Simple Summary
Exogenous glutamate administration to yearling-anestrous goats enhanced not only ovarian function (i.e., ovulation rate and antral follicle number) but also denoted undoubted effects at the hypothalamic-pituitary level, augmenting LH pulsatility. Our results show not only the use of glutamate as a clean alternative but also as an interesting reproductive strategy to amplify ovarian function, considering goats as an animal model. While this should enlarge the possibility of escalating our knowledge regarding the hypothalamic-pituitary-gonadal modulation by glutamate, such research outcomes may also embrace potential translational implications.
Abstract
The potential effect of intravenous administration of glutamate on the ovarian activity and the LH secretion pattern, considering the anestrous yearling goat as an animal model, were assessed. In late April, yearling goats (n = 20) were randomly assigned to either (1) Glutamate supplemented (GLUT; n = 10, Live Weight (LW) = 29.6 ± 1.02 kg, Body Condition (BCS) = 3.4 ± 0.2 units; i.v. supplemented with 7 mg GLUT kg−1 LW) or (2) Non-supplemented (CONT; n = 10; LW = 29.2 ± 1.07 kg, BCS = 3.5 ± 0.2 units; i.v. saline). The oats were estrus-synchronized; blood sampling (6 h × 15 min) was carried out for LH quantification. Response variables included pulsatility (PULSE), time to first pulse (TTFP), amplitude (AMPL), nadir (NAD), and area under the curve (AUC) of LH. Ovaries were ultra-sonographically scanned to assess ovulation rate (OR), number of antral follicles (AF), and total ovarian activity (TOA = OR + AF). LH-PULSE was quantified with the Munro algorithm; significant treatment x time interactions were evaluated across time. The variables LW and BCS did not differ (p > 0.05) between the experimental groups. Nevertheless, OR (1.77 vs. 0.87 ± 0.20 units), TOA (4.11 vs. 1.87 ± 0.47 units) and LH-PULSE (5.0 vs. 2.2 pulses 6 h-1) favored (p < 0.05) to the GLUT group. Our results reveal that targeted glutamate supplementation, the main central nervous system neurotransmitter, arose as an interesting strategy to enhance the hypothalamic–hypophyseal–ovarian response considering the anestrous-yearling goat as an animal model, with thought-provoking while promising translational applications.
1. Introduction
In goats, the seasonal variations in ovarian activity are the direct result of changes in the release of the gonadotropin-releasing hormone (GnRH) secretion, mainly exerted through the changing actions of the photoperiod [1,2]. Indeed, the photoperiod is the main environmental cue that modulates reproductive seasonality [3,4], altering the negative feedback exerted by estradiol (E2) upon the luteinizing hormone (LH) secretion [5,6]. The increased negative feedback of E2 on LH secretion has been accepted as the mechanism responsible for seasonal reproduction in most small ruminants [5,6]. In addition, glutamate (GLUT) has been recognized as the main, fast-acting excitatory neurotransmitter of the central nervous system (CNS), regulating most of the excitatory synaptic transmissions in the brain, while also involved in several biological processes [7]. GLUT-receptors have been localized in the diverse hypothalamic nuclei, some of which are key to the reproductive and neuroendocrine functions [8]. Moreover, the functional glutamatergic systems have been demonstrated in non-neural peripheral tissues, such as the heart, kidney, and gonads [7,8]. At a central level, the glutamatergic systems (i.e., ligand-receptors), have been involved in the control of pulsatile GnRH secretion, and the pre-ovulatory surge of gonadotropins (LH and FSH) [9]. Interestingly, the glutamatergic neurons that have been associated in the control of the GnRH neurons are also responsive to kisspeptin, an essential peptide in the regulation of the hypothalamic–pituitary–gonad (HPG) axis, promoting an increase in GnRH pulses [8,9,10]. While domestic sheep and goats have shown great potential as large animal models, sheep have been mainly used to perform preclinical and translational studies in reproductive research, while goats have been used in a more limited fashion [10]. We hypothesized a positive effect of the intravenous GLUT-supplementation upon ovarian function through an improved release profile of LH, considering the anestrous-yearling goat as the animal model. Thus, this study was designed to solve such a working hypothesis.
