1. Introduction
The Central Nervous System (CNS) is a basic biological system which includes three vital organs: neurons, synapses, and glias [
1,
2]. In this network, neurons are responsible for information processing and transformation of data in different parts of the human brain. By neurons switching, the essential informations are transferred to the brain parts [
3,
4,
5]. Neurons are high-speed organs that have high number of different connections for transferring data [
6]. On the other hand, synapses are the connections between two neurons. These connections causes the coupling of two connected neurons. These coupled neurons are basic submodules of the CNS that can be realized in real states [
7,
8,
9,
10]. Furthermore, glias are the protection-based cells for neurons and regulate the coupling behaviors between different neuron connections. One of the basic forms of the glias in the brain are astrocytes, which are responsible for protecting and regulating neurons behavior [
3,
4,
9]. From this standpoint, investigating neurons dynamic can be a vital requirements in case of neuromorphic engineering. Thus, modeling of neural dynamics and spiking neural network mechanisms have been a strong tool in analyzing and processing behavior of biological neural networks.
Interaction between these basic organs in the brain corresponds to transferring data, memory and learning [
11,
12,
13,
14]. In this approach, the important and basic block is neurons with a large number of connections. A large number of connected neurons with thousands of connection in the brain makes them vital organ in case of transferring data and informations. In this way, this important part must be investigated and analyzed to achieve an efficient system for designing real organs. These neurons have several functional roles, such as receiving, transmitting and analyzing data for producing voltage signals in all parts of the human brain [
3,
4,
5,
6]. These behaviors can be formulated by some mathematical Equations [
15,
16,
17,
18]. The basic signal behaviors of the biological neurons are modeled by these mathematical equations. In neuron modeling, two approaches are selected: first, the models which are based on the biological neurons behavior, such as Hodgkin-Huxley (HH) [
2] or the ADEX neuron model [
5,
6,
7,
8], and the second is based on spike state modeling, such as Izhikevich [
3,
4,
11,
12].
Among the above list, the Izhikevich model is a computationally reasonable neuronal model that is capable of reproducing all spiking patterns. This model generates all patterns of brain spikes, which is a very significant issue in the neural networks [
1,
3,
4]. On the other hand, the HH model is a full-biological neuron model with a high number of equations and terms and is a high-cost neuronal model [
2]. It may be not acceptable for implementing in hardware form because of its high overhead costs. On the other hand, the ADEX model is a cost-effective and biological neuron model that has the both behaviors of the Izhikevich and HH models at the same time, and it is an appropriate choice in case of digital design and realization [
5,
6].
To realize the neuronal models in hardware state, there are different choices. To achieve a hardware implementation of the neuronal models, we have two cases: analog implementation and digital realization. For realizing in the analog case, CMOS Components are used for designing an analog approach in case of following the mechanisms of neuronal models. This process is high performance in case of speed, but long development time is its disadvantage. Moreover, two factors in digital implementation, high-silicon area and power are required, but this procedure is efficient compared by other approaches [
1,
2,
3,
4,
5,
6]. The advantage of digital approach is its flexible attributes, time-down in processing and power supply. Using reconfigurable digital boards, such as FPGAs cause the speed-up and flexibility in this case [
8,
9,
10,
11,
12]. In the field of neural networks implementation, digital approach may be a suitable procedure [
1,
3,
4,
5,
6,
7,
8]. Indeed, because of high-speed switching in the neuronal networks, it is required to have a accelerate hardware, which is capable of regenerating the same behavior and performance of the human neuronal cells. Different approaches are considered for implementing the neural networks in hardware state in the literature [
10,
11,
12,
13,
14]. In the field of neuronal realization, since we have high-frequency networks, the digital way is a better solution. On the other hand, it is best suited for computing and digital electronics, and is less affected since noise response are analog in nature, digital hardware is flexible in implementation and digital instruments are free from observational errors such as parallax and approximation errors (compared to the analog approach). The implementation of the neuronal cells using configurable platforms such as FPGA is an attractive area in terms of low overhead costs and high-frequency digital systems [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10]. FPGA is an integrated circuit designed to be configured by a customer or a designer after manufacturing, hence the term field-programmable. The FPGA configuration is generally specified using a Hardware Description Language (HDL), similar to that used for an Application-Specific Integrated Circuit (ASIC). Circuit diagrams were previously used to specify the configuration, but this is increasingly rare due to the advent of electronic design automation tools. FPGAs are flexible and high-speed platforms which are suitable approaches for the digital realization of neuronal networks.
