Application of Optical Fluorescence Spectroscopy for Studying Bee Abundance in Tropaeolum majus L. (Tropaeolaceae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Procedures
2.2. Pigment Extraction
2.3. Fluorescence Measurements
2.4. Statistical Analysis
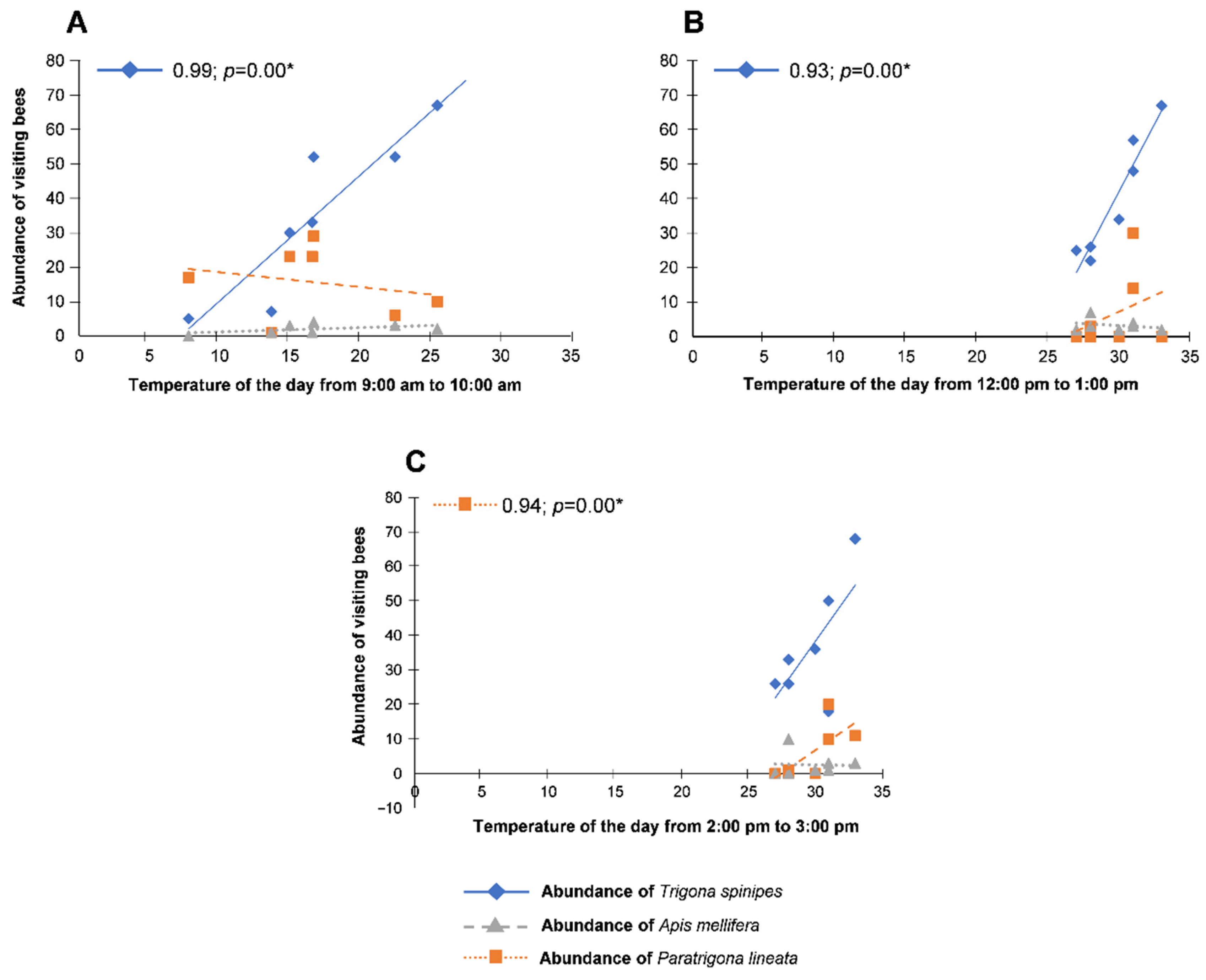
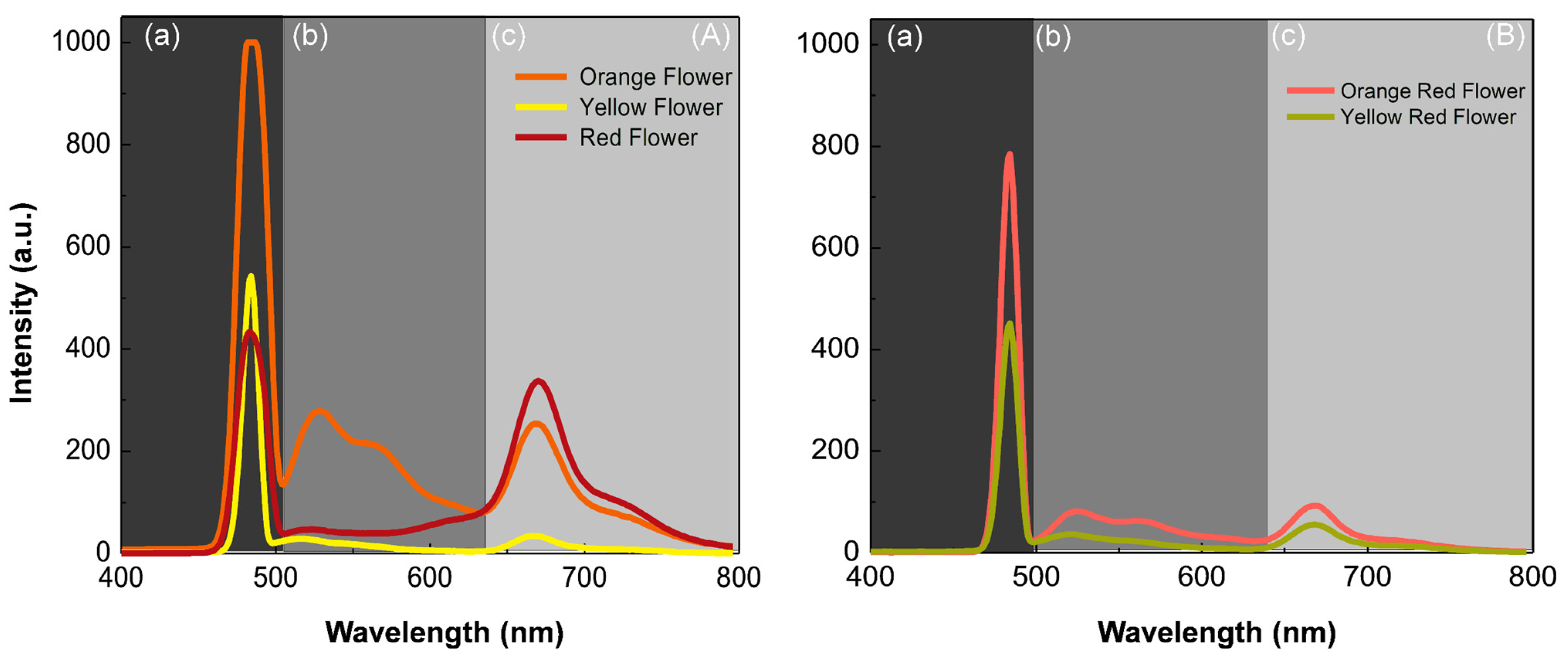
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dyer, A.G.; Whitney, H.M.; Arnold, S.E.; Glover, B.J.; Chittka, L. Mutations perturbing petal cell shape and anthocyanin synthesis influence bumblebee perception of Antirrhinum majus flower colour. Arthropod Plant Interact. 2007, 1, 45–55. [Google Scholar] [CrossRef]
- Whitney, H.M.; Kolle, M.; Andrew, P.; Chittka, L.; Steiner, U.; Blover, B.J. Floral iridescence, produced by diffractive optics, acts as a cue for animal pollinators. Science 2009, 323, 130–133. [Google Scholar] [CrossRef] [Green Version]
- Leonard, A.S.; Dornhaus, A.; Papaj, D.R. Flowers help bees cope with uncertainty: Signal detection and the function of floral complexity. J. Exp. Biol. 2011, 214, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, B.; Mou, F.; Sun, W.; Chen, S.; Peng, F.; Bradshaw, H.D.; Yuan, Y.W. A dominant-negative actin mutation alters corolla tube width and pollinator visitation in Mimulus lewisii. New Phytol. 2017, 2013, 1936–1944. [Google Scholar] [CrossRef] [Green Version]
- Gandía-Herrero, F.; Garcia-Carmona, F.; Escribano, J. Floral fluorescence effect. Nature 2005, 437, 334. [Google Scholar] [CrossRef]
- Thorp, R.W.; Briggs, D.L.; Estes, J.R.; Erickson, E.H. Nectar fluorescence under ultraviolet irradiation. Science 1975, 189, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.L.; Stpiczynska, M.; Gregg, A. Nectar-secreting floral stomata in Maxillaria anceps Ames & C. Schweinf. (Orchidaceae). Ann. Bot. 2005, 96, 217–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dafni, A.; Bernhardt, P.; Shmida, A.; Ivri, Y.; Greenbaum, S.; O’Toole, C.; Losito, L. Red bowl-shaped flowers: Convergence for beetle pollination in the Mediterranean region. Isr. J. Plant Sci. 1990, 39, 81–92. [Google Scholar] [CrossRef]