2. Materials and Methods
2.1. Location, Ethical-Welfare Issues, and Animal Management
The present study was carried out in northern Mexico (26° N, 103° W; 1120 m), in an intensive, commercial goat production system. Yearling anestrous Alpine-Saanen-Nubian × Criollo goats (n = 20), with an average live weight (LW) of 29.17 ± 1.02 kg and body condition score (BCS) of 3.45 ± 1.02 units, were involved. The experiment was conducted along the natural anestrous season, during April and May, i.e., under long-day photoperiodic conditions. The LW and BCS (from 1 = emaciated to 5 = obese) were weekly recorded before feeding by an experienced technician. All of the experimental procedures were completed following the recommendations for ethical use, care, and welfare of animals in research at global [11] and national [12] levels, and they were institutionally authorized (UACH-DGIP-REBIZA-IBIODEZA/15-510-400-2).
2.2. Experimental Design
At the end of April, the animals were individually housed in pens and they were randomly assigned to two experimental groups: (1) Glutamate (GLUT; n = 10) and (2) Control (CONT; n = 10). The LW and BCS were similar in both GLUT (29.1 ± 1.02 kg, 3.4 ± 0.2 units) and CONT (29.2 ± 1.07 kg, 3.5 ± 0.2 units) groups. All of the animals received a basal diet twice per day (07:00 and 16:00), consisting of alfalfa hay (14% crude protein, 4.7 MJ/kg), corn grain (11.2% crude protein, 9.9 MJ/kg), and corn silage (8.1% crude protein, 6.7 MJ/kg), balanced to cover their net energy requirements for maintenance [13]. Additionally, the GLUT-goats were supplemented every third day during the experimental period, with an intravenous injection of glutamate (L-glutamate, Merck-C5H9NO4-art-101791; from day 34 pre-estrus to day 17 post-estrus). Animals had ad libitum water access and shaded areas. The composition values of the components of the basal diet (Dry Matter (DM)% basis) were obtained from representative samples taken throughout the experimental period and analyzed based on the formerly defined techniques [14].
2.3. Estrus Synchronization, Blood Sampling, and LH Determinations
The estrus synchronization was initiated 23 days after the beginning of the experiment. Intravaginal progestogen-impregnated sponges containing 45 mg of fluorogestone acetate (Chronogest®; Intervet International B.V., Boxmeer, Holland) were inserted for 10 days; one day before the sponge withdrawal, the goats received an i.m. dose of 75 μg of D-cloprostenol (Prosolvin-C®, Intervet International B.V., Boxmeer, Holland). Five goats per group were randomly selected 24 h after the sponges’ withdrawal (−1 day); blood samples were collected at 3 h after the morning feeding. A volume of 10 mL of blood was collected every 15 min for 6 h by jugular venipuncture using sterile vacuum tubes (Corvac; Kendall Health Care, St. Louis, MO, USA) and allowed to clot at room temperature for 30 min. The blood was centrifuged (1500× g, 15 min) to obtain serum, and then it was decanted and stored into polypropylene microtubes (Axygen Scientific, Union City, CA, USA) at −20 °C until assayed. The peripheral serum LH concentrations were measured in duplicate by radioimmunoassay, as previously described [15]. The assay sensitivity was 0.2 ng/mL and intra-assay variation coefficient for LH quantification was 10%; the Munro algorithm was used to identify the LH pulses [16]. While the LH basal levels were quantified as the average of the lowest obtained values [17,18], the area under the curve (AUC) of LH was also determined.
2.4. Ultrasonographic Evaluation of Ovarian Activity
On day 17 post-estrus (coincident with the end of the luteal phase), an ultrasonographic assessment was carried out by a qualified operator to monitor the ovarian activity, using an ultrasound scanner (Toshiba Medical Systems Ltd., Crawley, UK), equipped with a 7.5 MHz linear-array transducer. The ovaries were scanned to record the number of corpus luteum (which indicate the ovulation rate, OR) and the antral follicles (AF; those ≥ 5 mm) [19]. Then, the total ovarian activity (TOA) was considered as AF + OR, recorded in both of the ovaries in each animal within the experimental group. The main activities performed during the experimental period are depicted in Figure 1.
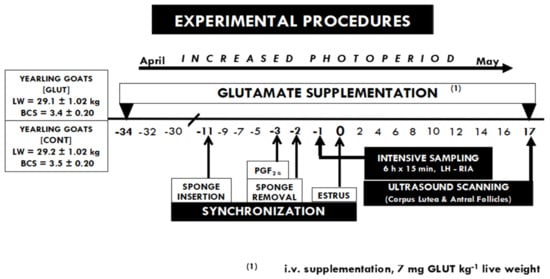
Figure 1.
