Maternal Age and Behavior during Pregnancy Affect the 2D:4D Digit Ratio in Polish Children Aged 6–13 Years
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Ethical Considerations
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manning, J.T.; Scutt, D.; Wilson, J.; Lewis-Jones, D.I. The ratio of 2nd to 4th digit length: A predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum. Reprod. 1998, 13, 3000–3004. [Google Scholar] [CrossRef] [PubMed]
- Lutchmaya, S.; Baron-Cohen, S.; Raggatt, P.; Knickmeyer, R.; Manning, J.T. 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Hum. Dev. 2004, 77, 23–28. [Google Scholar] [CrossRef]
- Malas, M.A.; Dogan, S.; Evcil, E.H.; Desdicioglu, K. Fetal development of the hand, digits and digit ratio (2D:4D). Early Hum. Dev. 2006, 82, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Richards, G. What is the evidence for a link between digit ratio (2D: 4D) and direct measures of prenatal sex hormones? Early Hum. Dev. 2017, 113, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.T.; Bundred, P.E.; Newton, D.J.; Flanagan, B.F. The second to fourth digit ratio and variation in the androgen receptor gene. Evol. Hum. Behav. 2003, 24, 399–405. [Google Scholar] [CrossRef]
- Huber, S.E.; Lenz, B.; Kornhuber, J.; Müller, C. Prenatal androgen-receptor activity has organizational morphological effects in mice. PLoS ONE 2017, 12, e0188752. [Google Scholar] [CrossRef]
- Hickey, M.; Doherty, D.A.; Hart, R.; Norman, R.J.; Mattes, E.; Atkinson, H.C.; Sloboda, D.M. Maternal and umbilical cord androgen concentrations do not predict digit ratio (2D:4D) in girls: A prospective cohort study. Psychoneuroendocrinology 2010, 35, 1235–1244. [Google Scholar] [CrossRef]
- Hollier, L.P.; Keelan, J.A.; Jamnadass, E.S.; Maybery, M.T.; Hickey, M.; Whitehouse, A.J. Adult digit ratio (2D:4D) is not related to umbilical cord androgen or estrogen concentrations, their ratios or net bioactivity. Early Hum. Dev. 2015, 91, 111–117. [Google Scholar] [CrossRef]
- Çetin, R.; Can, M.; Özcan, E. The relatıonshıp between testosterone and estrogen level of the cord blood and length of fıngers of newborns 2d:4d. Balıkesır Health Sci. J. 2016, 5, 75–82. [Google Scholar] [CrossRef]
- Richards, G.; Browne, W.V.; Constantinescu, M. Digit ratio (2D:4D) and amniotic testosterone and estradiol: An attempted replication of Lutchmaya et al. (2004). J. Dev. Origins Health Dis. 2020, 12, 859–864. [Google Scholar] [CrossRef]
- Richards, G.; Browne, W.V.; Aydin, E.; Constantinescu, M.; Nave, G.; Kim, M.S.; Watson, S.J. Digit ratio (2D:4D) and congenital adrenal hyperplasia (CAH): Systematic literature review and meta-analysis. Horm. Behav. 2020, 126, 104867. [Google Scholar] [CrossRef]
- Galis, F.; Ten Broek, C.M.; Van Dongen, S.; Wijnaendts, L.C. Sexual dimorphism in the prenatal digit ratio (2D:4D). Arch. Sex. Behav. 2010, 39, 57–62. [Google Scholar] [CrossRef]
