Ecophysiological Parameters of Medicinal Plant Filipendula vulgaris in Diverse Habitat Conditions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Study Area and Plants Material
2.2. Soil Analysis
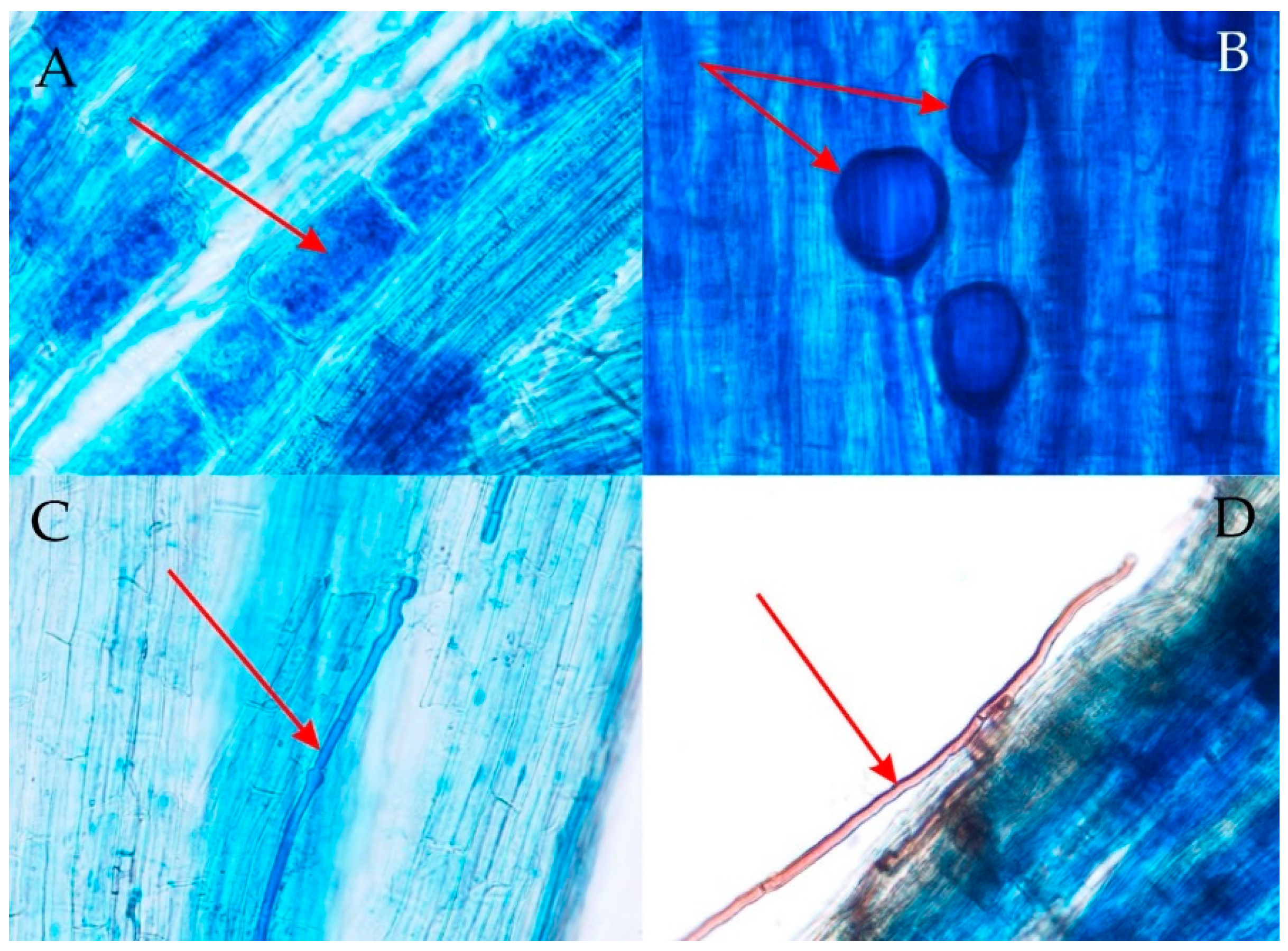
2.3. Root Staining and the Examination of Fungal Root Colonization
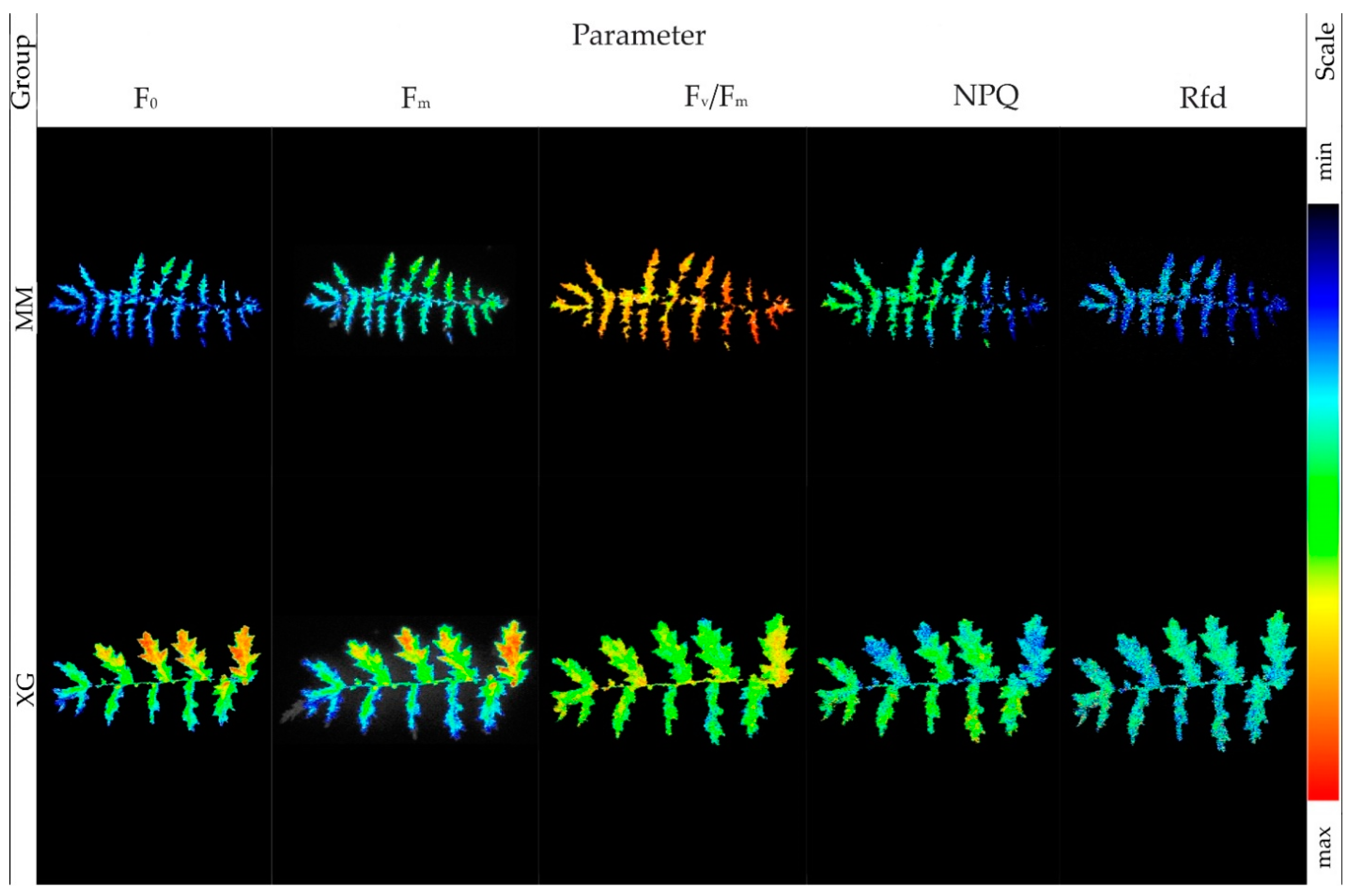
2.4. Chlorophyll a Fluorescence
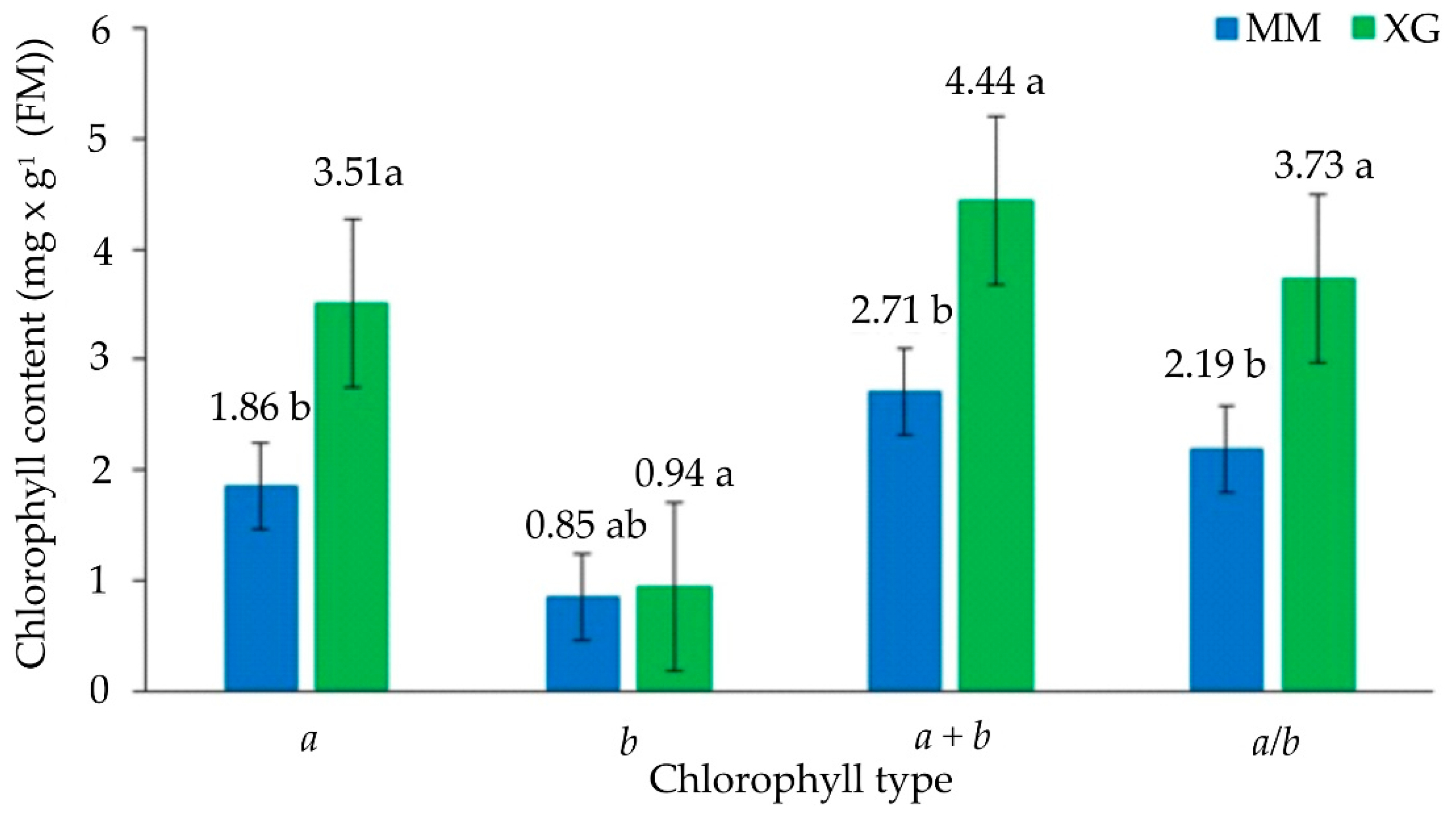
2.5. Chlorophyll Content
2.6. Chlorophyll Fluorescence Emission
2.7. Hydrogen Peroxide Content
2.8. Electrolyte Leakage
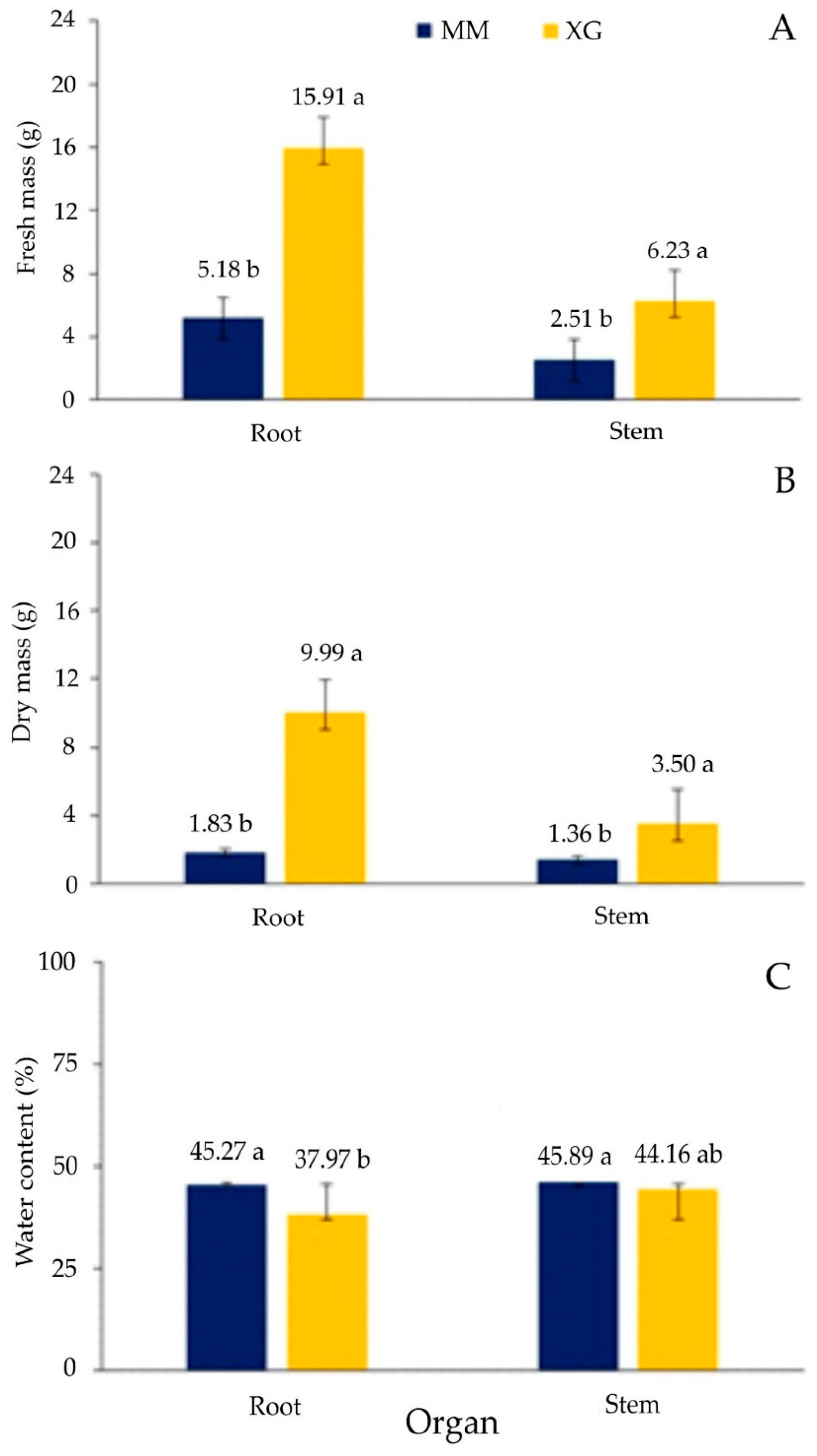
2.9. Plant Biomass
2.10. Statistical Analyses
3. Results
3.1. Soils
3.2. Degree of Arbuscular Mycorrhizal Fungi (AMF) Colonization of Roots and Morphology of Arbuscular Mycorrhiza (AM)
3.3. Physiological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rzepka, A. Ekofizjologiczne Aspekty Reakcji Różnych Gatunków Mchów na Abiotyczne Czynniki Stresowe; Scientific Publishers of the Pedagogical Academy in Krakow: Kraków, Poland, 2008; 92p, ISBN 978-83-7271-484-8. Available online: https://rep.up.krakow.pl/xmlui/handle/11716/986 (accessed on 5 September 2019). (In Polish)
- Nishiyama, Y.; Murata, N. Revised scheme for the mechanism of photoinhibition and its application to enhance the abiotic stress tolerance of the photosynthetic machinery. Appl. Microbiol Biotechnol. 2014, 98, 8777–8796. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S. Regulation of Photosynthesis during Abiotic Stress-Induced Photoinhibition. Mol. Plant. 2015, 8, 1304–1320. [Google Scholar] [CrossRef] [PubMed]
- Możdżeń, K. Wpływ Składu Spektralnego światła na Wybrane Procesy Fzjologiczne Mchów w Warunkach Stresu Ozonowego (Impact of the Spectral Composition of Light on Selected Physiological Processes of Mosses under Ozone Stress); Scientific Publisher of the Pedagogical University in Krakow: Kraków, Poland, 2019; 127p, (In Polish with English summary). [Google Scholar] [CrossRef]
- Marschall, M.; Proctor, M.C.F. Are bryophytes shade plants? Photosynthetic light responses and proportions of chlorophyll a, chlorophyll b and total carotenoids. Ann. Bot. 2004, 94, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Tallis, J.H. Studies in the biology and ecology of Rhacomitrium lanuginosum Brid. II. Growth reproduction and physiology. J. Ecol. 1959, 47, 325–350. [Google Scholar] [CrossRef]
- Rastorfer, J.R. Effects of light intensity and temperature on photosynthesis and respiration on two East Antarctic mosses, Bryum argenteum and Bryum antarcticum. Bryologist 1970, 73, 544–556. [Google Scholar] [CrossRef]
- Degenhardt, B.; Gimmler, H. Cell wall adaptations to multiple environmental stresses in maize roots. J. Exp. Bot. 2000, 51, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Young, J.E. Effects of spectral composition of light on the growth of a higher plant. In Light as an Ecological Factor; Evans, G.C., Bainbridge, R., Rackham, O., Eds.; Blackwell: Oxford, UK, 1975; pp. 135–159. [Google Scholar]
