A Decrease in the Staminode-Mediated Visitor Screening Mechanism in Response to Nectar Robbers Positively Affects Reproduction in Delphinium caeruleum Jacq. ex Camb. (Ranunculaceae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species and Site
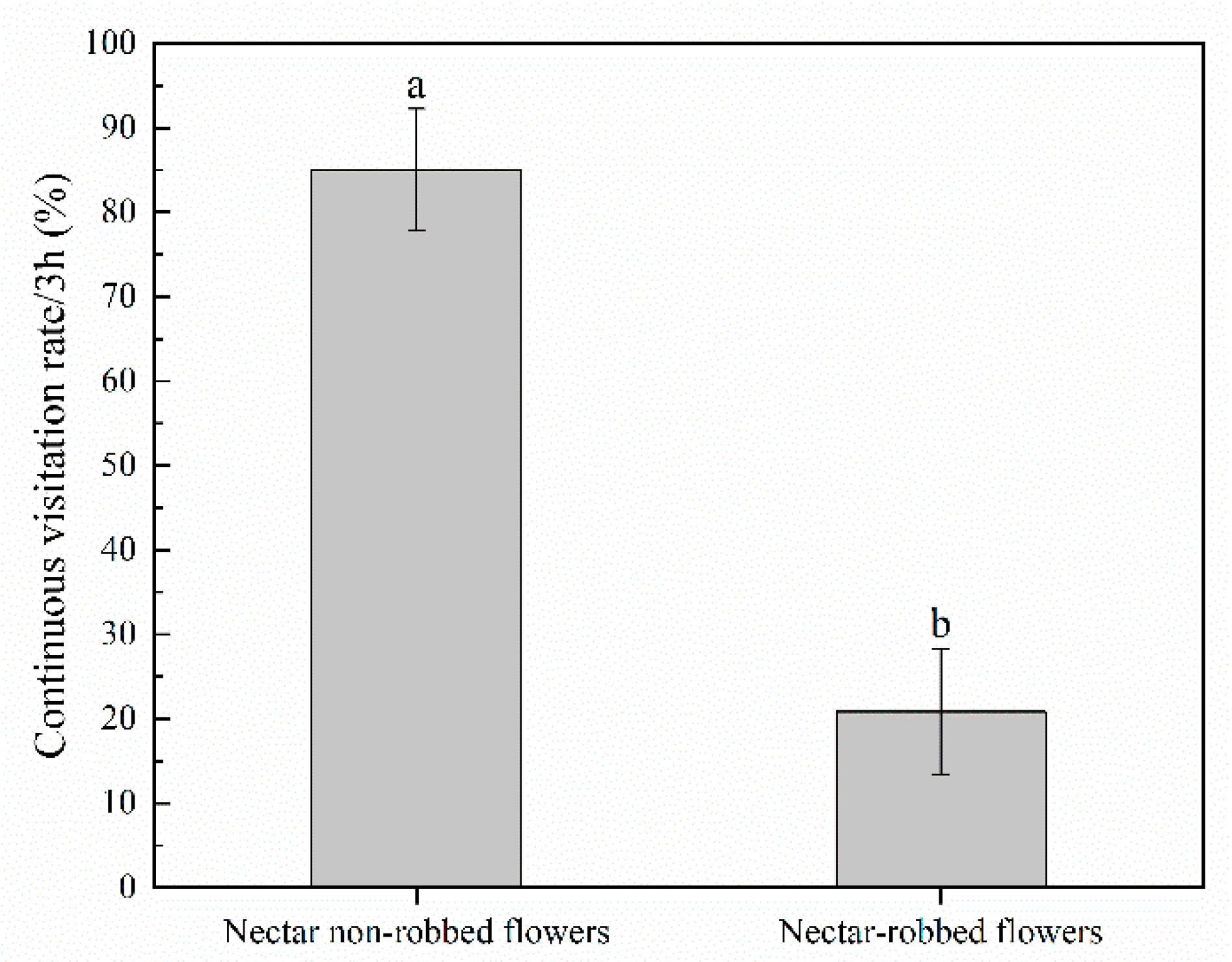
2.2. Effect of Nectar Robbing on Behavior of Visitors
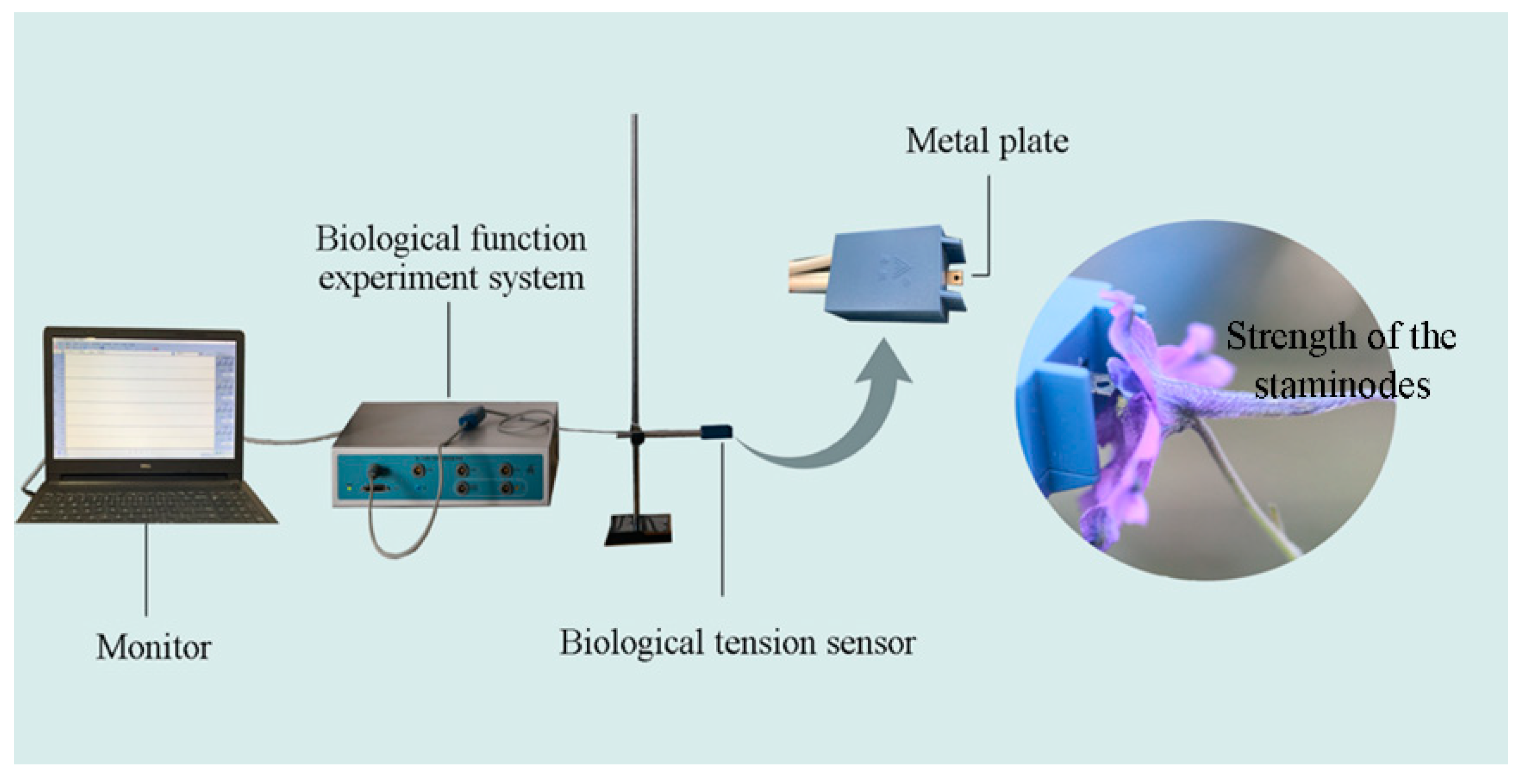
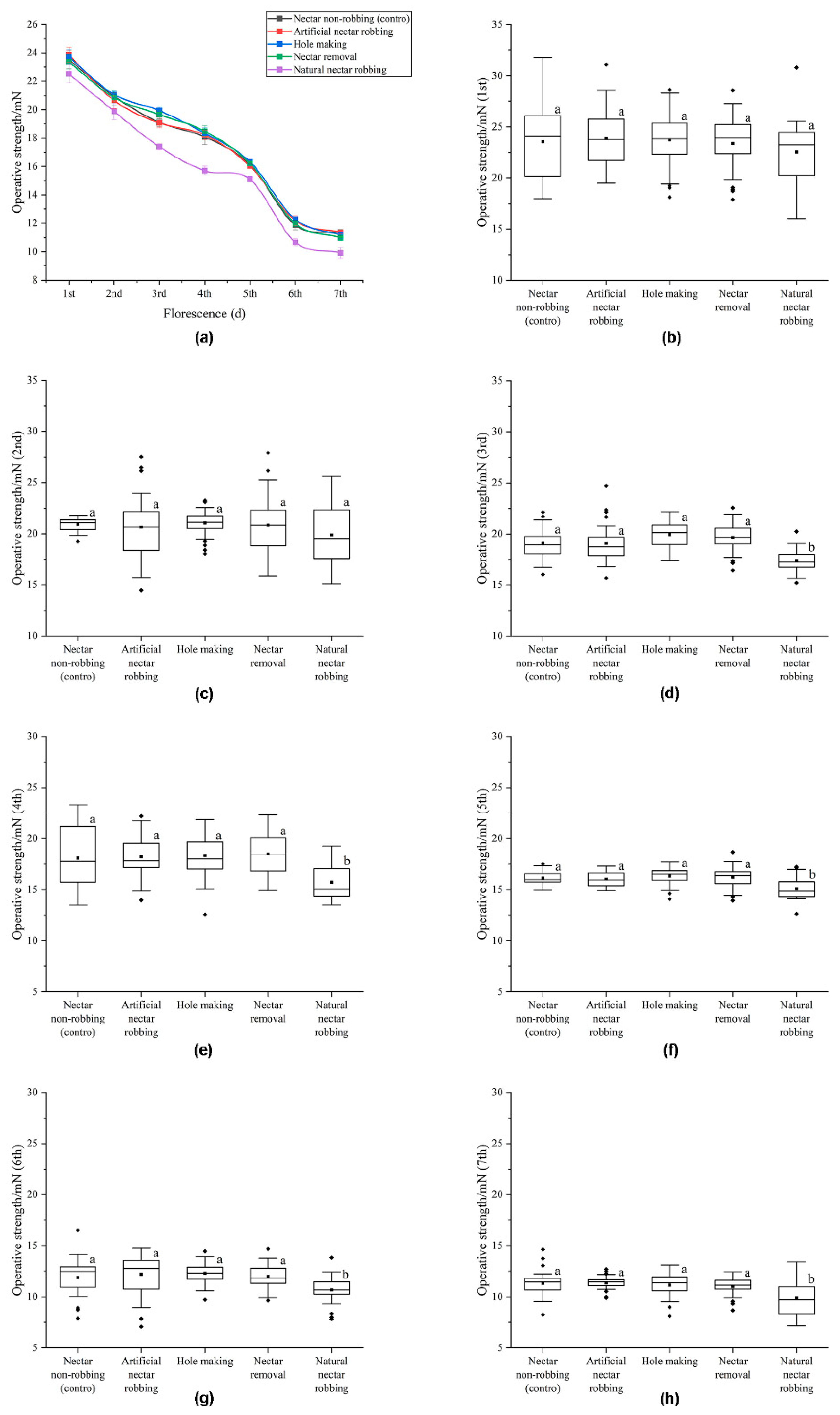
2.3. Biomechanical Studies
2.4. Effects of Nectar Robbing on Reproductive Output
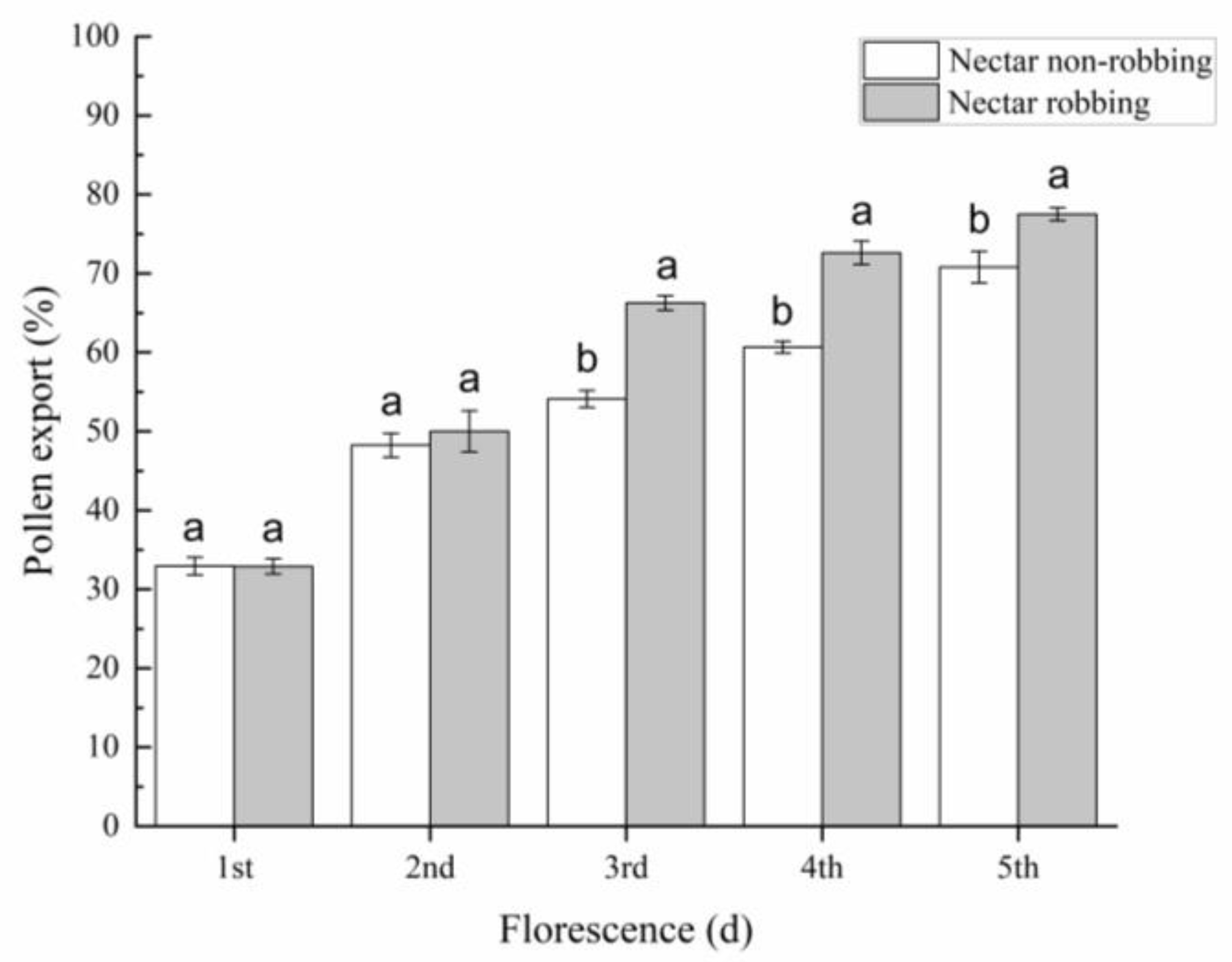
2.4.1. Pollen Export
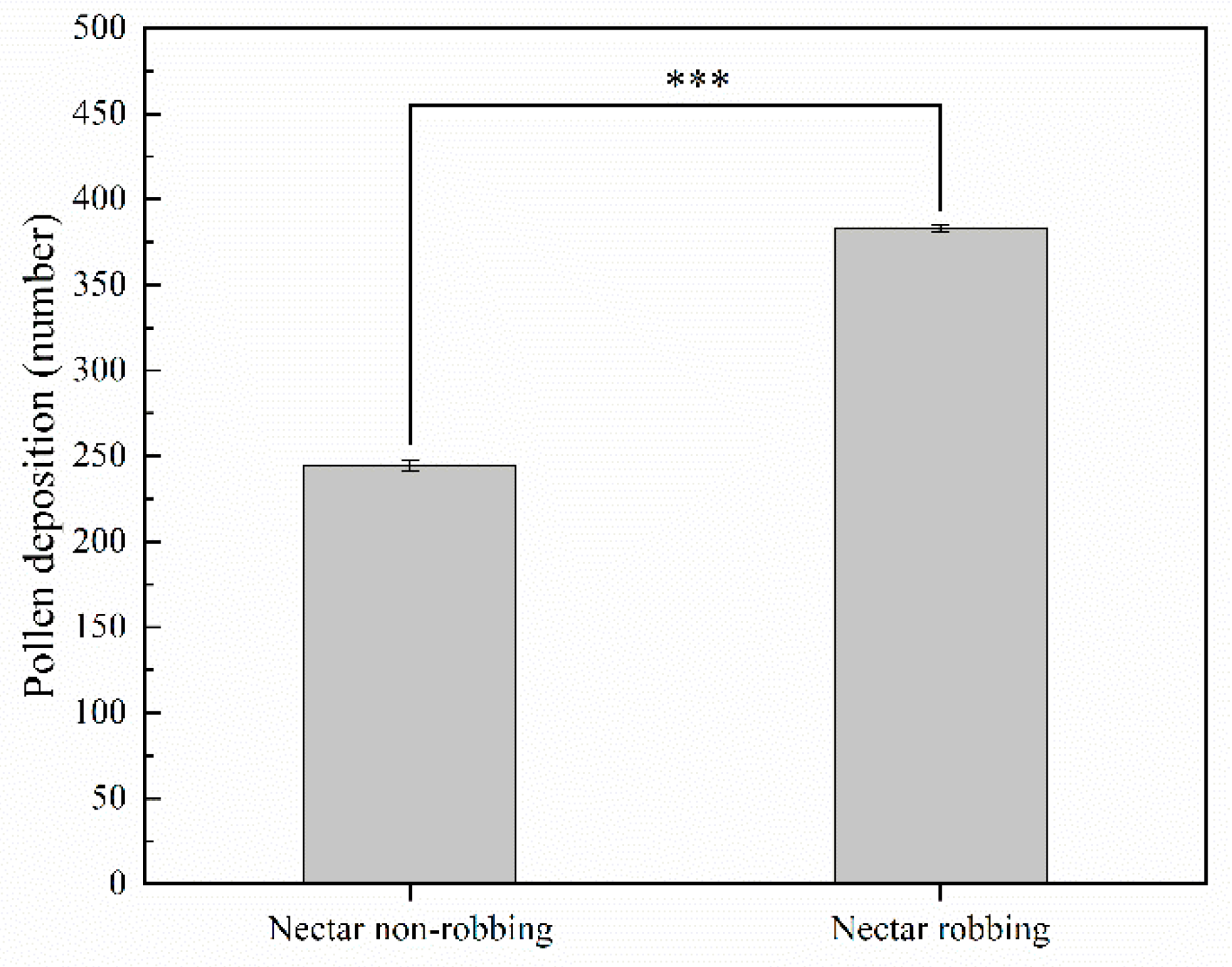
2.4.2. Pollen Deposition
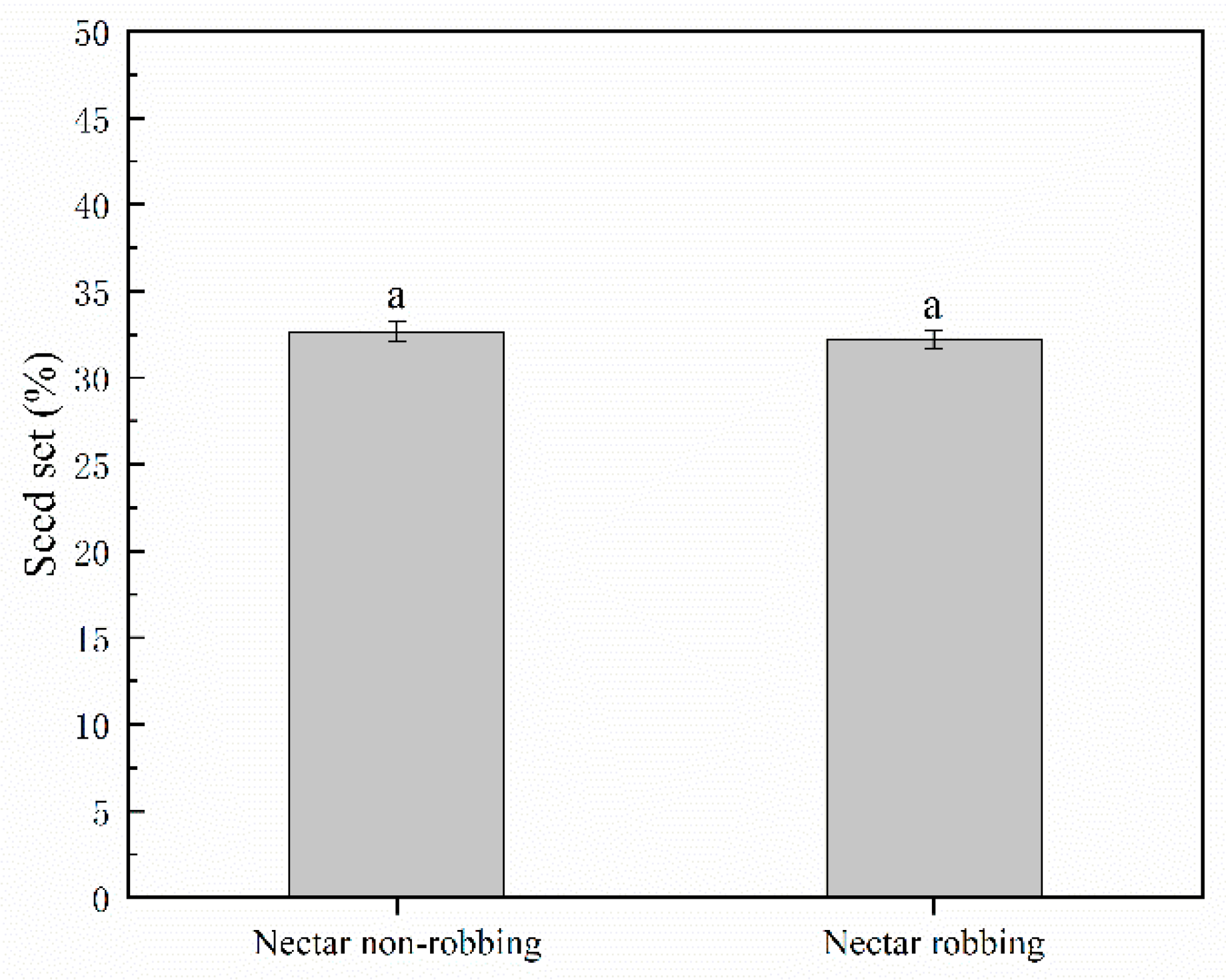
2.4.3. Seed Production
2.5. Data Analysis
3. Results
3.1. Effect of Nectar Robbing on the Behavior of Legitimate Pollinators
3.2. Biomechanical Studies
3.3. Effects of Nectar Robbing on Reproductive Success
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rojas-Nossa, S.V.; Sánchez, J.M.; Navarro, L. Nectar robbing: A common phenomenon mainly determined by accessibility constraints, nectar volume and density of energy rewards. Oikos 2016, 125, 1044–1055. [Google Scholar] [CrossRef]
- Navarro, L.; Medel, R. Relationship between floral tube length and nectar robbing in Duranta erecta L. (Verbenaceae). Biol. J. Linn. Soc. 2009, 96, 392–398. [Google Scholar] [CrossRef]
