Impaired Myocardial Mitochondrial Function in an Experimental Model of Anaphylactic Shock
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Preparation
2.2. Hemodynamic Measurements
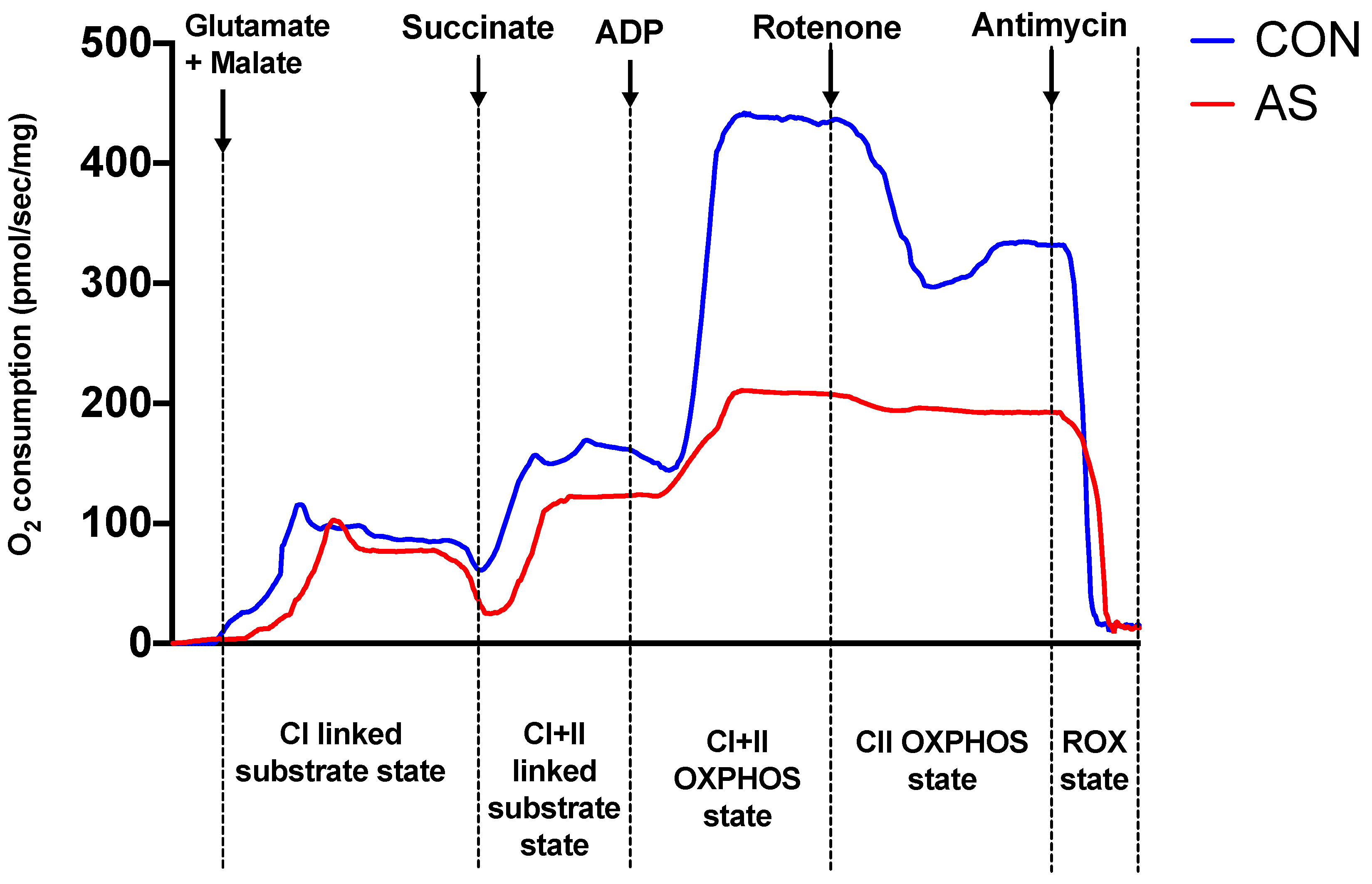
2.3. Myocardial Mitochondrial Respiration
2.4. Oxidative Stress Measurements
2.4.1. Production of Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS)
2.4.2. Superoxide Dismutases (SODs) Activity
2.5. Oxidative Damage: Lipid Peroxidation Measurement
2.6. Bradford Method to Determine Protein Concentration (mg Protein)
2.7. Transmission Electronic Microscopy (TEM)
2.8. Statistical Analysis
3. Results
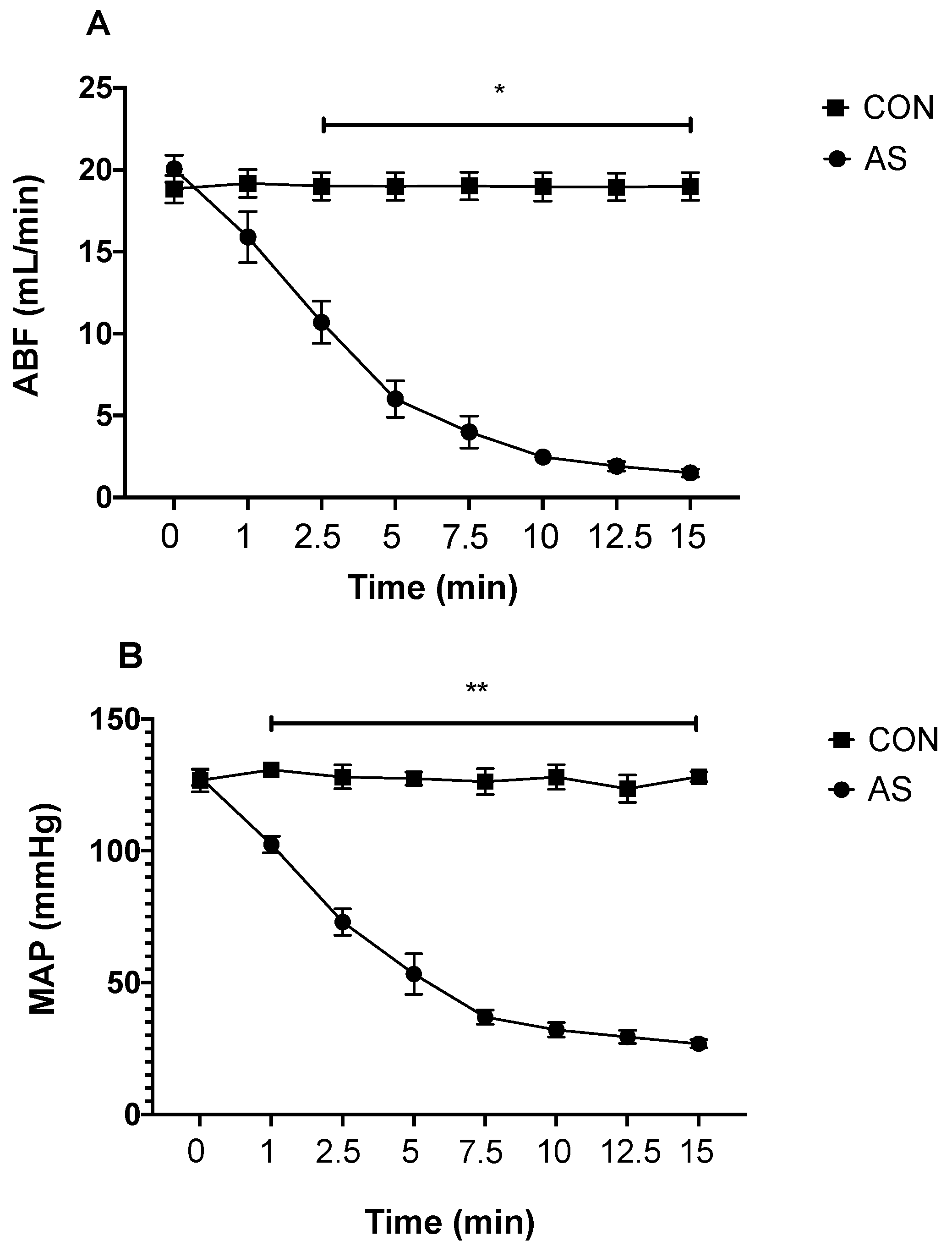
3.1. Hemodynamics during AS
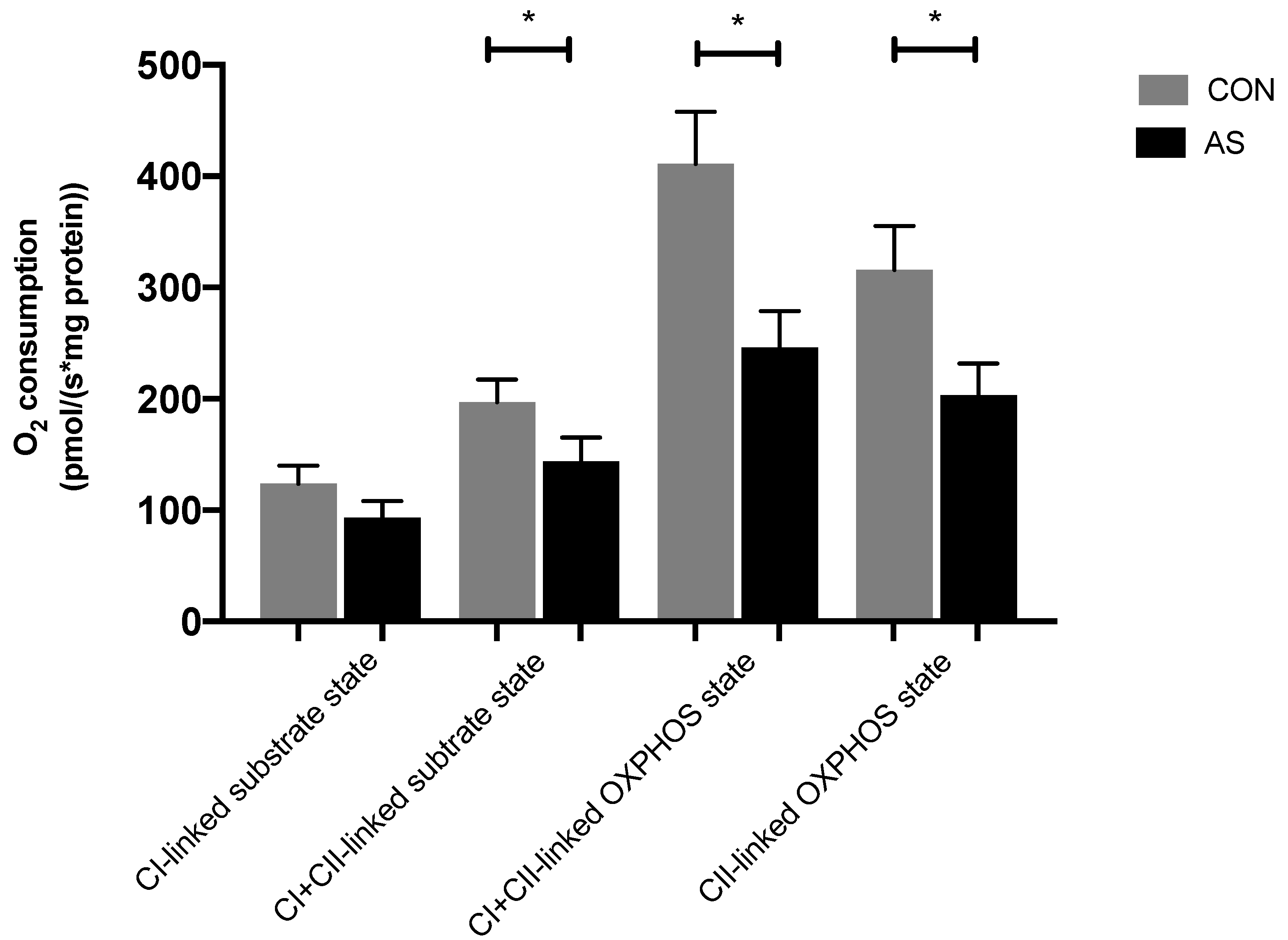
3.2. Myocardial Mitochondrial Respiration and Oxidative Stress in AS
3.3. Oxidative Stress Measurement
3.3.1. ROS and RNS Production
3.3.2. SODs Activity
3.4. Oxidative Damage with Lipid Peroxidation
3.5. Electron Microscopy Findings to Myocardial Mitochondria
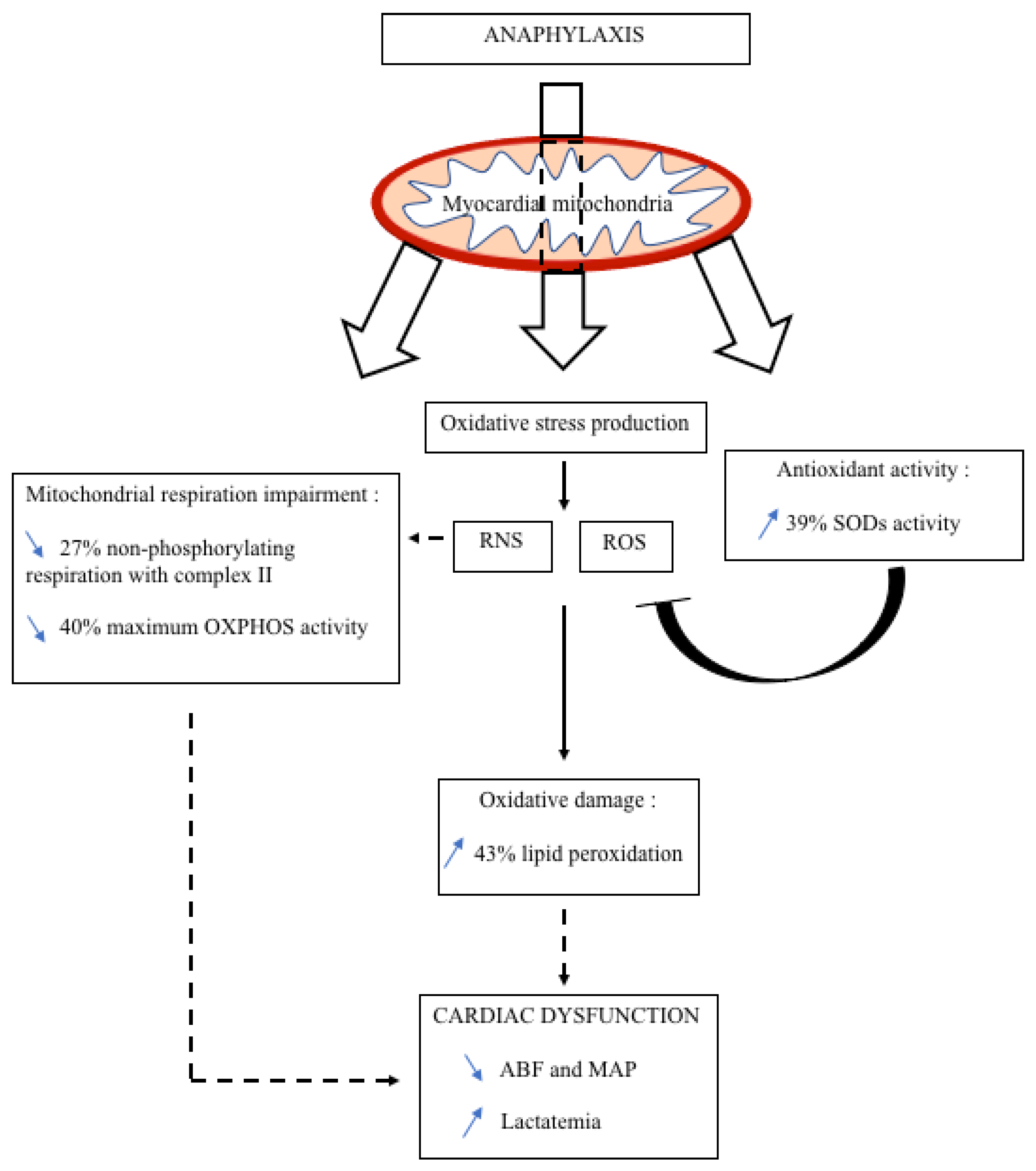
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerci, P.; Tacquard, C.; Chenard, L.; Millard, D.; Soufir, L.; Malinovsky, J.-M.; Garot, M.; Lalot, J.-M.; Besch, G.; Louis, G.; et al. Epidemiology and outcome of patients admitted to intensive care after anaphylaxis in France: A retrospective multicentre study. Br. J. Anaesth. 2020, 125, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Dewachter, P.; Jouan-Hureaux, V.; Franck, P.; Menu, P.; de Talance, N.; Zannad, F.; Laxenaire, M.-C.; Longrois, D.; Mertes, P.M. Anaphylactic shock: A form of distributive shock without inhibition of oxygen consumption. Anesthesiology 2005, 103, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Zavras, G.M. Histamine-induced coronary artery spasm: The concept of allergic angina. Br. J. Clin. Pract. 1991, 45, 121–128. [Google Scholar] [PubMed]
