The Use of Reproductive Indicators for Conservation Purposes: The Case Study of Palinurus elephas in Two Fully Protected Areas and Their Surrounding Zones (Central-Western Mediterranean)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
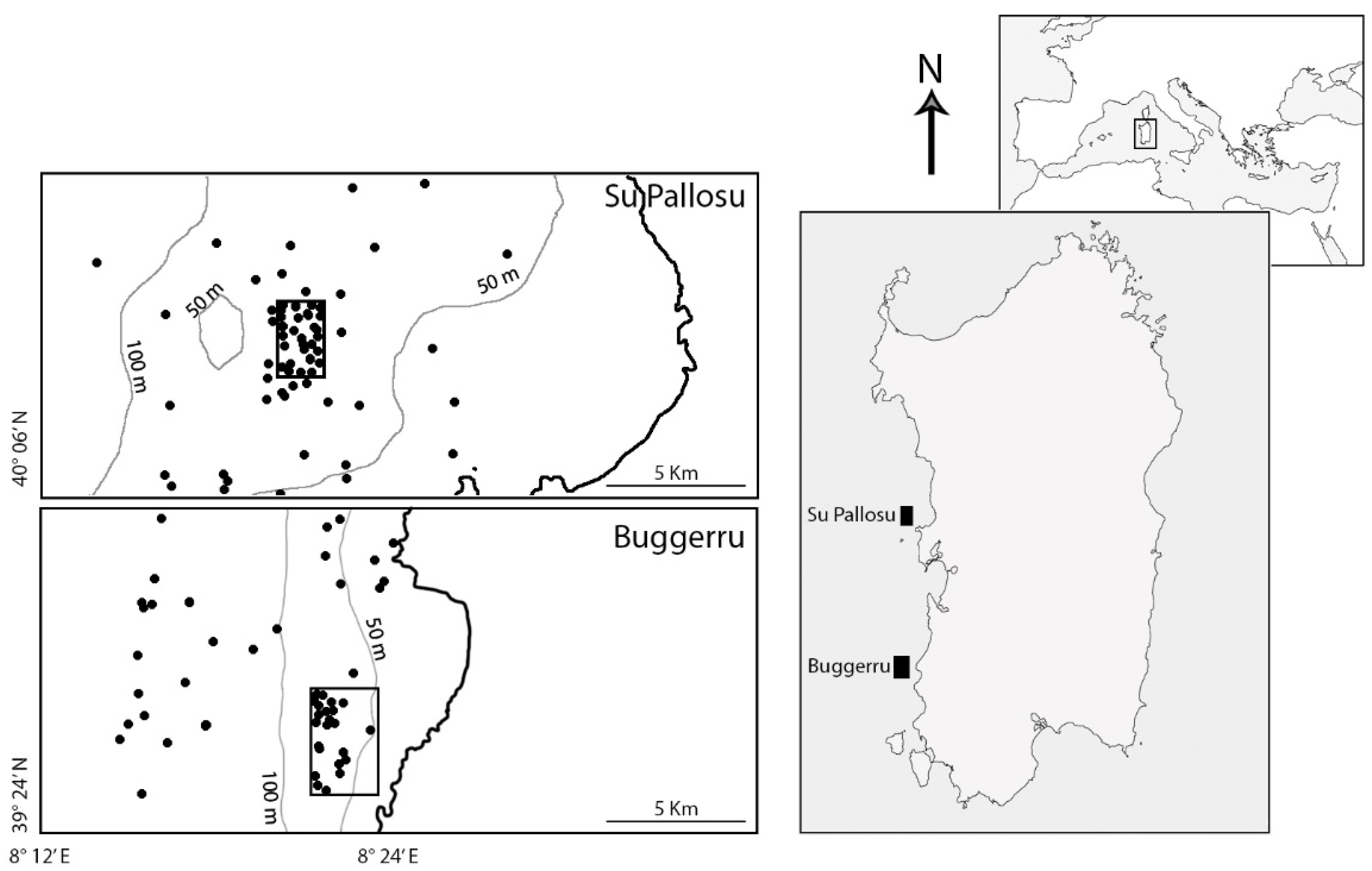
2.1. Study Area and Sampling
2.2. Sample Collection
2.3. Egg Stage, Number and Dimension
2.4. Fecundity Estimation
2.5. Functional Maturity
2.6. Relative Reproductive Potential
2.7. Vitellogenin Concentration
2.8. Index of Egg Production
3. Results
3.1. Egg Stage, Number and Dimension
3.2. Fecundity Estimation
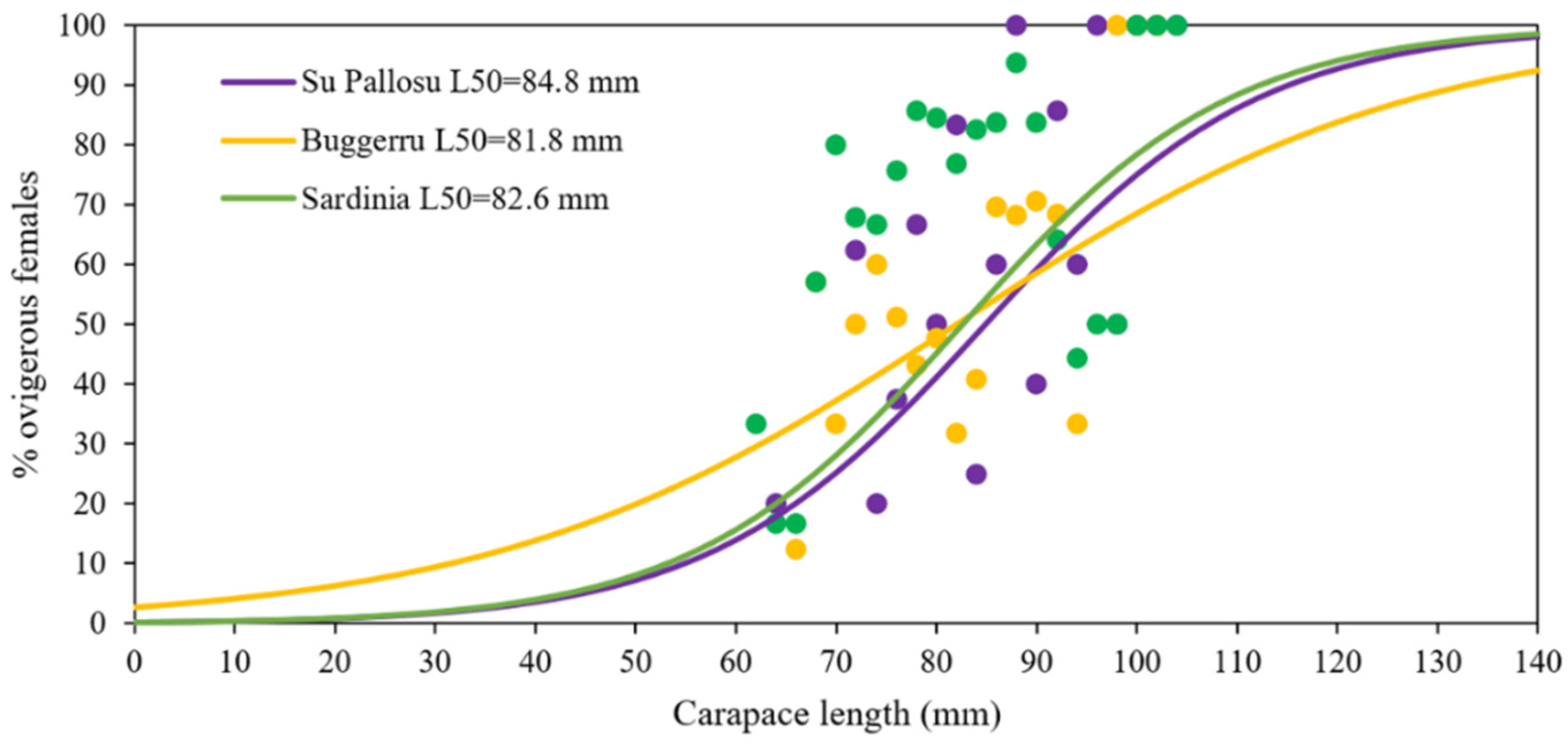
3.3. Functional Maturity
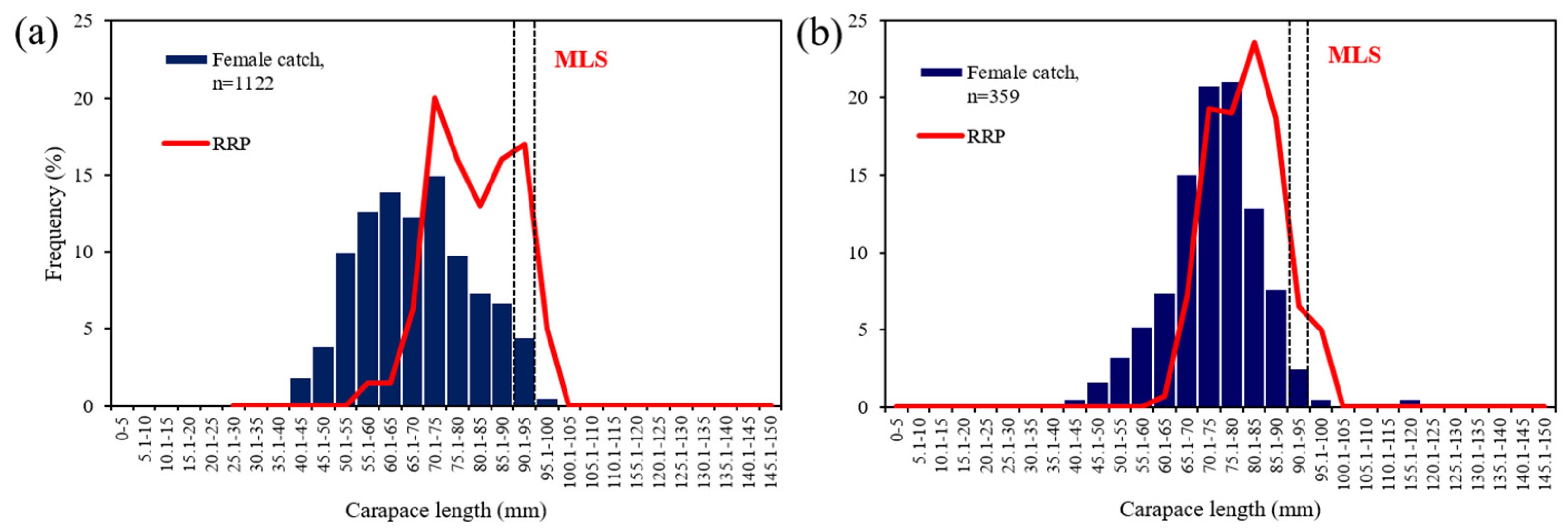
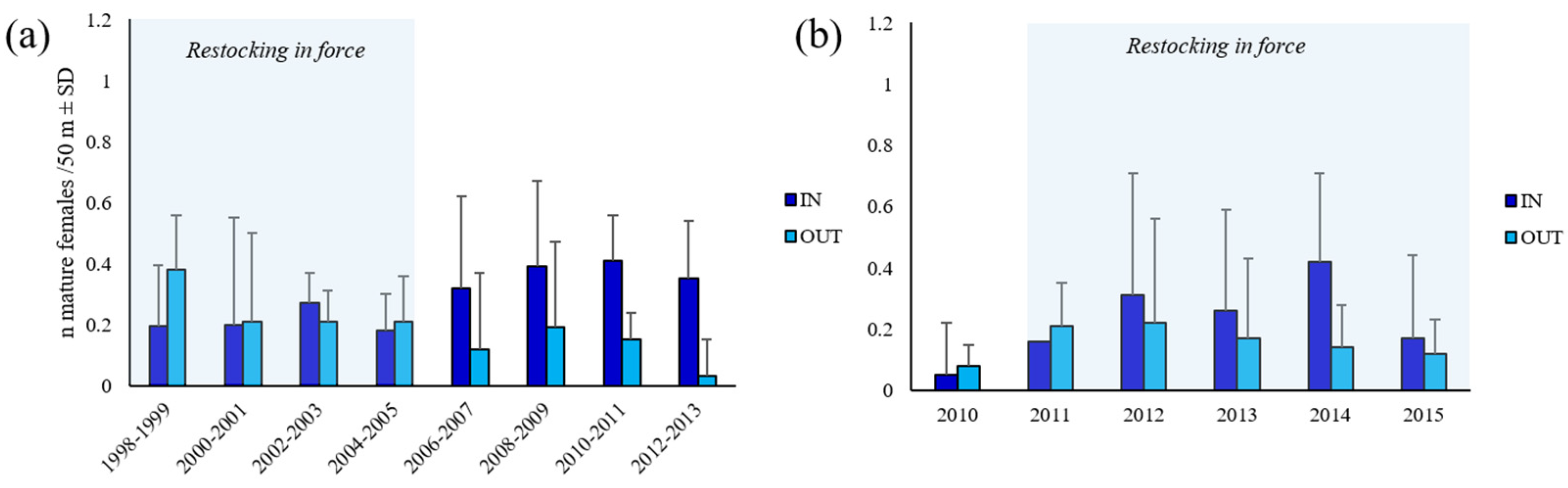
3.4. Relative Reproductive Potential
3.5. Vitellogenin Concentration
3.6. Index of Egg Production (IEP)