- Smith, H. Light quality, photoreception, and plant strategy. Annu. Rev. Plant Physiol. 1982, 33, 481–518. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, M.D.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.W.; Lewis, D.H.; Zhang, H.; Irving, L.J.; Jameson, P.E.; Davies, K.M. Light-induced vegetative anthocyanin pigmentation in Petunia. J. Exp. Bot. 2009, 60, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. Phenotypic Plasticity, Developmental Instability, and Robustness: The Concepts and How They Are Connected. Front. Ecol. Evol. 2019, 7, 56. [Google Scholar] [CrossRef]
- Sterck, F.J.; Duursma, R.A.; Valladares, F.; Cieslak, M.; Weemstra, M. Plasticity influencing the light compensation point offsets the specialization for light niches across shrub species in a tropical forest understorey. J. Ecol. 2013, 101, 971–980. [Google Scholar] [CrossRef]
- Stachurska-Swakoń, A.; Kuź, K. Phenotypic response of Doronicum austriacum Jacq. (Asteraceae) to diverse mountain and lowland conditions. Pol. J. Ecol. 2011, 59, 249–262. [Google Scholar]
- Stachurska-Swakoń, A.; Kostrakiewicz-Gierałt, K.; Świerczek, J. Variability of morphological traits of mountain Veratrum lobelianum in lowland locality. Ecol. Quest. 2018, 29, 61–69. [Google Scholar] [CrossRef]
- Xue, B.K.; Leibler, S. Benefits of phenotypic plasticity for population growth in varying environments. Proc. Natl. Acad. Sci. USA 2018, 115, 12745–12750. [Google Scholar] [CrossRef]
- Clapham, A.R.; Tutin, T.G.; Moore, D.M. Flora of the British Isles; Cambridge University Press: Cambridge, UK, 1987; 688p, ISBN 0-521-30985-9. [Google Scholar]
- Fecowicz, M.; Katarzyna Możdżeń, K.; Barabasz-Krasny, B.; Stachurska-Swakoń, A. Allelopathic influence of medicinal plant Filipendula vulgaris Moench on germination process. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 2032–2049. [Google Scholar] [CrossRef]
- Klimešová, J.; Klimeš, L. Clo-Pla 3 Database of Clonal Growth of Plants from Central Europe. 2006. Available online: http://clopla.butbn.cas.cz/ (accessed on 5 September 2019).
- Zając, M.; Zając, A. Elementy Geograficzne Rodzimej Flory Polski (The Geographical Elements of Native Flora of Poland); Institute of Botany, Jagiellonian University: Kraków, Poland, 2009; 93p, ISBN 9788392508090. (In Polish) [Google Scholar]
- Medwecka-Kornaś, A.; Kornaś, J.; Pawłowski, B. Survey of the most important plant associations in Poland. In The Vegetation of Poland; Szafer, W., Ed.; Pergamon Press: Oxford, UK, 1966; pp. 294–509. [Google Scholar]
- Towpasz, K.; Stachurska-Swakoń, A. Occurrence of Sesleria uliginosa (Poaceae) in the xerothermic grasslands (Festuco-Brometea) in the Nida Basin territory (Małopolska Upland). Fragm. Flor. Geobot. Polon. 2011, 18, 321–330. [Google Scholar]
- Towpasz, K.; Stachurska-Swakoń, A. Seslerio uliginosae-Scorzoneretum purpureae (Festuco-Brometea class) in the Nida Basin (Małopolska Upland) after 90 years. Acta Soc. Bot. Pol. 2012, 81, 167–173. [Google Scholar] [CrossRef]
- Dubiel, E.; Stachurska, A.; Gawroński, S. Nieleśne zbiorowiska roślinne Magurskiego Parku Narodowego (Beskid Niski) (Non-forest communities of the Magura National Park (Beskid Niski Mts.)). Zesz. Nauk. UJ Prace Bot. 1999, 33, 1–60. (In Polish) [Google Scholar]
- Towpasz, K.; Stachurska-Swakoń, A. Occurrence of Sesleria uliginosa (Poaceae) in the communities of the Caricetalia davallianae order in the Nida Basin territory (Małopolska Upland). Fragm. Flor. Geobot. Polon. 2009, 16, 305–316. [Google Scholar]