- Ômura, H.; Honda, K. Priority of color over scent during flower visitation by adult Vanessa indica butterflies. Oecologia Aust. 2005, 142, 588–596. [Google Scholar] [CrossRef]
- Dötterl, S.; Glück, U.; Jürgens, A.; Woodring, J.; Aas, G. Floral reward, advertisement and attractiveness to honey bees in dioecious Salix Caprea. PLoS ONE 2014, 9, e93421. [Google Scholar] [CrossRef]
- Arnold, K.E.; Owens, I.P.F.; Marshall, N.J. Fluorescent signaling in parrots. Science 2002, 295, 92. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.L.M.; Land, M.F.; Li, D. Sex-specific UV and fluorescence signals in jumping spiders. Science 2007, 315, 481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, K.; Reed, S.M.; Masta, S.E. Spiders fluoresce variably across many taxa. Biol. Lett. 2007, 3, 265–267. [Google Scholar] [CrossRef] [Green Version]
- Briscoe, A.D.; Chittka, L. The evolution of color vision in insects. Annu. Rev. Entomol. 2001, 46, 471–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, M.V. Honey bees as a model for vision, perception, and cognition. Annu. Rev. Entomol. 2010, 55, 267–284. [Google Scholar] [CrossRef]
- Ibarra, N.H.; Vorobyev, M.; Menzel, R. Mechanisms, functions and ecology of colour vision in the honeybee. J. Comp. Physiol. A 2014, 200, 411–433. [Google Scholar] [CrossRef] [Green Version]
- Dyer, A.G.; Garcia, J.E.; Shrestha, M.; Lunau, K. Seeing in colour: A hundred years of studies on bee vision since the work of the Nobel laureate Karl von Frisch. Proc. R. Soc. Vic. 2015, 127, 66–72. [Google Scholar] [CrossRef]
- Von Helversen, O. Zur spektralen Unterschiedsempfindlichkeit der Honigbiene. J. Comp. Physiol. A 1972, 80, 439–472. [Google Scholar] [CrossRef]
- Peitsch, D.; Fietz, A.; Hertel, H.; Souza, J.; Ventura, D.F.; Menzel, R. The spectral input systems of hymenopteran insects and their receptor-based colour vision. J. Comp. Physiol. A 1992, 170, 23–40. [Google Scholar] [CrossRef]
- Von Frisch, K. Bees: Their Vision, Chemical Senses and Language; Cornell University Press: London, UK, 1976; p. 154. [Google Scholar]
