebrafish immune function and improves resistance to Streptococcal infection. Front. Immunol. 2018, 9, 1960. [Google Scholar] [CrossRef] [PubMed]
- Chee, W.-L.; Turchini, G.M.; Teoh, C.-Y.; Ng, W.-K. Dietary arachidonic acid and the impact on growth performance, health and tissues fatty acids in Malabar red snapper (Lutjanus malabaricus) fingerlings. Aquaculture 2020, 519, 734757. [Google Scholar] [CrossRef]
- Rivero-Ramírez, F.; Torrecillas, S.; Betancor, M.B.; Izquierdo, M.S.; Caballero, M.J.; Montero, D. Effects of dietary arachidonic acid in European sea bass (Dicentrarchus labrax) distal intestine lipid classes and gut health. Fish Physiol. Biochem. 2020, 46, 681–697. [Google Scholar] [CrossRef]
- Farris, N.W.; Kim, D.; Hamidoghli, A.; Won, S.; Lee, S.; Bae, J.; Bai, S.C. Dietary α-Tocopheryl acetate and arachidonic acid synergistically improves superoxide dismutase activity in female Japanese eel broodstock, Anguilla japonica. Aquaculture 2020, 522, 735100. [Google Scholar] [CrossRef]
- Wei, C.; Liu, C.; Wang, X.; Zhou, H.; Mai, K.; He, G. Dietary arachidonic acid supplementation improves the growth performance and alleviates plant protein-based diet-induced inflammation in juvenile turbot (Scophthalmus maximus L.). Aquac. Nutr. 2021, 27, 533–543. [Google Scholar] [CrossRef]
- Fernandes, S.; São-José, C. Enzymes and mechanisms employed by tailed bacteriophages to breach the bacterial cell barriers. Viruses 2018, 10, 396. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, A.; Gerdol, M.; Pallavicini, A.; Greco, S.; Schepens, I.; Hamelin, R.; Armand, F.; Dyson, P.J.; Sava, G. Lysozyme-Induced Transcriptional Regulation of TNF-α Pathway Genes in Cells of the Monocyte Lineage. Int. J. Mol. Sci. 2019, 20, 5502. [Google Scholar] [CrossRef] [PubMed]
- Biller, J.D.; Takahashi, L.S. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. An. Acad. Bras. Cienc. 2018, 90, 3403–3414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabu, D.L.; Sahu, N.P.; Pal, A.K.; Dasgupta, S.; Narendra, A. Immunomodulation and interferon gamma gene expression in sutchi cat fish, Pangasianodon hypophthalmus: Effect of dietary fucoidan rich seaweed extract (FRSE) on pre and post challenge period. Aquac. Res. 2016, 47, 199–218. [Google Scholar] [CrossRef]
- Barros, M.M.; Falcon, D.R.; de Oliveira Orsi, R.; Pezzato, L.E.; Fernandes, A.C.; Guimarães, I.G.; Fernandes, A.; Padovani, C.R.; Sartori, M.M.P. Non-specific immune parameters and physiological response of Nile tilapia fed β-glucan and vitamin C for different periods and submitted to stress and bacterial challenge. Fish Shellfish Immunol. 2014, 39, 188–195. [Google Scholar] [CrossRef]
- Yan, M.; Liu, J.; Li, Y.; Wang, X.; Jiang, H.; Fang, H.; Guo, Z.; Sun, Y. Different concentrations of Edwardsiella tarda ghost vaccine induces immune responses in vivo and protects Sparus macrocephalus against a homologous challenge. Fish Shellfish Immunol. 2018, 80, 467–472. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Dietary astaxanthin augments disease resistance of Asian seabass, Lates calcarifer (Bloch, 1790), against Vibrio alginolyticus infection. Fish Shellfish Immunol. 2021, 114, 90–101. [Google Scholar] [CrossRef]
- Sankian, Z.; Khosravi, S.; Kim, Y.-O.; Lee, S.-M. Total replacement of dietary fish oil with alternative lipid sources in a practical diet for mandarin fish, Siniperca scherzeri, juveniles. Fish. Aquat. Sci. 2019, 22, 8. [Google Scholar] [CrossRef]