- Butovskaya, M.; Burkova, V.; Apalkova, Y.; Dronova, D.; Rostovtseva, V.; Karelin, D.; Batsevich, V. Sex, population origin, age and average digit length as predictors of digit ratio in three large world populations. Sci. Rep. 2021, 11, 8157. [Google Scholar] [CrossRef]
- Manning, J.T.; Barley, L.; Walton, J.; Lewis-Jones, D.I.; Trivers, R.L.; Singh, D.; Thornhill, R.; Rohde, P.; Bereczkei, T.; Henzi, P.; et al. The 2nd:4th digit ratio, sexual dimorphism, population differences, and reproductive success. Evidence for sexually antagonistic genes? Evol. Hum. Behav. 2000, 21, 163–183. [Google Scholar] [CrossRef]
- Trivers, R.; Manning, J.; Jacobson, A. A longitudinal study of digit ratio (2D:4D) and other finger ratios in Jamaican children. Horm Behav. 2006, 49, 150–156. [Google Scholar] [CrossRef]
- Hampson, E.; Ellis, C.L.; Tenk, C.M. On the relation between 2D:4D and sex-dimorphic personality traits. Arch. Sex. Behav 2008, 37, 133–144. [Google Scholar] [CrossRef]
- Richards, G.; Bellin, W.; Davies, W. Familial digit ratio (2D:4D) associations in a general population sample from Wales. Early Hum. Dev. 2017, 112, 14–19. [Google Scholar] [CrossRef]
- Velez, M.P.; Arbuckle, T.E.; Monnier, P.; Fraser, W.D. Is maternal periconceptional smoking associated with 2D:4D digit ratio in their children? J. Dev. Orig. Health Dis. 2017, 8, 597–603. [Google Scholar] [CrossRef]
- Ventura, T.; Gomes, M.C.; Pita, A.; Neto, M.T.; Taylor, A. Digit ratio (2D:4D) in newborns: Influences of prenatal testosterone and maternal environment. Early Hum. Dev. 2013, 89, 107–112. [Google Scholar] [CrossRef]
- Kozieł, S.; Kociuba, M.; Chakraborty, R.; Sitek, A.; Ignasiak, Z. Further evidence of an association between low second-to-fourth digit ratio (2D:4D) and selection for the uniformed services: A study among police personnel in Wrocław, Poland. J. Biosoc. Sci. 2018, 50, 527–539. [Google Scholar] [CrossRef]
- Barut, C.; Tan, U.; Dogan, A. Association of height and weight with second to fourth digit ratio (2D:4D) and sex differences. Percept. Mot. Skills 2008, 106, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Pruszkowska-Przybylska, P.; Sitek, A.; Rosset, I.; Sobalska-Kwapis, M.; Słomka, M.; Strapagiel, D.; Żądzińska, E. Association of the 2D:4D digit ratio with body composition among the Polish children aged 6-13 years. Early Hum. Dev. 2018, 124, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.T.; Bundred, P.E.; Mather, F.M. Second to fourth digit ratio, sexual selection and skin colour. Evol. Hum. Behav. 2004, 25, 38–50. [Google Scholar] [CrossRef]
- Sitek, A.; Kozieł, S.; Kasielska-Trojan, A.; Antoszewski, B. Do skin and hair pigmentation in prepubertal and early pubertal stages correlate with 2D:4D? Am. J. Hum. Biol. 2018, 30, e1263. [Google Scholar] [CrossRef] [PubMed]
- Koziel, S.; Kociuba, M.; Chakraborty, R.; Ignasiak, Z. Physical fitness and digit ratio (2D:4D) in male students from Wrocław, Poland. Coll. Antropol. 2017, 41, 31–37. Available online: https://pubmed.ncbi.nlm.nih.gov/29139646/ (accessed on 1 March 2017).