- Menzel, R.; Backhaus, W. Colour vision in insects. In Vision and Visual Dysfunction; Gouras, P., Ed.; MacMillan: London, UK, 1991; pp. 262–293. [Google Scholar]
- Iriel, A.; Lagorio, M.G. Is the flower fluorescence relevant in biocommunication? Sci. Nat. 2010, 97, 915–9240. [Google Scholar] [CrossRef]
- Alvares, C.L.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Melo, B.T.; Mota, T.; Schlindwein, C.; Antonini, Y.; Oliveira, R. Floral colour change in Byrsonima variabilis (Malpighiaceae) as a visual cue for pollen but not oil foraging by oil-collecting bees. Sci. Nat. 2018, 105, 46. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.E.P.F.; Mussury, R.M.; Silvestre, R.; Scalon, S.P.Q.; Braga, L.F.; Sousa, M.P. The entomology fauna visiting cultivated populations of Tropaeolum majus L. (Tropaeolaceae). Int. J. Sci. 2012, 3, 538–545. [Google Scholar]
- Malerbo-Souza, D.T.; Tasinafo, R.H. Sazonalidade das abelhas africanizadas Apis mellifera L. Cien. Cult. 2012, 8, 49–54. [Google Scholar]
- Barbosa, B.C.; Paschoalini, M.; Maciel, T.T.; Prezoto, F. Visitantes florais e seus padrões temporais de atividade em flores de Dombeya wallichii (Lindl.) K. Schum (Malvaceae). Entomotropica 2016, 31, 131–136. [Google Scholar]
- Malerbo-Souza, D.T.; de Toledo, V.D.A.A.; Couto, L.A.; Nogueira-Couto, R.H. Uso da tela excluidora de rainha no alvado e seus efeitos na atividade de coleta e no desenvolvimento de colônias de Apis Mellifera. Acta Sci. Anim. Sci. 1998, 20, 383–386. [Google Scholar] [CrossRef]
- Hilário, S.D.; Imperatriz-Fonseca, V.L.; Kleinert, A. Flight activity and colony strength in the stingless bee Melipona bicolor (Apidae, Meliponinae). Rev. Bras. Biol. 2000, 60, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Moreti, A.C.C.C.; Marchini, L.C.; Souza, V.C.; Rodrigues, R.R. Atlas do Pólen de Plantas Apícolas; Editora Papel Virtual: Rio de Janeiro, Brazil, 2002; p. 93. [Google Scholar]