Different neuronal models have been realized on FPGA hardware boards. In this approach, Yaghini Bonabi et al. [
2] proposed the FPGA implementation of the HH neuronal model using different methods. Moreover, Haghiri et al. [
3,
4], Nazari et al. [
14] and Soleimani et al. [
9] presented digital FPGA realization of coupled neurons with astrocyte cells. Furthermore, Soleimani et al. [
12] proposed a digital effective realization of Izhikevich neuron model in low-cost and high-frequency state. In other words, the ADEX neuron model is implemented in the literature [
5,
6,
7,
8]. In detail, Gomar et al. [
5] proposed power-2-based implementation of the ADEX model without any multiplications. Furthermore, Haghiri et al. [
6] presented a novel realization of the ADEX neuronal model using full-matching approximation and low-error calculations. Gomar et al. [
7] also proposed another approach for implementing this model using a CPG-based method and Heidarpour et al. [
8] presented a CORDIC approach for ADEX realization. In all of these papers, the authors use an approximation method to realize the final hardware. This may causes different levels of errors of two original and proposed approaches and affect the final digital hardware.
As a comparison between ADEX implementation, Gomar in [
5] proposes a power-2-based function for approximating the exponential term. This conversion may cause error levels between the original and proposed models, and also based on Table 7, speed-up and overall saving are reduced. Furthermore, in this approximation, the accuracy of fraction part of modification is low and this affects on the FPGA realization. On the other hand, Gomar in [
7] also implemented the ADEX model in a multiplierless state. As can be seen, in this approach, she also used the power-2-based method, which is low-accurate in case of fraction part calculation. In [
6], Haghiri proposed a high-accurate approximation of the ADEX model using a power-2-based function with a high degree of similarity. In their approach, the accuracy of matching is increased, but the FPGA cost is also increased; additionally, some levels of errors between the original and proposed spiking patterns can be observed. Finally, Heidarpour in [
8] presented the CORDIC algorithm for approximating the ADEX neuron model. In their way, although the accuracy will be increased, the overall saving in FPGA will be significantly decreased. This issue may affects on large number of implemented neurons on an FPGA core.
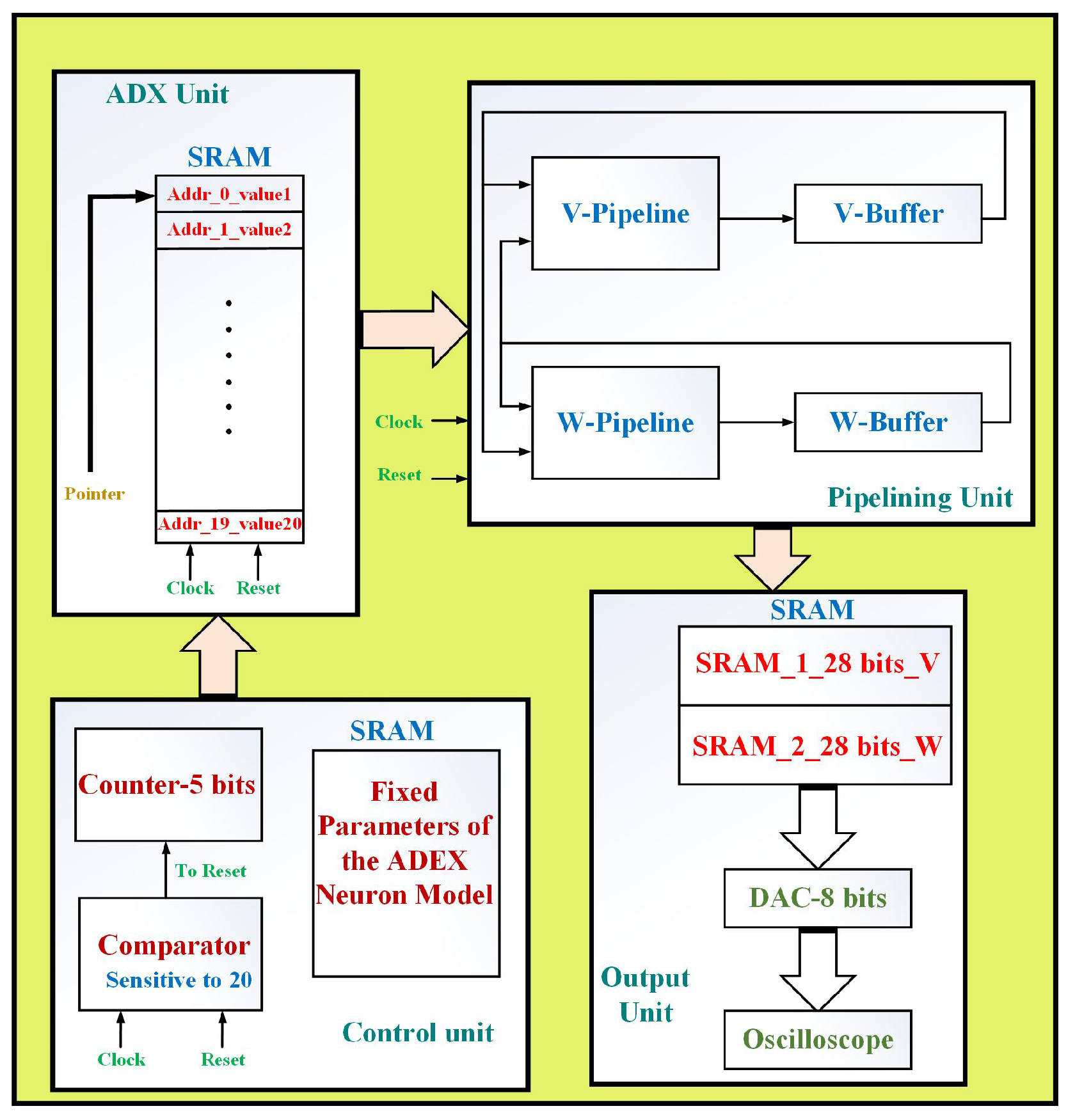