4. Discussion
4.1. Fecundity
4.2. Size at Maturity
4.3. Index of Egg Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of Mediterranean and Black Sea Fisheries 2020. General Fisheries Commission for the Mediterranean. 2020. Rome. Available online: https://doi.org/10.4060/cb2429en (accessed on 30 May 2022).
- STECF (Scientific, Technical and Economic Committee for Fisheries). CFP Monitoring—Expansion of indicators (STECF-18-15); JRC114754; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar] [CrossRef]
- Sala, E.; Giakoumi, S. No-take marine reserves are the most effective protected areas in the ocean. ICES J. Mar. Sci. 2018, 75, 1166–1168. [Google Scholar] [CrossRef]
- Maxwell, S.M.; Conners, M.; Gjerde, K.; Crowder, L.B. Mobile protected areas for biodiversity on the high seas. Science 2020, 367, 252–254. [Google Scholar] [CrossRef]
- Babcock, R.C.; Phillips, J.C.; Lourey, A.M.; Clapin, G. Increased density, biomass and egg production in an unfished population of western rock lobster (Panulirus cygnus) at Rottnest Island, Western Australia. Mar. Freshw. Res. 2007, 58, 286–292. [Google Scholar] [CrossRef]
- Cox, C.; Hunt, J.H. Change in size and abundance of Caribbean spiny lobsters Panulirus argus in a marine reserve in the Florida Keys National Marine Sanctuary, USA. Mar. Ecol. Prog. Ser. 2005, 294, 227–239. [Google Scholar] [CrossRef][Green Version]
- Jones, J.M. Assessing Marine Protected Areas as a Conservation Tool: A Decade Later, Are We Continuing to Enhance Lobster Populations at Eastport, Newfoundland? Canadian Technical Report of Fisheries and Aquatic Sciences; Fisheries and Oceans Canada: Ottawa, ON, Canada, 2009.
