Effect of an Oral Bivalent Vaccine on Immune Response and Immune Gene Profiling in Vaccinated Red Tilapia (Oreochromis spp.) during Infections with Streptococcus iniae and Aeromonas hydrophila
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Growth Condition
2.2. Ethical Approval
2.3. Fish Maintenance
2.4. Feed-Based Vaccine Preparation
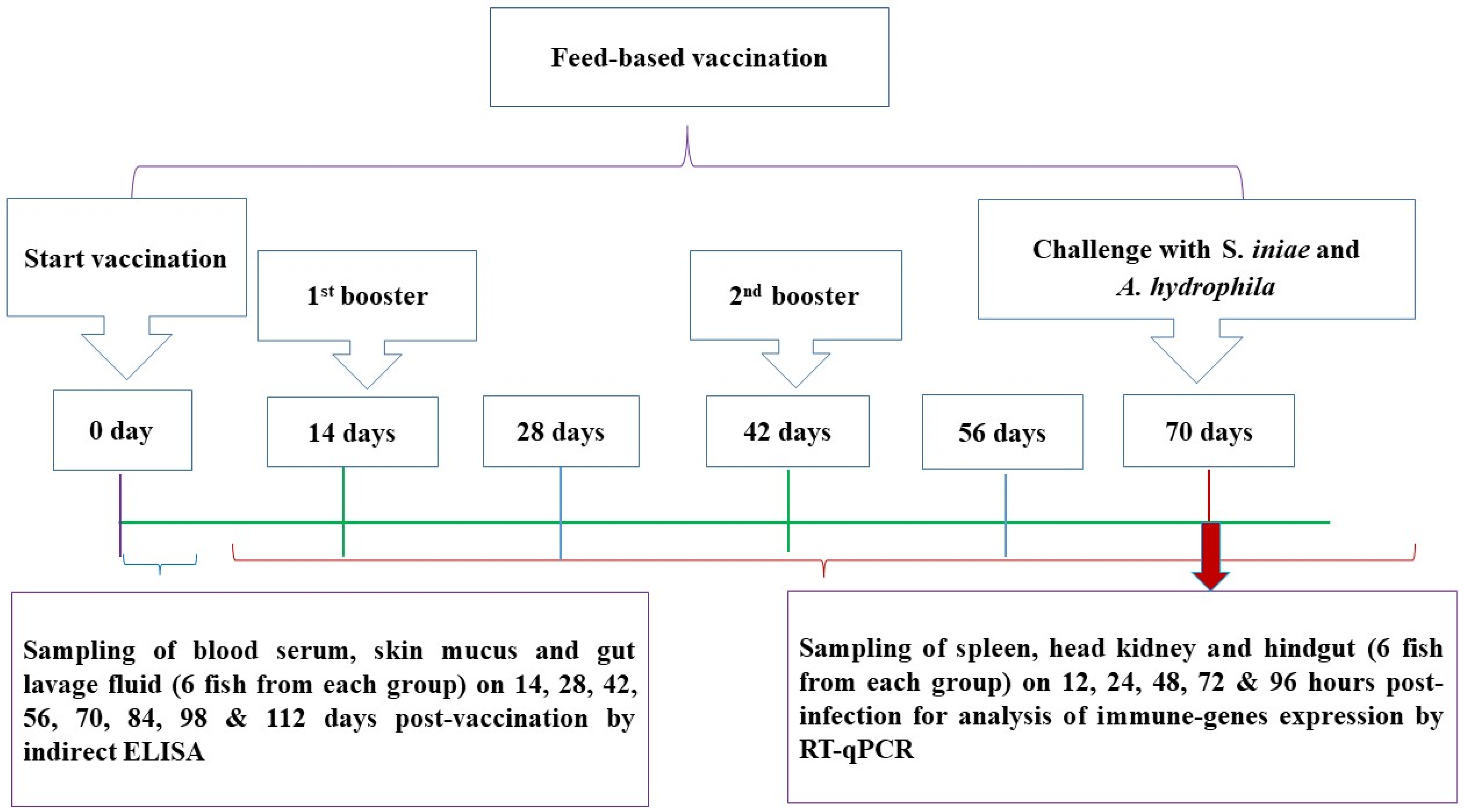
2.5. Feed-Based Vaccination
2.6. Challenge Tests
2.7. Sampling
2.8. Specific Antibody Detection by Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. RNA Extraction, cDNA Synthesis, and RT-qPCR
2.10. Statistical Analysis
3. Results
3.1. IgM Titers in Gut Lavage Fluid
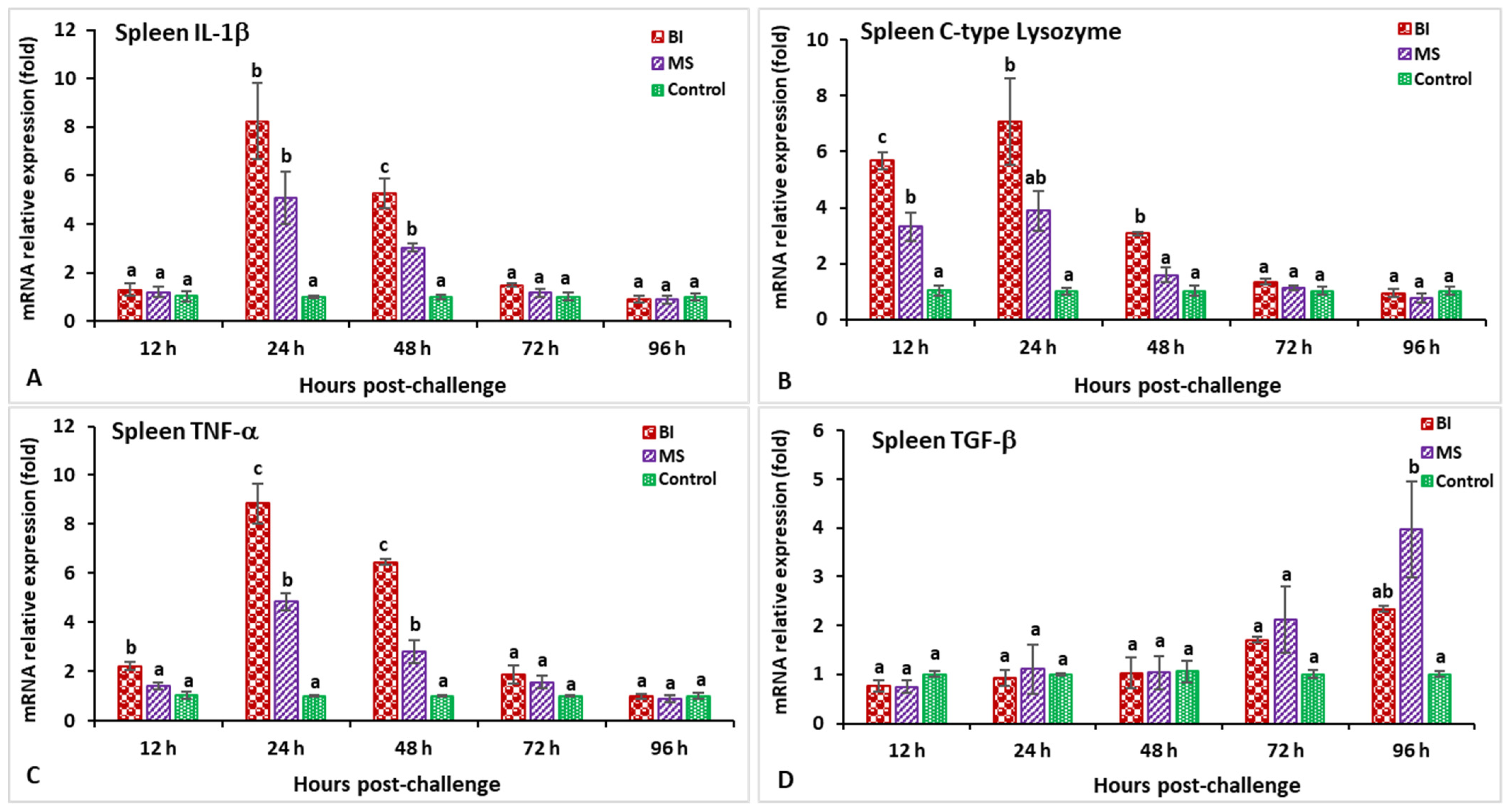
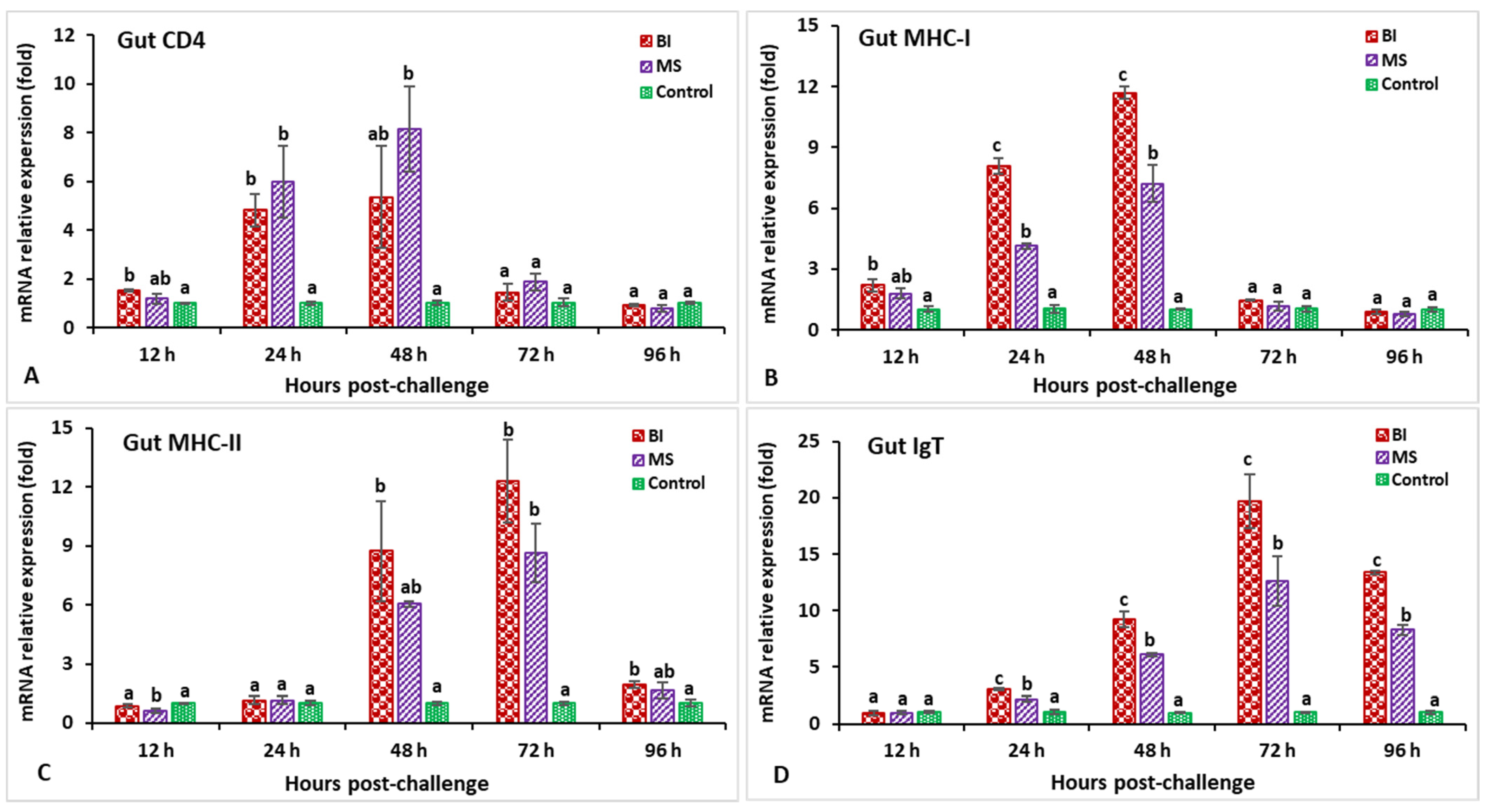
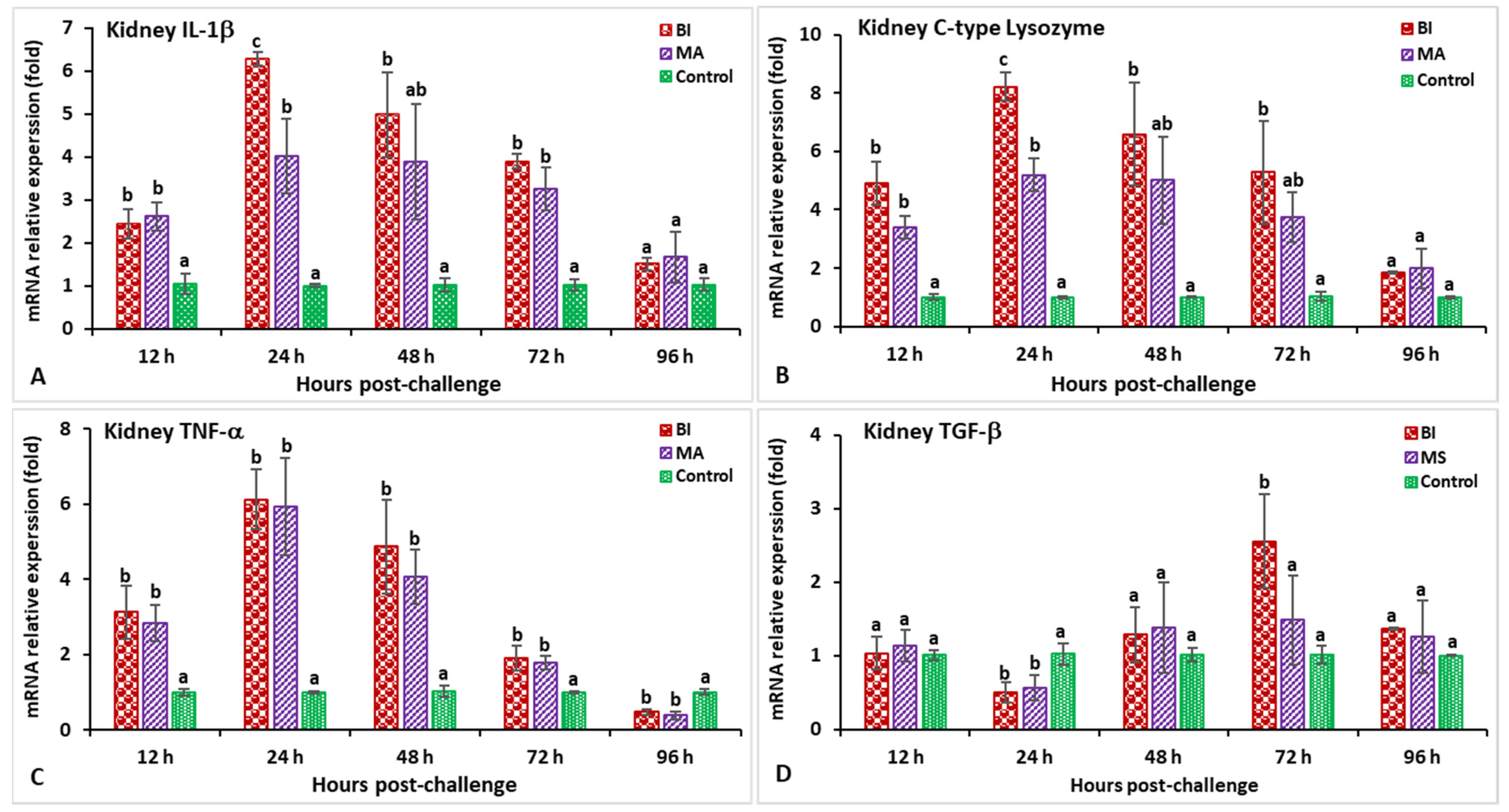
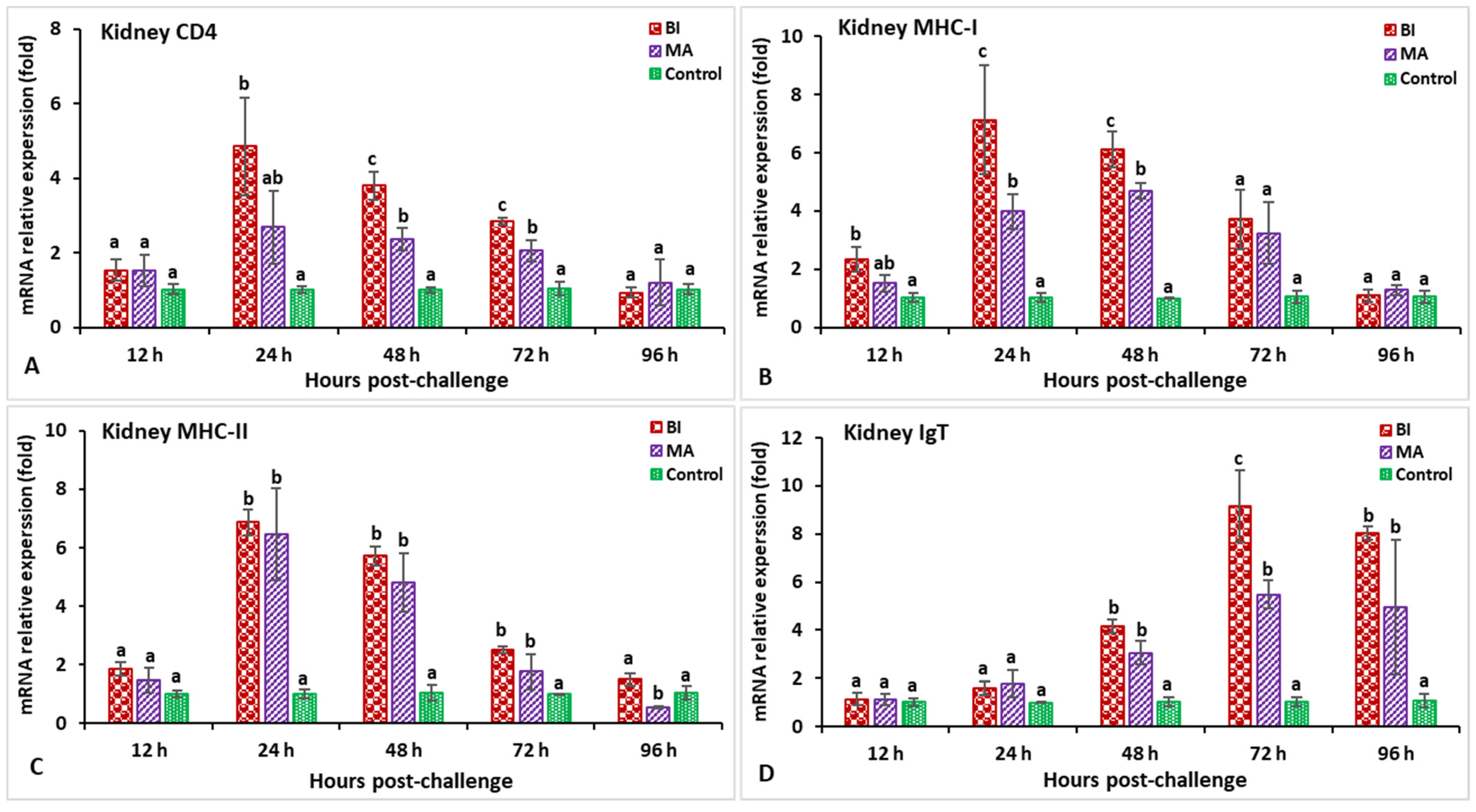
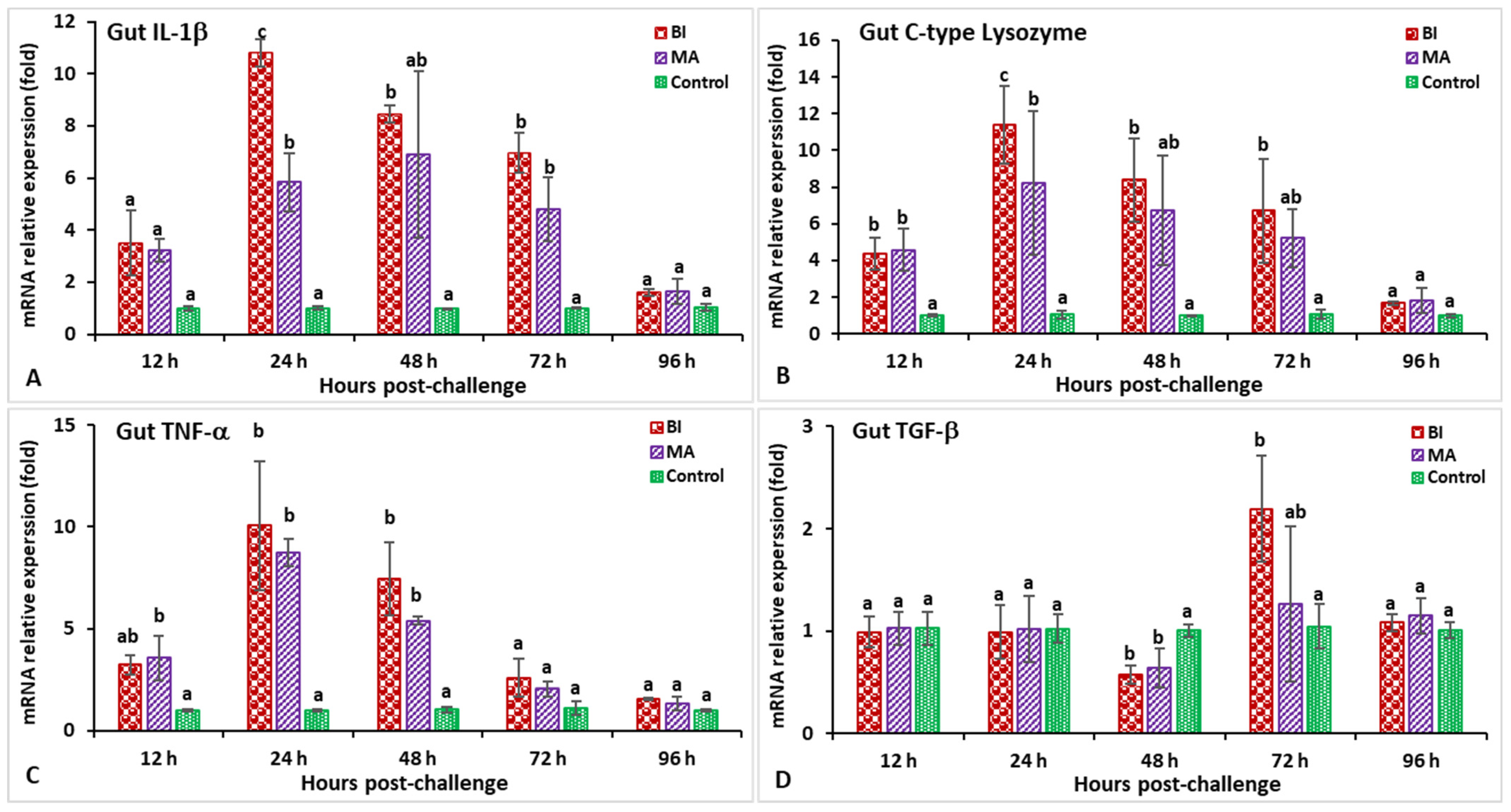
3.2. Expression Profiles of Immune-Related Genes Following Challenge with Streptococcus iniae
3.3. Expression Profiles of Immune-Related Genes Following Challenge with Aeromonas hydrophila
3.4. Protection of the Feed-Based Vaccinated Red Tilapia
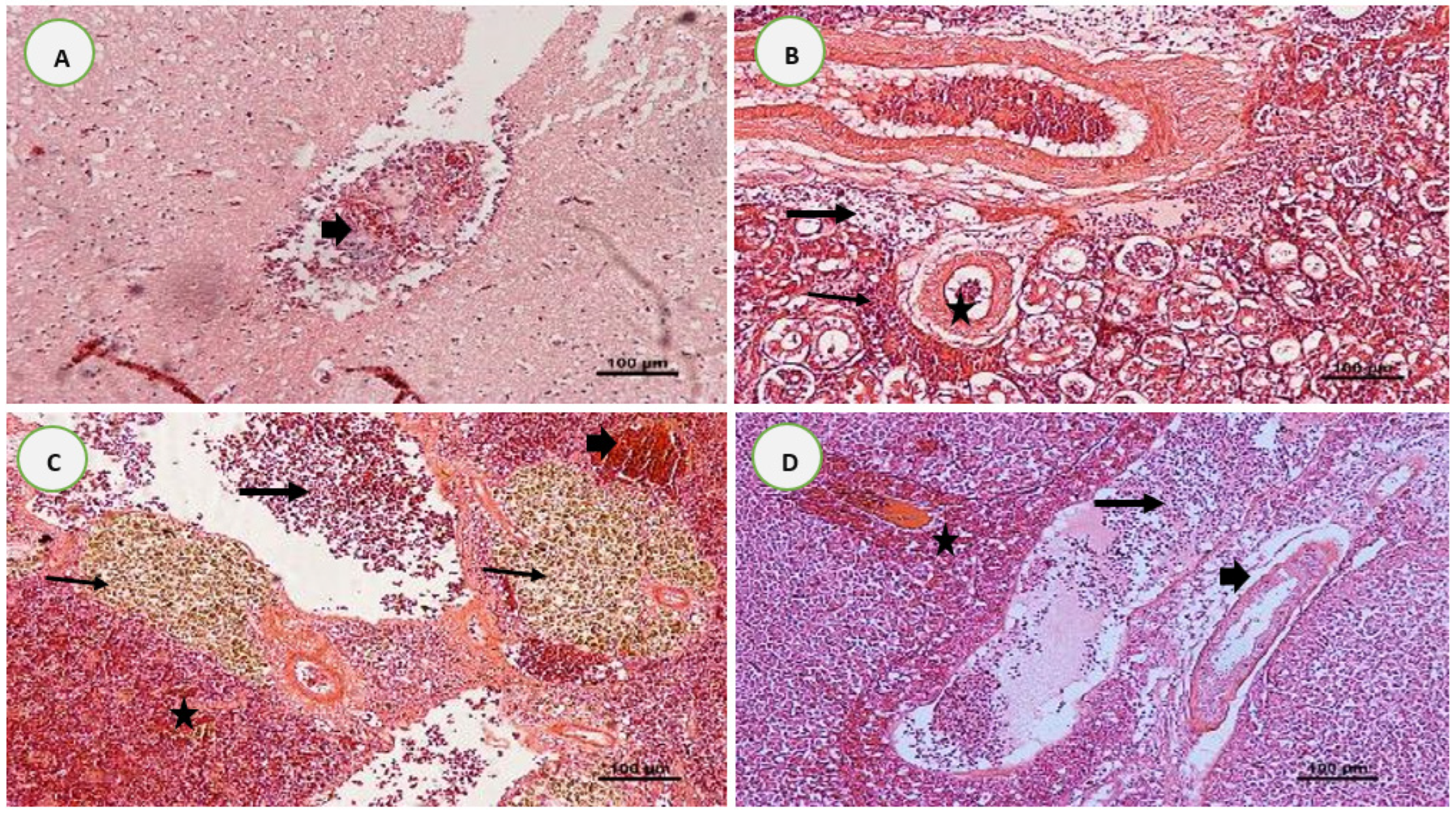
3.5. Histopathological Analysis after Experimental Infections
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Yu, A.; Lan, J.; Zhang, H.; Hu, M.; Cheng, J.; Zhao, L.; Lin, L.; Wei, S. GapA, a potential vaccine candidate antigen against Streptococcus agalactiae in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2017, 63, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Monir, M.S.; Yusoff, S.M.; Mohamad, A.; Ina-Salwany, M.Y. Vaccination of Tilapia against Motile Aeromonas Septicemia: A Review. J. Aquat. Anim. Health 2020, 32, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Rahmatullah, M.; Ariff, M.; Kahieshesfandiari, M.; Daud, H.M.; Zamri-Saad, M.; Sabri, M.Y.; Amal, M.N.A.; Ina-Salwany, M.Y. Isolation and pathogenicity of Streptococcus iniae in cultured red hybrid tilapia in Malaysia. J. Aquat. Anim. Health 2017, 29, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Carnelli, A.; Mauri, F.; Demarta, A. Characterization of genetic determinants involved in antibiotic resistance in Aeromonas spp. and fecal coliforms isolated from different aquatic environments. Res. Microbiol. 2017, 168, 461–471. [Google Scholar] [CrossRef]