The general technique for realizing the neuron models are clarified. Capable realization of biological neuronal networks are important. In biology approach, the experimental view of neuroscience are considered to have a basic inspiration of the brain architecture. Thus, studying hardware realization of these biological-like systems can be a necessary goal. At first, the neuronal modeling can be suited. Large number of neuron models are existed for SNNs based on different dynamical mechanisms. For example, the ADEX modeling is acceptable one and capable for duplicating different patterns of spikes in the brain. In the next step, the selected model can be validated in case of timing analysis and dynamics. Since the basic (original) models have high-cost functions, it is required that modification is done to achieve a low-area and cost modeling in hardware implementation. After that, the proposed model must be validated in terms of following the all aspects of original behaviors in MATLAB software. At the second step, to evaluate the proposed approach (in hardware consideration), the FPGA boards are applied in hardware case. Indeed, the Hardware Description Language (HDL) is considered for the proposed neuronal model in ModelSim and ISE Xilinx software’s. In this case, resource utilization and costs of the original and proposed models are compared in case of digital implementation. Two basic factors in this consideration (overall saving in FPGA resources and speed-up or frequency) are compared and the validated that the proposed model is in the better state of digital realization.
This paper presents the N-LUT-ADEX (Nyquist-Look Up Table-ADEX) model, which is based on accurate sampling of the original model. Since in this model, high-accuracy matching is achieved, it can recreate a large number of spike patterns in terms of the high similarity state with the original model and also reducing the final overhead costs compared to other papers. Indeed, by accurate sampling of the nonlinear terms of the original ADEX model, the final computational error will be significantly reduced. In this approach, the Nyquist frequency converts a continuous term to a discrete one. When the bandwidth of a signal is lower than the Nyquist frequency of the sampling, the equivalent sample rate is overhead the Nyquist ratio for that certain signal. Using this procedure, the nonlinear term of the original ADEX neuron model (which is an exponential term with high-cost state for digital hardware realization) can be replaced by LUT-based memories. This model can be implemented in hardware state without using any multipliers and other nonlinear terms. In other words, these nonlinear functions are high-cost and low-frequency blocks and by replacing them with some LUT-based terms, the frequency (speed) of digital system will be increased. Then, the overhead costs are significantly reduced. Two basic factors in neural networks implementations are: large-scale approach and high-speed switching. Indeed, to achieve a real and nature-inspired neural system, these two factors must be taken into account. Consequently, using this new model, we have an efficient and modified model that can be considered for implementation in biological neural networks.