- Kostrakiewicz-Gierałt, K.; Stachurska-Swakoń, A. The influence of habitat conditions on the abundance and selected traits of the rare medicinal plant species Filipendula vulgaris Moench. Ecol. Quest. 2017, 25, 9–18. [Google Scholar] [CrossRef][Green Version]
- Mowszowicz, J. Przewodnik Do Oznaczania Krajowych Roślin Zielarskich; PWRiL: Warszawa, Poland, 1985; 489p, ISBN 8309006829. (In Polish) [Google Scholar]
- Oszmianski, J.; Wojdylo, A.; Lamer-Zarawska, E.; Swiader, K. Antioxidant tannins from Rosaceae plant roots. Food Chem. 2007, 100, 579–583. [Google Scholar] [CrossRef]
- Imbrea, I.M.; Butnariu, M.; Nicolin, A.L.; Imbrea, F. Determining antioxidant capacity of extracts of Filipendula vulgaris Moench from south-western Romania. J. Food Agric. Environ. 2010, 8, 111–116. [Google Scholar]
- Olennikov, D.N.; Kruglova, M.Y. A new quercetin glycoside and other phenolic compounds from the genus Filipendula. Chem. Nat. Compd. 2013, 49, 610–616. [Google Scholar] [CrossRef]
- Tucakov, J. Lečenje Biljem: Fitoterapija; Izdavačko preduzeće Rad: Beograd, Serbia, 1997; 720p. (In Bosnian) [Google Scholar]
- Skiba, S.; Drewnik, M.; Kacprzak, A.; Żyła, M.; Żelazowska, E. Pokrywa Glebowa Rejonu Kampusu Uniwersytetu Jagiellońskiego; Domański, B., Skiba, S., Geografia i Sacrum, I., Eds.; Instytut Geografii i Gospodarki Przestrzennej: Kraków, Poland, 2005; pp. 161–169, (In Polish with English summary). [Google Scholar]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Kęsik, K.; Jadczyszyna, T.; Lipiński, W.; Jurga, B. Adaptacja testu Mehlicha 3 do rutynowych oznaczeń zawartości fosforu, potasu i magnezu w glebie (Adaptation of the Mehlich 3 procedure for routine determination of phosphorus, potassium and magnesium in soil). Przem. Chem. 2015, 94, 973–976. [Google Scholar] [CrossRef]
- Malý, S.; Zbíral, J.; Čižmárová, E. Is Mehlich 3 soil extraction a suitable screening method for determination of some risk elements? Plant Soil Environ. 2021, 67, 499–506. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Brit. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Zubek, S.; Błaszkowski, J.; Mleczko, P. Arbuscular mycorrhizal and dark septate endophyte associations of medicinal plants. Acta Soc. Bot. Polon. 2011, 80, 285–292. [Google Scholar] [CrossRef][Green Version]
- Dickson, S. The Arum-Paris continuum of mycorrhizal symbioses. New Phytol. 2004, 163, 187–200. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de methodes d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Zubek, S.; Majewska, M.L.; Błaszkowski, J.; Stefanowicz, A.M.; Nobis, M.; Kapusta, P. Invasive plants affect arbuscular mycorrhizal fungi abundance and species richness as well as the performance of native plants grown in invaded soils. Biol. Fertil. Soils 2016, 52, 841–852. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. Measurement of chlorophyll fluorescence kinetics (Kautsky effect) and the chlorophyll fluorescence decrease ratio (RFd–values) with the PAM–Fluorometer. In Analytical Methods in Plant Stress Biology; Filek, N., Biesaga-Kościelniak, J., Marcińska, I., Eds.; The Franciszek Gorski Institute of Plant Physiology of the Polish Academy of Sciences: Kraków, Poland, 2004; pp. 93–111. [Google Scholar]