- Pierrot, L.M.; Schlindwein, C. Variation in daily flight activity and foraging patterns in colonies of uruçu-Melipona scutellaris Latreille (Apidae, Meliponini). Rev. Bras. Zool. 2003, 20, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Pleasants, J.M. Bumblebee response to variation in nectar availability. Ecology 1981, 62, 1648–1661. [Google Scholar] [CrossRef]
- Real, L.A.; Rathcke, B.J. Individual variation in nectar production and its effect on fitness in Kalmia latifolia. Ecology 1991, 82, 149–155. [Google Scholar] [CrossRef]
- Lorenzon, M.C.A.; Matrangolo, C.A.R.; Schoereder, J.H. Flora visitada pelas abelhas eussociais (Hymenoptera, Apidae) na Serra da Capivara, em Caatinga do Sul do Piauí. Neotrop. Entomol. 2003, 32, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Ruedenauer, F.A.; Spaethe, J.; Leonhardt, S.D. How to know which food is good for you: Bumblebees use taste to discriminate between different concentrations of food differing in nutrient content. J. Exp. Biol. 2015, 218, 2233–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Kooi, C.J.; Kevan, P.G.; Koski, M.H. The thermal ecology of flower. Ann. Bot. 2019, 124, 343–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chittka, L.; Menzel, R. The evolutionary adaptation of flower colours and the insect pollinators’ colour vision. J. Comp. Physiol. A 1992, 171, 171–181. [Google Scholar] [CrossRef]
- Dyer, A.G.; Whitney, H.M.; Arnold, S.E.; Glover, B.J.; Chittka, L. Bees associate warmth with floral colour. Nature 2006, 442, 525. [Google Scholar] [CrossRef] [Green Version]
- Blacquière, T.; Van der Aa-Furnée, J.; Cornelissen, B.; Donders, J.N.L.C. Behaviour of honey bees and bumble bees beneath three different greenhouse claddings. Proc. Neth. Entomol. Soc. Meet. 2006, 17, 93–102. [Google Scholar]
- Morandin, L.A.; Laverty, T.M.; Gegear, R.J.; Kevan, P.G. Effect of greenhouse polyethelene covering on activity level and photoresponse of bumble bees. Can. Entomol. 2002, 134, 539–549. [Google Scholar] [CrossRef]
- Reis, M. Podem as abelhas resolver o problema causado pelo excesso de néctar na framboesa? Rev. Tec. 2019, 5–7. [Google Scholar]