- Inoue, M.; Ushijima, J.; Yokoyama, J. Effect of Weigela hortensis (Caprifoliaceae) floral morphology on pollinator behavior. Plant Spec. Biol. 2007, 22, 77–86. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Wang, Y.; Guo, Y.H. Effects of nectar-robbing on plant reproduction and evolution. Front. Biol. 2007, 2, 443–449. [Google Scholar] [CrossRef]
- Kjonaas, C.; Rengifo, C. Differential effects of avian nectar-robbing on fruit set of two Venezuelan Andean cloud forest plants. Biotropica 2006, 38, 276–279. [Google Scholar] [CrossRef]
- Irwin, R.E.; Bronstein, J.L.; Manson, J.S.; Richardson, L. Nectar robbing: Ecological and evolutionary perspectives. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 271–292. [Google Scholar] [CrossRef]
- Richman, S.K.; Irwin, R.E.; Nelson, C.J.; Bronstein, J.L. Facilitated exploitation of pollination mutualisms: Fitness consequences for plants. J. Ecol. 2017, 105, 188–196. [Google Scholar] [CrossRef]
- Varma, S.; Rajesh, T.P.; Manoj, K.; Asha, G.; Jobiraj, T.; Sinu, P.A. Nectar robbers deter legitimate pollinators by mutilating flowers. Oikos 2020, 129, 868–878. [Google Scholar] [CrossRef]
- Jayanthi, P.D.K.; Murthy, B.N.S.; Nagaraja, T.; Raghava, T.; Ravindra, M.A.; Kempraj, V.; Byatroy, H.; Sharma, P.; Verghese, A.; Krishnamoorthy, A. Nectar robbing by Purple Sunbird, Nectarinia asiatica (L.) in Pomegranate reduces reproductive success. Pest Manag. Hortic. Ecosyst. 2015, 21, 214–218. [Google Scholar]