- Pasaribu, W.; Sukenda, S.; Nuryati, S. The efficacy of Nile tilapia (Oreochromis niloticus) broodstock and larval immunization against Streptococcus agalactiae and Aeromonas hydrophila. Fishes 2018, 3, 16. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Wang, X.; Feng, R.; Luo, Q.; Huang, J. Generation of a novel Streptococcus agalactiae ghost vaccine and examination of its immunogenicity against virulent challenge in tilapia. Fish Shellfish Immunol. 2018, 81, 49–56. [Google Scholar] [CrossRef]
- Kayansamruaj, P.; Dong, H.T.; Pirarat, N.; Nilubol, D.; Rodkhum, C. Efficacy of α-enolase-based DNA vaccine against pathogenic Streptococcus iniae in Nile tilapia (Oreochromis niloticus). Aquaculture 2017, 468, 102–106. [Google Scholar] [CrossRef]
- Diab, A.M.; Khalil, R.H.; Khallaf, M. Autogenous bacterins cross-protection as a trial for Streptococcosis control in Oreochromis niloticus. Aquac. Int. 2019, 27, 1787–1800. [Google Scholar] [CrossRef]
- Wang, Q.; Fu, T.; Li, X.; Luo, Q.; Huang, J.; Sun, Y.; Wang, X. Cross-immunity in Nile tilapia vaccinated with Streptococcus agalactiae and Streptococcus iniae vaccines. Fish Shellfish Immunol. 2020, 97, 382–389. [Google Scholar] [CrossRef]
- Aly, S.M.; Albutti, A.S.; Rahmani, A.H.; Atti, N.M.A. The response of new-season Nile tilapia to Aeromonas hydrophila vaccine. Int. J. Clin. Exp. Med. 2015, 8, 4508–4514. [Google Scholar]
- Sukenda, S.; Carman, O.; Rahman, R.; Hidayatullah, D.; Yumaidawati, N.S. Vaccination in Nile tilapia broodstock with whole cell vaccine and disease resistance in its fry against Aeromonas hydrophila. J. Akuakultur Indones. 2017, 16, 279. [Google Scholar] [CrossRef][Green Version]
- Abu-Elala, N.M.; Samir, A.; Wasfy, M.; Elsayed, M. Efficacy of Injectable and Immersion Polyvalent Vaccine against Streptococcal Infections in Broodstock and Offspring of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 88, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhou, Y.; Fu, X.; Lin, Q.; Liu, L.; Liang, H.; Niu, Y.; Li, N. Transcriptomic profiles reveal that inactivated iridovirus and rhabdovirus bivalent vaccine elicits robust adaptive immune responses against lethal challenge in marbled sleepy goby. Fish Shellfish Immunol. 2020, 98, 429–437. [Google Scholar] [CrossRef]