Time-line of activities performed during the experiment, including the estrous synchronization protocol and the targeted glutamate i.v.—supplementation every third day along with the experimental period, (L-glutamate, from day 34 pre-estrus to day 17 post-estrus). Blood sampling (every 15 min × 6 h) for LH quantification was performed 24 h prior to the estrus day (day 0). Later, ovarian ultrasonographic assessment was carried out on day 17 post-estrus to study the relationship between the TOA and LH secretion pattern. All the experimental units had ad libitum water access and shaded areas in each pen.
2.5. Statistical Analyses
The variables LW, BCS, OR, TOA, and serum LH concentrations were evaluated using the PROC-MIXED of SAS (SAS Institute Inc., Cary, NC, USA), for repeated measures across time in the same animal. Each goat within the experimental group was defined as the experimental unit. The experimental treatment (i.e., GLUT or CONT) and the sampling day (i.e., Time) were analyzed using mixed linear model procedures and the estimation technique of restricted maximum likelihood (PROC MIXED). While time was considered as the repeated measure, the treated goats were defined as the repeated subject and regarded as the random error term [20]. In the case of mean significant differences of the analyzed response variables across time, these were solved through the LSMEANS-LSD option of PROC GLM. Since the LH pulse frequency showed a non-parametric distribution, a Kruskal–Wallis test was used to analyze the variable LH-PULSE. The response variables were evaluated for normality using the Shapiro–Wilk test, and log10, transformed for basal and mean LH concentrations as well as for LH pulse amplitude. When a significant effect of the treatment x time interaction occurred, the data were compared across time. Pearson’s correlations were used to check the associations among the variables LW, BCS, and OR. Non-transformed data are shown and expressed as least-square means ± standard error (SE). All of the statistical analyses were carried out using the procedures and options of SAS (SAS Inst. Inc., V9.1, Cary, NC, USA); significant differences between means were set at p < 0.05.
3. Results
The comparison of LW and BCS at the beginning (29.4 ± 1.02 kg and 3.4 ± 0.17) and the end of the experimental period (35.13 ± 1.07 kg and 3.4 ± 0.2) did not differ between the experimental groups (p > 0.05). Interestingly, however, the differences (p < 0.05) were observed for OR and TOA (1.77 vs. 0.87 ± 0.20) and (4.11 vs. 1.87 ± 0.47), respectively, favoring the GLUT-treated group. Moreover, an increased LH-PULSE (5.0 vs. 2.2 pulses 6 h−1, p < 0.05) also favored the GLUT-treated group (Table 1). The LH-pattern release across time, along with OR and TOA, are shown in Figure 2. Further, positive correlations occurred between LW1 and BCS1 (r2 = 0.71; p < 0.01), BCS and TOA (r2 = 0.7, p < 0.05), AF and OR (r2 = 0.61, p = 0.01), and OR and TOA (r2 = 0.87, p = 0.001).

Table 1.
Least-square means regarding LW and BCS at the onset of treatments (-initial) and at the ultrasound scanning (-ultrasound), ovulation rate (OR), total ovarian activity (TOA), and LH profile across time (pulsatility, time to first pulse, amplitude, nadir and AUC) in goats supplemented with glutamate (GLUT) and non-supplemented (CONT) groups.
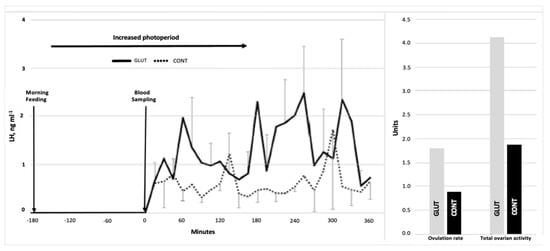
Figure 2.
Serum LH concentrations (ng/mL) across time (left), and OR (units) and TOA (units) (right) in glutamate-supplemented (GLUT) and non-supplemented (CONT) goats.
4. Discussion
The results obtained backs both our working hypothesis as well as our animal model; the intravenous supply of glutamate to anestrous-yearling goats as an animal model endorsed the increases in both ovulation rate and total ovarian activity, while it simultaneously generated rises in LH pulsatility, considering the anestrous-yearling goat as an animal model. Whereas, no differences occurred between the experimental groups regarding LW and BCS, in addition, the response variables time of the first LH pulse, the LH amplitude, LH-nadir, and LH-AUC were also not different between the groups. This neuroendocrine and physiologic scenario suggests that the glutamate supplementation to yearling-anestrous goats exerted a positive effect upon the hypothalamic centers responsible for GnRH, and, thereafter, upon the anterior pituitary, augmenting the LH pulsatility. The specific site of action of glutamate along the hypothalamic–pituitary–ovarian continuum, as well as its role within the neuronal pathway responsible for the inhibitory effects of E2 during seasonal anestrus in goats, await to be elucidated.