- Zhang, Y.W.; Robert, G.W.; Wang, Y.; Guo, Y.H. Nectar robbing of a carpenter bee and its effects on the reproductive fitness of Glechoma Longituba (Lamiaceae). Plant Ecol. 2007, 193, 1–13. [Google Scholar] [CrossRef]
- Irwin, R.E. Impact of nectar robbing on estimates of pollen flow: Conceptual predictions and empirical outcomes. Ecology 2003, 84, 485–495. [Google Scholar] [CrossRef]
- Thomson, J.D. Pollen transport and deposition by bumble bees in erythronium: Influences of floral nectar and bee grooming. J. Ecol. 1986, 74, 329–341. [Google Scholar] [CrossRef]
- Ellouise, L.; Lars, C. Social transmission of nectar-robbing behaviour in bumble-bees. Proc. R. Soc. B Biol. Sci. 2008, 275, 1669–1674. [Google Scholar]
- Sherry, D.F. Social learning: Nectar robbing spreads socially in bumble bees. Curr. Biol. 2008, 18, R608–R610. [Google Scholar] [CrossRef] [PubMed]
- Dedej, S.; Delaplane, K.S. Nectar-robbing carpenter bees reduce seed-setting capability of honey bees (Hymenoptera: Apidae) in rabbiteye blueberry, Vaccinium ashei ‘Climax’. Environ. Entomol. 2004, 33, 100–106. [Google Scholar] [CrossRef]
- Hou, Q.Z.; Ehmet, N.; Chen, D.W.; Wang, T.H.; Xu, Y.F.; Ma, J.; Sun, K. Corolla Abscission Triggered by Nectar Robbers Positively Affects Reproduction by Enhancing Self-Pollination in Symphytum officinale (Boraginaceae). Biology 2021, 10, 903. [Google Scholar] [CrossRef]