- Firdaus-Nawi, M.; Yusoff, S.M.; Yusof, H.; Abdullah, S.Z.; Zamri-Saad, M. Efficacy of feed-based adjuvant vaccine against Streptococcus agalactiae in Oreochromis spp. in Malaysia. Aquac. Res. 2014, 45, 87–96. [Google Scholar] [CrossRef]
- Ismail, M.; Syafiq, M.; Siti-Zahrah, A.; Fahmi, S.; Shahidan, H.; Hanan, Y.; Amal, M.; Saad, M.Z. The effect of feed-based vaccination on tilapia farm endemic for streptococcosis. Fish Shellfish Immunol. 2017, 60, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, L.; Li, S.; Li, G.; Mo, Z. The efficacy and side-effects of oil-based adjuvants emulsified Vibrio anguillarum bivalent inactivated vaccine in turbot (Scophthalmus maximus) under production mode. Aquaculture 2020, 524, 735259. [Google Scholar] [CrossRef]
- Xu, W.; Jiao, C.; Bao, P.; Liu, Q.; Wang, P.; Zhang, R.; Liu, X.; Zhang, Y. Efficacy of MontanideTM ISA 763 A VG as aquatic adjuvant administrated with an inactivated Vibrio harveyi vaccine in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2019, 84, 56–61. [Google Scholar] [CrossRef]
- Wang, E.; Wang, J.; Long, B.; Wang, K.; He, Y.; Yang, Q.; Chen, D.; Geng, Y.; Huang, X.; Ouyang, P.; et al. Molecular cloning, expression and the adjuvant effects of interleukin-8 of channel catfish (Ictalurus Punctatus) against Streptococcus iniae. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Monir, S.; Yusoff, S.B.M.; Zulperi, Z.B.M.; Abu Hassim, H.B.; Mohamad, A.; Ngoo, M.S.B.M.H.; Ina-Salwany, Y. Haemato-immunological responses and effectiveness of feed-based bivalent vaccine against Streptococcus iniae and Aeromonas hydrophila infections in hybrid red tilapia (Oreochromis mossambicus × O. niloticus). BMC Vet. Res. 2020, 16, 1–14. [Google Scholar] [CrossRef]
- Sa’aidatun Asyikin, A.; Farina, M.K.; Zamri-Saad, M.; Siti-Zahrah, A.; Mohd Syafiq, M.R.; Hanan, M.Y.; Shahidan, H.; Fahmi, S.; Ismail, M.S.; Suphia-Amiera, S. Effect of incorporating different concentrations of palm oil as adjuvant in fish vaccine. Int. J. Biosci. 2018, 12, 35–41. [Google Scholar] [CrossRef]