- Gorbe, E.; Calatayud, A. Applications of chlorophyll fluorescence imaging technique in horticultural research: A review. Sci. Hortic-Amst. 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Buschmann, C.; Langsdorf, G.; Lichtenthaler, H.K. Imaging of the blue, green and red fluorescence emission of plants: An overview. Photosynthetica 2000, 38, 483–491. [Google Scholar] [CrossRef]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 1992, 32, 83–100. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Knapp, M.; Buschmann, C. Recording chlorophyll fluorescence emission spectra with the Perkin Elmer fluorescence spectrometer LS 50. In Analytical Methods in Plant Stress Biology; Filek, N., Biesaga-Kościelniak, J., Marcińska, I., Eds.; The Franciszek Gorski Institute of Plant Physiology of the Polish Academy of Sciences: Kraków, Poland, 2004; pp. 112–124. [Google Scholar]
- Jena, S.; Acharya, S.; Mohapatra, P.K. Variation in effects of four OP insecticides on photosynthetic pigment fluorescence of Chlorella vulgaris Beij. Ecotoxicol. Environ. Saf. 2012, 80, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Daudi, A.; O’Brien, J. Detection of hydrogen peroxide by DAB staining in Arabidopsis leaves. Bio Protoc. 2016, 2, e263. [Google Scholar] [CrossRef]
- Wu, T.M.; Huang, J.Z.; Oung, H.M.; Hsu, Y.T.; Tsai, Y.C.; Hong, C.Y. H2O2-Based Method for Rapid Detection of Transgene-Free Rice Plants from Segregating CRISPR/Cas9 Genome-Edited Progenies. Int. J. Mol. Sci. 2019, 20, 3885. [Google Scholar] [CrossRef]
- Asaeda, T.; Jayasanka, S.M.D.H.; Xia, L.-P.; Barnuevo, A. Application of Hydrogen Peroxide as an Environmental Stress Indicator for Vegetation Management. Engineering 2018, 4, 610–616. [Google Scholar] [CrossRef]
- Możdżeń, K.; Barabasz-Krasny, B.; Zandi, P. Effect of Long-Term of He-Ne Laser Light Irradiation on Selected Physiological Processes of Triticale. Plants 2020, 9, 1703. [Google Scholar] [CrossRef]
- Zenglu, X.; Congru, M.; Senzhao, L.; Weiqi, M. Characteristics of natural contents of chemical elements in profiles of some soil types in china and their relationship. Sci. China Chem. 1985, 28, 1333–1344. [Google Scholar] [CrossRef]
- Pakuła, K.; Kalembasa, D. Makroelementy w glebach ornych Wysoczyzny siedleckiej (Macroelements in arable soils of the Siedlce Upland). Acta Agrophys. 2012, 19, 803–814. (In Polish) [Google Scholar]
- Zhukovskaya, N.; Lukashev, O. The chemical elements associations in the soils of natural and urban areas. Vestnik BGU. Ser. 2. Khimiya Biol. Geogr. 2016, 1, 3–13. (In Russian) [Google Scholar]
- Semenkov, I.N.; Koroleva, T.V.; Sharapova, A.V.; Terskaya, E.V. Standard Rates of Content of Chemical Elements in the Soil: International Experience and Use for Western Siberia. Geogr. Nat. Resour. 2020, 41, 9–17. [Google Scholar] [CrossRef]
- Nedelkoska, T.V.; Doran, P.M. Characteristics of heavy metal uptake by plant species with potential for phytoremediation and phytomining. Miner. Eng. 2000, 13, 549–561. [Google Scholar] [CrossRef]
- Turisová, I.; Kviatková, T.; Możdżeń, K.; Barabasz-Krasny, B. Effects of natural sorbents on the germination and early growth of grasses on soils contaminated by potentially toxic elements. Plants 2020, 9, 1591. [Google Scholar] [CrossRef] [PubMed]
