Effect of Nitric Oxide Pathway Inhibition on the Evolution of Anaphylactic Shock in Animal Models: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
Narrative Review
2. Materials and Methods
2.1. Study Design
- -
- Population: animal species with AS.
- -
- Intervention: blockade of NO production and guanylate cyclase activation.
- -
- Comparator: animal model with AS undergoing no treatment, epinephrine, or baseline measurements.
- -
- Outcomes: survival and normalization of blood pressure.
- -
- Study design: experimental.
2.2. Search Strategy
2.3. Selection Process
2.4. Eligibility Criteria
2.5. Data Extraction
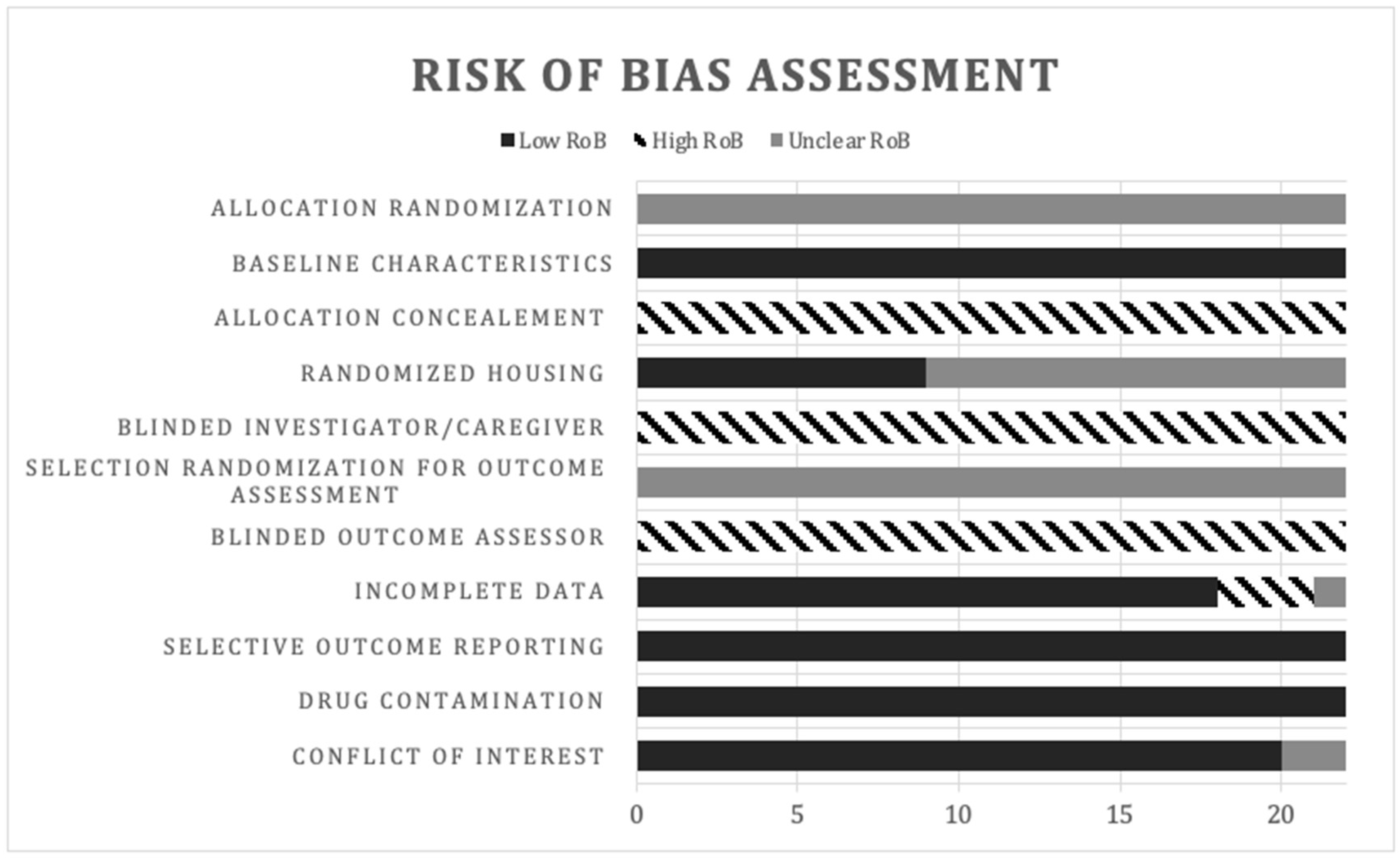
2.6. Risk of Bias
2.7. Data Synthesis and Statistical Analysis
3. Results
- Overview
3.1. Characteristics of Included Studies
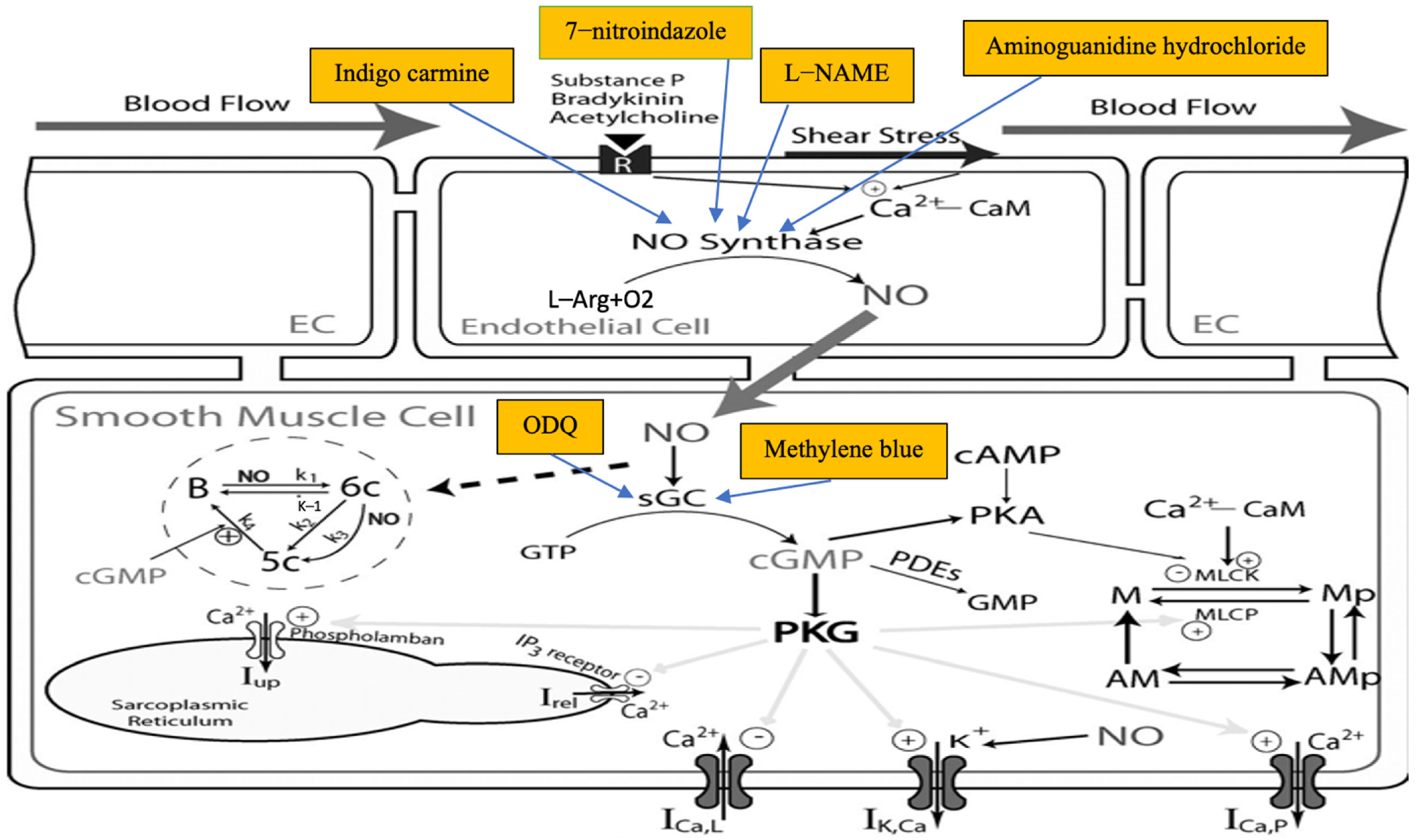
3.2. Descriptive Data Synthesis of the Effects of INOP on Arterial Blood Pressure and Survival
3.3. Pre-Treatment
3.3.1. L-NAME
3.3.2. Methylene Blue (MB)
3.3.3. 1H-[1,2,4] Oxadiazole [4,3-a] quinoxalin-1-one (ODQ)
3.3.4. Indigo Carmine (IC)
3.3.5. Aminoguanidine (AG)
3.3.6. 7-Nitroindazole (7-NI)
3.4. Post-Treatment
3.4.1. L-NAME
3.4.2. Methylene Blue (MB)
3.4.3. Indigo Carmine (IC)
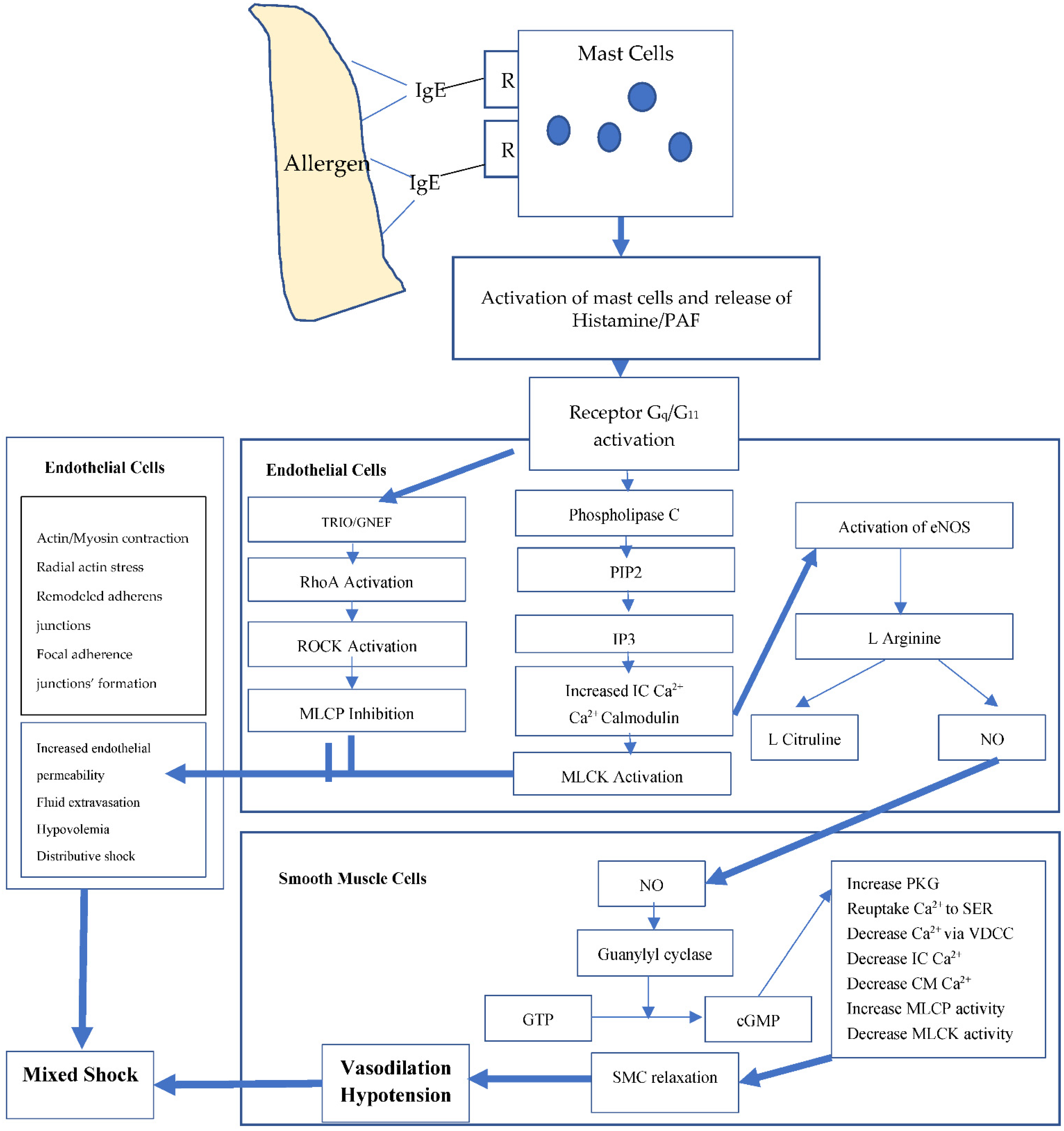
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AG | Aminoguanidine hydrochloride |
| AS | Anaphylactic shock |
| cGMP | Cyclic guanosine monophosphate |
| eNOS | Endothelial nitric oxide synthase |
| EAACI | European Academy of Allergy and Clinical Immunology |
| IC | Indigo carmine |
| iNOS | Induced nitric oxide synthase |
| INOP | Inhibition of nitric oxide pathway (INOP) |
| MAP | Mean arterial pressure |
| MB | Methylene blue |
| nNOS | Neuronal nitric oxide synthase |
| L-NAME | NG-nitro-L-arginine methyl ester |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| PI3K | Phosphatidylinositol 3-kinase |
| PAF | Platelet activating factor |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PK | Protein kinase |
| RoB | Risk of bias |
| sGC | Soluble guanylate cyclase |
| SYRCLE | SYstematic Review Center for Laboratory animal Experimentation |
| ODQ | 1H-[1,2,4] Oxadiazole [4,3-a] quinoxalin-1-one |
| 7-NI | 7-Nitroindazole |
Appendix A
| Source | Search String | Results | Notes |
|---|---|---|---|
| PubMed (NLM) Coverage: From inception- search date Search Date: 24 March 2022 | ((“anaphylactic shock”[Title/Abstract] OR “anaphylactic shocks”[Title/Abstract] OR “Anaphylaxis”[Mesh] OR Anaphylaxis[Title/Abstract] OR “anaphylactic reaction”[Title/Abstract] OR “anaphylactic reactions”[Title/Abstract] OR “shock, anaphylactic”[Title/Abstract]) AND (murine*[Title/Abstract] OR rat[Title/Abstract] OR rats[Title/Abstract] OR canine*[Title/Abstract] OR dog[Title/Abstract] OR dogs[Title/Abstract] OR rabbit*[Title/Abstract] OR animal*[Title/Abstract] OR mouse[Title/Abstract] OR mice[Title/Abstract] OR “Models, Animal”[Mesh:NoExp] OR “Murinae”[Mesh] OR Murinae[Title/Abstract] OR “Mice”[Mesh] OR “Dogs”[Mesh] OR “Rats”[Mesh] OR “Rabbits”[Mesh] OR monkey*[Title/Abstract] OR “Haplorhini”[Mesh] OR sheep [Title/Abstract] OR “Sheep”[Mesh] OR pig[Title/Abstract] OR “Swine”[Mesh] OR pigs[Title/Abstract] OR guinea-pig[Title/Abstract] OR guinea-pigs[Title/Abstract] OR rattus[Title/Abstract] OR metazoa[Title/Abstract]) AND (“nitric oxide”[Title/Abstract] OR “oxide, nitric”[Title/Abstract] OR “nitrogen monoxide”[Title/Abstract] OR “endogenous nitrate vasodilator”[Title/Abstract] OR “monoxide, mononitrogen”[Title/Abstract] OR “nitric-oxide”[Title/Abstract] OR “Ntric Oxide”[Mesh] OR “methylene blue”[Title/Abstract] OR “blue, methylene”[Title/Abstract] OR “methylthioninium chloride”[Title/Abstract] OR “methylthionine chloride”[Title/Abstract] OR “swiss blue”[Title/Abstract] OR “basic blue 9”[Title/Abstract] OR “methylene blue N”[Title/Abstract] OR chromosmon[Title/Abstract] OR “Methylene Blue”[Mesh] OR “ENOS enzyme”[Title/Abstract] OR “ECNOS enzyme”[Title/Abstract] OR “Nitric Oxide Synthase Type III”[Mesh] OR “oxide synthase, nitric”[Title/Abstract] OR “NO synthase”[Title/Abstract] OR “Nitric Oxide Synthase”[Mesh] OR “NG-nitroarginine methyl ester”[Title/Abstract] OR “NG nitroarginine methyl Ester”[Title/Abstract] OR “N omega-nitro-L-arginine methyl ester”[Title/Abstract] OR “N omega nitro L arginine methyl ester”[Title/Abstract] OR “NG-Nitro-L-arginine methyl ester”[Title/Abstract] OR “methyl ester, NG-nitro-L-arginine”[Title/Abstract] OR “NG nitro L arginine methyl ester”[Title/Abstract] OR “N(G)-nitroarginine methyl ester”[Title/Abstract] OR “L-NAME”[Title/Abstract] OR “N(G)-nitro-L-arginine methyl ester”[Title/Abstract] OR “NG nitroarginine methyl ester, D orn isomer”[Title/Abstract] OR “NG-Nitroarginine Methyl Ester”[Mesh] OR “omega-N-methylarginine”[Title/Abstract] OR “omega N methylarginine”[Title/Abstract] OR “NG-monomethyl-L-arginine”[Title/Abstract] OR “NG monomethyl L arginine”[Title/Abstract] OR “L-NMMA”[Title/Abstract] OR “L-monomethylarginine”[Title/Abstract] OR “L monomethylarginine”[Title/Abstract] OR “D-NMMA”[Title/Abstract] OR “L-NG-monomethyl arginine”[Title/Abstract] OR “arginine, L-NG-monomethyl”[Title/Abstract] OR “L NG monomethyl arginine”[Title/Abstract] OR “inhibitor of NO synthase”[Title/Abstract] OR “iNOS”[Title/Abstract] OR “cNOS”[Title/Abstract] OR “nNOS”[Title/Abstract] OR “endothelial NOS”[Title/Abstract] OR “neuronal NOS”[Title/Abstract] OR “NOS1”[Title/Abstract] OR “NOS2”[Title/Abstract] OR “NOS3”[Title/Abstract] OR “inducible NOS”[Title/Abstract] OR “inhibitor of NOS”[Title/Abstract] OR “nitric-oxide synthase”[Title/Abstract] OR “endothelium-derived relaxation factor-forming enzyme”[Title/Abstract] OR “endothelium-derived relaxation factor synthase”[Title/Abstract] OR “NADPH-diaphorase”[Title/Abstract] OR “constitutive NOS”[Title/Abstract] OR “NOSII”[Title/Abstract] OR “NOSIII”[Title/Abstract] OR “L-NNA”[Title/Abstract] OR nitroarginine[Title/Abstract] OR “Nitroarginine”[MeSH Terms] OR “NOARG”[Title/Abstract] OR “omega-nitroarginine”[Title/Abstract] OR “omega nitroarginine”[Title/Abstract] OR “NO2Arg”[Title/Abstract] OR “NG-nitro-L-arginine”[Title/Abstract] OR “NG nitro L arginine”[Title/Abstract] OR “NG-nitroarginine”[Title/Abstract] OR “NG nitroarginine”[Title/Abstract] OR “N omega-nitro-L-arginine”[Title/Abstract] OR “N omega nitro L arginine”[Title/Abstract] OR tilarginine[Title/Abstract] OR “NG-methyl-L-arginine”[Title/Abstract] OR “L-NMA”[Title/Abstract] OR dimethylarginine[Title/Abstract] OR “ADMA”[Title/Abstract] OR “L-NIL”[Title/Abstract] OR “L-NIO”[Title/Abstract] OR “L-NIO dihydrochloride”[Title/Abstract])) | 191 | All search terms are searched in the search fields “title” and “abstract” (here marked with TI/AB) and in MeSH (when available). No filters or limitations applied An asterisk * is used to search for different variations of a word. |