- Dash, P.; Yadav, S.K.; Garg, L.C.; Dixit, A.; Sahoo, P.K. Post-challenge immune gene expression profiling in rohu, Labeo rohita vaccinated with modified adjuvant-based Aeromonas hydrophila outer membrane protein R formulation. Vet. Arh. 2017, 87, 607–622. [Google Scholar] [CrossRef]
- Hoare, R.; Ngo, T.P.H.; Bartie, K.L.; Adams, A. Efficacy of a polyvalent immersion vaccine against Flavobacterium psychrophilum and evaluation of immune response to vaccination in rainbow trout fry (Onchorynchus mykiss L.). Vet. Res. 2017, 48, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-H.; Moreira, G.S.; Shoemaker, C.A.; Zhang, D.; Beck, B. Expression of immune genes in systemic and mucosal immune tissues of channel catfish vaccinated with live theronts of Ichthyophthirius multifiliis. Fish. Shellfish Immunol. 2017, 66, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Monir, S.; Yusoff, S.M.; Zulperi, Z.M.; Abu Hassim, H.; Zamri-Saad, M.; Amal, M.N.A.; Salleh, A.; Mohamad, A.; Yie, L.J.; Ina-Salwany, Y. Immuno-protective efficiency of feed-based whole-cell inactivated bivalent vaccine against Streptococcus and Aeromonas infections in red hybrid tilapia (Oreochromis niloticus × Oreochromis mossambicus). Fish. Shellfish Immunol. 2021, 113, 162–175. [Google Scholar] [CrossRef]
- Kahieshesfandiari, M.; Sabri, M.Y.; Hassan, M.D.; Noraini, O. Streptococcosis in Oreochromis sp.: Is feed-based biofilm vaccine of Streptococcus agalactiae effective? Aquac. Int. 2019, 27, 817–832. [Google Scholar] [CrossRef]
- Qiang, J.; He, J.; Yang, H.; Xu, P.; Habte-Tsion, H.M.; Ma, X.Y.; Zhu, Z.X. The changes in cortisol and expression of immune genes of GIFT tilapia Oreochromis niloticus (L.) at different rearing densities under Streptococcus iniae infection. Aquac. Int 2016, 24, 1365–1378. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, Y.; Li, Q.; Ke, X.; Liu, Z.; Lu, M.; Shi, C. An effective live attenuated vaccine against Streptococcus agalactiae infection in farmed Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 98, 853–859. [Google Scholar] [CrossRef]
- Yao, Y.-Y.; Chen, D.-D.; Cui, Z.-W.; Zhang, X.-Y.; Zhou, Y.-Y.; Guo, X.; Li, A.-H.; Zhang, Y.-A. Oral vaccination of tilapia against Streptococcus agalactiae using Bacillus subtilis spores expressing Sip. Fish Shellfish Immunol. 2019, 86, 999–1008. [Google Scholar] [CrossRef]