- Możdżeń, K.; Barabasz-Krasny, B.; Kviatková, T.; Zandi, P.; Turisová, I. Effect of Sorbent Additives to Copper-Contaminated Soils on Seed Germination and Early Growth of Grass Seedlings. Molecules 2021, 26, 5449. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Brooks, R.R. Terrestrial higher plants which hyperaccumulate metallic elements—A review of their distribution, ecology and phytochemistry. BioRecovery 1989, 1, 81–126. [Google Scholar] [CrossRef]
- Solanki, R.; Dhankhar, R. Biochemical changes and adaptive strategies of plants under heavy metal stress. Biologia 2011, 66, 195–204. [Google Scholar] [CrossRef]
- Możdżeń, K.; Barabasz-Krasny, B.; Stachurska-Swakoń, A.; Zandi, P.; Puła, J. Effect of aqueous extracts of peppermint (Mentha × piperita L.) on the germination and the growth of selected vegetable and cereal seeds. Not. Bot. Horti. Agrobo. 2019, 47, 412–417. [Google Scholar] [CrossRef]
- Likar, M.; Regvar, M.; Mandic-Mulec, I.; Stres, B.; Bothe, H. Diversity and seasonal variations of mycorrhiza and rhizosphere bacteria in three common plant species at the Slovenian Ljubljana Marsh. Biol. Fertil. Soils 2009, 45, 573–583. [Google Scholar] [CrossRef]
- Grzyś, E. The Effect of Some Biologically Active Substances on Maize Grown under Stress Conditions; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu ELMA: Wrocław, Poland, 2012; 100p, ISBN 978-83-7717-085-4. (In Polish) [Google Scholar]
- Stahl, R.R.; Smith, W.K. Effects of different geographic isolates of Glomus on the water relations of Agrophyron smithii. Mycologia 1984, 76, 261–267. [Google Scholar] [CrossRef]
- Dehn, B.; Schüepp, H. Influence of VA mycorrhiza on the uptake and distribution of heavy metals in plants. Agric. Ecosyst. Environ. 1990, 29, 79–83. [Google Scholar] [CrossRef]
- Griffioen, W.A.J.; Ernst, W.H.O. The role of VA mycorrhiza in the heavy metal tolerance of Agrostis capillaries L. Agric. Ecosyst. Environ. 1989, 29, 173–177. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008; ISBN 9780080559346. [Google Scholar]
- Koske, R.E.; Sutton, J.C.; Sheppard, B.R. Ecology of Endogone in Lake Huron sand dunes. Can. J. Bot. 1975, 53, 87–93. [Google Scholar] [CrossRef]
- Sutton, J.C.; Sheppard, B.R. Aggregation of sand dune soil by endomycorrhizal fungi. Can. J. Bot. 1976, 54, 326–333. [Google Scholar] [CrossRef]
- Kotilínek, M.; Hiiesalu, I.; Košnar, J.; Šmilauerová, M.; Šmilauer, P.; Altman, J.; Dvorský, M.; Kopecký, M.; Doležal, J. Fungal root symbionts of high-altitude vascular plants in the Himalayas. Sci. Rep. 2017, 7, 6562. [Google Scholar] [CrossRef] [PubMed]
- Starck, Z.; Chołuj, D.; Niemyska, B. Fizjologiczne Reakcje Roślin Na Niekorzystne Czynniki Środowiska; Wyd. SGGW: Warszawa, Poland, 1993; 116p. (In Polish) [Google Scholar]
- Starck, Z. Wpływ warunków stresowych na koordynację wytwarzania i dystrybucji fotoasymilatów. Post. Nauk Rol. 2010, 1, 9–26. (In Polish) [Google Scholar]
- Lin, W.C.; Jolliffe, P.A. Chlorophyll fluorescence of long english cucumber affected by storage conditions. Acta Hortic. 2000, 517, 449–456. [Google Scholar] [CrossRef]
- Oxborough, K. Imaging of chlorophyll a fluorescence: Theoretical and practical aspects of an emerging technique for the monitoring of photosynthetic performance. J. Exp. Bot. 2004, 55, 1195–1205. [Google Scholar] [CrossRef]