- Marone, G.; Genovese, A.; Varricchi, G.; Granata, F. Human heart as a shock organ in anaphylaxis. Allergo J. Int. 2014, 23, 60–66. [Google Scholar] [CrossRef]
- Tacquard, C.; Oulehri, W.; Collange, O.; Garvey, L.H.; Nicoll, S.; Tuzin, N.; Geny, B.; Mertes, P.M. Treatment with a platelet-activating factor receptor antagonist improves hemodynamics and reduces epinephrine requirements, in a lethal rodent model of anaphylactic shock. Clin. Exp. Allergy 2019, 50, 383–390. [Google Scholar] [CrossRef]
- Bisaccia, G.; Ricci, F.; Gallina, S.; Di Baldassarre, A.; Ghinassi, B. Mitochondrial Dysfunction and Heart Disease: Critical Appraisal of an Overlooked Association. Int. J. Mol. Sci. 2021, 22, 614. [Google Scholar] [CrossRef]
- Filardi, T.; Ghinassi, B.; Di Baldassarre, A.; Tanzilli, G.; Morano, S.; Lenzi, A.; Basili, S.; Crescioli, C. Cardiomyopathy Associated with Diabetes: The Central Role of the Cardiomyocyte. Int. J. Mol. Sci. 2019, 20, 3299. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Scaglia, F. Mitochondrial Cardiomyopathies. Front. Cardiovasc. Med. 2016, 3, 25. [Google Scholar] [CrossRef]
- Schirone, L.; Forte, M.; D’Ambrosio, L.; Valenti, V.; Vecchio, D.; Schiavon, S.; Spinosa, G.; Sarto, G.; Petrozza, V.; Frati, G.; et al. An Overview of the Molecular Mechanisms Associated with Myocardial Ischemic Injury: State of the Art and Translational Perspectives. Cells 2022, 11, 1165. [Google Scholar] [CrossRef]