- Jebali, A.; Noroozi Karimabad, M.; Ahmadi, Z.; Khorramdel, H.; Kaeidi, A.; Mirzaei, M.; Zare-Bidaki, M.; Ahmadinia, H.; Vakilian, A.; Darekordi, A.; et al. Attenuation of inflammatory response in the EAE model by PEGlated nanoliposome of pistachio oils. J. Neuroimmunol. 2020, 347, 577352. [Google Scholar] [CrossRef]
- Leyton, Y.; Riquelme, C. Oleic Acid and Diketopiperazines Produced by Marine Bacteria Reduce the Load of the Pathogen Vibrio parahaemolyticus in Argopecten purpuratus. J. Aquac. Res. Dev. 2013, 4. [Google Scholar] [CrossRef]
- Librán-Pérez, M.; Pereiro, P.; Figueras, A.; Novoa, B. Antiviral activity of palmitic acid via autophagic flux inhibition in zebrafish (Danio rerio). Fish Shellfish Immunol. 2019, 95, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.-H.; Hwang, Y.; Kim, K.-N.; Jun, H.-S. Palmitate induces nitric oxide production and inflammatory cytokine expression in zebrafish. Fish Shellfish Immunol. 2018, 79, 163–167. [Google Scholar] [CrossRef]
- Jubie, S.; Ramesh, P.N.; Dhanabal, P.; Kalirajan, R.; Muruganantham, N.; Shanish Antony, A. Synthesis, antidepressant and antimicrobial activities of some novel stearic acid analogues. Eur. J. Med. Chem. 2012, 54, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Senthilkumar, A.; Venkatesalu, V. Antibacterial and antifungal efficacy of fatty acid methyl esters from the leaves of Sesuvium portulacastrum L. Eur. Rev. Med. Pharmacol. 2011, 15, 775–780. [Google Scholar]
| Experimental Diets | |||||
|---|---|---|---|---|---|
| Ingredients | Control | Oleic Acid | Stearic Acid | Behenic Acid | Palmitic Acid |
| Fish meal | 11.5 | 11.5 | 11.5 | 11.5 | 11.5 |
| Soybean meal | 50.5 | 50.5 | 50.5 | 50.5 | 50.5 |
| Vegetable oil | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| Corn flour | 24.0 | 24.0 | 24.0 | 24.0 | 24.0 |
| Vitamin mixture a | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Mineral mixture b | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Oleic acid c | 0.0 | 2.0 | 0.0 | 0.0 | 0.0 |
| Stearic acid d | 0.0 | 0.0 | 2.0 | 0.0 | 0.0 |
| Behenic acid e | 0.0 | 0.0 | 0.0 | 2.0 | 0.0 |
| Palmitic acid f | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 |
| α-Cellulose g | 2.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Proximate composition h | |||||
| Total ash | 6.9 | 6.7 | 6.8 | 6.7 | 6.7 |
| Moisture | 6.7 | 6.8 | 6.8 | 8.7 | 7.6 |
| Protein | 31.3 | 31.4 | 31.0 | 30.8 | 31.3 |
| Lipid | 9.4 | 11.4 | 11.3 | 11.1 | 11.2 |
| Carbohydrate | 45.7 | 43.7 | 44.1 | 42.7 | 43.2 |
| Energy (kcl/100 g) | 393 | 403 | 402 | 394 | 399 |
| Control | Oleic Acid | Stearic Acid | Behenic Acid | Palmitic Acid | |
|---|---|---|---|---|---|
| Saturated fatty acids | |||||
| C4:0 | 0.19 | 0.65 | 0.00 | 0.16 | 0.22 |
| C8:0 | 0.58 | 0.81 | 0.61 | 0.66 | 0.61 |
| C10:0 | 0.51 | 0.76 | 0.50 | 0.51 | 0.61 |
| C11:0 | 0.54 | 1.00 | 0.59 | 0.64 | 0.64 |
| C12:0 | 3.41 | 15.11 | 4.26 | 4.59 | 3.78 |
| C13:0 | 0.00 | 1.06 | 0.20 | 0.20 | 0.31 |
| C14:0 | 19.45 | 30.39 | 19.43 | 26.60 | 19.60 |
| C15:0 | 5.55 | 7.61 | 5.50 | 6.50 | 5.67 |
| C16:0 | 947.56 | 877.36 | 1311.84 | 1302.02 | 1245.07 |
| C17:0 | 9.14 | 10.34 | 10.83 | 9.80 | 10.04 |
| C18:0 | 204.02 | 221.62 | 257.93 | 263.62 | 250.52 |
| C20:0 | 49.43 | 48.15 | 61.76 | 64.91 | 61.03 |
| C22:0 | 21.27 | 23.25 | 29.58 | 38.31 | 27.21 |
| C23:0 | 14.70 | 23.58 | 18.56 | 9.74 | 11.39 |
| C24:0 | 32.13 | 83.99 | 39.74 | 53.53 | 34.80 |
| Total | 1308.47 | 1345.68 | 1761.34 | 1781.81 | 1671.49 |
| Monounsaturated fatty acids | |||||
| C14:1 | 1.39 | 2.77 | 0.89 | 0.97 | 0.86 |