- Matchock, R.L. Low digit ratio (2D:4D) is associated with delayed menarche. Am. J. Hum. Biol. 2008, 20, 487–489. [Google Scholar] [CrossRef]
- Manning, J.T.; Fink, B. Is low digit ratio linked with late menarche? Evidence from the BBC internet study. Am. J. Hum. Biol. 2011, 23, 527–533. [Google Scholar] [CrossRef]
- Hönekopp, J. Digit Ratio 2D:4D in Relation to Autism Spectrum Disorders, Empathizing, and Systemizing: A Quantitative Review. Autism Res. 2012, 5, 221–230. [Google Scholar] [CrossRef]
- Lippa, R.A. Are 2D:4D finger-length ratios related to sexual orientation? Yes A for men, no for women. J. Personal. Soc. Psychol. 2003, 85, 179–188. [Google Scholar] [CrossRef]
- Lippa, R.A. Finger lengths, 2D:4D ratios, and their relation to gender-related personality traits and the Big Five. Biol. Psychol. 2006, 71, 116–121. [Google Scholar] [CrossRef]
- Lenz, B.; Bouna-Pyrrou, P.; Mühle, C.; Kornhuber, J. Low digit ratio (2D:4D) and late pubertal onset indicate prenatal hyperandrogenziation in alcohol binge drinking. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 86, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.T.; Fink, B. Digit ratio (2D:4D), dominance, reproductive success, asymmetry, and sociosexuality in the BBC Internet Study. Am. J. Hum. Biol. 2008, 20, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Pruszkowska-Przybylska, P.; Kobus, M.; Iljin, A.; Wiktorska, J.A.; Żądzińska, E.; Sitek, A. Thyroid diseases and second to fourth digit ratio in Polish adults. Sci. Rep. 2021, 11, 18979. [Google Scholar] [CrossRef]
- Pruszkowska-Przybylska, P.; Sitek, A.; Rosset, I.; Sobalska-Kwapis, M.; Słomka, M.; Strapagiel, D.; Żądzińska, E.; Morling, N. Cortisol concentration affects fat and muscle mass among Polish children aged 6–13 years. BMC Pediatr. 2021, 21, 365. [Google Scholar] [CrossRef]
- Kobus, M.; Sitek, A.; Antoszewski, B.; Rożniecki, J.; Pełka, J.; Żądzińska, E. Prenatal oestrogen-testosterone balance as a risk factor of migraine in adults. J. Headache Pain 2021, 22, 119. [Google Scholar] [CrossRef] [PubMed]
- Kobus, M.; Sitek, A.; Rosset, I.; Pruszkowska–Przybylska, P.; Żądzińska, E. Association of prenatal sex steroid exposure estimated by the digit ratio (2D:4D) with birth weight, BMI and muscle strength in 6- to 13-yearold Polish children. PLoS ONE 2021, 16, e0258179. [Google Scholar] [CrossRef]
- Manning, J.; Wood, D. Fluctuating asymmetry and aggression in boys. Hum. Nat. 1998, 9, 53–65. [Google Scholar] [CrossRef]