- Camara, A.K.S.; Lesnefsky, E.J.; Stowe, D.F. Potential Therapeutic Benefits of Strategies Directed to Mitochondria. Antioxid. Redox Signal. 2010, 13, 279–347. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Song, Y.S.; Yee, S.-B.; Lee, B.G.; Seo, S.Y.; Park, Y.C.; Kim, J.-M.; Kim, H.M.; Yoo, Y.H. Phospho-ser 15-p53 translocates into mitochondria and interacts with Bcl-2 and Bcl-xL in eugenol-induced apoptosis. Apoptosis 2005, 10, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-T.; Shen, Y.-F.; Gong, J.-M.; Yang, Y.-J. Effect of Sophoridine on Ca2+ Induced Ca2+ Release During Heart Failure. Physiol. Res. 2016, 65, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Palmerio, S.; Bianchi, F.; Volpe, M.; Rubattu, S. Mitochondrial complex I deficiency and cardiovascular diseases: Current evidence and future directions. J. Mol. Med. 2019, 97, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Collange, O.; Davidson, J.; Barthel, G.; Oulehri, W.; Thornton, S.N.; Longrois, D.; Levy, B.; Audibert, G.; Malinovsky, J.-M.; et al. Epinephrine, compared with arginine vasopressin, is associated with similar haemodynamic effects but significantly improved brain oxygenation in the early phase of anaphylactic shock in rats: An experimental study. Eur. J. Anaesthesiol. 2015, 32, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Bellou, A.; Lambert, H.; Gillois, P.; Montémont, C.; Gerard, P.; Vauthier, E.; Sainte-Laudy, J.; Longrois, D.; Guéant, J.L.; Mallié, J.P. Constitutive Nitric Oxide Synthase Inhibition Combined with Histamine and Serotonin Receptor Blockade Improves the Initial Ovalbumin-Induced Arterial Hypotension but Decreases the Survival Time in Brown Norway Rats Anaphylactic Shock. Shock 2003, 19, 71–78. [Google Scholar] [CrossRef]