- Puła, J.; Zandi, P.; Stachurska-Swakoń, A.; Barabasz-Krasny, B.; Możdżeń, K.; Wang, Y. Influence of alcoholic extracts from Helianthus annnus L. roots on the photosynthetic activity of Sinapis alba L. cv. Barka plants. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2020, 70, 8–13. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Łoboda, T. Fluorescencja Chlorofilu W Badaniach Stanu Fizjologicznego Roślin; Wydawnictwo SGGW: Warszawa, Poland, 2010; 116p, ISBN 9788375831191. (In Polish) [Google Scholar]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Murkowski, A. Ocena wrażliwości roślin uprawnych na wybrane stresy środowiska przy użyciu metody fluorescencyjnej. Inż. Roln. 2005, 9, 37–45. (In Polish) [Google Scholar]
- Drożak, A.; Romanowska, E. Acclimation of mesophyll and bundle sheath chloroplasts of maize to different irradiances during growth. BBA Bioenerg. 2006, 1757, 1539–1546. [Google Scholar] [CrossRef][Green Version]
- Strihz, I.G.; Lysenko, G.G.; Neverov, K.V. Photoreduction of molecular oxygen in preparation of photosystem II under photoinhibitory conditions. Rus. J. Plant Physiol. 2005, 52, 717–723. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III; Logan, B.A.; Verhoeven, A.S. Xantophyllcycle–dependend energy dissipation and flexible photosystem II efficiency in plants acclimated to light stress. Austr. J. Plant Physiol. 1995, 22, 249–260. [Google Scholar] [CrossRef]
- Murkowski, A.; Skórska, E. Chlorophyll fluorescence in research of chill and light stress in cucumber plants from in vitro culture during acclimation. Hortic. Veget. Grow. 2004, 23, 192–198. [Google Scholar]
- Sulkiewicz, M.; Ciereszko, I. Fluorescencja chlorofilu a–historia odkrycia i zastosowanie w badaniach roślin. Kosmos 2016, 65, 103–115. (In Polish) [Google Scholar]
- Heber, U.; Bilger, W.; Shuvalov, V.A. Thermal energy dissipation in reaction centres and in the antenna of photosystem II protects desiccated poikilohydric mosses against photo-oxidation. J. Exp. Bot. 2006, 57, 2993–3006. [Google Scholar] [CrossRef]
- Costa, A.C.; Rezende-Silva, S.L.; Megguer, C.A.; Moura, L.M.F.; Rosa, M.; Silva, A.A. The effect of irradiance and water restriction on photosynthesis in young jatobá-do-cerrado (Hymenaea stigonocarpa) plants. Photosynthetica 2015, 53, 118–127. [Google Scholar] [CrossRef]
- Frosch, S.; Bergfeld, R.; Mehnert, C.; Wagner, E. Ribulose bisphosphate carboxylase capacity and chlorophyll content in developing seedlings of Chenopodium rubrum L. growing under light of different qualities and fluence rates. Photosynth. Res. 1985, 7, 41–57. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Zepka, L.Q.; Queiroz, M.I. Chlorophyll; IntechOpen: London, UK, 2017; 132p, Available online: https://www.intechopen.com/books/5841 (accessed on 28 April 2022).
- Cetner, M.D.; Dąbrowski, P.; Samborska, I.A.; Łukasik, I.; Swoczyna, T.; Pietkiewicz, S.; Bąba, W.; Kalaji, H.M. Zastosowanie pomiarów fluorescencji chlorofilu w badaniach środowiskowych. Kosmos 2016, 65, 197–205. (In Polish) [Google Scholar]
- Buschmann, C.; Lichtenthaler, H.K. Principles and characteristics of multi-colour fluorescence imaging of plants 2. J. Plant Physiol. 1998, 152, 297–314. [Google Scholar] [CrossRef]