- Maloof, J.E.; Inouye, D.W. Are nectar robbers cheaters or mutualists? Ecology 2000, 81, 2651–2661. [Google Scholar] [CrossRef]
- Hazlehurst, J.A.; Karubian, J.O. Nectar robbing impacts pollinator behavior but not plant reproduction. Oikos 2016, 125, 1668–1676. [Google Scholar] [CrossRef]
- Carrió, E.; Güemes, J. Nectar robbing does not affect female reproductive success of an endangered Antirrhinum species, Plantaginaceae. Plant Ecol. Divers. 2019, 12, 159–168. [Google Scholar] [CrossRef]
- Ye, Z.M.; Jin, X.F.; Wang, Q.F.; Yang, C.F.; Inouye, D.W. Nectar replenishment maintains the neutral effects of nectar robbing on female reproductive success of Salvia przewalskii (Lamiaceae), a plant pollinated and robbed by bumble bees. Ann. Bot. 2017, 119, 1053–1059. [Google Scholar] [CrossRef]
- Wang, W.C.; Warnock, M.J. Flora of China; Science Press: Beijing, China, 2001; Volume 6, pp. 223–274. [Google Scholar]
- Chang, H.L.; Downie, S.R.; Peng, H.L.; Sun, F.J. Floral organogenesis in three members of the tribe Delphinieae (Ranunculaceae). Plants 2001, 8, 493. [Google Scholar] [CrossRef] [PubMed]
- Endress, P.K. The role of inner staminodes in the floral display of some relic Magnoliales. Plant Syst. Evol. 1984, 146, 269–282. [Google Scholar] [CrossRef]
- Armstrong, J.E.; Irvine, A.K. Functions of staminodia in the beetle-pollinated flowers of Eupomatia laurina. Biotropica 1990, 22, 429–431. [Google Scholar] [CrossRef]