- Charles, A.-L.; Guilbert, A.-S.; Guillot, M.; Talha, S.; Lejay, A.; Meyer, A.; Kindo, M.; Wolff, V.; Bouitbir, J.; Zoll, J.; et al. Muscles Susceptibility to Ischemia-Reperfusion Injuries Depends on Fiber Type Specific Antioxidant Level. Front. Physiol. 2017, 8, 52. [Google Scholar] [CrossRef]
- Paradis, S.; Charles, A.-L.; Georg, I.; Goupilleau, F.; Meyer, A.; Kindo, M.; Laverny, G.; Metzger, D.; Geny, B. Aging Exacerbates Ischemia-Reperfusion-Induced Mitochondrial Respiration Impairment in Skeletal Muscle. Antioxidants 2019, 8, 168. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zheng, F.; Barthel, G.; Collange, O.; Montémont, C.; Thornton, S.N.; Longrois, D.; Levy, B.; Audibert, G.; Malinovsky, J.-M.; Mertes, P.-M. Methylene Blue and Epinephrine: A synergetic association for anaphylactic shock treatment. Crit. Care Med. 2013, 41, 195–204. [Google Scholar] [CrossRef]
- Davidson, J.; Zheng, F.; Tajima, K.; Barthel, G.; Alb, I.; Tabarna, A.; Thornton, S.N.; Lambert, M.; Longrois, D.; Audibert, G.; et al. Anaphylactic Shock decreases cerebral blood flow more than what would be expected from severe arterial hypotension. Shock 2012, 38, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Dewachter, P.; Jouan-Hureaux, V.; Lartaud, I.; Bello, G.; De Talancé, N.; Longrois, D.; Mertes, P.M. Comparison of Arginine Vasopressin, Terlipressin, or Epinephrine to Correct Hypotension in a Model of Anaphylactic Shock in Anesthetized Brown Norway Rats. Anesthesiology 2006, 104, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Kapin, M.A.; Ferguson, J.L. Hemodynamic and regional circulatory alterations in dog during anaphylactic challenge. Am. J. Physiol. Circ. Physiol. 1985, 249, H430–H437. [Google Scholar] [CrossRef] [PubMed]