- Dyer, A.G.; Chittka, L. Bumblebee search time without ultraviolet Light. J. Exp. Biol. A 2004, 207, 1683–1688. [Google Scholar] [CrossRef] [Green Version]
- Dey, P.M.; Harborne, J.B. Plant Biochemistry; Editora Academic Press: London, UK, 1997. [Google Scholar]
- Raven, P.H.; Evert, R.F.; Eichhorn, S.E. Biologia Vegetal, 7th ed.; Editora Guanabara-Koogan: Rio de Janeiro, Brazil, 2007. [Google Scholar]
- Costa, L.C.; Ribeiro, W.S.; Barbosa, J.A. Compostos bioativos e alegações de potencial antioxidante de flores de maracujá, cravo amarelo, rosa e capuchinha. Rev. Bras. Prod. Agroindus. 2014, 16, 279–289. [Google Scholar] [CrossRef]
- Gonçalves, J.; Silva, G.C.O.; Carlos, L.A. Compostos bioativos em flores comestíveis. Persp. Online Biol. Saúde 2019, 9, 11–20. [Google Scholar] [CrossRef]
- Qian, H.; Liu, T.; Deng, M.; Miao, H.; Cai, C.; Shen, W. Effects of light quality on main health-promoting compounds and antioxidant capacity of Chinese kale sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef]
- Burrill, R.M.; Dietz, A. The response of honey bees to variations in solar radiation and temperature. Apidologie 1981, 12, 319–328. [Google Scholar] [CrossRef]
- Morato, E.F.; Campos, L.A.D.O. Partição de recursos florais de espécies de Sida Linnaeus e Malvastrum coromandelianum (Linnaeus) Garcke (Malvaceae) entre Cephalurgus anomalus Moure & Oliveira (Hymenoptera, Andrenidae, Panurginae) e Melissoptila cnecomala (Moure) (Hymenoptera, Apidae, Eucerini). Rev. Bras. Zool. 2000, 17, 705–727. [Google Scholar] [CrossRef]
- Putschögl, M.; Zirak, P.; Penzkofer, A. Absorption and emission behaviour of trans-p-coumaric acid in aqueous solutions and some organic solvents. J. Chem. Phys. 2008, 343, 107–120. [Google Scholar] [CrossRef]
- Garzoón, G.A.; Manns, D.C.; Riedl, K.; Schwartz, S.J.; Padilla-Zakour, O. Identification of phenolic compounds in petals of nasturtium flowers (Tropaeolum majus) by high-performance liquid chromatography coupled to mass spectrometry and determination of oxygen radical absorbance capacity (ORAC). J. Agric. Food Chem. 2015, 63, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Cherry, R.J.; Chapman, D.; Langelaar, J. Fluorescence and phosphorescence of β-carotene. Trans. Faraday Soc. 1968, 64, 2304–2307. [Google Scholar] [CrossRef]