The ability of E2 to inhibit the release of GnRH and LH is the mechanism responsible for the variation in ovarian activity during anestrous [21]. Such E2-negative action upon the LH-pulse generator is facilitated by a group of neurons located in the retrochiasmatic area of the hypothalamus, known as the A15 dopaminergic neurons [22]. These neurons release dopamine and through the dopamine type 2 receptor (D2R), which are present in the kisspeptinergenic neurons of the arcuate nucleus (ARC), inhibit the release of the peptide kisspeptin, resulting in a decrease in GnRH and LH pulses during the anestrous season [5]. Most of the brain cells use glutamate as the main fast-acting excitatory neurotransmitter [7]. This neuroexcitatory amino acid and its receptors are distributed throughout the CNS and diverse parts of the body, including the ovary [23]. Likewise, glutamatergic and kisspeptinergenic neurons have been involved in different physiological processes, including reproductive ones, such as the activation of the hypothalamic axis, particularly in the production of the preovulatory GnRH-LH peak [24].
The GnRH neurons are stimulated by glutamate through the activation of the ionotropic receptors, as N-methyl-D-aspartate (NMDA), and amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) [25]. In goats, the glutamate administration has shown positive effects upon diverse reproductive outcomes. The previous studies of our group have exposed that the glutamate supply diminishes the E2-negative feedback exerted on the hypothalamus–pituitary axis, modulating ovarian function and metabolic hormone synthesis [10]. The results of our study suggest that the responses generated by glutamate administration could have acted upon the ionotropic ovarian receptors [26,27], increasing the ovulatory response. Besides, the glutamate supply may have promoted the increase in metabolic hormones and growth factors key to ovarian function, increasing follicular cell proliferation, and augmenting E2 synthesis [10]. Such a neurophysiological and endocrine scenario should have promoted a positive E2-effect upon the hypothalamus–pituitary axis, increasing the GnRH-LH pulsatility, and activating, in turn, ovarian activity.
Indeed, these results suggest that, in yearling-anestrous goats supplemented with intravenous glutamate, the inhibitory E2-feedback upon the hypothalamus–pituitary axis would be considerably diminished. Such an administration of glutamate probably acted somewhere along the neuronal continuum responsible for blocking GnRH secretion during the natural anestrous season, decreasing the action of dopamine on the kisspeptin-producing neurons, while enhancing the activation of the hypothalamic centers responsible for GnRH synthesis and secretion. The last, in turn, may have increased the LH-pulse, while augmenting the ovarian activity. Whereas diverse studies support the positive effects of glutamate administration upon the reproductive outcomes in diverse species, either male [28] or female [10,24,29,30], other studies have also demonstrated an interesting interplay between glutamatergic signaling and sexual male-to-female behavior [28,31].
Some components of the rams’ sexual behavior such as ano-genital sniffing, vocalizations, mounting, intromission, and ejaculation are also observed in rodents [32,33,34]. Preceding studies of our research group demonstrated that parenteral glutamate supply enhanced male sexual behavior, both appetitive and consummatory, either in adult [32] or pubertal [33] rams. Recent reports have also shown that glutamatergic neurons in the ventral tegmental area of the lateral hypothalamus are activated by, and required for, innate defensive reactions [35]. Moreover, GnRH stimulates the LH release via glutamatergic receptors expressed in the hypothalamic kisspeptin neurons in both peripubertal [36] and adult [37] females. Such amplified glutamatergic neuron transmission effect is enhanced because of the co-expression of kisspeptin, neurokinin-B, and dynorphin, the so-called glutamatergic KNDy neurons, which drive the episodic release of GnRH [38,39]. Besides, increases in the ARC Kiss1, and Pdyn expression were positively correlated not only with gonadotropin secretion and follicular growth, but also with an augmented positive energy balance [40].