2. ADEX Neuron
Adaptive Exponential integrate-and-fire (ADEX) neuron is based on coupling mathematical formulation for the voltage variable and the recovery one [
5,
6,
7,
8]. This biological neuron model is described by two coupled differential equations as follows:
By the following reset equations:
where
The first equation denotes the dynamics of membrane potential and includes an activation term with an exponential voltage dependence. Voltage is coupled to a second equation, which describes adaptation. Both variables are reset if an action potential has been triggered. The combination of adaptation and exponential voltage dependence gives rise to the name adaptive exponential integrate-and-fire model. The adaptive exponential integrate-and-fire model is capable of describing known neuronal firing patterns, e.g., adapting, bursting, delayed spike initiation, initial bursting, fast spiking and regular spiking. Different ADEX parameters are described as:
C: Capacitance of the membrane voltage (pF);
: Leakage conductance (ns);
: Potential of rest in effective state (mV);
: Threshold factor (mV);
: Potential of threshold (mV);
: Potential of rest (mV);
: Time of adaptation (ms);
a: Adaptation of subthreshold (ns);
b: Adaptation of splike-trigger (pA);
I: Stimulus current (pA).
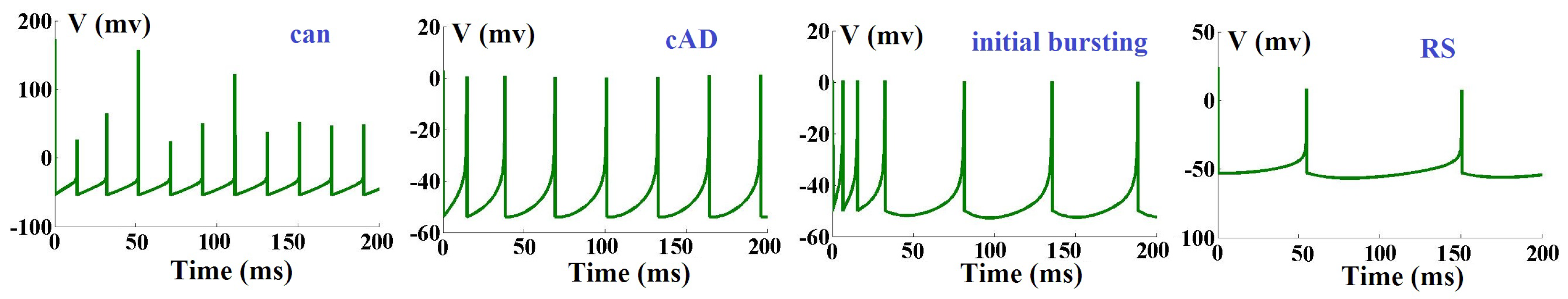
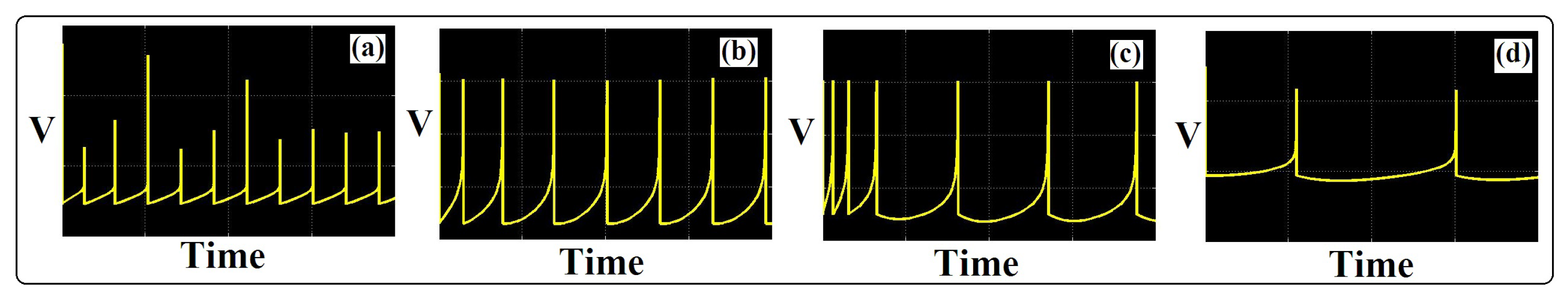
Furthermore, as depicted in
Table 1, based on the variation of different ADEX parameters, we have eight spiking patterns. Based on
Table 1, some spiking behaviors of the original ADEX neuron model are illustrated in
Figure 1.