- Edwards, J.; Whitaker, D.; Klionsky, S.; Laskowski, M. A record-breaking pollen catapult. Nature 2005, 435, 164. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, S.A.; Cocucci, A.A. Flower power: Its association with bee power and floral functional morphology in papilionate legumes. Ann. Bot. 2011, 108, 919–931. [Google Scholar] [CrossRef]
- Carlos, L.; Juan, O. Preferential nectar robbing of flowers with long corollas: Experimental studies of two hummingbird species visiting three plant species. Oecologia 2001, 128, 263–273. [Google Scholar]
- Miller, T.E.; Travis, J. The evolutionary role of indirect effects in communities. Proc. Natl. Acad. Sci. USA 1996, 77, 1329–1335. [Google Scholar] [CrossRef]
- Bosch, M.; Simon, J.; Molero, J.; Blanché, C. Reproductive biology, genetic variation and conservation of the rare endemic dysploid Delphinium bolosii (ranunculaceae). Biol. Conserv. 1998, 86, 57–66. [Google Scholar] [CrossRef]
- Bosch, M.; Waser, N.M. Experimental manipulation of plant density and its effect on pollination and reproduction of two confamilial montane herbs. Oecologia 2001, 126, 76–83. [Google Scholar] [CrossRef]
- Duan, Y.W.; He, Y.P.; Liu, J.Q. Reproductive ecology of the qinghai-tibet plateau endemic Gentiana straminea (Gentianaceae), a hermaphrodite perennial characterized by herkogamy and dichogamy. Acta Oecol. 2005, 27, 225–232. [Google Scholar] [CrossRef]