- Velázquez, J.; Acosta, J.; Lugo, J.M.; Reyes, E.; Herrera, F.; González, O.; Morales, A.; Carpio, Y.; Estrada, M.P. Discovery of immunoglobulin T in Nile tilapia (Oreochromis niloticus): A potential molecular marker to understand mucosal immunity in this species. Dev. Comp. Immunol. 2018, 88, 124–136. [Google Scholar] [CrossRef]
- Thu Lan, N.G.; Salin, K.R.; Longyant, S.; Senapin, S.; Dong, H.T. Systemic and mucosal antibody response of freshwater cultured Asian seabass (Lates calcarifer) to monovalent and bivalent vaccines against Streptococcus agalactiae and Streptococcus iniae. Fish Shellfish Immunol. 2020, 108, 7–13. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, C.; Zhao, Y.; Kong, X.; Pei, C.; Li, L.; Nie, G.; Li, X. Immune effects of the vaccine of live attenuated Aeromonas hydrophila screened by rifampicin on common carp (Cyprinus carpio L). Vaccine 2016, 34, 3087–3092. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Zhou, X.; Tang, X.; Sheng, X.; Zhan, W. FlaC supplemented with VAA, OmpK or OmpR as bivalent subunit vaccine candidates induce immune responses against Vibrio anguillarum in flounder (Paralichthys olivaceus). Vaccine 2018, 36, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.; Sahoo, P.K.; Gupta, P.K.; Garg, L.C.; Dixit, A. Immune responses and protective efficacy of recombinant outer membrane protein R (rOmpR)-based vaccine of Aeromonas hydrophila with a modified adjuvant formulation in rohu (Labeo rohita). Fish Shellfish Immunol. 2014, 39, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, T.; Alishahi, M.; Tabandeh, M.R.; Ghorbanpoor, M.; Gharibi, D. Changes in Immunity, Expression of some Immune-Related Genes of Shabot Fish, Tor grypus, Following Experimental Infection with Aeromonas hydrophila: Effects of Autochthonous Probiotics. Probiotics Antimicrob. Proteins 2017, 10, 616–628. [Google Scholar] [CrossRef]
- Guo, Q.; Zheng, H.; Liu, X.; Chi, S.; Xu, Z.; Wang, Q. Nutrient sensing signaling functions as the sensor and regulator of immunometabolic changes in grass carp during Flavobacterium columnare infection. Fish Shellfish Immunol. 2019, 93, 278–287. [Google Scholar] [CrossRef]