- Triggiani, M.; Patella, V.; Staiano, R.I.; Granata, F.; Marone, G. Allergy and the cardiovascular system. Clin. Exp. Immunol. 2008, 153 (Suppl. S1), 7–11. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.S.; Kim, H.; Bang, M.H.; Kim, O.H.; Kim, H.I.; Cha, K.; Lee, K.H.; Hwang, S.O. Evaluation of myocardial injury through serum troponin I and echocardiography in anaphylaxis. Am. J. Emerg. Med. 2016, 34, 140–144. [Google Scholar] [CrossRef]
- Ferdinandy, P.; Hausenloy, D.J.; Heusch, G.; Baxter, G.F.; Schulz, R. Interaction of Risk Factors, Comorbidities, and Comedications with Ischemia/Reperfusion Injury and Cardioprotection by Preconditioning, Postconditioning, and Remote Conditioning. Pharmacol. Rev. 2014, 66, 1142–1174. [Google Scholar] [CrossRef]
- Seferović, P.M.; Polovina, M.; Bauersachs, J.; Arad, M.; Ben Gal, T.B.; Lund, L.H.; Felix, S.B.; Arbustini, E.; Caforio, A.L.P.; Farmakis, D.; et al. Heart failure in cardiomyopathies: A position paper from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 553–576. [Google Scholar] [CrossRef]
- Gambardella, J.; Sorriento, D.; Ciccarelli, M.; Del Giudice, C.; Fiordelisi, A.; Napolitano, L.; Trimarco, B.; Iaccarino, G.; Santulli, G. Functional Role of Mitochondria in Arrhythmogenesis. Adv. Exp. Med. Biol. 2017, 982, 191–202. [Google Scholar] [CrossRef]
- Gellerich, F.N.; Trumbeckaite, S.; Hertel, K.; Zierz, S.; Müller-Werdan, U.; Werdan, K.; Redl, H.; Schlag, G. Impaired energy metabolism in hearts of septic baboons: Diminished activities of Complex I and Complex II of the mitochondrial respiratory chain. Shock 1999, 11, 336–341. [Google Scholar] [CrossRef]
- Jain-Ghai, S.; Cameron, J.M.; Al Maawali, A.; Blaser, S.; MacKay, N.; Robinson, B.; Raiman, J. Complex II deficiency—A case report and review of the literature. Am. J. Med Genet. A 2013, 161, 285–294. [Google Scholar] [CrossRef]