- Hell, B.; Päßler, K. Are occupational interests hormonally influenced? The 2D:4D-interest nexus. Personal. Individ. Differ. 2011, 51, 376–380. [Google Scholar] [CrossRef]
- Entringer, S.; de Punder, K.; Buss, C.; Wadhwa, P.D. The fetal programming of telomere biology hypothesis: An update. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 5, 373. [Google Scholar] [CrossRef]
- Rizwan, S.; Manning, J.T.; Brabin, B.J. Maternal smoking during pregnancy and possible effects of in utero testosterone: Evidence from the 2D:4D finger length ratio. Early Hum. Dev. 2007, 83, 87–90. [Google Scholar] [CrossRef]
- Kandel, D.B.; Udry, J.R. Prenatal effects of maternal smoking on daughters’ smoking: Nicotine or testosterone exposure. Am. J. Public Health 1999, 89, 1377–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowers, M.F.; Beebe, J.L.; McConnell, D.; Randolph, J.; Jannausch, M. Testosterone concentrations in women aged 25—50 years: Associations with lifestyle, body composition and ovarian status. Am. J. Epidemiol. 2001, 153, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Kitawaki, J.; Inoue, S.; Tamura, T.; Yamamoto, T.; Honjo, H.; Higashiyama, T. Cigarette smoking during pregnancy lowers aromatase cytochrome P-450 in the human placenta. J. Steroid Biochem. Mol. Biol. 1998, 45, 485–491. [Google Scholar] [CrossRef]
- Manning, J.T.; Fink, B. Digit ratio, nicotine and alcohol intake and national rates of smoking and alcohol consumption. Pers. Indiv. Dif. 2011, 50, 344–348. [Google Scholar] [CrossRef]
- Anand-Ivell, R.; Cohen, A.; Nørgaard-Pedersen, B.; Jönsson, B.A.; Bonde, J.P.; Hougaard, D.M.; Ivell, R. Amniotic Fluid INSL3 Measured During the Critical Time Window in Human Pregnancy Relates to Cryptorchidism, Hypospadias, and Phthalate Load: A Large Case–Control Study. Front. Physiol. 2018, 9, 406. [Google Scholar] [CrossRef]
- Brañas-Garza, P.; Galizzi, M.; Nieboer, J. Experimental and self-reported measures of risk taking and digit ratio (2D: 4D): Evidence from a large, systematic study. Int. Econ. Rev. 2017, 59, 1131–1157. [Google Scholar] [CrossRef]
- Manning, J.T. Digit Ratio: A Pointer to Fertility, Behaviour and Health; Rutgers University Press: New Brunswick, NJ, USA, 2002. [Google Scholar]
- Wilson, G.D. Finger-length as an index of assertiveness in women. Personal. Individ. Differ. 1983, 4, 111–112. [Google Scholar] [CrossRef]