- Jinendiran, S.; Nathan, A.A.; Ramesh, D.; Vaseeharan, B.; Sivakumar, N. Modulation of innate immunity, expression of cytokine genes and disease resistance against Aeromonas hydrophila infection in goldfish (Carassius auratus) by dietary supplementation with Exiguobacterium acetylicum S01. Fish Shellfish Immunol. 2019, 84, 458–469. [Google Scholar] [CrossRef]
- Ling, X.-D.; Dong, W.-T.; Zhang, Y.; Qian, X.; Zhang, W.-D.; He, W.-H.; Zhao, X.-X.; Liu, J.-X. Comparative transcriptomics and histopathological analysis of crucian carp infection by atypical Aeromonas salmonicida. Fish Shellfish Immunol. 2019, 94, 294–307. [Google Scholar] [CrossRef]
- Pridgeon, J.W.; Klesius, P.H.; Dominowski, P.J.; Yancey, R.J.; Kievit, M.S. Chicken-type lysozyme in channel catfish: Expression analysis, lysozyme activity, and efficacy as immunostimulant against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2013, 35, 680–688. [Google Scholar] [CrossRef]
- He, L.; Wu, L.; Tang, Y.; Lin, P.; Zhai, S.; Xiao, Y.; Guo, S. Immunization of a novel outer membrane protein from Aeromonas hydrophila simultaneously resisting A. hydrophila and Edwardsiella anguillarum infection in European eels (Angullia angullia). Fish Shellfish Immunol. 2020, 97, 300–312. [Google Scholar] [CrossRef]
- Jaafar, R.M.; Chettri, J.K.; Dalsgaard, I.; Al-Jubury, A.; Kania, P.W.; Skov, J.; Buchmann, K. Effects of adjuvant Montanide TM ISA 763 A VG in rainbow trout injection vaccinated against Yersinia ruckeri. Fish Shellfish Immunol. 2015, 47, 797–806. [Google Scholar] [CrossRef]
- Ravindra; Pradhan, P.; Paria, A.; Pande, V.; Verma, D.K.; Arya, P.; Rathore, G.; Sood, N. Expression of immune genes in Indian major carp, Catla catla challenged with Flavobacterium columnare. Fish Shellfish Immunol. 2019, 94, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Kole, S.; Qadiri, S.S.N.; Shin, S.M.; Kim, W.S.; Lee, J.; Jung, S.J. Nanoencapsulation of inactivated-viral vaccine using chitosan nanoparticles: Evaluation of its protective efficacy and immune modulatory effects in olive flounder (Paralichthys olivaceus) against viral haemorrhagic septicaemia virus (VHSV) infection. Fish Shellfish Immunol. 2019, 91, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.K.; Al-Saari, N.; Zulperi, Z.; Mohd-Aris, A.; Salleh, A.; Silvaraj, S.; Mohamad, A.; Lee, J.; Zamri-Saad, M.; Ina-Salwany, Y. Efficacy of bath vaccination with a live attenuated Vibrio harveyi against vibriosis in Asian seabass fingerling, Lates calcarifer. Aquac. Res. 2019, 51, 389–399. [Google Scholar] [CrossRef]