- Bezawork-Geleta, A.; Rohlena, J.; Dong, L.; Pacak, K.; Neuzil, J. Mitochondrial Complex II: At the Crossroads. Trends Biochem. Sci. 2017, 42, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, C.-L.; Kang, P.T.; Garg, V.; Hu, K.; Green-Church, K.B.; Chen, Y.-R. Peroxynitrite-Mediated Oxidative Modifications of Complex II: Relevance in Myocardial Infarction. Biochemistry 2010, 49, 2529–2539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, X.; Chen, Y.-R.; He, G.; Zhang, A.; Druhan, L.J.; Strauch, A.R.; Zweier, J.L. Endothelial nitric oxide synthase (NOS3) knockout decreases NOS2 induction, limiting hyperoxygenation and conferring protection in the postischemic heart. Am. J. Physiol. Circ. Physiol. 2007, 292, H1541–H1550. [Google Scholar] [CrossRef][Green Version]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxid. Med. Cell. Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 1991, 288, 481–487. [Google Scholar] [CrossRef]
- Moris, D.; Spartalis, M.; Tzatzaki, E.; Spartalis, E.; Karachaliou, G.-S.; Triantafyllis, A.S.; Karaolanis, G.I.; Tsilimigras, D.I.; Theocharis, S. The role of reactive oxygen species in myocardial redox signaling and regulation. Ann. Transl. Med. 2017, 5, 324. [Google Scholar] [CrossRef]
- Galatro, A.; González, P.M.; Malanga, G.; Robello, E.; Piloni, N.E.; Puntarulo, S. Nitric oxide and membrane lipid peroxidation in photosynthetic and non-photosynthetic organisms under several stress conditions. Front. Physiol. 2013, 4, 276. [Google Scholar] [CrossRef]
- Horton, J.W.; White, D.J. Lipid peroxidation contributes to cardiac deficits after ischemia and reperfusion of the small bowel. Am. J. Physiol. Circ. Physiol. 1993, 264, H1686–H1692. [Google Scholar] [CrossRef]