- Lang, M.; Stober, F.; Lichtenthaler, H.K. Fluorescence emission spectra of plant leaves and plant constituents. Radiat. Environ. Biophys. 1991, 30, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Saja, D.; Rys, M.; Stawoska, I.; Skoczowski, A. Metabolic response of cornflower (Centaurea cyanus L.) exposed to tribenuron-methyl- one of active substance of sulfonylurea herbicides. Acta Physiol. Plant. 2016, 38, 168. [Google Scholar] [CrossRef]
- Randi, A.M.; Freitas, M.C.A.; Rodrigues, A.C.; Maraschin, M.; Torres, M.A. Acclimation and photoprotection of young gametophytes of Acrostichum danaeifolium to UV-B stress. Photosynthetica 2014, 52, 50–56. [Google Scholar] [CrossRef]
- Gitelson, A.; Buschmann, C.; Lihtentahler, H.K. Leaf chlorophyll fluorescence corrected for re-absorption by means of absorption and reflectance measurements. J. Plant Physiol. 1998, 152, 283–296. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Rinderle, U. The role of chlorophyll fluorescence in the detection of stress conditions in plants. Crit. Rev. Anal. Chem. 1988, 19, 29–85. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Hak, R.; Rinderle, U. The chlorophyll fluorescence ratio F690/F730 in leaves of different chlorophyll content. Photosynth. Res. 1990, 25, 295–298. [Google Scholar] [CrossRef]
- Zandi, P.; Barabasz-Krasny, B.; Stachurska-Swakoń, A.; Puła, J.; Możdżeń, K. Allelopathic effect of invasive Canadian goldenrod (Solidago canadensis L.) on early growth of red clover (Trifolium pratense L.). Not. Bot. Horti. Agrobo. Cluj-Napoca 2020, 48, 2060–2071. [Google Scholar] [CrossRef]
- Chojnacka-Ożga, L.; Lorenc, H. (Eds.) Współczesne Problemy Klimatu Polski; IMGW-PIB: Warszawa, Poland, 2019; 260p, ISBN 978-83-64979-33-0. (In Polish) [Google Scholar]
- Mishra, K.B.; Iannacone, R.; Petrozza, A.; Mishra, A.; Armentano, N.; La Vecchia, G.; Trtílekm, M.; Cellini, F.; Nedbal, L. Engineered drought tolerance in tomato plants is reflected in chlorophyll fluorescence emission. Plant Sci. 2012, 182, 79–86. [Google Scholar] [CrossRef]
- Hochmal, A.K.; Schulze, S.; Trompelt, K.; Hippler, M. Calcium-dependent regulation of photosynthesis. Biochim. Biophys. Acta 2015, 1847, 993–1003. [Google Scholar] [CrossRef]
- Sai, J.; Johnson, C.H. Dark-stimulated calcium ion fluxes in the chloroplast stroma and cytosol. Plant Cell. 2002, 14, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Amir, M.; Manzoor, H.; Rasul, S.; Athar, H. Role of Calcium in Conferring Abiotic Stress Tolerance. In Plant Tolerance to Environmental Stress; Hasanuzzaman, M., Fujita, M., Oku, H., Islam, M.T., Eds.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Huang, K.; Peng, L.; Liu, Y.; Yao, R.; Liu, Z.; Li, X.; Yang, Y.; Wang, J. Arabidopsis calcium-dependent protein kinase AtCPK1 plays a positive role in salt/drought-stress response. Biochem. Biophys. Res. Commun. 2018, 498, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Naeem, M.S.; Ahmad, R.; Ihsan, M.Z.; Ashraf, M.Y.; Hussain, Y.; Fahad, S. Foliar calcium spray confers drought stress tolerance in maize via modulation of plant growth, water relations, proline content and hydrogen peroxide activity. Arch. Agron. Soil Sci. 2018, 64, 116–131. [Google Scholar] [CrossRef]