Based on the obtained research outcomes from our study, and merged with those generated by others previously discussed, a sensible question is, how to align these outcomes with a translational perspective? In humans, female infertility is one of the main public health problems worldwide [41]; whereas female infertility represents 37% of the causes in infertile couples [42], the most common factors causing women reproductive dysfunctions mainly involve ovulatory disorders (25%) and ovarian dysfunctions (50%) [43]. Such women’s reproductive failures have also been linked, unfortunately, to mental, emotional, and physical issues [44]. The clinical management of infertility has included active ovarian stimulation treatments [45] to lessen a poor ovarian response or primary ovarian insufficiency; the key aim is to diagnose and then be able to manage women’s infertility [46]. As noted, ovine and, to a lesser extent, caprine, have been used as large animal models for diverse research purposes. The use of the former ranges from the generation of therapeutic agents in the mammary glands, up to their use in human genetic disorders and regenerative medicine, as well as the edition of their genomes using CRISPR-based systems for diverse translational purposes [47]. Hence, building on the diverse research outcomes generated from the various goat research models presented along with this discussion, and considering the need to develop basic biomedical research for studying the neurophysiological functions and dysfunctions along with the hypothalamic–pituitary–ovarian axis to upgrade women’s reproductive fitness, our research outcomes undoubtedly unveiled the interesting role that goats can play as a successful experimental animal model.
5. Conclusions
The targeted glutamate intravenous administration to anestrous-yearling goats positively influenced not only the ovarian function (i.e., ovulation rate and antral follicle number) but also undeniably denoted effects at the hypothalamic–pituitary level (i.e., an augmented LH pulse). Such a neurophysiological complex scenario was certainly not observed in the control group. Our results show the use of glutamate to be a clean, green, and ethical alternative, substituting the use of exogenous hormones, while providing an interesting reproductive strategy to augment the activity of the hypothalamic–pituitary–ovarian continuum (HPOC). Our research outcomes should enlarge the possibility of escalating the knowledge about the action of a targeted neurotransmitter supply, such as glutamate, upon the endocrine and physiological mechanisms involved in the enhancement of the HPOC response, through the use of a goat animal model. Certainly, the research outcomes that stemmed from this study, using the goat as an experimental model, should augment the confidence in the design of biomedical reproductive studies. Besides, a potential glutamate-modulated cross-talk among the CNS and the HPOC requires clarification. Undoubtedly, the obtained results from this study await and deserve to be tested in clinical trials with potential translational applications.
Author Contributions
Conceptualization, L.A.L.-G. and C.A.M.-H.; Data curation, L.A.L.-G., C.A.M.-H. and C.C.P.-M.; Formal analysis, L.A.L.-G., C.A.M.-H., C.C.P.-M. and R.C.; Funding acquisition, C.A.M.-H., R.C. and F.G.V.-D.; Investigation, L.A.L.-G., J.R.L.-O., R.D.-G., R.R.-V., C.A.R.-N., J.A.B.-A. and U.N.G.-G.; Methodology, J.R.L.-O., F.G.V.-D., R.D.-G., C.A.R.-N. and J.A.B.-A.; Project administration, R.D.-G., R.R.-V., J.A.B.-A. and U.N.G.-G.; Resources, R.C., J.R.L.-O., F.G.V.-D., R.D.-G., R.R.-V., C.A.R.-N., J.A.B.-A. and U.N.G.-G.; Software, C.A.R.-N.; Supervision, C.A.M.-H.; Writing—original draft, C.A.M.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by diverse International Collaborative Projects from the National Council of Science and Technology (CONACYT, Mexico): CONACYT-FOMIX-DURANGO: DGO-2008-C01-87559 and DGO-2009-C02-116746, and CONACYT-SIVILLA-1998-0401010, as well as the ALFA-III-ALAS/ALFA-III-82, supported by the European Union. We also acknowledge the Research Sectorial Fund SAGARPA-CONACYT: 2017-4-291691, which also contributed to the generation of most of the information presented in this study.
Institutional Review Board Statement
All of the experimental procedures were completed in accordance with the recommendations for ethical use, care and welfare of animals in research at global (USA; 11) and national (Mexico; 12) levels, and they were institutionally authorized by Chapingo Autonomous University; the animal study protocol was authorized by the Institutional Review Board, with approval reference number: UACH-DGIP-REBIZA-IBIODEZA/20-510-400-2.
Informed Consent Statement
Not applicable.
Data Availability Statement
None of the data were deposited in an official repository, yet, information can be made available upon request.
Acknowledgments
L.A.L.-G. is a double-degree doctoral student at Chapingo Autonomous University-URUZA (UACH-URUZA, Mexico) and at the University of Cordoba (UCO, Spain), supported by a CONACYT-Scholarship Grant, CVU-934555. (In Loving Memory, Dr. Santiago Zuñiga-García, 1985–2020; M.Sc. Sergio Yong-Wong, 1964–2020).