- Trippel, E.A.; Kjesbu, O.S.; Solemdal, P. Effects of adult age and size structure on reproductive output in marine fishes. In Early Life History and Recruitment in Fish Populations; Chambers, R.C., Trippel, E.A., Eds.; Chapman and Hall: New York, NY, USA, 1997; pp. 31–62. [Google Scholar]
- Vallin, L.; Nissling, A. Maternal effects on egg size and egg buoyancy of the Baltic Cod, Gadus morhua; implications for stock structure effects on recruitment. Fish. Res. 2000, 49, 21–37. [Google Scholar] [CrossRef]
- Nazari, R.M.; Sohrabnejad, M.; Ghomi, M.R.; Modanloo, M.; Ovissipour, M.; Katantarian, H. Correlation between egg size and dependent variables related to larval stage in Persian sturgeon Acipenser persicus. Mar. Freshw. Behav. Physiol. 2009, 42, 147–155. [Google Scholar] [CrossRef]
- Murawski, S.A.; Rago, P.J.; Trippel, E.A. Impacts of demographic variation in spawning characteristics as reference points for fishery management. ICES J. Mar. Sci. 2001, 58, 1002–1014. [Google Scholar] [CrossRef]
- Goñi, R.; Quetglas, A.; Renones, O. Spillover of spiny lobsters Palinurus elephas from a marine reserve to an adjoining fishery. Mar. Ecol. Prog. Ser. 2006, 306, 207–219. [Google Scholar] [CrossRef][Green Version]
- Bertelsen, R.D.; Maxwell, K.E.; Matthews, T.R.; Hunt, J.H. Caribbean spiny lobster (Panulirus argus) movement and population metrics at the Western Sambo Ecological Reserve (Florida USA). In Proceedings of the Linking Science to Management: A Conference & Workshop on the Florida Keys Marine Ecosystem, Duck Key, FL, USA, 19–22 October 2010. [Google Scholar]
- Jack, L.; Wing, S.R. Maintenance of old-growth size structure and fecundity of the red rock lobster Jasus edwardsii among marine protected areas in Fiordland, New Zealand. Mar. Ecol. Prog. Ser. 2010, 404, 161–172. [Google Scholar] [CrossRef]
- Cau, A.; Bellodi, A.; Cannas, R.; Fois, M.; Guidetti, P.; Moccia, D.; Porcu, C.; Pusceddu, A.; Follesa, M.C. European Spiny lobster recovery from overfishing enhanced through active restocking in Fully Protected Areas. Sci. Rep. 2019, 9, 13025. [Google Scholar] [CrossRef] [PubMed]
- Carbonara, P.; Intini, S.; Modugno, E.; Maradonna, F.; Spedicato, M.T.; Lembo, G.; Zupa, W.; Carnevali, O. Reproductive biology characteristics of red mullet (Mullus barbatus L., 1758) in Southern Adriatic Sea and management implications. Aquat. Living Resour. 2015, 28, 21–31. [Google Scholar] [CrossRef][Green Version]
- Jackson, R.L.; Lin, H.Y.; Mao, J.T.; Chan, L.; Means, A.R. Estrogen induction of plasma vitellogenin in the cockerel: Studies with a phosvitin antibody. Endocrinology 1977, 101, 849–857. [Google Scholar] [CrossRef]
- Flouriot, G.; Pakdel, F.; Ducouret, B.; Valotaire, Y. Influence of xenobiotics on rainbow trout liver estrogen receptor and vitellogenin gene expression. J. Mol. Endocrinol. 1995, 15, 143–151. [Google Scholar] [CrossRef]
- Palmer, B.D.; Palmer, S.K. Vitellogenin induction by xenobiotic estrogens in theredeared turtle and African clawed frog. Environ. Health Perspect. 1995, 103, 19–25. [Google Scholar] [PubMed]
- Tahara, D.; Suitoh, K.; Hattori, H. Hemolymph vitellogenin levels during final maturation and postspawning in the female kuruma prawn Marsupenaeus japonicus. Aquaculture 2005, 245, 311–319. [Google Scholar] [CrossRef]
- Ibarra, A.M.; Famula, T.R.; Arcos, F.G. Heritability of vitellogenin in hemolymph, a pre-spawning selectable trait in Penaeus (Litopenaeus) vannamei, has a large genetic correlation with ovary maturity measured as oocytes mean diameter. Aquaculture 2009, 297, 64–69. [Google Scholar] [CrossRef]
- Denslow, N.D.; Chow, M.C.; Green, K.; Green, L. Vitellogenin as a Biomarker of Exposure for Estrogen or Estrogen Mimics. Ecotoxicology 1999, 8, 385–398. [Google Scholar] [CrossRef]
- Barot, S.; Heino, M.; O’Brien, L.; Dieckman, U. Estimation reaction norm for age and size at maturation when age at first reproduction is unknown. Evol. Ecol. Res. 2004, 6, 659–678. [Google Scholar]
- Essington, T.E.; Beaudreau, A.H.; Wiedenmann, J. Fishing through marine food webs. Proc. Natl. Acad. Sci. USA 2006, 103, 3171–3175. [Google Scholar] [CrossRef]
- Gephart, J.A.; Henriksson, P.J.G.; Parker, R.W.R.; Shepon, A.; Gorospe, K.D.; Bergman, K.; Eshel, G.; Golden, C.D.; Halpern, B.S.; Hornborg, S.; et al. Environmental performance of blue foods. Nature 2021, 597, 360–365. [Google Scholar] [CrossRef]
- Groeneveld, J.C. Fecundity of spiny lobster Palinurus gilchristi (Decapoda: Palinuridae) off South Africa. Afr. J. Mar. Sci. 2005, 27, 231–237. [Google Scholar] [CrossRef]
- Spanier, E.; Lavalli, K.L.; Goldstein, J.S.; Groeneveld, J.C.; Jordaan, G.L.; Jones, C.M.; Phillips, B.F.; Bianchini, M.L.; Kibler, R.; Díaz, D.; et al. A concise review of lobster utilization by worldwide human populations from prehistory to the modern era. ICES J. Mar. Sci. 2015, 72, i7–i21. [Google Scholar] [CrossRef]
- Groeneveld, J.C.; Goñi, R.; Díaz, D. Palinurus species. In Lobsters: Biology, Management, Aquaculture & Fisheries, 2nd ed.; Phillips, B.F., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2013; pp. 326–356. [Google Scholar] [CrossRef]
- Santos, R.; Peixoto, U.I.; Medeiros-Leal, W.; Sequeira, R.M.; Novoa-Pabon, A.; Pinho, M. Demographics and Yield–Per–Recruit Assessment of the Vulnerable Spiny Lobster Palinurus elephas in the Azores—Implications for Conservation and Fisheries Management. Biology 2022, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Goñi, R. Palinurus elephas. The IUCN Red List of Threatened Species 2014: e.T169975A1281221. Available online: https://doi.org/10.2305/IUCN.UK.2014-1.RLTS.T169975A1281221.en (accessed on 30 May 2022).