| Scopus (Elsevier) Coverage: From inception- search date Search Date: 24 March 2022 | ((ABS (murine* OR rat OR rattus OR rats OR canine* OR dog OR dogs OR rabbt* OR animal* OR mouse OR mice OR murinae OR monkey*. OR sheep OR pig OR pigs OR “guinea-pig” OR “guinea pigs” OR metazoa) OR TITLE (murine* OR rat OR rattus OR rats OR canine* OR dog OR dogs OR rabbit* OR animal* OR mouse OR mice OR murinae OR monkey* OR sheep OR pig OR pigs OR “guinea pig” OR “guinea pigs” OR metazoa)) AND (ABS (“nitric oxide” OR “oxide, nitric” OR “nitrogen monoxide” OR “endogenous nitrate vasodilator” OR “monoxide, mononitrogen” OR “nitric. oxide” OR “methylene blue” OR “blue, methylene” OR “methylthioninium chloride” OR “methylthionine chloride” OR “swiss blue” OR “basic blue 9” OR “methylene blue N” OR “chromosmon” OR “ENOS enzyme” OR “ECNOS enzyme” OR “oxide synthase, nitric” OR “NO synthase” OR “NG-nitroarginine methyl ester” OR “NG nitroarginine methyl ester” OR “N omega-nitro-L-arginine methyl ester” OR “N omega nitro L arginine methyl ester” OR “NG-nitro-L-arginine methyl ester” OR “methyl ester, NG-nitro-L-arginine” OR “NG nitro L arginine methyl ester” OR “N(G)-nitroarginine methyl ester” OR “L NAME” OR “N(G)-nitro-L-arginine methyl ester” OR “NG nitroarginine methyl ester, D orn isomer” OR “omega-N-methylarginine” OR “omega N methylarginine” OR “NG-monomethyl-L-arginine” OR “NG monomethyl L arginine” OR “L-NMMA” OR “L-monomethylarginine” OR “L monomethylarginine” OR “D-NMMA” OR “L-NG-monomethyl arginine” OR “arginine, L-NG-monomethyl” OR “L NG monomethyl arginine” OR “inhibitor of NO synthase” OR “iNOS” OR “cNOS” OR “nNOS” OR “endothelial NOS” OR “neuronal NOS” OR “NOS1” OR “NOS2” OR “NOS3” OR “inducible NOS” OR “inhibitor of NOS” OR “nitric-oxide synthase” OR “endothelium-derived relaxation factor-forming enzyme” OR “endothelium-derived relaxation factor synthase” OR “NADPH diaphorase” OR “constitutive NOS” OR “NOSII” OR “NOSIII” OR “LNNA” OR nitroarginine OR “NOARG” OR “omega-nitroarginine” OR “omega nitroarginine” OR “NO2Arg” OR “NG-nitro-L-arginine” OR “NG nitro L arginine” OR “NG-nitroarginine” OR “NG nitroarginine” OR “N omega-nitro-L-arginine” OR “N omega nitro L arginine” OR tilarginine OR “NG-methyl-L-arginine” OR “L-NMA” OR dimethylarginine OR “ADMA” OR “L-NIL” OR “L-NIO” OR “L-NIO dihydrochloride”) OR TITLE (“nitric oxide” OR “oxide, nitric” OR “nitrogen monoxide” OR “endogenous nitrate vasodilator” OR “monoxide, mononitrogen” OR “nitric-oxide” OR “methylene blue” OR “blue, methylene” OR “methylthioninium chloride” OR “methylthionine chloride” OR “swiss blue” OR “basic blue 9” OR “methylene blue N” OR “chromosmon” OR “ENOS enzyme”OR “ECNOS enzyme” OR “oxide synthase, nitric” OR “NO synthase” OR “NG-nitroarginine methyl ester” OR “NG nitroarginine methyl ester” OR “N omega-nitro-L-arginine methyl ester” OR “N omega nitro L arginine methyl ester” OR “NG-nitro-L-arginine methyl ester” OR “methyl ester, NG-nitro-L-arginine” OR “NG nitro L arginine methyl ester” OR “N(G)-nitroarginine methyl ester” OR “L-NAME” OR “N(G)-nitro-L-arginine methyl ester” OR “NG nitroarginine methyl ester, D orn isomer” OR “omega-N methylarginine” OR “omega N methylarginine”OR “NG-monomethyl-L-arginine” OR “NG monomethyl L arginine” OR “L-NMMA” OR “L. monomethylarginine” OR “L monomethylarginine” OR “D-NMMA” OR “L-NG-monomethyl arginine” OR “arginine, L-NG-monomethyl” OR “L NG monomethyl arginine” OR “inhibitor of NO synthase” OR “iNOS” OR “cNOS” OR “nNOS” OR “endothelial NOS” OR “neuronal NOS” OR “NOS1”OR “NOS2”OR “NOS3” OR “inducible NOS” OR “inhibitor of NOS” OR “nitric-oxide synthase” OR “endothelium-derived relaxation factor-forming enzyme” OR “endothelium-derived relaxation factor synthase” OR “NADPH diaphorase” OR “constitutive NOS” OR “NOSII” OR “NOSIII” OR “LNNA” OR nitroarginine OR “NOARG” OR “omega-nitroarginine” OR “omega nitroarginine” OR “NO2Arg” OR “NG-nitro-L-arginine” OR “NG nitro L arginine” OR “NG-nitroarginine” OR “NG nitroarginine” OR “N omega-nitro-L-arginine” OR “N omega nitro L arginine” OR tilarginine OR “NG-methyl-L-arginine” OR “L NMA” OR dimethylarginine OR “ADMA” OR “L-NIL” OR “L-NIO” OR “L-NIO dihydrochloride”)) AND (ABS (“anaphylactic shock” OR “anaphylactic shocks” OR anaphylaxis OR “anaphylactic reaction” OR “anaphylactic reactions” OR “shock, anaphylactic”) OR TITLE (“anaphylactic shock” OR “anaphylacticshocks” OR anaphylaxis OR “anaphylactic reaction” OR “anaphylactic reactions” OR “shock, anaphylactic”))) | 102 | All search terms are searched in the search fields “Title” OR “Abstract. No thesaurus available. No filters or limitations applied |
| Embase (Elsevier) Source: “Embase” and Embase and (Medline” Coverage: From inception- search date Search Date: 24 March 2022 | ((‘anaphylactic shock’:ab,ti OR ‘anaphylactic shocks’:ab,ti OR anaphylaxis: ab,ti OR ‘anaphylactic reaction’:ab,ti OR ‘anaphylactic reactions’:ab,ti OR ‘shock, anaphylactic’:ab,ti OR ‘anaphylaxis’/exp) AND (‘nitric oxide’:ab,ti OR ‘oxide, nitric’:ab,ti OR ‘nitrogen monoxide’:ab,ti OR ‘endogenous nitrate vasodilator’:ab,ti OR ‘monoxide, mononitrogen’:ab,ti OR ‘nitric-oxide’:ab,ti OR ‘methylene blue’:ab,ti OR ‘blue, methylene’:ab,ti OR ‘methylthioninium chloride’:ab,ti OR ‘methylthionine chloride’:ab,ti OR ‘swiss blue’:ab,ti OR ‘basic blue 9’:ab,ti OR ‘methylene blue n’:ab,ti OR ‘chromosmon’:ab,ti OR ‘enos enzyme’:ab,ti OR ‘ecnos enzyme’:ab,ti OR ‘oxide synthase, nitric’:ab,ti OR ‘no synthase’:ab,ti OR ‘ng-nitroarginine methyl ester’:ab,ti OR ‘ng nitroarginine methyl ester’:ab,ti OR ‘n omega-nitro-l-arginine methyl ester’:ab,ti OR ‘n omega nitro l arginine