On the other hand, the first equation determines the membrane voltage and the injected DC current represents the input to the neuron. The variable represents the membrane potential of neuron and denotes an adaptation current variable. When the membrane voltage crosses its apex
, the voltage and adaptation variables will be reset according to an auxiliary. Furthermore, by scaling the parameters, the model can reproduce different types of spiking patterns, such as adapting, bursting, delayed spike initiation, initial bursting, fast spiking and regular spiking. The data presented in this study are available in
Supplementary Material.
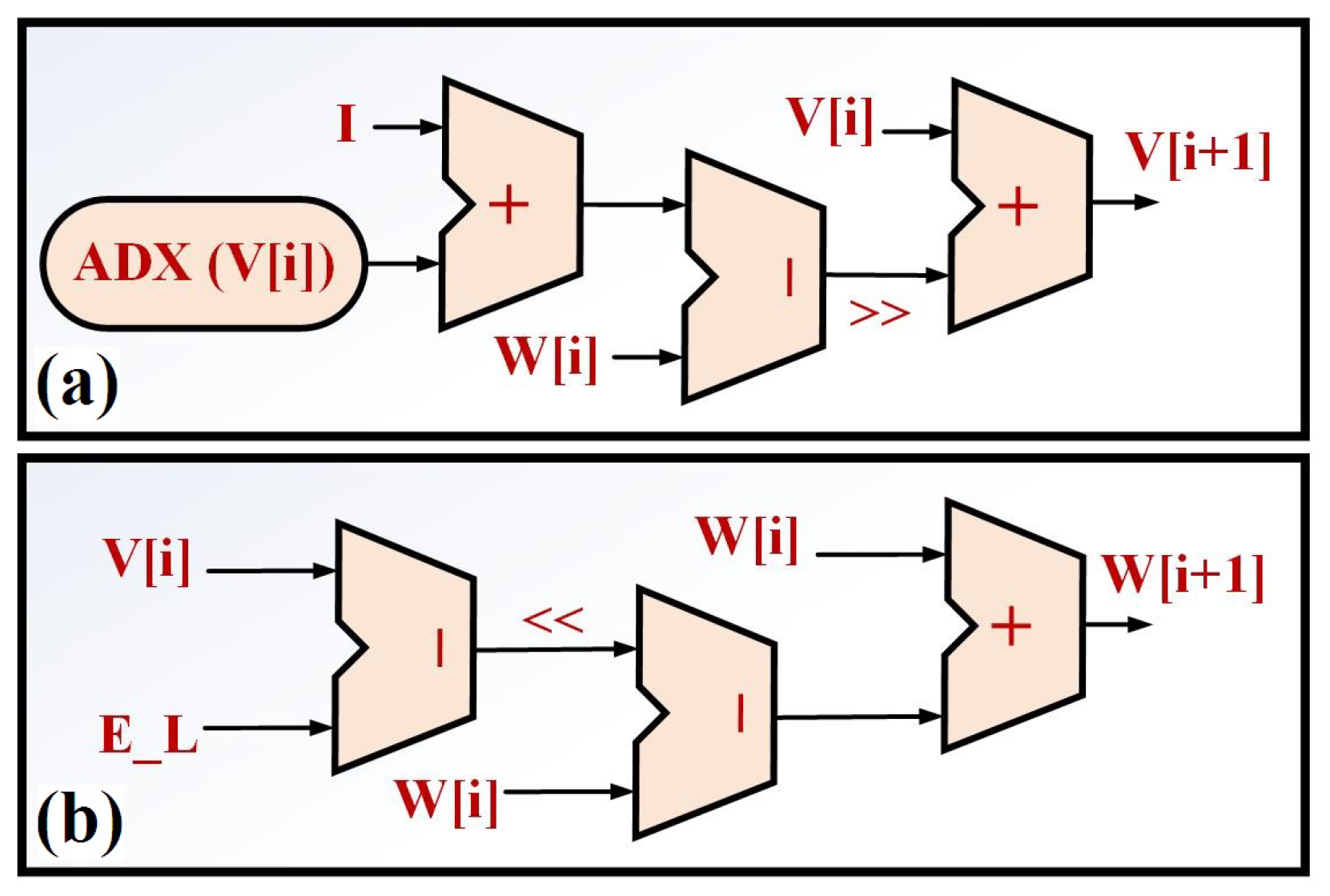
3. N-LUT-ADEX Modeling
To have a reduced-cost and high-speed biological neural network, the original nonlinear terms of the neuronal models must be approximated or replaced by simple and efficient terms. In this methodology, if the original high-cost model is modified to modified modeling (such the original model behaviors in all consideration), the large-scale implementation can be realized. In this paper, we have presented the N-LUT-ADEX (Nyquist-Look Up Table-ADEX) model, which is an accurate and cost-reduced approach compared to the original ADEX model. In the new approach, ADEX neuronal modeling is reformulated as below:
where
After a continuous signal,
is sampled at a constant ratio,
samples. There is a continual limitless numeral of additional continuous terms that fits a similar set of samples. In terms of the terms bandwidth
, the Nyquist standard is regularly specified as
. In this method,
is named the Nyquist rate for functions with bandwidth
[
19].
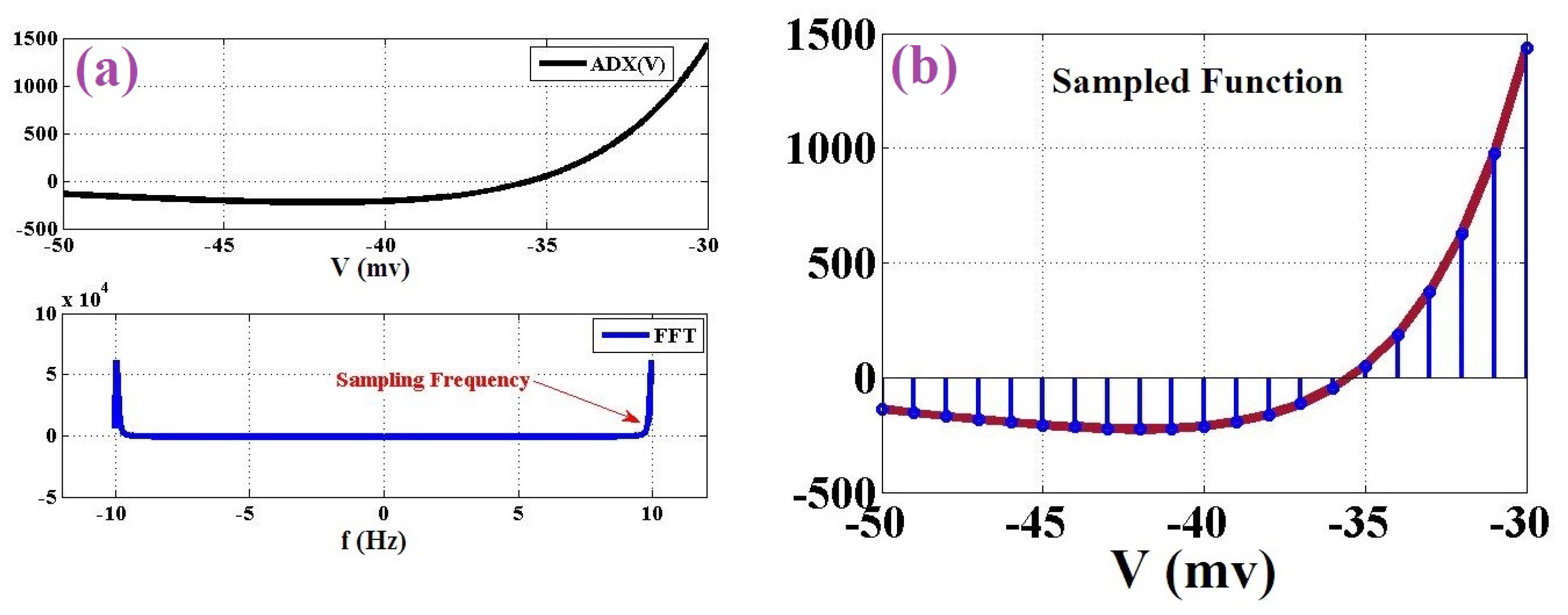
In our method, as can be seen in Equation (
7), the