- Hepper, B.T. The fisheries of Crawfish, Palinurus elephas, off the coast of Cornwall. J. Mar. Biol. Assoc. U. K. 1977, 57, 925–941. [Google Scholar] [CrossRef]
- Hunter, E.; Shackley, S.E.; Bennett, D.B. Recent studies on the crawfish Palinurus elephas in South Wales and Cornwall. J. Mar. Biol. Assoc. U. K. 1996, 76, 963–983. [Google Scholar] [CrossRef]
- Goñi, R.; Latrouite, D. Review of the biology, ecology and fisheries of Palinurus species of European waters: Palinurus elephas (Fabricius, 1787) and Palinurus mauritanicus (Gruvel, 1911). Cah. Biol. Mar. 2005, 46, 127–142. [Google Scholar]
- Bevacqua, D.; Melià, P.; Follesa, M.C.; De Leo, G.A.; Gatto, M.; Cau, A. Body growth and mortality of the spiny lobster Palinurus elephas within and outside a small marine protected area. Fish. Res. 2010, 106, 543–554. [Google Scholar] [CrossRef]
- Follesa, M.C.; Cuccu, D.; Cannas, R.; Cabiddu, S.; Murenu, M.; Sabatini, A.; Cau, A. Effects of protection on spiny lobster abundance and size (Palinurus elephas Fabr. 1787) in a Central Western Mediterranean Area. Hydrobiologia 2008, 606, 63–68. [Google Scholar] [CrossRef]
- Follesa, M.C.; Cannas, R.; Cau, A.; Cuccu, D.; Gastoni, A.; Ortu, A.; Pedoni, C.; Porcu, C.; Cau, A. Spillover effects of a Mediterranean marine protected area on the European spiny lobster Palinurus elephas resource. Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 564–572. [Google Scholar] [CrossRef]
- Follesa, M.C.; Cannas, R.; Cau, A.; Cuccu, D.; Mulas, A.; Porcu, C.; Saba, S.; Cau, A. Homing and orientation of Palinurus elephas (Fabricius) in three no-take areas of the central-western Mediterranean: Implications for marine reserve design. Mar. Freshw. Res. 2014, 66, 1–9. [Google Scholar] [CrossRef]
- Secci, E.; Cuccu, D.; Follesa, M.C.; Sabatini, A.; Cau, A. Restocking of Palinurus elephas (Fabr, 1787) in a Western Central Sardinian area. Biol. Mar. Mediterr. 1999, 6, 614–616. [Google Scholar]
- Follesa, M.C.; Cuccu, D.; Cannas, R.; Sabatini, A.; Deiana, A.M.; Cau, A. Movement patterns of the spiny lobster Palinurus elephas (Fabricius, 1787) from a central western Mediterranean protected area. Sci. Mar. 2009, 73, 499–506. [Google Scholar] [CrossRef][Green Version]
- Ballesteros, E. Mediterranean coralligenous assemblages: A synthesis of present knowledge. Oceanogr. Mar. Biol. 2006, 44, 123–195. [Google Scholar]
- Ingrosso, G.; Abbiati, M.; Badalamenti, F.; Bavestrello, G.; Belmonte, G.; Cannas, R.; Benedetti-Cecchi, L.; Bertolino, M.; Bevilacqua, S.; Bianchi, C.N.; et al. Chapter Three–Mediterranean Bioconstructions Along the Italian Coast. Adv. Mar. Biol. 2018, 79, 61–136. [Google Scholar] [CrossRef]
- Goñi, R.; Quetglas, A.; Reñones, O. Size at maturity, fecundity and reproductive potential of a protected population of the spiny lobster Palinurus elephas (Fabricius, 1787) from the Western Mediterranean. Mar. Biol. 2003, 143, 583–592. [Google Scholar] [CrossRef]
- Beyers, C.D.B.; Goosen, P.C. Variation in fecundity of female rock lobster Jasus lalandi in the Benguela ecosystem. S. Afr. J. Mar. Sci. 1987, 5, 513–522. [Google Scholar] [CrossRef]
- Rohlf, F.J. TpsDig, Digitize Landmarks and Outlines, Version 2.16. In Department of Ecology and Evolution; State University of New York at Stony Brook: New York, NY, USA, 2005. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1999; p. 663. [Google Scholar]
- Annala, J.H.; Bycroft, B.L. Fecundity of the New Zealand red rock lobster, Jasus edwardsii. N. Z. J. Mar. Freshw. Res. 1987, 21, 591–597. [Google Scholar] [CrossRef]
- Freitas, R.; Medina, A.; Correia, S.; Castro, M. Reproductive biology of spiny lobster Panulirus regius from the northwestern Cape Verde Islands. Afr. J. Mar. Sci. 2007, 29, 201–208. [Google Scholar] [CrossRef]
- Follesa, M.C.; Cuccu, D.; Cannas, R.; Cau, A. On the growth of the European spiny lobster, Palinurus elephas (Fabricius, 1787) in Sardinian seas (central western Mediterranean). N. Z. J. Mar. Freshw. Res. 2007, 41, 377–383. [Google Scholar] [CrossRef][Green Version]