methyl ester’:ab,ti OR ‘ng-nitro-l-arginine methyl ester’:ab,ti OR ‘methyl ester, ng-nitro-l-arginine’:ab,ti OR ‘ng nitro l arginine methyl ester’:ab,ti OR ‘l-name’:ab,ti OR ‘ng nitroarginine methyl ester, d orn isomer’:ab,ti OR ‘omega-n-methylarginine’:ab,ti OR ‘omega n methylarginine’:ab,ti OR ‘ng-monomethyl-l-arginine’:ab,ti OR ‘ng monomethyl l arginine’:ab,ti OR ‘l-nmma’:ab,ti OR ‘l-monomethylarginine’:ab,ti OR ‘l monomethylarginine’:ab,ti OR ‘d-nmma’:ab,ti OR ‘l-ng-monomethyl arginine’:ab,ti OR ‘arginine, l-ng-monomethyl’:ab,ti OR ‘l ng monomethyl arginine’:ab,ti OR ‘inhibitor of no synthase’:ab,ti OR ‘inos’:ab,ti OR ‘cnos’:ab,ti OR ‘nnos’:ab,ti OR ‘endothelial nos’:ab,ti OR ‘neuronal nos’:ab,ti OR ‘nos1’:ab,ti OR ‘nos2’:ab,ti OR ‘nos3’:ab,ti OR ‘inducible nos’:ab,ti OR ‘inhibitor of nos’:ab,ti OR ‘nitric-oxide synthase’:ab,ti OR ‘endothelium-derived relaxation factor-forming enzyme’:ab,ti OR ‘endothelium-derived relaxation factor synthase’:ab,ti OR ‘nadph-diaphorase’:ab,ti OR ‘constitutive nos’:ab,ti OR ‘nosii’:ab,ti OR ‘nosiii’:ab,ti OR ‘l-nna’:ab,ti OR nitroarginine:ab,ti OR ‘noarg’:ab,ti OR ‘omega-nitroarginine’:ab,ti OR ‘omega nitroarginine’:ab,ti OR ‘no2arg’:ab,ti OR ‘ng-nitro-l-arginine’:ab,ti OR ‘ng nitro l arginine’:ab,ti OR ‘ng-nitroarginine’:ab,ti OR ‘ng nitroarginine’:ab,ti OR ‘n omega-nitro-l-arginine’:ab,ti OR ‘n omega nitro l arginine’:ab,ti OR tilarginine:ab,ti OR ‘ng-methyl-l-arginine’:ab,ti OR ‘l-nma’:ab,ti OR dimethylarginine:ab,ti OR ‘adma’:ab,ti OR ‘l-nil’:ab,ti OR ‘l-nio’:ab,ti OR ‘l-nio dihydrochloride’:ab,ti OR ‘n(g)-nitro-l-arginine methyl ester’:ab,ti OR ‘nitric oxide’/exp OR ‘methylene blue’/exp OR ‘endothelial nitric oxide synthase’/exp OR ‘nitric oxide synthase’/exp OR ‘n(g) nitroarginine methyl ester’/exp OR ‘n(g) nitroarginine’/exp) AND (murine*:ab,ti OR rat:ab,ti OR rattus:ab,ti OR rats:ab,ti OR canine*:ab,ti OR dog:ab,ti OR dogs:ab,ti OR rabbit*:ab,ti OR animal*:ab,ti OR mouse:ab,ti OR mice:ab,ti OR murinae:ab,ti OR monkey*:ab,ti OR sheep:ab,ti OR pig:ab,ti OR pigs:ab,ti OR ‘guinea pig’:ab,ti OR ‘guinea-pigs’:ab,ti OR metazoa:ab,ti OR ‘animal model’/exp OR ‘haplorhini’/exp OR ‘sheep’/exp OR ‘murine’/exp OR ‘pig’/exp OR ‘mouse’/exp OR ‘dog’/exp OR ‘rat’/exp OR ‘leporidae’/exp)) | 517 | All search terms are searched in the fields: “title” and “abstract” (here marked with “:ab,ti”) and in the “thesaurus” (here marked with “/de”) when available. No filters or limitations applied Thesauru (Emtree) variations compared with PubMed’s MeSH is applied as per availability and recommendation in Embase. |
| Cochrane Library (Cochrane Collaboration) Coverage: From inception- search date Search Date: 24 March 2022 | ((“anaphylactic shock”:ti,ab,kw OR “anaphylactic shocks”:ti,ab,kw OR “Anaphylaxis”[Mesh] OR Anaphylaxis:ti,ab,kw OR “anaphylacticreaction”:ti,ab,kw OR “anaphylactic reactions”:ti,ab,kw OR “shock, anaphylactic”:ti,ab,kw) AND (murine*:ti,ab,kw OR rat:ti,ab,kw OR rats:ti,ab,kw OR canine*:ti,ab,kw OR dog:ti,ab,kw OR dogs:ti,ab,kw OR rabbit*:ti,ab,kw OR animal*:ti,ab,kw OR mouse:ti,ab,kw OR mice:ti,ab,kw OR “Models, Animal”[Mesh:NoExp] OR “Murinae”[Mesh] OR Murinae:ti,ab,kw OR “Mice”[Mesh] OR “Dogs”[Mesh] OR “Rats”[Mesh] OR “Rabbits”[Mesh] OR monkey*:ti,ab,kw OR “Haplorhini”[Mesh] OR sheep:ti,ab,kw OR “Sheep”[Mesh] OR pig:ti,ab,kw OR “Swine”[Mesh] OR pigs:ti,ab,kw OR guinea-pig:ti,ab,kw OR guinea-pigs:ti,ab,kw OR rattus:ti,ab,kw OR metazoa:ti,ab,kw) AND (“nitric oxide”:ti,ab,kw OR “oxide, nitric”:ti,ab,kw OR “nitrogen monoxide”:ti,ab,kw OR “endogenous nitrate vasodilator”:ti,ab,kw OR “monoxide, mononitrogen”:ti,ab,kw OR “nitric-oxide”:ti,ab,kw OR “Ntric Oxide”[Mesh] OR “methylene blue”:ti,ab,kw OR “blue, methylene”:ti,ab,kw OR “methylthioninium chloride”:ti,ab,kw OR “methylthionine chloride”:ti,ab,kw OR “swiss blue”:ti,ab,kw OR “basic blue 9”:ti,ab,kw OR “methylene blue N”:ti,ab,kw OR chromosmon:ti,ab,kw OR “Methylene Blue”[Mesh] OR “ENOS enzyme”:ti,ab,kw OR “ECNOS enzyme”:ti,ab,kw OR “Nitric Oxide Synthase Type III”[Mesh] OR “oxide synthase, nitric”:ti,ab,kw OR “NO synthase”:ti,ab,kw OR “Nitric Oxide Synthase”[Mesh] OR “NG-nitroarginine methyl ester”:ti,ab,kw OR “NG nitroarginine methyl Ester”:ti,ab,kw OR “N omega-nitro-L-arginine methyl ester”:ti,ab,kw OR “N omega nitro L arginine methyl ester”:ti,ab,kw OR “NG-Nitro-L-arginine methyl ester”:ti,ab,kw OR “methyl ester, NG-nitro-L-arginine”:ti,ab,kw OR “NG nitro L arginine methyl ester”:ti,ab,kw OR “N(G)-nitroarginine methyl ester”:ti,ab,kw OR “L-NAME”:ti,ab,kw OR “N(G)-nitro-L-arginine methyl ester”:ti,ab,kw OR “NG nitroarginine methyl ester, D orn isomer”:ti,ab,kw OR “NG-Nitroarginine Methyl Ester”[Mesh] OR “omega-N-methylarginine”:ti,ab,kw OR “omega N methylarginine”:ti,ab,kw OR “NG-monomethyl-L-arginine”:ti,ab,kw OR “NG monomethyl L arginine”:ti,ab,kw OR “L-NMMA”:ti,ab,kw OR “L-monomethylarginine”:ti,ab,kw OR “L monomethylarginine”:ti,ab,kw OR “D-NMMA”:ti,ab,kw OR “L-NG-monomethyl arginine”:ti,ab,kw OR “arginine, L-NG-monomethyl”:ti,ab,kw OR “L NG monomethyl arginine”:ti,ab,kw OR “inhibitor of NO synthase”:ti,ab,kw OR “iNOS”:ti,ab,kw OR “cNOS”:ti,ab,kw OR “nNOS”:ti,ab,kw OR “endothelial NOS”:ti,ab,kw OR “neuronal NOS”:ti,ab,kw OR “NOS1”:ti,ab,kw OR “NOS2”:ti,ab,kw OR “NOS3”:ti,ab,kw OR “inducible NOS”:ti,ab,kw OR “inhibitor of NOS”:ti,ab,kw OR “nitric-oxide synthase”:ti,ab,kw OR “endothelium-derived relaxation factor-forming enzyme”:ti,ab,kw OR “endothelium-derived relaxation factor synthase”:ti,ab,kw OR “NADPH-diaphorase”:ti,ab,kw OR “constitutive NOS”:ti,ab,kw OR “NOSII”:ti,ab,kw OR “NOSIII”:ti,ab,kw OR “L-NNA”:ti,ab,kw OR nitroarginine:ti,ab,kw OR “Nitroarginine”[MeSH Terms] OR “NOARG”:ti,ab,kw OR “omega-nitroarginine”:ti,ab,kw OR “omega nitroarginine”:ti,ab,kw OR “NO2Arg”:ti,ab,kw OR “NG-nitro-L-arginine”:ti,ab,kw OR “NG nitro L arginine”:ti,ab,kw OR “NG-nitroarginine”:ti,ab,kw OR “NG nitroarginine”:ti,ab,kw OR “N omega-nitro-L-arginine”:ti,ab,kw OR “N omega nitro L arginine”:ti,ab,kw OR tilarginine:ti,ab,kw OR “NG-methyl-L-arginine”:ti,ab,kw OR “L-NMA”:ti,ab,kw OR dimethylarginine:ti,ab,kw OR “ADMA”:ti,ab,kw OR “L-NIL”:ti,ab,kw OR “L-NIO”:ti,ab,kw OR “L-NIO dihydrochloride”:ti,ab,kw)) | 4 trials | All search terms searched in the fields: “title”, “abstract” and “keywords” (here marked with “ti,ab,kw”) and in the “MeSH” when available. No MeSH variations compared with PubMed’s MeSH. |