function is considered the objective function. At first, the Fast Fourier Transform (FFT) of this function is evaluated in case of sampling frequency. Based on the Nyquist rate, if we have the peak frequency of the function, it can be discretized to some points in the range of the voltage variable, and then, this digitized points will eventually reconstruct the objective function. The objective function (
) and its FFT have been illustrated in
Figure 2a. As can be seen in this figure, the base frequency of the objective function is about
. Thus, this function is sampled by the Nyquist rate method for at least twice as much of this rate (at least 20 points of sampling). In this modification, the basic criteria for design and development of the final system are achieving a low-resources-cost, high-frequency (speed) and low-error digital design in comparison to the original basic modeling. As a result, by modifying the original ADEX neuron model, we have new high-speed, low-error and low-area hardware that can be implemented on FPGA boards as a compact digital design. In large-scale realization, this system can be considered for achieving real neural networks. These networks have the same biological behaviors as real neural networks.
The final sampling result is shown in
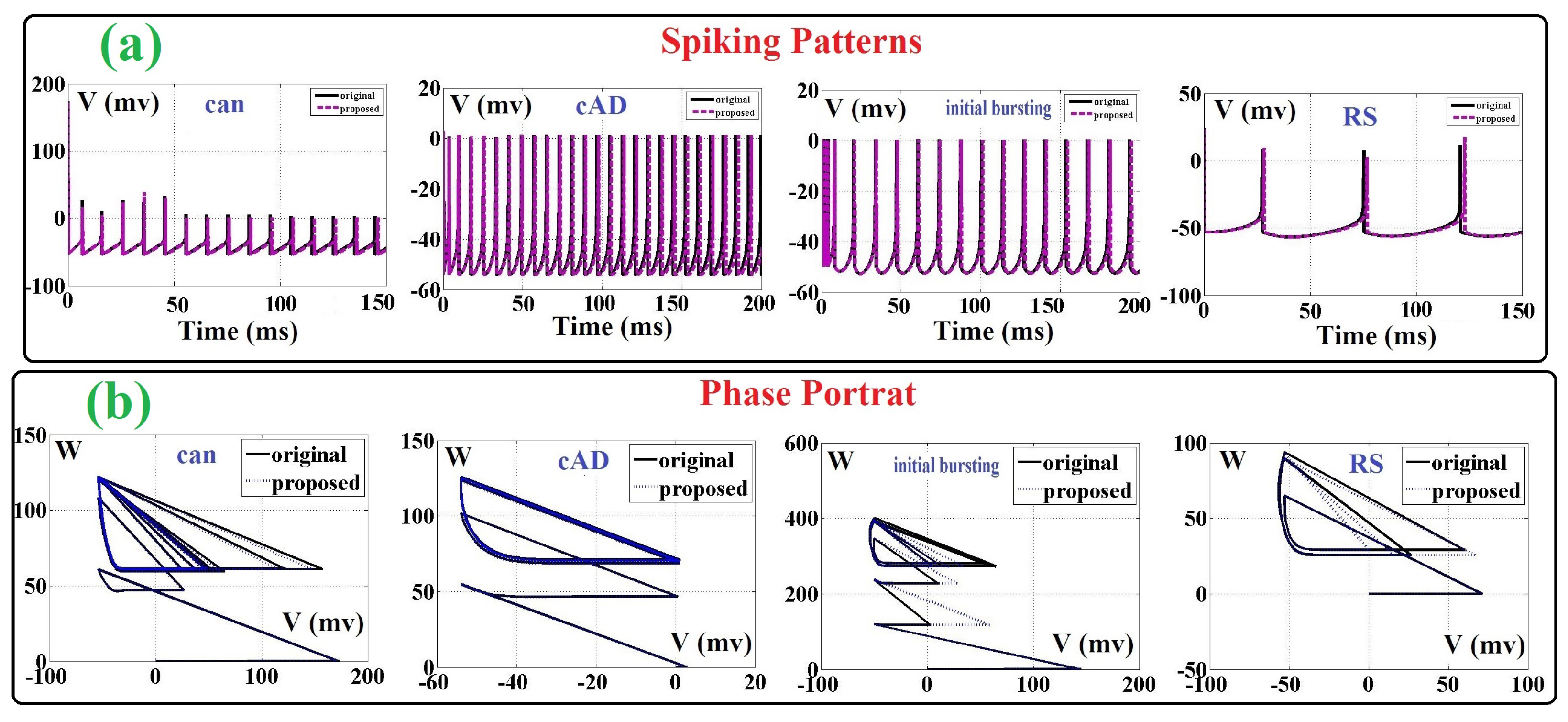
Figure 2b. As can be depicted in this figure, 20 points are selected based on the Nyquist rate. Thus, this continuous function is replaced by 20 values, and then, the nonlinearity of the ADEX neuron model can be solved. By this modification, the final overhead costs will be significantly reduced. Furthermore, the spiking shapes of two modeling (original and proposed) are compared in
Figure 3a. It is showed that differences between the ADEX neuron and the proposed method is in the low-state, considerably so. For further consideration, the phase portrait of the original and proposed models are illustrated in