- Fabens, A.J. Properties and fitting of the von Bertalanffy growth curve. Growth 1965, 29, 265–289. [Google Scholar]
- Gayanilo, F.C.; Sparre, P.; Pauly, D. FISAT FAO-ICLARM Stock Assessment Tools. User’s Manual; FAO Computerised Information Series; Food and Agriculture Organization of the United Nations: Rome, Italy, 1997; 262 p. [Google Scholar]
- Gulland, J.A.; Holt, S.J. Estimation of growth parameters for data at unequal time intervals. J. Cons. Int. Explor. Mer. 1959, 25, 47–49. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2017. Available online: https://www.R-project.org/ (accessed on 30 May 2022).
- ICES. Report of the Workshop on Maturity Ogive Estimation for Stock Assessment (WKMOG). In Proceedings of the Workshop on Maturity Ogive Estimation for Stock Assessment (WKMOG), Lisbon, Portugal, 3–6 June 2008; p. 72. [Google Scholar]
- Hobday, D.K.; Ryan, T.J. Contrasting sizes at sexual maturity of southern rock lobsters (Jasus edwardsii) in two Victorian fishing zones: Implications for total egg production and management. Mar. Ecol. Prog. Ser. 1997, 48, 1009–1014. [Google Scholar] [CrossRef]
- Tsukimura, B.; Waddy, S.L.; Vogel, J.M.; Linder, C.J.; Bauer, D.K.; Borst, D.W. Characterization and Quantification of Yolk Proteins in the Lobster, Homarus americanus. J. Exp. Zool. 2002, 292, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Cannea, F.B.; Follesa, M.C.; Porcu, C.; Rossino, R.; Olianas, A.; Rescigno, A.; Padiglia, P. Antibodies targeting the European lobster (Palinurus elephas) vitellogenin developed by mRNA isolation and in-silico-designed antigenic peptides. Biol. Open 2022, 11, bio059019. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA + for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Lenihan, H.S.; Gallagher, J.P.; Peters, J.R.; Stier, A.C.; Hofmeister, J.K.K.; Reed, D.C. Evidence that spillover from Marine Protected Areas benefits the spiny lobster (Panulirus interruptus) fishery in southern California. Sci. Rep. 2021, 11, 2663. [Google Scholar] [CrossRef] [PubMed]
- Russ, G.R. Yet another review of marine reserves as reef fishery management tools. In Coral Reef Fishes; Sale, P., Ed.; Academic Press: San Diego, CA, USA, 2002; p. 549. [Google Scholar]
- Rogers-Bennett, L.; Leaf, R.T. Elasticity analyses of size-based red and white Abalone matrix models: Management and conservation. Ecol. Appl. 2006, 16, 213–224. [Google Scholar] [CrossRef]
- Diíaz, D.; Mallol, S.; Parma, A.M.; Gonñi, R. Decadal trend in lobster reproductive output from a temperate marine protected area. Mar. Ecol. Prog. Ser. 2011, 433, 149–157. [Google Scholar] [CrossRef]
- Mercer, J.P. Studies on the Spiny Lobsters (Crustacea: Decapoda: Palinuridae) of the West Coast of Ireland with Particular Reference to Palinurus elephas, Fabricius 1787. Ph.D. Thesis, University College Galway, Galway, Ireland, 1973. [Google Scholar]
- Campillo, A. Premières données sur la pêche et la biologie de la langouste de Corse, Palinurus elephas, Fabricius. Quad. Lab. Tecnol. Pesca 1982, 3, 115–139. [Google Scholar]
- DeMartini, E.E.; DiNardo, G.T.; Williams, H.A. Temporal changes in population density, fecundity, and egg size of the Hawaiian spiny lobster (Panulirus marginatus) at Necker Bank, northwestern Hawaiian Islands. Fish. Bull. 2003, 101, 22–31. [Google Scholar]
- Chang, Y.J.; Sun, C.L.; Chen, Y.; Yeh, S.Z.; Chiang, W.C. Reproductive biology of the spiny lobster, Panulirus penicillatus, in the southeastern coastal waters off Taiwan. Mar. Biol. 2007, 151, 553–564. [Google Scholar] [CrossRef]
- Green, B.S.; Gardner, C.; Kennedy, R.B. Generalised linear modelling of fecundity at length in southern rock lobsters, Jasus edwardsii. Mar. Biol. 2009, 156, 1941–1947. [Google Scholar] [CrossRef]