- Kadmon, R.; Shmida, A. Departure rules used by bees foraging for nectar: A field test. Evol. Ecol. 1992, 6, 142–151. [Google Scholar] [CrossRef]
- Klinkhamer, P.G.L.; de Jong, T.J. Attractiveness to pollinators: A plant’s dilemma. Oikos 1993, 66, 180–184. [Google Scholar] [CrossRef]
- Singh, V.K.; Barman, C.; Tandon, R. Nectar robbing positively influences the reproductive success of Tecomella undulata (Bignoniaceae). PLoS ONE 2014, 9, e102607. [Google Scholar] [CrossRef] [PubMed]
- Mattiacci, L.; Dicke, M.; Posthumus, M.A. beta-Glucosidase: An elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc. Natl. Acad. Sci. USA 1995, 92, 2036–2040. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Leclere, S.; Carroll, M.J.; Alborn, H.T.; Teal, P.E.A. Cowpea chloroplastic ATP synthase is the source of multiple plant defense elicitors during insect herbivory. Plant Physiol. 2007, 144, 793–805. [Google Scholar] [CrossRef]
- Guo, H.J.; Wielsch, N.; Hafke, J.B.; Svatos, A.; Mithofer, A.; Boland, W. A porin-like protein from oral secretions of Spodoptera littoralis larvae induces defense-related early events in plant leaves. Insect Biochem. Mol. Biol. 2013, 43, 849–858. [Google Scholar] [CrossRef]
- Chaudhary, R.; Atamian, H.S.; Shen, Z.X.; Briggs, S.P.; Kaloshian, I. GroEL from the endosymbiont Buchnera aphidicola betrays the aphid by triggering plant defense. Proc. Natl. Acad. Sci. USA 2014, 111, 8919–8924. [Google Scholar] [CrossRef]