- Kociuba, M.; Kozieł, S.; Chakraborty, R.; Ignasiak, Z. Sports preference and digit ratio (2D:4D) among female students in Wrocław, Poland. J. Biosoc. Sci. 2017, 49, 623–633. [Google Scholar] [CrossRef]
- Klimek, M.; Galbarczyk, A.; Nenko, I.; Jasienska, G. Women with more feminine digit ratio (2D:4D) have higher reproductive success. Am. J. Phys. Anthropol. 2016, 160, 549–553. [Google Scholar] [CrossRef]
- Höenekopp, J.; Watson, S. Meta-analysis of digit ratio 2D:4D shows greater sex difference in the right hand. Am. J. Hum. Biol. 2010, 22, 619–630. [Google Scholar] [CrossRef]
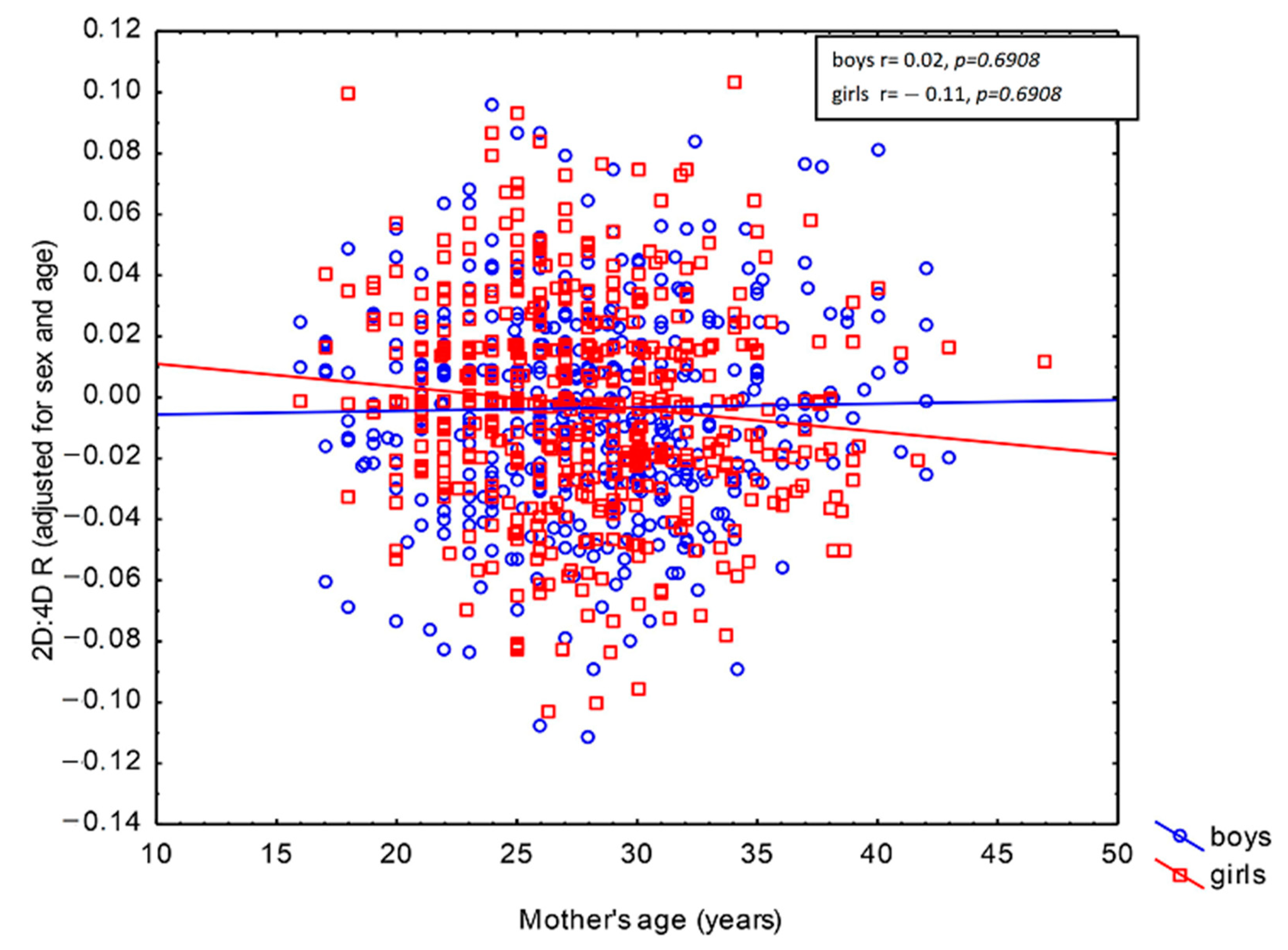
| Digit Ratio | Boys n = 537 | Girls n = 646 | p | ||
|---|---|---|---|---|---|
| SD | SD | ||||
| 2D:4D R | 0.9740 | 0.0336 | 0.9842 | 0.0323 | <0.0001 |
| 2D:4D L | 0.9725 | 0.0309 | 0.9802 | 0.0306 | <0.0001 |
| Correlation | Boys n = 537 | Girls n = 646 | ||
|---|---|---|---|---|
| r | p | r | p | |
| 2D:4D R × child age | 0.08 | 0.0590 | 0.01 | 0.7759 |
| 2D:4D L × child age | 0.08 | 0.0625 | 0.09 | 0.0185 |
| Maternal Prenatal Factors | Total | Boys | Girls | p | |
|---|---|---|---|---|---|
| Maternal age at childbirth | 27.97 | 28.05 | 27.89 | 0.6311 | |
| SD | 4.97 | 5.16 | 4.81 | ||
| n | 990 | 462 | 528 | ||
| Use of medications for high-risk pregnancy | Yes | 407 | 191 | 216 | 0.9780 |
| No | 808 | 380 | 428 | ||
| Total | 1215 | 571 | 644 | ||
| Maternal illness during pregnancy | Yes | 188 | 83 | 105 | 0.4310 |
| No | 993 | 474 | 522 | ||
| Total | 1181 | 554 | 627 | ||
| Maternal active smoking during pregnancy | Yes | 138 | 65 | 73 | 0.9469 |
| No | 1081 | 508 | 573 | ||
| Total | 1219 | 573 | 646 | ||
| Maternal passive smoking during pregnancy | Yes | 422 | 196 | 226 | 0.8549 |
| No | 780 | 368 | 412 | ||
| Total | 1202 | 564 | 638 | ||
| Maternal work during pregnancy | Yes | 575 | 272 | 303 | 0.8295 |
| No | 632 | 294 | 338 | ||
| Total | 1207 | 566 | 641 | ||
| Maternal psychological trauma during pregnancy | Yes | 103 | 42 | 61 | 0.2315 |
| No | 1102 | 523 | 579 | ||
| Total | 1205 | 565 | 640 |
| Prenatal Factors (Independent Variables) | Direct Effect of Prenatal Factor | Prenatal Factor × Child Sex Interaction | |||||
|---|---|---|---|---|---|---|---|
| β | t | p | ω2 (%) | F | p | ω2 (%) | |
| Maternal age at childbirth | −0.05 | −1.47 | 0.1410 | 0.12 | 4.14 | 0.0421 | 0.32 |
| Use of medications for high-risk pregnancy—Yes vs. No | −0.01 | −0.37 | 0.7149 | 0.00 | 0.05 | 0.8147 | 0.00 |
| Maternal illness during pregnancy—Yes vs. No | 0.01 | 0.35 | 0.7250 | 0.00 | 0.03 | 0.8643 | 0.00 |