| Web of Science (Clarivate) Source: Core Collection Coverage: From inception- search date Search Date: 24 March 2022 | ((TOPIC:(“anaphylactic shock” OR “anaphylactic shocks” OR anaphylaxis OR “anaphylactic reaction” OR “anaphylactic reactions” OR “shock, anaphylactic”) AND (TOPIC:(“nitric oxide” OR “oxide, nitric” OR “nitrogen monoxide” OR “endogenous nitrate vasodilator” OR “monoxide, mononitrogen” OR “nitric-oxide” OR “methylene blue” OR “blue, methylene” OR “methylthioninium chloride” OR “methylthionine chloride” OR “swiss blue” OR “basic blue 9” OR “methylene blue N” OR “chromosmon” OR “ENOS enzyme” OR “ECNOS enzyme” OR “oxide synthase, nitric” OR “NO synthase” OR “NG-nitroarginine methyl ester” OR “NG nitroarginine methyl ester” OR “N omega-nitro-L-arginine methyl ester” OR “N omega nitro L arginine methyl ester” OR “NG-nitro-L-arginine methyl ester” OR “methyl ester, NG-nitro-L-arginine” OR “NG nitro L arginine methyl ester” OR “N(G) nitroarginine methyl ester” OR “L-NAME” OR “N(G)-nitro-L arginine methyl ester” OR “NG nitroarginine methyl ester, D orn isomer” OR “omega-N methylarginine” OR “omega N methylarginine” OR “NG-monomethyl-L-arginine” OR “NG monomethyl L arginine” OR “L-NMMA” OR “L-monomethylarginine” OR “L monomethylarginine” OR “D-NMMA” OR “L-NG-monomethyl arginine” OR “arginine, L-NG monomethyl” OR “L NG monomethyl arginine” OR “inhibitor of NO synthase” OR “iNOS” OR “cNOS” OR “nNOS” OR “endothelial NOS” OR “neuronal NOS” O “NOS1” OR “NOS2” OR “NOS3” OR “inducible NOS” OR “inhibitor o NOS” OR “nitric-oxide synthase” OR “endothelium derived relaxation factor-forming enzyme” OR “endothelium derived relaxation factor synthase” OR “NADPH diaphorase” OR “constitutive NOS” OR “NOSII” OR “NOSIII” OR “LNNA” OR nitroarginine OR “NOARG” OR “omega-nitroarginine” OR “omega nitroarginine” OR “NO2Arg” OR “NG-nitro-L-arginine” OR “NG nitro L arginine” OR “NG-nitroarginine” OR “NG nitroarginine” OR “N omega-nitro-L-arginine” OR “N omega nitro L arginine” OR tilarginine OR “NG-methyl-L-arginine” OR “L-NMA” OR dimethylarginine OR “ADMA” OR “L-NIL” OR “L-NIO” OR “L NIO dihydrochloride”)) AND (TOPIC: (murine* OR rat OR rattus OR rats OR canine* OR dog OR dogs OR rabbit* OR animal* OR mouse OR mice OR murinae OR monkey* OR sheep OR “pig” OR “pigs” OR guinea-pig OR “guinea-pigs” OR metazoa))) | 176 | All search terms are searched in the field “topic” (including title, abstract and author supplied keywords, here marked with “TOPIC”). No filters or limitations applied No thesaurus available. |
| Total no. of records identified: | 990 | ||
| Total no. or unique records identified after automatic de-duplication in Covidence | 345 | ||
Appendix B
| Section/Topic | # | Checklist Item | Location(s) Reported |
|---|---|---|---|
| Information Sources and Methods | |||
| Database name | 1 | Name each individual database searched, stating the platform for each. | 5 |
| Multi-database searching | 2 | If databases were searched simultaneously on a single platform, state the name of the platform, listing all of the databases searched. | 5 |
| Study registries | 3 | List any study registries searched. | 7 |
| Online resources and browsing | 4 | Describe any online or print source purposefully searched or browsed (e.g., tables of contents, print conference proceedings, web sites), and how this was done. | 6 |
| Citation searching | 5 | Indicate whether cited references or citing references were examined, and describe any methods used for locating cited/citing references (e.g., browsing reference lists, using a citation index, setting up email alerts for references citing included studies). | 6 |
| Contacts | 6 | Indicate whether additional studies or data were sought by contacting authors, experts, manufacturers, or others. | 6 |
| Other methods | 7 | Describe any additional information sources or search methods used. | 6 |
| Search Strategies | |||
| Full search strategies | 8 | Include the search strategies for each database and information source, copied and pasted exactly as run. | Appendix A |
| Limits and restrictions | 9 | Specify that no limits were used, or describe any limits or restrictions applied to a search (e.g., date or time period, language, study design) and provide justification for their use. | 6 |
| Search filters | 10 | Indicate whether published search filters were used (as originally designed or modified), and if so, cite the filter(s) used. | 6 |
| Prior work | 11 | Indicate when search strategies from other literature reviews were adapted or reused for a substantive part or all of the search, citing the previous review(s). | - |
| Updates | 12 | Report the methods used to update the search(es) (e.g., rerunning searches, email alerts). | |
| Dates of searches | 13 | For each search strategy, provide the date when the last search occurred. | Appendix A |
| Peer Review | |||
| Peer review | 14 | Describe any search peer review process. | - |
| Managing Records | |||
| Total Records | 15 | Document the total number of records identified from each database and other information sources. | 7 |
| Deduplication | 16 | Describe the processes and any software used to deduplicate records from multiple database searches and other information sources. | 6 |
| PRISMA-S: An Extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews | |||
| Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, Koffel JB, PRISMA-S Group. | |||
| Last updated 27 February 2020. | |||
Appendix C
| Section and Topic | Item # | Checklist Item | Location where Item Is Reported |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review. | 1 |
| Abstract | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | - |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | 5 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | 5 |