- Pashalidou, F.G.; Lambert, H.; Peybernes, T.; Mescher, M.C.; De Moraes, C.M. Bumble bees damage plant leaves and accelerate flower production when pollen is scarce. Science 2020, 368, 881–884. [Google Scholar] [CrossRef]
- Winsor, J.A.; Peretz, S.; Stephenson, A.G. Pollen competition in a natural population of Cucurbita foetidissima (Cucurbitaceae). Am. J. Bot. 2000, 87, 527–532. [Google Scholar] [CrossRef]
- Colling, G.; Reckinger, C.; Matthies, D. Effects of pollen quantity and quality on reproduction and offspring vigor in the rare plant Scorzonera humilis (Asteraceae). Am. J. Bot. 2004, 91, 1774–1782. [Google Scholar] [CrossRef]
- Zhang, C.; Zha, S.Q.; Yang, Y.P.; Duan, Y.W. Effects of the yellow barbs of the staminodes on reproductive success of Delphinium caeruleum (Ranunculaceae). Biodivers. Sci. 2012, 20, 348–353. [Google Scholar]
- Shi, J.; Luo, Y.B.; Bernhardt, P.; Ran, J.C.; Liu, Z.J.; Zhou, Q. Pollination by deceit in Paphiopedilum barbigerum (orchidaceae): A staminode exploits the innate colour preferences of Hoverflies (syrphidae). Plant Biol. 2008, 11, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Kirchoff, B.K. Homeosis in the flowers of the zingiberales. Am. J. Bot. 1991, 78, 833–837. [Google Scholar] [CrossRef]