- Gao, Y.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. Antigen uptake and expression of antigen presentation-related immune genes in flounder (Paralichthys olivaceus) after vaccination with an inactivated Edwardsiella tarda immersion vaccine, following hyperosmotic treatment. Fish Shellfish Immunol. 2016, 55, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, T.; Balcazar, J.L.; Merrifield, D.; Carnevali, O.; Gioacchini, G.; de Blas, I.; Ruiz-Zarzuela, I. Expression of immune-related genes in rainbow trout (Oncorhynchus mykiss) induced by probiotic bacteria during Lactococcus garvieae infection. Fish Shellfish Immunol. 2011, 31, 196–201. [Google Scholar] [CrossRef]
- Bao, P.; Sun, X.; Liu, Q.; Zhang, Y.; Liu, X. Synergistic effect of a combined live Vibrio anguillarum and Edwardsiella piscicida vaccine in turbot. Fish Shellfish Immunol. 2019, 88, 84–90. [Google Scholar] [CrossRef]
- Adikesavalu, H.; Banerjee, S.; Patra, A.; Abraham, T.J. Meningoencephalitis in farmed monosex Nile tilapia (Oreochromis niloticus L.) caused by Streptococcus agalactiae. Arch. Polish. Fish. 2017, 25, 187–200. [Google Scholar] [CrossRef]
- Ortega CE SA, R.; García, I.; Irgang, R.; Fajardo, R.; Tapia-Cammas, D.; Acosta, J.; Avendaño-Herrera, R. First identification and characterization of Streptococcus iniae obtained from tilapia (Oreochromis aureus) farmed in Mexico. J. Fish Dis. 2018, 41, 773–782. [Google Scholar] [CrossRef]








| Target mRNA | Sequence (5′-3′) | Amplicon Size (bp) | Accession | References |
|---|---|---|---|---|
| IL-1B-F | CAAGGATGACGACAAGCCAACC | 149 | XM_019365844.2 | Qiang et al. [26] |
| IL-1B-R | AGCGGACAGACATGAGAGTGC | |||
| C-type lysozyme-F | AAGGGAAGCAGCAGCAGTTGTG | 151 | XM_019361339.1 | Qiang et al. [26] |
| C-type lysozyme-R | CGTCCATGCCGTTAGCCTTGAG | |||
| TNF-α-F | GGAAGCAGCTCCACTCTGATGA | 137 | NM_001279533.1 | Qiang et al. [26] |
| TNF-α-R | CACAGCGTGTCTCCTTCGTTCA | |||
| TGF-β-F | TGCGGCACCCAATCACACAAC | 105 | XM_025897821.1 | Wang et al. [6] |