| Methods | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | 6 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | 5 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | Appendix A |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | 6 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | 6 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | 6 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | 6 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | 7 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | No meta-analysis |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | 12 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | 12 | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | 12 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | No meta-analysis | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | No meta-analysis | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | No meta-analysis | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | No meta-analysis |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | No meta-analysis |
| Results | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | 7 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | - | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | 9 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | 7 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | No meta-analysis |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | No meta-analysis |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | No meta-analysis | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | 8 | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | No meta-analysis | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | - |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | No meta-analysis |
| Discussion | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | 21 |
| 23b | Discuss any limitations of the evidence included in the review. | 22 | |
| 23c | Discuss any limitations of the review processes used. | 22 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | 22 | |
| Other Information | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | 5 |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | 5 | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | - | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | - |
| Competing interests | 26 | Declare any competing interests of review authors. | 23 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | - |
References
- De Bisschop, M.-B.; Bellou, A. Anaphylaxis. Curr. Opin. Crit. Care 2012, 18, 308–317. [Google Scholar] [CrossRef]
- Cauwels, A. Nitric oxide in shock. Kidney Int. 2007, 72, 557–565. [Google Scholar] [CrossRef]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125 (Suppl. 2), S73–S80. [Google Scholar] [CrossRef]
- Nguyen, S.M.; Rupprecht, C.P.; Haque, A.; Pattanaik, D.; Yusin, J.; Krishnaswamy, G. Mechanisms Governing Anaphylaxis: Inflammatory Cells, Mediators, Endothelial Gap Junctions and Beyond. Int. J. Mol. Sci. 2021, 22, 7785. [Google Scholar] [CrossRef]
- Yu, J.E.; Lin, R.Y. The Epidemiology of Anaphylaxis. Clin. Rev. Allergy Immunol. 2018, 54, 366–374. [Google Scholar] [CrossRef]
- Muraro, A.; Worm, M.; Alviani, C.; Cardona, V.; DunnGalvin, A.; Garvey, L.H.; Riggioni, C.; de Silva, D.; Angier, E.; Arasi, S.; et al. EAACI guideline: Anaphylaxis (2021 update). Allergy 2021, 77, 357–377. [Google Scholar] [CrossRef]
- Halken, S.; Muraro, A.; de Silva, D.; Khaleva, E.; Angier, E.; Arasi, S.; Arshad, H.; Bahnson, H.T.; Beyer, K.; Boyle, R.; et al. EAACI guideline: Preventing the development of food allergy in infants and young children (2020 update). Pediatric Allergy Immunol. 2021, 32, 843–858. [Google Scholar] [CrossRef]
- Al-Salam, S.; Aburawi, E.H.; Al-Hammadi, S.; Dhanasekaran, S.; Shafiuallah, M.; Yasin, J.; Sudhadevi, M.; Awwad, A.; Alper, S.L.; Kazzam, E.E.; et al. Cellular and Immunohistochemical Changes in Anaphylactic Shock Induced in the Ovalbumin-Sensitized Wistar Rat Model. Biomolecules 2019, 9, 101. [Google Scholar] [CrossRef]
- Jönsson, F.; Mancardi, D.A.; Kita, Y.; Karasuyama, H.; Iannascoli, B.; Van Rooijen, N.; Shimizu, T.; Daëron, M.; Bruhns, P. Mouse and human neutrophils induce anaphylaxis. J. Clin. Investig. 2011, 121, 1484–1496. [Google Scholar] [CrossRef]
- Cauwels, A.; Janssen, B.; Buys, E.; Sips, P.; Brouckaert, P. Anaphylactic shock depends on PI3K and eNOS-derived NO. J. Clin. Investig. 2006, 116, 2244–2251. [Google Scholar] [CrossRef]
- Tacquard, C.; Oulehri, W.; Collange, O.; Garvey, L.H.; Nicoll, S.; Tuzin, N.; Geny, B.; Mertes, P.M. Treatment with a platelet-activating factor receptor antagonist improves hemodynamics and reduces epinephrine requirements, in a lethal rodent model of anaphylactic shock. Clin. Exp. Allergy 2020, 50, 383–390. [Google Scholar] [CrossRef]
- Masini, E.; Zagli, G.; Ndisang, J.F.; Solazzo, M.; Mannaioni, P.F.; Bani, D. Protective effect of relaxin in cardiac anaphylaxis: Involvement of the nitric oxide pathway. Br. J. Pharmacol. 2002, 137, 337–344. [Google Scholar] [CrossRef]
- Ahrens, R.; Osterfeld, H.; Wu, D.; Chen, C.-Y.; Arumugam, M.; Groschwitz, K.; Strait, R.; Wang, Y.-H.; Finkelman, F.D.; Hogan, S.P. Intestinal Mast Cell Levels Control Severity of Oral Antigen-Induced Anaphylaxis in Mice. Am. J. Pathol. 2012, 180, 1535–1546. [Google Scholar] [CrossRef]
- Mukai, K.; Kuda, Y.; Shibamoto, T.; Tanida, M.; Kurata, Y.; Yokoyama, H. Renal response to anaphylaxis in anesthetized rats and isolated perfused rat kidneys: Roles of nitric oxide. J. Physiol. Sci. JPS 2018, 68, 689–697. [Google Scholar] [CrossRef]
- Nathan, C. Inducible nitric oxide synthase: What difference does it make? J. Clin. Investig. 1997, 100, 2417–2423. [Google Scholar] [CrossRef]
- Mitsuhata, H.; Shimizu, R.; Mitsuo Yokoyama, M. Role of nitric oxide in anaphylactic shock. J. Clin. Immunol. 1995, 15, 277–283. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Cabell’s Scholarly Analytics. 2020. Available online: https://www2.cabells.com/about-predatory (accessed on 5 January 2022).
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; Blunt, H.; Brigham, T.; Chang, S.; et al. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software, version; Veritas Health Innovation: Melbourne, Australia. Available online: www.covidence.org (accessed on 24 March 2022).
- Huwaldt, J.A. Plot Digitizer. Version 2.6.8. Available online: https://plotdigitizer.sourceforge.net/ (accessed on 9 June 2020).