- Plitmann, U.; Raven, P.H.; Breedlove, D.E. The Systematics of Lopezieae (Onagraceae). Ann. Mo. Bot. Gard. 1973, 60, 478–563. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Q.; Wang, T.; Yang, G.; Shao, W.; Min, W.; Zhong, Y. A Decrease in the Staminode-Mediated Visitor Screening Mechanism in Response to Nectar Robbers Positively Affects Reproduction in Delphinium caeruleum Jacq. ex Camb. (Ranunculaceae). Biology 2022, 11, 1203. https://doi.org/10.3390/biology11081203
Hou Q, Wang T, Yang G, Shao W, Min W, Zhong Y. A Decrease in the Staminode-Mediated Visitor Screening Mechanism in Response to Nectar Robbers Positively Affects Reproduction in Delphinium caeruleum Jacq. ex Camb. (Ranunculaceae). Biology. 2022; 11(8):1203. https://doi.org/10.3390/biology11081203
Chicago/Turabian StyleHou, Qinzheng, Taihong Wang, Guang Yang, Wenjuan Shao, Wenrui Min, and Yuqin Zhong. 2022. "A Decrease in the Staminode-Mediated Visitor Screening Mechanism in Response to Nectar Robbers Positively Affects Reproduction in Delphinium caeruleum Jacq. ex Camb. (Ranunculaceae)" Biology 11, no. 8: 1203. https://doi.org/10.3390/biology11081203
APA StyleHou, Q., Wang, T., Yang, G., Shao, W., Min, W., & Zhong, Y. (2022). A Decrease in the Staminode-Mediated Visitor Screening Mechanism in Response to Nectar Robbers Positively Affects Reproduction in Delphinium caeruleum Jacq. ex Camb. (Ranunculaceae). Biology, 11(8), 1203. https://doi.org/10.3390/biology11081203