Conflicts of Interest
The authors declare that there are no conflict of interest that could be perceived as prejudicing the impartiality of the research reported in this manuscript. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Szpręgiel, I.; Wrońska, D. The role of photoperiod and melatonin in the control of seasonal reproduction in mammals. Rocz. Nauk. Pol. Tow. Zootech. 2020, 16, 39–47. [Google Scholar] [CrossRef]
- Fabre-Nys, C.; Chanvallon, A.; Dupont, J.; Lardic, L.; Lomet, D.; Martinet, S.; Scaramuzzi, R.J. The “Ram Effect”: A “non-classical” mechanism for inducing LH surges in sheep. PLoS ONE 2016, 11, e0158530. [Google Scholar] [CrossRef]
- Escareño, L.M.; Wurzinger, M.; López, F.P.; Salinas, H.; Sölkner, J.; Iñiguez, L. La cabra y los sistemas de producción caprina de los pequeños productores de la Comarca Lagunera en el norte de México. Rev. Chapingo Serie Cienc. For. Ambiente 2011, 17, 235–246. [Google Scholar] [CrossRef]
- Dardente, H.; Lomet, D.; Robert, V.; Decourt, C.; Beltramo, M.; Pellicer-Rubio, M.T. Seasonal breeding in mammals: From basic science to applications and back. Theriogenology 2016, 86, 324–332. [Google Scholar] [CrossRef]
- Weems, P.W.; Goodman, R.L.; Lehman, M.N. Neural mechanisms controlling seasonal reproduction: Principles derived from the sheep model and its comparison with hamsters. Front. Neuroendocrinol. 2015, 37, 43–51. [Google Scholar] [CrossRef]
- Duarte, G.; Nava-Hernández, M.P.; Malpaux, B.; Delgadillo, J.A. Ovulatory activity of female goats adapted to the subtropics is responsive to photoperiod. Anim. Reprod. Sci. 2010, 120, 65–70. [Google Scholar] [CrossRef]
- Iremonger, K.J.; Constantin, S.; Liu, X.; Herbison, A.E. Glutamate regulation of GnRH neuron excitability. Brain Res. 2010, 1364, 35–43. [Google Scholar] [CrossRef]
- Meza-Herrera, C.A.; Ross, T.; Hallford, D.; Hawkins, D.; González-Bulnes, A. Effects of body condition and protein supplementation on LH secretion and luteal function in sheep. Reprod. Domest. Anim. 2007, 42, 461–465. [Google Scholar] [CrossRef]
- Dhandapani, K.M.; Brann, D.W. The role of glutamate and nitric oxide in the reproductive neuroendocrine system. Biochem. Cell Biol. 2000, 78, 165–179. [Google Scholar] [CrossRef]
- Meza-Herrera, C.A.; Vergara-Hernández, H.P.; Paleta-Ochoa, A.; Álvarez-Ruíz, A.R.; Véliz-Deras, F.G.; Arellano-Rodríguez, G.; Rosales-Nieto, C.A.; Macías-Cruz, U.; Rodríguez-Martínez, R.; Carrillo, E. Glutamate supply reactivates ovarian function while increases serum insulin and triiodothyronine concentrations in criollo x saanen-alpine yearlings’ goats during the anestrous season. Animals 2020, 10, 234. [Google Scholar] [CrossRef]
- FASS. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching, 3rd ed.; Federation Animal Science Society: Champaing, IL, USA, 2010; p. 177. [Google Scholar]
- NAM-National Academy of Medicine. Guide for the Care and Use of Laboratory Animals. Co-Produced by the National Academy of Medicine—Mexico and the Association for Assessment and Accreditation of Laboratory Animal Care International, 1st ed.; Harlan: Mexico City, Mexico, 2002. [Google Scholar]
- NRC—National Research Council. Nutrient Requirements of Goats: Angora, Dairy and Meat Goats in Temperature and Tropical Countries; National Academy Press: Washington, DC, USA, 1998. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 15th ed.; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Hoefler, H.; Hallford, D.M. Influence of suckling status and type of birth on serum hormone profiles and return to estrus early pospartum spring lambing ewes. Theriogenology 1987, 27, 887–892. [Google Scholar] [CrossRef]
- Merriam, G.R.; Wachter, K.W. Algorithms for the study of episodic hormone secretion. Am. J. Physiol.-Endocrinol. Metab. 1982, 243, E310–E318. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.B.; Oldham, C.M.; Lindsay, D.R. Increased plasma LH levels in seasonally anovular Merino ewes following the introduction of rams. Anim. Reprod. Sci. 1980, 3, 125–132. [Google Scholar] [CrossRef]