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Takano, H.; Liu, W.; Zhao, Z.; Cui, S.; Zhang, W.; Shibamoto, T. NG-nitro-L-arginine methyl ester, but not methylene blue, attenuates anaphylactic hypotension in anesthetized mice. J. Pharmacol. Sci. 2007, 104, 212–217. [Google Scholar] [CrossRef]
- Shinomiya, S.; Shibamoto, T.; Kurata, Y.; Kuda, Y.; Zhang, W.; Tanida, M.; Toga, H. Nitric oxide and β2-adrenoceptor activation attenuate pulmonary vasoconstriction during anaphylactic hypotension in anesthetized BALB/c mice. Exp. Lung Res. 2013, 39, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, A.A.S.; Ferreira, L.G.; Carvalho, M.T.M.; Capellini, V.K.; Evora, P.R.B.; Celotto, A.C. Effects of NO/cgmp inhibitors in a rat model of anaphylactoid shock. Braz. J. Med. Biol. Res. 2020, 53, e8853. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, A.A.S.; Celotto, A.C.; Becari, C.; Prandi, M.; Barbosa, J.M.; Moreira, F.N.; Jordani, M.C.; Evora, P.R.B. Indigo Carmine Hemodynamic Studies to Treat Vasoplegia Induced by Compound 48/80 in a Swine Model of Anaphylaxis. Braz. J. Cardiovasc. Surg. 2022, 37, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Buzato, M.A.S.; Viaro, F.; Piccinato, C.E.; Evora, P.R.B. The use of methylene blue in the treatment of anaphylactic shock induced by compound 48/80: Experimental studies in rabbits. Shock 2005, 23, 582–587. [Google Scholar]
- Menardi, A.C.; Capellini, V.K.; Celotto, A.C.; Albuquerque, A.A.S.; Viaro, F.; Vicente, W.V.A.; Rodrigues, A.J.; Evora, P.R.B. Methylene blue administration in the compound 48/80-induced anaphylactic shock. Hemodynamic study in pigs. Acta Cir. Bras. 2011, 26, 481–489. [Google Scholar] [CrossRef]
- Mitsuhata, H.; Takeuchi, H.; Saitoh, J.; Hasome, N.; Horiguchi, Y.; Shimizu, R. An inhibitor of nitric oxide synthase, N omega-nitro-L-arginine-methyl ester, attenuates hypotension but does not improve cardiac depression in anaphylaxis in dogs. Shock 1995, 3, 447–453, discussion 454. [Google Scholar]
- Shibamoto, T.; Wang, H.G.; Tanaka, S.; Miyahara, T.; Koyama, S. Participation of nitric oxide in the sympathetic response to anaphylactic hypotension in anesthetized dogs. Neurosci. Lett. 1996, 212, 99–102. [Google Scholar] [CrossRef]
- Bellou, A.; Lambert, H.; Gillois, P.; Montémont, C.; Gerard, P.; Vauthier, E.; Sainte-Laudy, J.; Longrois, D.; Guéant, J.L.; Mallié, J.P. Constitutive nitric oxide synthase inhibition combined with histamine and serotonin receptor blockade improves the initial ovalbumin-induced arterial hypotension but decreases the survival time in brown norway rats anaphylactic shock. Shock 2003, 19, 71–78. [Google Scholar] [CrossRef]
- Zhang, W.; Shibamoto, T.; Cui, S.; Takano, H.; Kurata, Y. 7-Nitroindazole, but not L-NAME or aminoguanidine, attenuates anaphylactic hypotension in conscious rats. Shock 2009, 31, 201–206. [Google Scholar] [CrossRef]
- Zheng, F.; Barthel, G.; Collange, O.; Montémont, C.; Thornton, S.N.; Longrois, D.; Levy, B.; Audibert, G.; Malinovsky, J.M.; Mertes, P.M. Methylene blue and epinephrine: A synergetic association for anaphylactic shock treatment. Crit. Care Med. 2013, 41, 195–204. [Google Scholar] [CrossRef]
- Amir, S.; English, A.M. An inhibitor of nitric oxide production, N(G)-nitro-L-arginine-methyl ester, improves survival in anaphylactic shock. Eur. J. Pharmacol. 1991, 203, 125–127. [Google Scholar] [CrossRef]
- Albuquerque, A.A.S.; Margarido, E.A.; Menardi, A.C.; Scorzoni Filho, A.; Celotto, A.C.; Rodrigues, A.J.; Vicente, W.V.A.; Evora, P.R.B. Methylene blue to treat protamine-induced anaphylaxis reactions. An experimental study in pigs. Braz. J. Cardiovasc. Surg. 2016, 31, 226–231. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mitsuhata, H.; Saitoh, J.; Hasome, N.; Takeuchi, H.; Horiguchi, Y.; Shimizu, R. Nitric oxide synthase inhibition is detrimental to cardiac function and promotes bronchospasm in anaphylaxis in rabbits. Shock 1995, 4, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Osada, S.; Ichiki, H.; Oku, H.; Ishiguro, K.; Kunitomo, M.; Semma, M. Participation of nitric oxide in mouse anaphylactic hypotension. Eur. J. Pharmacol. 1994, 252, 347–350. [Google Scholar] [CrossRef]
- Yang, J.; Clark, J.W.; Bryan, R.M.; Robertson, C.S. Mathematical modeling of the nitric oxide/cGMP pathway in the vascular smooth muscle cell. Am. J. Physiol. -Heart Circ. Physiol. 2005, 289, H886–H897. [Google Scholar] [CrossRef]
- Kopincová, J.; Púzserová, A.; Bernátová, I. L-NAME in the cardiovascular system—Nitric oxide synthase activator? Pharmacol. Rep. 2012, 64, 511–520. [Google Scholar] [CrossRef]
- Stawicki, S.P.; Sims, C.; Sarani, B.; Grossman, M.D.; Gracias, V.H. Methylene blue and vasoplegia: Who, when, and how? Mini-Rev. Med. Chem. 2008, 8, 472–490. [Google Scholar] [CrossRef]
- Chang, K.S.; Zhong, M.Z.; Davis, R.F. Indigo carmine inhibits endothelium-dependent and -independent vasodilation. Hypertension 1996, 27, 228–234. [Google Scholar] [CrossRef]
- Sakuma, I.; Togashi, H.; Yoshioka, M.; Saito, H.; Yanagida, M.; Tamura, M.; Kobayashi, T.; Yasuda, H.; Gross, S.S.; Levi, R. NG-methyl-L-arginine, an inhibitor of L-arginine-derived nitric oxide synthesis, stimulates renal sympathetic nerve activity in vivo. A role for nitric oxide in the central regulation of sympathetic tone? Circ. Res. 1992, 70, 607–611. [Google Scholar] [CrossRef]
- De Caterina, R.; Libby, P.; Peng, H.B.; Thannickal, V.J.; Rajavashisth, T.B.; Gimbrone, M.A., Jr.; Shin, W.S.; Liao, J.K. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J. Clin. Investig. 1995, 96, 60–68. [Google Scholar] [CrossRef]
- Pemmaraju, D.; Kallur, L.; Roach, K.; King, A.; Bryant, K.; Lindsey, J.; Sahebazamani, M. Use of methylene blue in refractory anaphylaxis may prevent a catastrophic intubation. Chest 2019, 156, A1892–A1893. [Google Scholar] [CrossRef]
- Bauer, C.S.; Vadas, P.; Kelly, K.J. Methylene Blue for the Treatment of Refractory Anaphylaxis without Hypotension. Am. J. Emerg. Med. 2013, 31, e263–e264. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Neto, A.M.; Duarte, N.M.; Vicente, W.V.A.; Viaro, F.; Evora, P.R.B. Methylene blue: An effective treatment for contrast medium-induced anaphylaxis. Med. Sci. Monit. 2003, 9, CS102–CS106. [Google Scholar] [PubMed]
- Rodrigues, J.M.; Pazin Filho, A.; Rodrigues, A.J.; Vicente, W.V.D.A.; Evora, P.R.B. Methylene blue for clinical anaphylaxis treatment: A case report. Sao Paulo Med. J. 2007, 125, 60–62. [Google Scholar] [CrossRef]
- Stocche, R.M.; Garcia, L.V.; Paulino Dos Reis, M.; Klamt, J.G.; Évora, P.R.B. Methylene blue to treat anaphylaxis during anesthesia. Case report. Rev. Bras. Anestesiol. 2004, 54, 809–814. [Google Scholar] [CrossRef]
- Evora, P.R.B.; Roselino, C.H.C.; Schiaveto, P.M. Methylene blue in anaphylactic shock. Ann. Emerg. Med. 1997, 30, 240. [Google Scholar]
- Da Silva, P.S.; Furtado, P. Methylene blue to treat refractory latex-induced anaphylactic shock: A case report. A&A Pract. 2018, 10, 57–60. [Google Scholar]
- Avontuur, J.A.M.; Nolthenius, R.P.T.; Buijk, S.L.C.E.; Kanhai, K.J.K.; Braining, H.A. Effect of L-NAME, an Inhibitor of Nitric Oxide Synthesis, on Cardiopulmonary Function in Human Septic Shock. Chest 1998, 113, 1640–1646. [Google Scholar] [CrossRef]
- Petros, A.; Bennett, D.; Vallance, P. Effect of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet 1991, 338, 1557–1558. [Google Scholar] [CrossRef]
- Cotter, G.; Kaluski, E.; Milo, O.; Blatt, A.; Salah, A.; Hendler, A.; Krakover, R.; Golick, A.; Vered, Z. LINCS: L-NAME (a NO synthase inhibitor) In the treatment of refractory Cardiogenic Shock: A prospective randomized study. Eur. Heart J. 2003, 24, 1287–1295. [Google Scholar] [CrossRef]
- Bellou, A.; Al-Hammadi, S.; Aburawi, E.H.; Dhanasekaran, S.; Nemmar, A.; Oulhaj, A.; Shafiuallah, M.; Zerrouki, M.; Yasin, J.; Bellou, L.; et al. 4-Aminopyridine, a Blocker of Voltage-Dependent K+ Channels, Restores Blood Pressure and Improves Survival in the Wistar Rat Model of Anaphylactic Shock. Crit. Care Med. 2016, 44, e1082–e1089. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, S.; Nemmar, A.; Aburawi, E.H.; Kazzam, E.E.; Abdulle, A.; Bellou, M.; Bellou, A. Glyburide, a K+ATP channel blocker, improves hypotension and survival in anaphylactic shock induced in Wistar rats sensitized to ovalbumin. Eur. J. Pharmacol. 2013, 720, 166–173. [Google Scholar] [CrossRef] [PubMed]
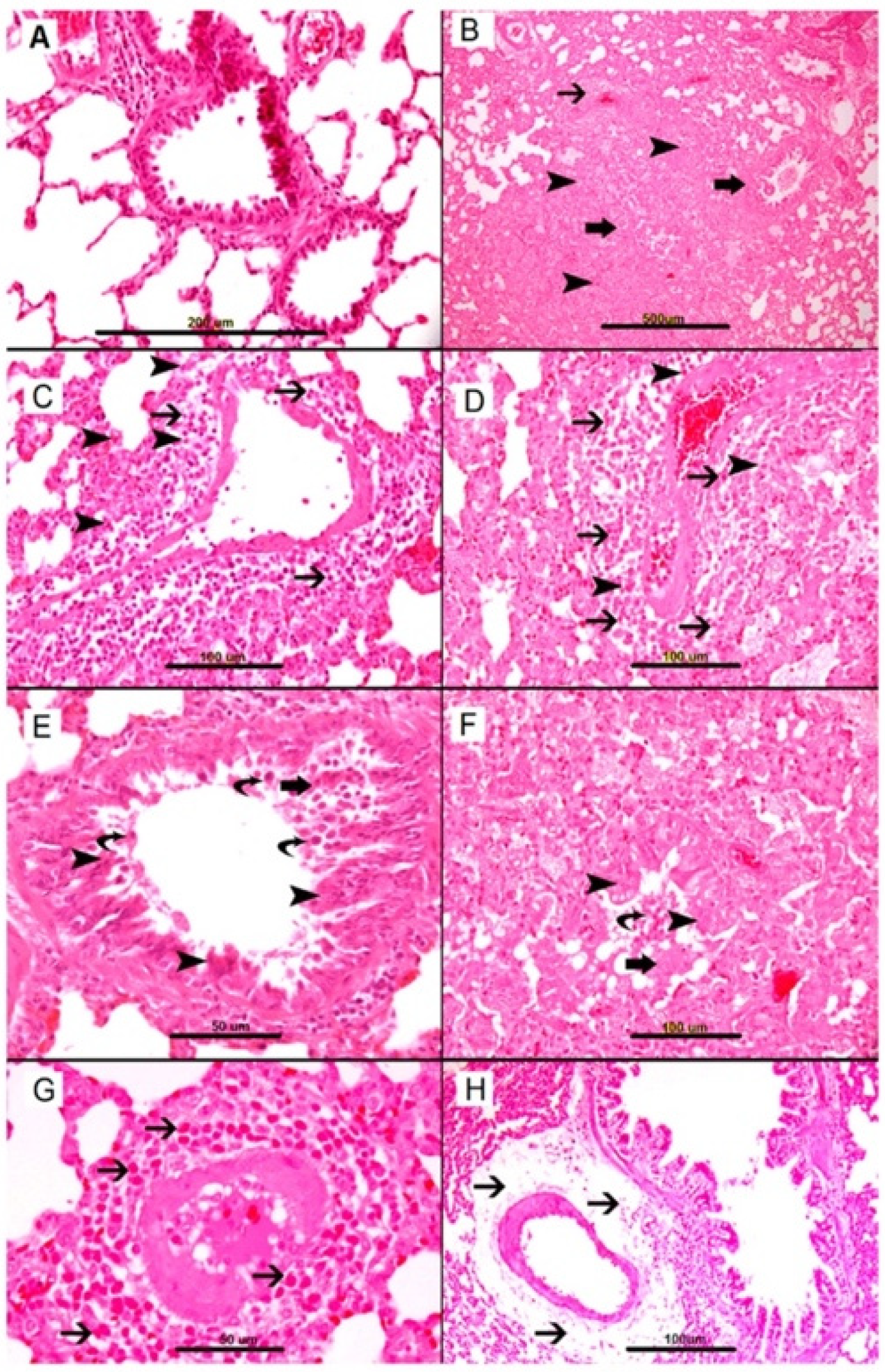

| Authors, Year | Title | Animal Species | AS Sensitization and Induction | Intervention and Dose | Pathophysiology Suspected | ||
|---|---|---|---|---|---|---|---|