| C15:1 | 0.97 | 1.69 | 0.95 | 1.20 | 1.11 |
| C16:1 | 32.01 | 40.31 | 30.70 | 42.16 | 31.89 |
| C17:1 | 7.67 | 13.04 | 7.96 | 7.80 | 8.61 |
| C18:1n9 cis | 3144.73 | 4536.41 | 3576.96 | 3548.18 | 3601.36 |
| C20:1n9 | 46.22 | 57.29 | 51.91 | 55.10 | 52.15 |
| C22:1n9 | 2.32 | 6.63 | 8.52 | 9.81 | 4.24 |
| C24:1 | 13.83 | 135.28 | 27.23 | 48.09 | 18.57 |
| Total | 3249.15 | 4793.43 | 3705.12 | 3713.31 | 3718.78 |
| Polyunsaturated fatty acids | |||||
| C18:2n6 cis | 4633.96 | 5001.67 | 5601.85 | 5363.93 | 5570.41 |
| C18:3n6 | 10.60 | 7.23 | 13.56 | 9.28 | 12.40 |
| C18:3n3 | 116.47 | 134.44 | 133.04 | 130.87 | 142.08 |
| C20:2 | 3.34 | 4.96 | 3.43 | 3.65 | 3.43 |
| C20:3n3 | 8.41 | 16.99 | 8.27 | 9.22 | 7.82 |
| C20:4n6 | 4.77 | 5.67 | 4.58 | 4.58 | 6.69 |
| C20:5n3 | 15.11 | 23.45 | 15.61 | 21.17 | 17.45 |
| C22:2 | 10.82 | 7.62 | 13.82 | 12.75 | 7.79 |
| C22:6n3 | 38.89 | 58.86 | 39.36 | 49.43 | 41.67 |
| Total | 4842.38 | 5260.89 | 5833.54 | 5604.88 | 5809.73 |
| Control | Oleic Acid | Stearic Acid | Behenic Acid | Palmitic Acid | |
|---|---|---|---|---|---|
| Initial weight (g) | 11.37 ± 0.33 a | 12.03 ± 0.07 a | 11.30 ± 0.45 a | 11.82 ± 0.42 a | 11.73 ± 0.23 a |
| Final weight (g) | 14.32 ± 0.22 a | 16.13 ± 0.15 b | 14.64 ± 0.35 ab | 15.49 ± 0.12 bc | 14.96 ± 0.32 ac |
| Weight gain (%) | 26.00 ± 1.40 a | 34.10 ± 1.00 b | 29.60 ± 3.10 ab | 31.10 ± 1.00 ab | 27.50 ± 1.20 ab |
| SGR (%/day) | 0.55 ± 0.03 a | 0.70 ± 0.02 a | 0.62 ± 0.06 a | 0.64 ± 0.02 a | 0.58 ± 0.02 a |
| FCR | 3.1 a | 2.1 b | 2.8 ab | 2.3 ab | 2.9 a |
| Control | Oleic Acid | Stearic Acid | Behenic Acid | Palmitic Acid | |
|---|---|---|---|---|---|
| Total survival/initial fish used | 13/30 | 19/30 | 16/30 | 15/30 | 16/30 |
| Survival rate (%) | 43.30 ± 5.77 a | 63.30 ± 8.82 a | 53.30 ± 3.33 a | 50.00 ± 5.77 a | 53.30 ± 3.33 a |
| RBC count (×106)/mm3 | 1.12 ± 0.07 a | 1.32 ± 0.08 a | 1.18 ± 0.01 a | 1.22 ± 0.06 a | 1.21 ± 0.03 a |
| WBC count (×103)/mm3 | 3.10 ± 0.10 a | 3.98 ± 0.18 a | 3.45 ± 0.25 a | 3.50 ± 0.20 a | 3.18 ± 0.13 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natnan, M.E.; Low, C.F.; Chong, C.M.; Daud, N.I.N.A.A.; Om, A.D.; Baharum, S.N. Comparison of Different Dietary Fatty Acids Supplement on the Immune Response of Hybrid Grouper (Epinephelus fuscoguttatus × Epinephelus lanceolatus) Challenged with Vibrio vulnificus. Biology 2022, 11, 1288. https://doi.org/10.3390/biology11091288
Natnan ME, Low CF, Chong CM, Daud NINAA, Om AD, Baharum SN. Comparison of Different Dietary Fatty Acids Supplement on the Immune Response of Hybrid Grouper (Epinephelus fuscoguttatus × Epinephelus lanceolatus) Challenged with Vibrio vulnificus. Biology. 2022; 11(9):1288. https://doi.org/10.3390/biology11091288
Chicago/Turabian StyleNatnan, Maya Erna, Chen Fei Low, Chou Min Chong, Nur Iwani Nasuha Akiko Ahmad Daud, Ahmad Daud Om, and Syarul Nataqain Baharum. 2022. "Comparison of Different Dietary Fatty Acids Supplement on the Immune Response of Hybrid Grouper (Epinephelus fuscoguttatus × Epinephelus lanceolatus) Challenged with Vibrio vulnificus" Biology 11, no. 9: 1288. https://doi.org/10.3390/biology11091288
APA StyleNatnan, M. E., Low, C. F., Chong, C. M., Daud, N. I. N. A. A., Om, A. D., & Baharum, S. N. (2022). Comparison of Different Dietary Fatty Acids Supplement on the Immune Response of Hybrid Grouper (Epinephelus fuscoguttatus × Epinephelus lanceolatus) Challenged with Vibrio vulnificus. Biology, 11(9), 1288. https://doi.org/10.3390/biology11091288