- Martin, G.B.; Scaramuzzi, R.J.; Oldham, C.M.; Lindsay, D.R. Effects of progesterone on the responses of Merino ewes to the introduction of rams during anoestrus. Aust. J. Biol. Sci. 1983, 36, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Dickie, A.M.; Paterson, C.; Anderson, L.M.; Boyd, J.S. Determination of corpora lutea numbers in Booroola—Texel ewes using transrectal ultrasound. Theriogenology 1999, 51, 1209–1224. [Google Scholar] [CrossRef]
- Littell, C.R.; Henry, P.R.; Ammermam, C.B. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar] [CrossRef]
- Weems, P.; Smith, J.; Clarke, I.J.; Coolen, L.M.; Goodman, R.L.; Lehman, M.N. Effects of season and estradiol on KNDy neuron peptides, colocalization with D2 dopamine receptors, and dopaminergic inputs in the ewe. Endocrinology 2017, 158, 831–841. [Google Scholar] [CrossRef]
- Lehman, M.N.; Ladha, Z.; Coolen, L.M.; Hileman, S.M.; Connors, J.M.; Goodman, R.L. Neuronal plasticity and seasonal reproduction in sheep. Eur. J. Neurosci. 2010, 32, 2152–2164. [Google Scholar] [CrossRef]
- Gérard, N.; Loiseau, S.; Duchamp, G.; Seguin, F. Analysis of the variations of follicular fluid composition during follicular growth and maturation in the mare using proton nuclear magnetic resonance (^1H NMR). Reproduction 2002, 124, 241–248. [Google Scholar] [CrossRef]
- Meza-Herrera, C.A.; González-Velázquez, A.; Véliz-Deras, F.G.; Rodríguez-Martínez, R.; Arellano-Rodríguez, G.; Serradilla, J.M.; García-Martínez, A.; Avendaño-Reyes, L.; Macías-Cruz, U. Short-term glutamate administration positively affects the number of antral follicles and the ovulation rate in cycling adult goats. Reprod. Biol. 2014, 13, 298–301. [Google Scholar] [CrossRef]
- Kuehl-Kovarik, M.C.; Pouliot, W.A.; Halterman, G.L.; Handa, R.J.; Dudek, F.E.; Partin, K.M. Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein. J. Neurosci. 2002, 22, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Sasaki, S.; Watanabe, T.; Nishimura, S.; Ideta, A.; Yamazaki, M. Ionotropic glutamate receptor AMPA1 is associated with ovulation rate. PLoS ONE 2010, 5, e13817. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, N.; Kinoshita, M.; Saito, Y.; Ikeda, S. Identification of the N-Methyl-D-aspartate receptor (NMDR)-related epitope, NR2B, in the normal human ovary: Implication for the pathogenesis of anti-NMDR encephalitis. Tohoku J. Exp. Med. 2013, 230, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Kayode, O.T.; Rotimi, D.E.; Kayode, A.A.A.; Olaolu, T.D.; Adeyemi, O.S. Monosodium glutamate (MSG)-induced male reproductive dysfunction: A mini review. Toxics 2020, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Meza-Herrera, C.A.; Torres-Moreno, M.; López-Medrano, J.L.; González-Bulnes, A.; Véliz-Deras, F.G.; Mellado, M.; Wurzinger, M.; Soto-Sanchez, M.J.; Calderón-Leyva, M.G. Glutamate supply positively affects serum release of triiodothyronine and insulin across time without increases of glucose during the onset of puberty in the female goat. Anim. Reprod. Sci. 2011, 125, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Meza-Herrera, C.A.; Calderón-Leyva, G.; Soto-Sánchez, M.J.; Serradilla, J.M.; García-Martínez, A.; Mellado, M.; Véliz-Deras, F.G. Glutamate supply positively affects cholesterol concentrations without increases in total protein and urea around the onset of puberty in goats. Anim. Reprod. Sci. 2014, 147, 106–111. [Google Scholar] [CrossRef]
- Moore, K.M.; Oelberg, W.L.; Glass, M.R.; Johnson, M.D.; Been, L.E.; Meisel, R.L. Glutamate afferents from the medial prefrontal cortex mediate nucleus accumbens activation by female sexual behavior. Front. Behav. Neurosci. 2019, 13, 227. [Google Scholar] [CrossRef]