| Osada et al., 1994 [37] | Participation of Nitric Oxide in mouse anaphylactic hypotension | ddY mice | Subcutaneous sensitization by 50 ug of hen egg-white lysozyme in Freund’s complete adjuvant on day 0. After 9 days, AS induced by 1 ug of intravenous (IV) lysozyme in saline | L-NAME 1 mg/kg 30 min before AS | Histamine released from sensitized mast cells stimulates vascular endothelial cells via H1 receptors. This leads to activation of NOS. The subsequent release of NO causes peripheral vasodilation through blood vessel smooth muscle stimulation, resulting in AS. | ||
| Mitsuhata et al., 1995 [36] | Nitric oxide synthase inhibition is detrimental to cardiac function and promotes bronchospasm in anaphylaxis in rabbits | Japanese white rabbits | Sensitized to horse serum with an initial 2 mL subcutaneous dose followed 2 days later by IV dose. After the second dose (14 days), AS induced by IV challenge with 2 mL horse serum over 10 s | L-NAME 30 mg/kg 15 min before AS | NOS inhibition may accentuate cardiac depression more than it increases venous return, therefore lowering the survival rate in L-NAME pretreated animals. | ||
| Shibamoto et al., 1996 [30] | Participation of nitric oxide in the sympathetic response to anaphylactic hypotension in anesthetized dogs | Mongrel dogs | Naturally sensitized to Ascaris antigen and shock induced by IV bolus 10 mg Ascaris suum diluted in 1 mL of saline | L-NAME 20 mg/kg bolus 15 min before anaphylactic shock and continuous infusion of 0.05 mg/kg per min (0.3 mg/min) over 75 min | NO is involved in the anaphylaxis-induced renal sympathoinhibitory response but not hypotension in anesthetized dogs. | ||
| Bellou et al., 2003 [31] | Constitutive nitric oxide synthase inhibition combined with histamine and serotonin receptor blockade improves initial ovalbumin-induced arterial hypotension but decreases the survival time in brown norway rats anaphylactic shock | Brown Norway Rats | SC 1 mg of OVA + 3.5 mg of aluminum hydroxide (Al OH) in 1 mL of 0.9% NaCl suspension given on day 0, 5 and 21. Shock induced by IV 1 mg OVA suspended in 1 mL of 0.9% saline | L-NAME, IV 100 mg/kg. 30 min before AS | Overall: imbalance between vasoconstrictor and vasodilator PG. NO synthase inhibition aggravates cardiac dysfunction and promotes bronchospasm. Inhibition of NOS3 by L-NAME could promote the activity of vasoconstrictor prostaglandins and/or leukotrienes, therefore decreasing HR by coronary vasoconstriction. | ||
| Buzato et al., 2005 [27] | The use of methylene blue in the treatment of anaphylactic shock induced by compound C48/80: experimental study in rabbits | New Zealand rabbits | No sensitization. AS induced by C48/80 intravenous bolus infusion (4/5 mg/kg) | MB 3 mg/kg intravenous bolus infusion 1–2 min before C48/80 infusion | The use of MB post-treatment reversed the AS hypotension but not when used as pre-treatment. Hypothesized pathophysiology involves the improvement of blood pressure by vasoconstriction. This proposes that MB has a role in increasing the smooth muscle cGMP, caused by NO released by histamine. | ||
| Cauwels et al., 2006 [10] | Anaphylactic shock depends on PI3K and eNOS derived NO | C57BL/6 mice | AS was induced by PAF. It was diluted in 200 μL endotoxin-free phosphate buffered saline (PBS) supplemented with 0.25% BSA and injected IV | L-NAME (Tempol was injected IP at 6 mg 1 h before PAF) 100 mg/kg IV | 1 h before AS | The role of eNOS is important in regulating vascular function in shock. Downstream sGC is the main mediator for NO-induced vascular smooth muscle vasodilation. | |
| 2 h before AS | |||||||
| 4 h before AS | |||||||
| L-NAME (Tempol was injected IP at 6 mg 1 h before PAF) 100 mg/kg, IV, 2 h before AS | |||||||
| MB, in glucose solution suitable for IV injection at a dose of 15 mg/kg | 1 h before AS | ||||||
| 2 h before AS | |||||||
| 4 h before AS | |||||||
| 6 h before AS | |||||||
| ODQ was used i.p. in 50 μL DMSO at 20, 15, 10 or 5 mg/kg | 0.5 h before AS | ||||||
| 2 h before AS | |||||||
| 4 h before AS | |||||||
| Single IP injection of 1 mg BSA mixed with 300 ng pertussis toxin. AS was induced 15 days after by: | BSA induced anaphylaxis with dose not mentioned | L-NAME, 200 mg/kg IV 2 h before AS | |||||
| IV injection of 0.1 mg of BSA | |||||||
| IV injection of 2 mg of BSA | |||||||
| Takano et al., 2007 [23] | NG-nitro-L arginine methyl-ester, but not methylene blue, attenuates anaphylactic hypotension in anesthesized mice | BALB/c mice | SC injection of an emulsion made by mixing aluminum potassium sulfate adjuvant (2 mg) with 0.01 mg ovalbumin, dissolved in saline (0.2 mL). The antigen emulsion was injected a second time, 7 days after the first antigen injection. AS was induced 1 week after the second injection. AS was induced by 0.01 mg of ovalbumin antigen (in 100 μL saline) | L-NAME, 1.0 mg/kg, 25 μL IV 10 min prior to AS | AS causes hepatic venoconstriction and portal hypertension, resulting in congestion of the upstream splanchnic organs. This decreases venous return and effective circulating blood volume exacerbates anaphylactic hypotension. L-NAME seems to increase systemic arterial blood pressure through sympathetic nerve activity stimulation of systemic arterioles but has no effect on hepatic circulation. Therefore, it was concluded that NO partially contributes to anaphylactic hypotension. The lack of improvement with MB or ODQ use suggests that there are sGC independent events downstream from NO production in AS that explain the beneficial effect of INOP. | ||
| MB, 3.0 mg/kg, 25 μL IV 2 min prior to AS | |||||||
| Same as above but intraperitoneal for AS induction | ODQ, IP 10 mg/kg in 50 μL DMSO 1.5 h prior to AS | ||||||
| Naturally sensitized by C48/80, AS induced by C48/80 (4.0 mg/kg, 100 μL); IV | MB, 3.0 mg/kg, 25 μL IV 2 min before AS | ||||||
| Zhang et al., 2009 [32] | 7-Nitroindazole, but not L-NAME or aminoguanidine, attenuates anaphylactic hypotension in conscious rats | Sprague-Dawley rats | SC injection of an emulsion made by mixing equal volumes of complete Freund adjuvant (0.5 mL) with 1 mg ovalbumin dissolved in physiological saline (0.5 mL). Two weeks after, AS was induced by IV 0.6 mg of ovalbumin antigen in 300 μL saline | L-NAME, IV 10 mg/kg, 100 μL, 20 min before AS | 7-NI (nNOS inhibitor) significantly attenuated the antigen-induced MAP decrease. Beneficial effect of 7-NI: nNOS inhibition might have counteracted the anaphylaxis-related sympathoinhibition, which preserved vasoconstriction of the resistance arteries and attenuated the antigen-induced systemic hypotension. L-NAME led to shorter survival time, most likely due to cardiac dysfunction and coronary vasoconstriction causing left heart failure and pulmonary congestion and edema. AG (iNOS inhibitor) did not affect the anaphylactic response. | ||
| iNos inhibitor, IV Aminoguanidine hydrochloride (AG), 20 min before AS | |||||||
| nNos inhibitor, 7-Nitroindazole (7-NI), IP 50 mg/kg, 1 mL, 20 min before AS | |||||||
| Menardi AC et al., 2011 [28] | Methylene blue administration in the compound 48/80-induced anaphylactic shock. Hemodynamic study in pigs | Dalland pigs | No sensitization. AS induced by bolus injection of C48/80 (4 mg/kg) | MB 2 mg/g bolus injection 3 min before AS | MB did not prevent or reverse the C48/80-induced anaphylactic shock; but the epidermal alterations did disappear after MB infusion. Pre-treatment had little to no effect on either. | ||
| Shinomiya et al., 2013 [24] | Nitric oxide and B2-adrenoceptor activation attenuate pulmonary vasoconstriction during anaphylactic hypotension in anesthetized BALB/c mice | BALB/c mice | Subcutaneous injection of an emulsion made by mixing aluminum potassium sulfate adjuvant 2 mg) with 0.01 mg ovalbumin dissolved in saline (0.2 mL). A second antigen injection was given 7 days after the first injection. The AS was induced one week after the second injection. | L-NAME 50 mg/kg; 50 μL 10 min before AS | Anaphylaxis causes pulmonary vasoconstriction, resulting in increased right heart afterload, and then a decrease in venous return, which finally contributes to anaphylactic hypotension. In this study, it was observed that L-NAME pre-treatment enhanced anaphylactic pulmonary vasoconstriction evidenced by the greater increases in systolic PAP. | ||