| Maternal active smoking during pregnancy—Yes vs. No | 0.10 | 3.36 | 0.0008 | 0.84 | 0.06 | 0.8143 | 0.00 |
| Maternal passive smoking during pregnancy—Yes vs. No | 0.04 | 1.40 | 0.1628 | 0.08 | 0,09 | 0.7607 | 0.00 |
| Maternal work during pregnancy—Yes vs. No | −0.07 | −2.27 | 0.0233 | 0.34 | 0.09 | 0.7696 | 0.00 |
| Maternal psychological trauma during pregnancy—Yes vs. No | 0.03 | 1.07 | 0.2860 | 0.01 | 0.41 | 0.5220 | 0.00 |
| Prenatal Factors (Independent Variables) | Direct Effect of Prenatal Factor | Prenatal Factor × Child Sex Interaction | |||||
|---|---|---|---|---|---|---|---|
| β | t | p | ω2 (%) | F | p | ω2 (%) | |
| Maternal age at childbirth | −0.04 | −1.30 | 0.1951 | 0.07 | 0.75 | 0.3855 | 0.00 |
| Use of medications for high-risk pregnancy—Yes vs. No | −0.02 | −0.81 | 0.4208 | 0.00 | 0.31 | 0.5749 | 0.00 |
| Maternal illness during pregnancy—Yes vs. No | 0.01 | 0.37 | 0.7111 | 0.00 | 0.14 | 0.7105 | 0.00 |
| Maternal active smoking during pregnancy—Yes vs. No | 0.04 | 1.22 | 0.2216 | 0.04 | 0.52 | 0.4702 | 0.00 |
| Maternal passive smoking during pregnancy—Yes vs. No | −0.01 | −0.20 | 0.8437 | 0.00 | 0.03 | 0.8726 | 0.00 |
| Maternal work during pregnancy—Yes vs. No | −0.02 | −0.67 | 0.5053 | 0.00 | 0.17 | 0.6838 | 0.00 |
| Maternal psychological trauma during pregnancy—Yes vs. No | −0.01 | −0.42 | 0.6743 | 0.00 | 1.66 | 0.1982 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitek, A.; Rosset, I.; Kobus, M.; Pruszkowska-Przybylska, P.; Żądzińska, E. Maternal Age and Behavior during Pregnancy Affect the 2D:4D Digit Ratio in Polish Children Aged 6–13 Years. Biology 2022, 11, 1286. https://doi.org/10.3390/biology11091286
Sitek A, Rosset I, Kobus M, Pruszkowska-Przybylska P, Żądzińska E. Maternal Age and Behavior during Pregnancy Affect the 2D:4D Digit Ratio in Polish Children Aged 6–13 Years. Biology. 2022; 11(9):1286. https://doi.org/10.3390/biology11091286
Chicago/Turabian StyleSitek, Aneta, Iwona Rosset, Magdalena Kobus, Paulina Pruszkowska-Przybylska, and Elżbieta Żądzińska. 2022. "Maternal Age and Behavior during Pregnancy Affect the 2D:4D Digit Ratio in Polish Children Aged 6–13 Years" Biology 11, no. 9: 1286. https://doi.org/10.3390/biology11091286
APA StyleSitek, A., Rosset, I., Kobus, M., Pruszkowska-Przybylska, P., & Żądzińska, E. (2022). Maternal Age and Behavior during Pregnancy Affect the 2D:4D Digit Ratio in Polish Children Aged 6–13 Years. Biology, 11(9), 1286. https://doi.org/10.3390/biology11091286