- Calderón-Leyva, G.; Meza-Herrera, C.A.; Rodríguez-Martínez, R.; Ángel-García, O.; Rivas-Muñoz, R.; Delgado-Bermejo, J.V. Influence of sexual behavior of Dorper rams treated with glutamate and/or testosterone on reproductive performance of anovulatory ewes. Theriogenology 2018, 106, 79–86. [Google Scholar] [CrossRef]
- Calderón-Leyva, G.; Meza-Herrera, C.A.; Rodríguez-Martínez, R.; Ángel-García, O.; Rivas-Muñoz, R.; Delgado-Bermejo, J.V.; Véliz-Deras, F.G. Effect of glutamate and/or testosterone administration upon appetitive and consummatory sexual behaviors in pubertal rams and their influence upon the reproductive performance of nulliparous anovulatory ewes. J. Vet. Behav. Clin. Appl. Res. 2019, 30, 96–102. [Google Scholar] [CrossRef]
- Chiang, V.S.-C.; Park, J.H. Glutamate in Male and Female Sexual Behavior: Receptors, Transporters and Steroid Independence. Front. Behav. Neurosci. 2020, 14, 589882. [Google Scholar] [CrossRef]
- Barbano, F.; Wang, H.-L.; Zhang, S.; Miranda-Barrientos, J.; Estrin, D.J.; Figueroa-Gonzalez, A.; Liu, B.; Barker, D.J.; Morales, M. VTA glutamatergic neurons mediate innate defensive behaviors. Neuron 2020, 107, 368–382.e8. [Google Scholar] [CrossRef] [PubMed]
- Naulé, L.; Maione, L.; Kaise, U.B. Puberty, a sensitive window of hypothalamic development and plasticity. Endocrinology 2021, 162, bqaa209. [Google Scholar] [CrossRef] [PubMed]
- Ieda, N.; Assadullah; Minabe, S.; Ikegami, K.; Watanabe, Y.; Sugimoto, Y.; Sugimoto, A.; Kawai, N.; Ishii, H.; Inoue, N.; et al. GnRH (1–5), a metabolite of gonadotropin-releasing hormone, enhances luteinizing release via activation of kisspeptin neurons in female rats. Endocr. J. 2020, 67, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yeo, S.H.; McQuillan, J.; Herde, H.J.; Hessler, S.; Cheong, I.; Porteous, R.; Herbison, A.E. Highly redundant neuropeptide volume co-transmission underlying episodic activation of the GnRH neuron dendron. Elife 2021, 10, e62455. [Google Scholar] [CrossRef]
- Wakabayashi, Y.; Okamura, H.; Yakamura, T. Local administration of Neurokinin B in the arcuate nucleus accelerates the neural activity of the GnRH pulse generator in goats. J. Reprod. Dev. 2021, 67, 352–358. [Google Scholar] [CrossRef]
- Majarune, S.; Nima, P.; Sugimoto, A.; Nagae, M.; Inoue, N.; Tsukamura, H.; Uenoyoma, Y. Ad libitum feeding triggers puberty onset associated with increases in arcuate Kiss1 and Pdyn expression in growth-retarded rats. J. Reprod. Dev. 2019, 65, 397–406. [Google Scholar] [CrossRef]
- Indarwati, I.; Hastuti, U.R.B.; Dewi, Y.L.R. Analysis of factors influencing female infertility. J. Matern. Child Health 2017, 2, 150–161. [Google Scholar] [CrossRef][Green Version]
- Weiss, R.V.; Clapauch, R. Female infertility of endocrine origin. Arq. Bras. Endocrinol. Metabol. 2014, 58, 144–152. [Google Scholar] [CrossRef]
- Unuane, D.; Tournaye, H.; Velkeniers, B.; Poppe, K. Endocrine disorders & female infertility. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 861–873. [Google Scholar] [CrossRef]
- Ghajari, G.; Heydari, A.; Ghorbani, M. Mesenchymal stem cell-based therapy and female infertility: Limitations and advances. Curr. Stem Cell Res. Ther. 2022. online ahead print. [Google Scholar] [CrossRef]
- Wang, R.; Danhof, N.A.; Tjon-Kon-Fat, R.I.; Eijkemans, M.J.; Bossuyt, P.M.; Mochtar, M.H.; van Wely, M. Interventions for unexplained infertility: A systematic review and network meta-analysis. Cochrane Database Syst. Rev. 2019, 9, CD012692. [Google Scholar] [CrossRef] [PubMed]
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility: A Review. J. Am. Med. Assoc. 2021, 326, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Kalds, P.; Gao, Y.; Zhou, S.; Bei Cai, B.; Xingxu Huang, X.; Wang, X.; Chen, Y. Redesigning small ruminant genomes with CRISPR toolkit: Overview & perspectives. Theriogenology 2020, 147, 25–33. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).