- Linnane, A.; Penny, S.; Hawthorne, P.; Hoare, M. Spatial differences in size of maturity and reproductive potential between inshore and offshore fisheries for southern rock lobster (Jasus edwardsii) in South Australia. Fish. Res. 2009, 96, 238–243. [Google Scholar] [CrossRef]
- Aiken, D.E.; Waddy, S.L. Reproductive biology. In The Biology and Management of Lobsters; Cobb, J.S., Phillips, B.F., Eds.; Academic Press: New York, NY, USA, 1980; pp. 215–276. [Google Scholar]
- Marin, J. La langouste Rouge: Biologie et Exploitation; Pêche Maritime: Paris, France, 1985; pp. 105–113. [Google Scholar]
- Gonñi, R.; Hilborn, R.; Diíaz, D.; Mallol, S.; Adlerstein, S. Net contribution of spillover from a marine reserve to fishery catches. Mar. Ecol. Prog. Ser. 2010, 400, 233–243. [Google Scholar] [CrossRef]
- Marin, J. Exploitation, Biologie et Dynamique du Stock de Langouste Rouge de Corse. Ph.D. Thesis, Universitéd’Aix-Marseille II, Marseille, France, 1987. [Google Scholar]
- Polovina, J.J. Density dependence in spiny lobster, Panulirus marginatus, in the northwestern Hawaiian Islands. Can. J. Fish. Aquat. Sci. 1989, 46, 660–665. [Google Scholar] [CrossRef]
- Chubb, C.F. Reproductive biology: Issues for management. In Spiny Lobsters: Fisheries and Culture, 2nd ed.; Phillips, B.F., Kittaka, J., Eds.; Fishing New Books (Blackwell): Oxford, UK, 2000; pp. 245–275. [Google Scholar]
- Kanciruk, P. Ecology of juvenile and adult spiny lobsters. In The Biology and Management of Lobsters. Ecology and Management; Cobb, J.S., Phillips, B.F., Eds.; Academic Press: New York, NY, USA, 1980; Volume 2, pp. 59–92. [Google Scholar]
- Annala, J.H. Factors influencing fecundity and population egg production of Jasus species. In Crustacean Egg Production; Wenner, A., Kuris, A., Eds.; Balkema: Rotterdam, The Netherlands, 1991; Volume 7, pp. 301–315. [Google Scholar]
- Waddy, S.L.; Aiken, D.E. Egg production in American lobster, Homarus americanus. In Crustacean Egg Production; Wenner, A., Kuris, A., Eds.; Balkema: Rotterdam, The Netherlands, 1991; Volume 7, pp. 267–290. [Google Scholar]
- DeMartini, E.E.; Ellis, D.M.; Honda, V.A. Comparisons of spiny lobsters Panulirus marginatus fecundity, egg, size, and spawning frequency before and after exploitation. Fish. Bull. 1993, 91, 1–7. [Google Scholar]
- MacDiarmid, A.B.; Breen, P.A. Spiny lobster population change in a marine reserve. In Proceedings of the Second International Temperate Reef Symposium, Auckland, New Zealand, 7–10 January 1992; Battershill, C.N., Schiel, D.R., Jones, G.P., Creese, R.G., MacDiarmid, A.B., Eds.; National Institute of Water and Atmospheric Research: Wellington, New Zealand, 1992; pp. 47–56. [Google Scholar]
- Follesa, M.C.; Locci, I.; Pesci, P.; Floris, E.; Cau, A. GSA 11—Sardinian seas. First section. Chapter 2—Ecological aspects. In The State of Italian Marine Fisheries and Aquaculture; Cautadella, S., Spagnolo, M., Eds.; Ministero delle Politiche Agricole, Alimentari e Forestali (MIPAAF): Rome, Italy, 2011; p. 618. [Google Scholar]
- Butler, M.; Bertelsen, R.; MacDiarmid, A. Mate choice in temperate and tropical spiny lobsters with contrasting reproductive systems. ICES J. Mar. Sci. 2015, 72, 101–114. [Google Scholar] [CrossRef]
- Whomersley, P.; Van der Molen, J.; Holt, D.; Trundle, C.; Clark, S.; Fletcher, D. Modeling the Dispersal of Spiny Lobster (Palinurus elephas) Larvae: Implications for Future Fisheries Management and Conservation Measures. Front. Mar. Sci. 2018, 5, 58. [Google Scholar] [CrossRef]
- Babbucci, M.; Buccoli, S.; Cau, A.; Cannas, R.; Goñi, R.; Díaz, D.; Marcato, S.; Zane, L.; Patarnello, T. Population structure, demographic history, and selective processes: Contrasting evidences from mitochondrial and nuclear markers in the European spiny lobster Palinurus elephas (Fabricus, 1787). Mol. Phylogenet. Evol. 2010, 56, 1040–1050. [Google Scholar] [CrossRef]
- Planes, S.; Jones, G.P.; Thornold, S.R. Larval dispersal connects fish populations in a network of marine protected areas. Proc. Natl. Acad. Sci. USA 2009, 106, 5693–5697. [Google Scholar] [CrossRef] [PubMed]