| TGF-β-R | GTTAGCATAGTAACCCGTTGGC | |||
| MHC-I-F | TTCTCACCAACAATGACGGG | 188 | XM_019355579.2 | Zhang et al. [27] |
| MHC-I-R | AGGGATGATCAGGGAGAAGG | |||
| MHC-II-F | AGTGTGGGGAAGTTTGTTGGAT | 207 | NM_001279562.1 | Yao et al. [28] |
| MHC-II-R | ATGGTGACTGGAGAGAGGCG | |||
| CD4-F | TTCAGTGGCACTTTGCTCCTAA | 277 | XM_005455490.4 | Yao et al. [28] |
| CD4-R | TGGGCGATGATTTCCAACA | |||
| IgT-F | TCCCACACACTGACCTGTAC | 151 | XM_025904470.1 | Velázquez et al. [29] |
| IgT-R | GGCCTTGGACTGACTGAGAA | |||
| Control gene | 111 | NM_001101.5 | Qiang et al. [26] | |
| β-Actin-F | CCACACAGTGCCCATCTACGA | |||
| β-Actin-R | CCACGCTCTGTCAGGATCTTCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monir, M.S.; Yusoff, M.S.M.; Zamri-Saad, M.; Amal, M.N.A.; Mohamad, A.; Azzam-Sayuti, M.; Ina-Salwany, M.Y. Effect of an Oral Bivalent Vaccine on Immune Response and Immune Gene Profiling in Vaccinated Red Tilapia (Oreochromis spp.) during Infections with Streptococcus iniae and Aeromonas hydrophila. Biology 2022, 11, 1268. https://doi.org/10.3390/biology11091268
Monir MS, Yusoff MSM, Zamri-Saad M, Amal MNA, Mohamad A, Azzam-Sayuti M, Ina-Salwany MY. Effect of an Oral Bivalent Vaccine on Immune Response and Immune Gene Profiling in Vaccinated Red Tilapia (Oreochromis spp.) during Infections with Streptococcus iniae and Aeromonas hydrophila. Biology. 2022; 11(9):1268. https://doi.org/10.3390/biology11091268
Chicago/Turabian StyleMonir, Md Shirajum, Md Sabri Mohd Yusoff, Mohd Zamri-Saad, Mohammad Noor Azmai Amal, Aslah Mohamad, Mohamad Azzam-Sayuti, and Md Yasin Ina-Salwany. 2022. "Effect of an Oral Bivalent Vaccine on Immune Response and Immune Gene Profiling in Vaccinated Red Tilapia (Oreochromis spp.) during Infections with Streptococcus iniae and Aeromonas hydrophila" Biology 11, no. 9: 1268. https://doi.org/10.3390/biology11091268
APA StyleMonir, M. S., Yusoff, M. S. M., Zamri-Saad, M., Amal, M. N. A., Mohamad, A., Azzam-Sayuti, M., & Ina-Salwany, M. Y. (2022). Effect of an Oral Bivalent Vaccine on Immune Response and Immune Gene Profiling in Vaccinated Red Tilapia (Oreochromis spp.) during Infections with Streptococcus iniae and Aeromonas hydrophila. Biology, 11(9), 1268. https://doi.org/10.3390/biology11091268