| Albuquerque AAS et al., 2016 [35] | Methylene blue to treat protamine-induced anaphylaxis reactions. An experimental study in pigs | Dalland pigs | No sensitization. AS induced by protamine IV infusion (dose not mentioned) | MB 3 mg/kg IV infusion (time not mentioned) | Protamine binds to an endothelial cell receptor that signals conversion of L-arginine to NO. NO activates sGC in the vascular smooth muscle to cause cGMP-mediated vasodilation. The resultant vasodilation decreases pulmonary vascular resistance and blood pressure. MB reversed the hypotension caused by protamine by acting on the NO/endothelium-dependent mechanism. | ||
| Mukai et al., 2018 [14] | Renal response to anaphylaxis in anesthetized rats and isolated perfused rat kidneys: roles of nitric oxide | Sprague Dawley rats | SC injection of an emulsion made by mixing equal volumes of complete Freund’s adjuvant (0.5 mL) and 0.5 mg ovalbumin. Two weeks after injection, shock induced by IV challenge with 0.6 mg of antigen | L-NAME, 10 mg/kg, 100 μL, IV. 10 min before AS | NO is produced in AS by different mechanisms that lead to hypotension and shock state. Proposed mechanisms of NO production are by the anaphylactic mediators inducing the vascular endothelium or by increased shear stress on the vascular endothelium. NO inhibitors reverse the AS by counteracting this hypotension. | ||
| Albuquerque et al., 2020 [25] | Effects of NO/cGMP inhibitors in a rat model of anaphylactoid shock | Male Wistar rats | Naturally sensitized by C48/80. AS was induced by C48/80 (3 mg/kg) IV bolus injection | L-NAME, 1 mg/kg IV 5 min before AS | The beneficial effect of L-NAME could be attributed to the blockage of eNOS. Removing NO production caused an SBP increase. MB is a non-selective GC inhibitor. When GC is inhibited, cGMP will not increase to cause vasodilation and hypotension. It is difficult to interpret the mechanism of IC’s effect on BP due to the ambiguous results. | ||
| MB, 3 mg/kg 5 min before AS | |||||||
| IC, 3 mg/kg 5 min before AS | |||||||
| Albuquerque et al., 2022 [26] | Indigo Carmine Hemodynamic Studies to Treat Vasoplegia Induced by Compound 48/80 in a Swine Model of Anaphylaxis | Male Daland Pigs | Naturally sensitized by C48/80. | IC 3 mg/kg 10 min before AS | IC inhibits endothelium-dependent relaxation specifically in relation to cGMP release. Additional effectiveness of IC was expected due to its alpha-adrenergic stimulation, which should counteract systemic hypotension. However, the vasoconstrictive effect was not apparent in this study. | ||
| Authors, Year | Title | Animal Species | AS Sensitization and Induction | Intervention and Dose | Pathophysiology Suspected | |
|---|---|---|---|---|---|---|
| Amir and English, 1991 [34] | An inhibitor of nitric oxide production, NG-nitro-L-arginine-methyl ester, improves survival in anaphylactic shock | Swiss Webster mice | IP with 2 mg bovine serum albumin (BSA) in 0.2 mL aluminum hydroxide gel. AS induced by IV 0.2 mL saline containing 100 ug BSA | L-NAME | 30 mg/kg | The principal mediators of AS, histamine and bradykinin, stimulate NO release from vascular endothelial cells. NO relaxed vascular smooth muscle to cause venous dilation and systemic hypotension. Blocking NO production using L-NAME prevented vasorelaxation and improved the hypotension caused by AS. |
| 60 mg/kg | ||||||
| AS induced by C48/80 | 30 mg/kg | |||||
| 60 mg/kg | ||||||
| Mitsuhata et al., 1995 [29] | An inhibitor of nitric oxide production, NG-nitro-L-arginine-methyl ester, attenuates hypotension but does not improve cardiac depression in anaphylaxis in dogs | Dog | Intradermal 0.1 mL of 1:100 dilution of an aqueous extract of Ascaris suum antigen with N2 concentration of 2.5 mg/mL. AS induced by 1 mL of A suum antigen into systemic circulation over 30 s | L-NAME 60 mg/kg (in 10 mL saline solution) | NO released by antigen challenge may be responsible (in part) for the hypotension due to vasodilation and fluid loss into the tissue space resulting from increased capillary permeability in anaphylaxis. NOS inhibitor did not improve cardiac function, which implies that production of NO in anaphylaxis may have a protective effect regarding cardiac function. | |
| Buzato et al., 2005 [27] | The use of methylene blue in the treatment of anaphylactic shock induced by compound C48/80: experimental studies in rabbits | New Zealand Rabbits | AS induction by C48/80 IV bolus infusion (4.5 mg/kg) | MB 3 mg/kg venous bolus infusion | The use of MB post-treatment reversed the AS hypotension but not when used as pre-treatment. Hypothesized pathophysiology involves the improvement of blood pressure by vasoconstriction. This proposes that MB has a role in increasing the smooth muscle cGMP, caused by NO released by histamine. | |
| Menardi AC et al., 2011 [28] | Methylene blue administration in the compound 48/80-induced anaphylactic shock: hemodynamic study in pigs | Dalland pigs | No sensitization. AS induced by bolus injection of C48/80 (4 mg/kg) | MB 2 mg/g bolus injection followed by continuous infusion of MB (2.66 mg/kg/h) delivered by syringe infusion pump | MB acts as an sGC inhibitor that abolishes the NO/cGMP-dependent smooth muscle vasodilatation. | |
| Zheng et al., 2013 [33] | Methylene blue and epinephrine: a synergetic association for anaphylactic shock treatment | Brown-Norway rats | Sensitization by 1 mg grade VI chicken egg albumin (ovalbumin) and 4 mg aluminum hydroxide in adjuvant diluted in 1 mL 0.9% saline solution. Subcutaneous injection given on days 0, 4 and 14. AS induced on day 21 by IV injection of 1 mg ovalbumin. | A single bolus of 3 mg/kg MB | When MB was administered alone, there was disparity between the improved survival and the lack of tissue perfusion correction. This can be attributed to NO-independent pathway effects. | |
| Albuquerque AAS et al., 2016 [35] | Methylene blue to treat protamine-induced anaphylaxis reactions. An experimental study in pigs | Dalland pigs | No sensitization. AS induced by protamine IV infusion (dose not mentioned) | MB 3 mg/kg IV infusion | Protamine binds to an endothelial cell receptor that signals conversion of L-arginine to NO. NO activates sGC in the vascular smooth muscle to cause cGMP-mediated vasodilation. The resultant vasodilation decreases pulmonary vascular resistance and blood pressure. MB reversed the hypotension caused by protamine, by acting on the NO/endothelium-dependent mechanism. | |
| Albuquerque et al., 2020 [25] | Effects of NO/cGMP inhibitors in a rat model of anaphylactoid shock | Male Wistar rats | AS induction by C48/80 (3 mg/kg) IV bolus injection | L-NAME 1 mg/kg | The beneficial effect of L-NAME could be attributed to the blockage of eNOS. Removing NO production caused an SBP increase. MB is a non-selective GC inhibitor. When GC is inhibited, cGMP will not increase to cause vasodilation and hypotension. It is difficult to interpret the mechanism of IC’s effect on BP due to the ambiguous results | |
| MB 3 mg/kg | ||||||
| IC 3 mg/kg | ||||||
| Albuquerque et al., 2022 [26] | Indigo carmine hemodynamic studies to treat vasoplegia induced by compound 48/80 in a swine model of anaphylaxis | Male Dalland Pigs | Naturally sensitized by C48/80. | IC 3 mg/kg 10 min after AS. | IC inhibits endothelium-dependent relaxation specifically in relation to cGMP release. Additional effectiveness of IC was expected due to its alpha-adrenergic stimulation, which should counteract systemic hypotension. However, the vasoconstrictive effect was not apparent in this study. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfalasi, M.; Alzaabi, S.; Östlundh, L.; Al-Rifai, R.H.; Al-Salam, S.; Mertes, P.M.; Alper, S.L.; Aburawi, E.H.; Bellou, A. Effect of Nitric Oxide Pathway Inhibition on the Evolution of Anaphylactic Shock in Animal Models: A Systematic Review. Biology 2022, 11, 919. https://doi.org/10.3390/biology11060919
Alfalasi M, Alzaabi S, Östlundh L, Al-Rifai RH, Al-Salam S, Mertes PM, Alper SL, Aburawi EH, Bellou A. Effect of Nitric Oxide Pathway Inhibition on the Evolution of Anaphylactic Shock in Animal Models: A Systematic Review. Biology. 2022; 11(6):919. https://doi.org/10.3390/biology11060919
Chicago/Turabian StyleAlfalasi, Maryam, Sarah Alzaabi, Linda Östlundh, Rami H. Al-Rifai, Suhail Al-Salam, Paul Michel Mertes, Seth L. Alper, Elhadi H. Aburawi, and Abdelouahab Bellou. 2022. "Effect of Nitric Oxide Pathway Inhibition on the Evolution of Anaphylactic Shock in Animal Models: A Systematic Review" Biology 11, no. 6: 919. https://doi.org/10.3390/biology11060919
APA StyleAlfalasi, M., Alzaabi, S., Östlundh, L., Al-Rifai, R. H., Al-Salam, S., Mertes, P. M., Alper, S. L., Aburawi, E. H., & Bellou, A. (2022). Effect of Nitric Oxide Pathway Inhibition on the Evolution of Anaphylactic Shock in Animal Models: A Systematic Review. Biology, 11(6), 919. https://doi.org/10.3390/biology11060919