- Cauwels, A. Nitric oxide in shock. Kidney Int. 2007, 72, 557–565. [Google Scholar] [CrossRef]
- Thors, B.; Halldórsson, H.; Thorgeirsson, G. Thrombin and histamine stimulate endothelial nitric-oxide synthase phosphorylation at Ser1177 via an AMPK mediated pathway independent of PI3K-Akt. FEBS Lett. 2004, 573, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.L.; Davies, M.J. Detection and characterisation of radicals in biological materials using EPR methodology. Biochim. Biophys. Acta 2014, 1840, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, K.; Dikalov, S.; Matuschka, S.; Mainka, L.; Hofmann, M.; Khramtsov, V.; Zimmer, G. Oxygen radical generation and enzymatic properties of mitochondria in hypoxia/reoxygenation. Arzneimittelforschung 1998, 48, 629–636. [Google Scholar] [PubMed]
- Dikalov, S.I.; Polienko, Y.F.; Kirilyuk, I. Electron Paramagnetic Resonance Measurements of Reactive Oxygen Species by Cyclic Hydroxylamine Spin Probes. Antioxid. Redox Signal. 2018, 28, 1433–1443. [Google Scholar] [CrossRef]
- Zhao, H.; Joseph, J.; Fales, H.M.; Sokoloski, E.A.; Levine, R.L.; Vasquez-Vivar, J.; Kalyanaraman, B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc. Natl. Acad. Sci. USA 2005, 102, 5727–5732. [Google Scholar] [CrossRef]
- Hopper, R.K.; Carroll, S.; Aponte, A.M.; Johnson, D.T.; French, S.; Shen, R.F.; Witzmann, F.A.; Harris, R.A.; Balaban, R.S. Mitochondrial Matrix Phosphoproteome: Effect of Extra Mitochondrial Calcium. Biochemistry 2006, 45, 2524–2536. [Google Scholar] [CrossRef]
- Lee, Y.S.; Choi, J.-H.; Lee, J.-H.; Lee, H.-W.; Lee, W.; Kim, W.T.; Kim, T.-Y. Extracellular superoxide dismutase ameliorates house dust mite-induced atopic dermatitis-like skin inflammation and inhibits mast cell activation in mice. Exp. Dermatol. 2016, 25, 630–635. [Google Scholar] [CrossRef]