Figure 3b. In this figure, the original and proposed models are similar at four spike patterns.
6. Hardware Results
In this section, the proposed ADEX neuron model has been implemented on an FPGA board. The XC2VP30 FPGA board (which consists of required elements) is used for realizing the final hardware. The final hardware is synthesized and the overhead costs are presents in
Table 7. As can be seen, our proposed neuron model in comparison to the original model is in a better state (frequency and costs). Moreover, the proposed model is more efficient compared to other, similar papers.
As illustrated in this table, our proposed model provides the best results in comparison with similar papers. Two basic factors in this approach are: speed (frequency) and large-scale implementation. The proposed neuron model has high-speed behaviors, and also in the case of large-scale neuron realization, based on the overall saving factor (
), the saving of our proposed model is the best in comparison with other works. By this modification, using one FPGA board, more implemented neurons can be realized in hardware form.
Figure 8 displays oscilloscope photographs of the proposed ADEX neuron type implementations. As can be seen, hardware signals have the same behaviors of the original simulation voltage signals in all spiking patterns. The device utilization for the implementation of about 60 neurons based on the proposed ADEX model can be realized (on an FPGA core). Consequently, our proposed neuron model is more efficient compared to the original model and other similar works in case of high-speed digital system and large-scale biological neural networks implementation.