- Sørdalen, T.K.; Halvorsen, K.T.; Harrison, H.B.; Ellis, C.D.; Asbjørn Vøllestad, L.; Knutsen, H.; Moland, E.; Olsen, E.M. Harvesting changes mating behaviour in European lobster. Evol. Appl. 2018, 11, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.N.; Butler, M.J., IV. Mate choice and sperm limitation in the spotted spiny lobster, Panulirus guttatus. Mar. Biol. Res. 2013, 9, 69–76. [Google Scholar] [CrossRef]
- MacDiarmid, A.B.; Butler, M.J., IV. Sperm Economy and Limitation in Spiny Lobsters. Behav. Ecol. Sociobiol. 1999, 46, 14–24. [Google Scholar] [CrossRef]




| FPA | Depth Range (m) | Year | N Fishing Sets | N F Released |
|---|---|---|---|---|
| Buggerru | 40–70 | 2010 | 6 | - |
| 2011 | 9 | 567 | ||
| 2012 | 41 | 603 | ||
| 2013 | 14 | 167 | ||
| 2014 | 16 | 251 | ||
| 2015 | 33 | 249 | ||
| Su Pallosu | 50–80 | 1998 | 22 | 479 |
| 1999 | 40 | 324 | ||
| 2000 | 39 | 450 | ||
| 2001 | 33 | 173 | ||
| 2002 | 32 | 35 | ||
| 2003 | 26 | 9 | ||
| 2004 | 32 | 199 | ||
| 2005 | 26 | 63 | ||
| 2006 | 24 | - | ||
| 2007 | 36 | - | ||
| 2008 | 186 | - | ||
| 2009 | 57 | - | ||
| 2010 | 47 | - | ||
| 2011 | 110 | - | ||
| 2012 | 101 | - | ||
| 2013 | 32 | - |
| FPA | N | Range CL (mm) | CL–Fecundity Relationship | r2 |
|---|---|---|---|---|
| Su Pallosu | ||||
| IN | 34 | 63.6–98.1 | F = 1882 × CL − 108,073 | 0.84 |
| OUT | 38 | 66.4–100.4 | F = 2414.8 × CL − 148,291 | 0.82 |
| Buggerru | ||||
| IN | 29 | 64.0–96.7 | F = 1790 × CL − 98,190 | 0.85 |
| OUT | 29 | 71.5–97.2 | F = 2227.6 × CL − 133,389 | 0.78 |
| FPA | L50 (CL, mm) ± s.e. | MR ± s.e |
|---|---|---|
| Su Pallosu | ||
| IN | 85.4 ± 3.2 | 29.4 ± 8.6 |
| OUT | 83.0 ± 5.3 | 16.7 ± 6.4 |
| IN + OUT | 84.8 ± 4.1 | 20.3 ± 7.5 |
| Buggerru | ||
| IN | 81.2 ± 19.9 | 25.6 ± 5.6 |
| OUT | 80.0 ± 14.0 | 23.4 ± 4.1 |
| IN + OUT | 81.8 ± 15.0 | 32.88 ± 6.1 |
| Sardinia | 82.6 ± 1.63 | 26.4 ± 4.5 |
| Area | N | CL Range (mm) | Eggs Stage | VTG Range (ng/mL) | VTG Mean ± SD (ng/mL) |
|---|---|---|---|---|---|
| IN | 16 | 60.9–84.8 | 2 | 120.93–252.71 | 177.01 ± 43.28 |
| OUT | 14 | 68.8–100.3 | 2 | 78.88–256.45 | 187.44 ± 49.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcu, C.; Carugati, L.; Bellodi, A.; Carbonara, P.; Cau, A.; Cuccu, D.; Cannea, F.B.; Marongiu, M.F.; Mulas, A.; Padiglia, A.; et al. The Use of Reproductive Indicators for Conservation Purposes: The Case Study of Palinurus elephas in Two Fully Protected Areas and Their Surrounding Zones (Central-Western Mediterranean). Biology 2022, 11, 1188. https://doi.org/10.3390/biology11081188
Porcu C, Carugati L, Bellodi A, Carbonara P, Cau A, Cuccu D, Cannea FB, Marongiu MF, Mulas A, Padiglia A, et al. The Use of Reproductive Indicators for Conservation Purposes: The Case Study of Palinurus elephas in Two Fully Protected Areas and Their Surrounding Zones (Central-Western Mediterranean). Biology. 2022; 11(8):1188. https://doi.org/10.3390/biology11081188
Chicago/Turabian StylePorcu, Cristina, Laura Carugati, Andrea Bellodi, Pierluigi Carbonara, Alessandro Cau, Danila Cuccu, Faustina Barbara Cannea, Martina Francesca Marongiu, Antonello Mulas, Alessandra Padiglia, and et al. 2022. "The Use of Reproductive Indicators for Conservation Purposes: The Case Study of Palinurus elephas in Two Fully Protected Areas and Their Surrounding Zones (Central-Western Mediterranean)" Biology 11, no. 8: 1188. https://doi.org/10.3390/biology11081188
APA StylePorcu, C., Carugati, L., Bellodi, A., Carbonara, P., Cau, A., Cuccu, D., Cannea, F. B., Marongiu, M. F., Mulas, A., Padiglia, A., Pascale, N., Pesci, P., & Follesa, M. C. (2022). The Use of Reproductive Indicators for Conservation Purposes: The Case Study of Palinurus elephas in Two Fully Protected Areas and Their Surrounding Zones (Central-Western Mediterranean). Biology, 11(8), 1188. https://doi.org/10.3390/biology11081188