- Tenório, M.C.D.S.; Graciliano, N.G.; Moura, F.A.; de Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef]
- Pei, Y.; Liu, H.; Yang, Y.; Yang, Y.; Jiao, Y.; Tay, F.R.; Chen, J. Biological Activities and Potential Oral Applications of N-Acetylcysteine: Progress and Prospects. Oxid. Med. Cell. Longev. 2018, 2018, 2835787. [Google Scholar] [CrossRef]
- Sochman, J. N-acetylcysteine in acute cardiology: 10 years later: What do we know and what would we like to know?! J. Am. Coll. Cardiol. 2002, 39, 1422–1428. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oulehri, W.; Collange, O.; Tacquard, C.; Bellou, A.; Graff, J.; Charles, A.-L.; Geny, B.; Mertes, P.-M. Impaired Myocardial Mitochondrial Function in an Experimental Model of Anaphylactic Shock. Biology 2022, 11, 730. https://doi.org/10.3390/biology11050730
Oulehri W, Collange O, Tacquard C, Bellou A, Graff J, Charles A-L, Geny B, Mertes P-M. Impaired Myocardial Mitochondrial Function in an Experimental Model of Anaphylactic Shock. Biology. 2022; 11(5):730. https://doi.org/10.3390/biology11050730
Chicago/Turabian StyleOulehri, Walid, Olivier Collange, Charles Tacquard, Abdelouahab Bellou, Julien Graff, Anne-Laure Charles, Bernard Geny, and Paul-Michel Mertes. 2022. "Impaired Myocardial Mitochondrial Function in an Experimental Model of Anaphylactic Shock" Biology 11, no. 5: 730. https://doi.org/10.3390/biology11050730
APA StyleOulehri, W., Collange, O., Tacquard, C., Bellou, A., Graff, J., Charles, A.-L., Geny, B., & Mertes, P.-M. (2022). Impaired Myocardial Mitochondrial Function in an Experimental Model of Anaphylactic Shock. Biology, 11(5), 730. https://doi.org/10.3390/biology11050730