As mentioned, the original ADEX neuron model is a nonlinear high-cost model. In this paper, the proposed model has been presented, which is capable for reproducing all patterns of the original model with a high degree of similarity. In this paper, a new ADEX modeling is applied to follow the main nervous system, that is capable to reinventing different mechanisms of the brain. The proposed approach can regenerate all mechanisms of the original ADEX neuron in high levels of performance and similarity. The new proposed case is used the Look-Up Table (LUT) modules to model the mathematical-based neurological structures, that can be implemented in high-level of equality with the original neuronal modeling. In other words, if the high-cost functions and terms are ignored, the final proposed model the will be realized and well implemented with such attributes as high-performances, low-overhead costs, and low-level computational errors. To evaluate the final digital circuit in case of validation with the original one, we have used the FPGA reconfigurable boards (Xilinx Virtex-II). After digital synthesize of digital HDL codes, it is showed that our proposed modeling is low-cost in case of FPGA resource utilization consumption and significantly follows the basic ADEX model. Finally, digital hardware results demonstrate the increasing overall saving of the presented work to in FPGA and a higher frequency (speed-up) of the proposed model of about 212 MHz, that is higher than the basic model designing, 34 MHz. Indeed, based on these two basic factors (FPGA saving and speed-up), the proposed model is better than the original model in all aspects. Moreover, in large-scale state, the presented modeling is in the best form in comparison with the original model (in case of the maximum number of realized neurons on an FPGA board).