- Larré, C.F.; Fernando, J.A.; Marini, P.; Bacarin, M.A.; Peters, J.A. Growth and chlorophyll a fluorescence in Erythrina crista-galli L. plants under flooding conditions. Acta Physiol. Plant. 2013, 35, 1463–1471. [Google Scholar] [CrossRef]



| Stands | pH (in KCl) | (mg/kg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | P | K | Mn | Mg | Fe | Cu | Zn | Cd | Pb | NO3-N | ||
| MM | 5.05 b | 5218.32 b | 42.26 b | 86.97 b | 16.75 b | 179.10 b | 354.04 a | 3.51 a | 97.33 a | 0.96 a | 12.45 a | 8.19 a |
| XG | 6.67 a | 6644.33 a | 178.94 a | 281.27 a | 186.84 a | 217.21 a | 77.28 b | 2.50 b | 59.35 b | 0.85 b | 11.74 b | 7.73 a |
| Stands | F440/F530 | F440/F690 | F440/F735 | F690/F735 | PSI | PSII | PSI/PSII |
|---|---|---|---|---|---|---|---|
| MM | 1.06 a ±0.04 | 4.14 a ±0.76 | 7.69 a ±0.56 | 1.91 a ±0.17 | 1.48 a ±0.11 | 1.07 a ±0.14 | 1.41 a ±0.26 |
| XG | 0.95 b ±0.04 | 2.41 b ±0.28 | 4.02 b ±0.39 | 1.68 a ±0.18 | 1.31 ab ±0.06 | 1.05 a ±0.11 | 1.31 ab ±0.12 |
| Successive No. | Parameter | Molinia Meadows | Xerothermic Grasslands |
|---|---|---|---|
| Soils | |||
| 1. | pH in KCl | ||
| 2. | macronutrients | ||
| 3. | heavy metals | ||
| Parameters of AMF colonization | |||
| 4. | F—mycorrhizal frequency | ||
| 5. | M—relative mycorrhizal root length | ||
| 6. | A—relative arbuscular richness | ||
| 7. | DSE presence | ||
| Chlorophyll a fluorescence | |||
| 8. | F0—zero fluorescence | ||
| 9. | Fm—maximum fluorescence | ||
| 10. | Fv/Fm—maximum photochemical efficiency of PSII | ||
| 11. | NPQ—non-photochemical quenching | ||
| 12. | Rfd—PSII vitality indicator | ||
| Chlorophyll content | |||
| 13. | Chl a | ||
| 14. | Chl b | ||
| 15. | Sum a + b | ||
| 16. | Ratio a/b | ||
| Chlorophyll fluorescence emission | |||
| 17. | F440/F530 | ||
| 18. | F440/F6950 | ||
| 19. | F440/F735 | ||
| 20. | F690/F735 | ||
| 21. | PSI | ||
| 22. | PSII | ||
| 23. | PSI/PSII | ||
| Others | |||
| 24. | Hydrogen peroxide content | ||
| 25. | Electrolyte leakage | ||
| 26. | Fresh mass | ||
| 27. | Dry mass | ||
| 28. | Water content | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barabasz-Krasny, B.; Możdżeń, K.; Tatoj, A.; Rożek, K.; Zandi, P.; Schnug, E.; Stachurska-Swakoń, A. Ecophysiological Parameters of Medicinal Plant Filipendula vulgaris in Diverse Habitat Conditions. Biology 2022, 11, 1198. https://doi.org/10.3390/biology11081198
Barabasz-Krasny B, Możdżeń K, Tatoj A, Rożek K, Zandi P, Schnug E, Stachurska-Swakoń A. Ecophysiological Parameters of Medicinal Plant Filipendula vulgaris in Diverse Habitat Conditions. Biology. 2022; 11(8):1198. https://doi.org/10.3390/biology11081198
Chicago/Turabian StyleBarabasz-Krasny, Beata, Katarzyna Możdżeń, Agnieszka Tatoj, Katarzyna Rożek, Peiman Zandi, Ewald Schnug, and Alina Stachurska-Swakoń. 2022. "Ecophysiological Parameters of Medicinal Plant Filipendula vulgaris in Diverse Habitat Conditions" Biology 11, no. 8: 1198. https://doi.org/10.3390/biology11081198
APA StyleBarabasz-Krasny, B., Możdżeń, K., Tatoj, A., Rożek, K., Zandi, P., Schnug, E., & Stachurska-Swakoń, A. (2022). Ecophysiological Parameters of Medicinal Plant Filipendula vulgaris in Diverse Habitat Conditions. Biology, 11(8), 1198. https://doi.org/10.3390/biology11081198