- Drabent, R.; Pliszka, B.; Olszewska, T. Fluorescence properties of plant anthocyanin pigments. I. Fluorescence of anthocyanins in Brassica oleracea L. extracts. J. Photochem. Photobiol. B Biol. 1999, 50, 53–58. [Google Scholar] [CrossRef]
- Falco, W.F.; Botero, E.R.; Falcão, E.A.; Santiago, E.F.; Bagnato, V.S.; Caires, A.R.L. In vivo observation of chlorophyll fluorescence quenching induced by gold nanoparticle. J. Photochem. Photobiol. A Chem. 2011, 225, 65–71. [Google Scholar] [CrossRef]
- Monago-Maraña, O.; Durán-Merás, I.; Galeano-Díaz, T.; de la Peña, A.M. Fluorescence properties of flavonoid compounds. Quantification in paprika samples using spectrofluorimetry coupled to second order chemometric tools. Food Chem. 2016, 196, 1058–1065. [Google Scholar] [CrossRef]
- Chittka, L.; Shmida, A.; Troje, N.; Menzel, R. Ultraviolet as a component of flower reflections, and the colour perception of Hymenoptera. Vis. Res. 1994, 34, 1489–1508. [Google Scholar] [CrossRef]
- Reverté, S.; Retana, J.; Gómez, J.M.; Bosch, J. Pollinators show flower colour preferences but flowers with similar colours do not attract similar pollinators. Ann. Bot. 2016, 118, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taiz, L.; Zeiger, E. Fisiologia Vegetal, 3rd ed.; Editora Artmed: Porto Alegre, Brazil, 2004; pp. 449–484. [Google Scholar]
- Claro, P.R. Cores de outono. Rev. Ciência Elem. 2019, 7, 38. [Google Scholar] [CrossRef] [Green Version]
- Taiz, L.; Zeiger, E. Fisiologia Vegetal, 5th ed.; Editora Artmed: Porto Alegre, Brazil, 2013; p. 954. [Google Scholar]
- Brito, V.L.G.; Telles, F.; Lunau, K. Ecologia cognitiva da polinização. In Biologia da Polinização; Rech, A.R., Agostini, K., Oliveira, P.E.A.M., Machado, I.C., Eds.; Editora Projeto Cultural: Rio de Janeiro, Brazil, 2014; pp. 417–438. [Google Scholar]


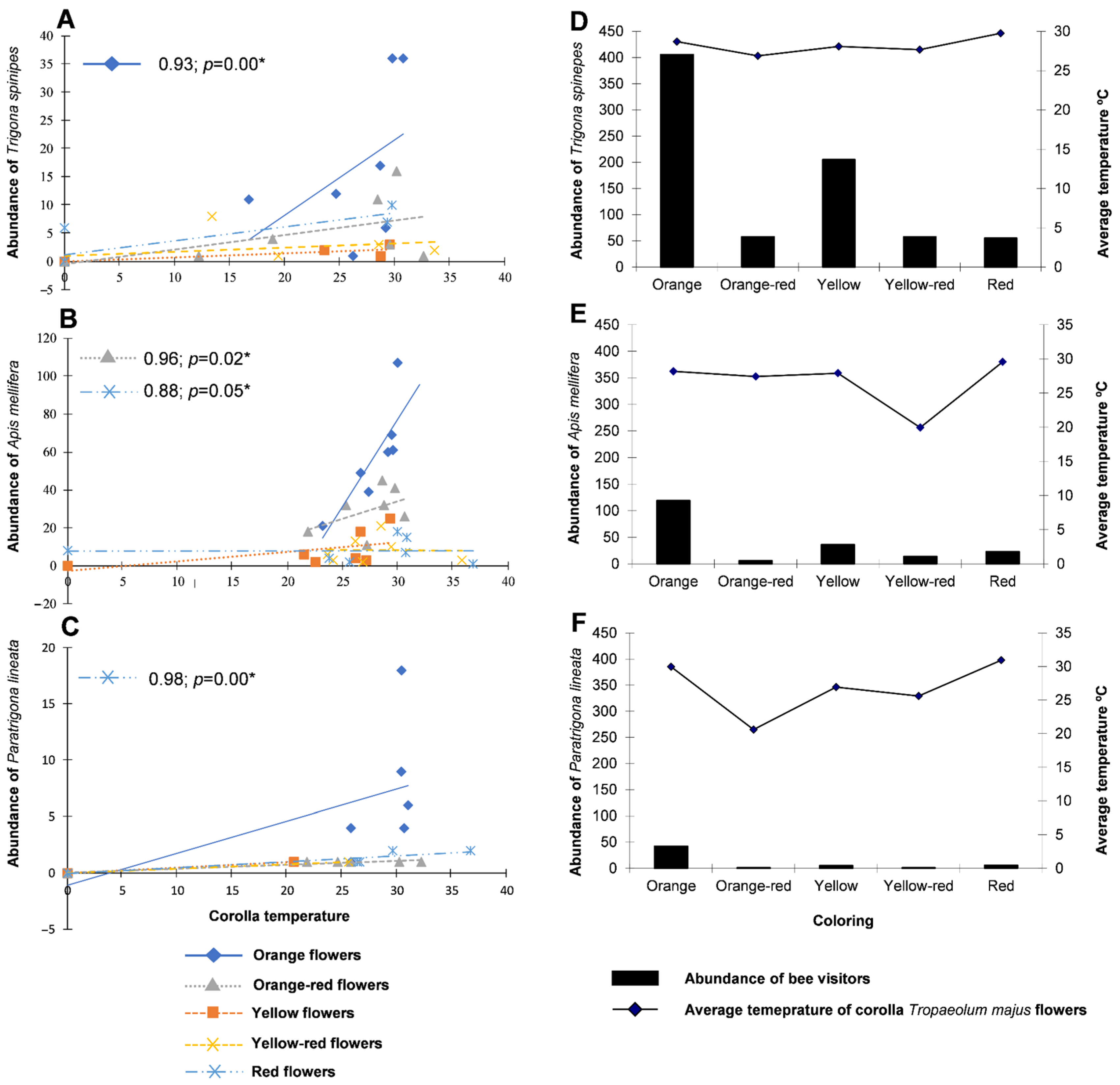
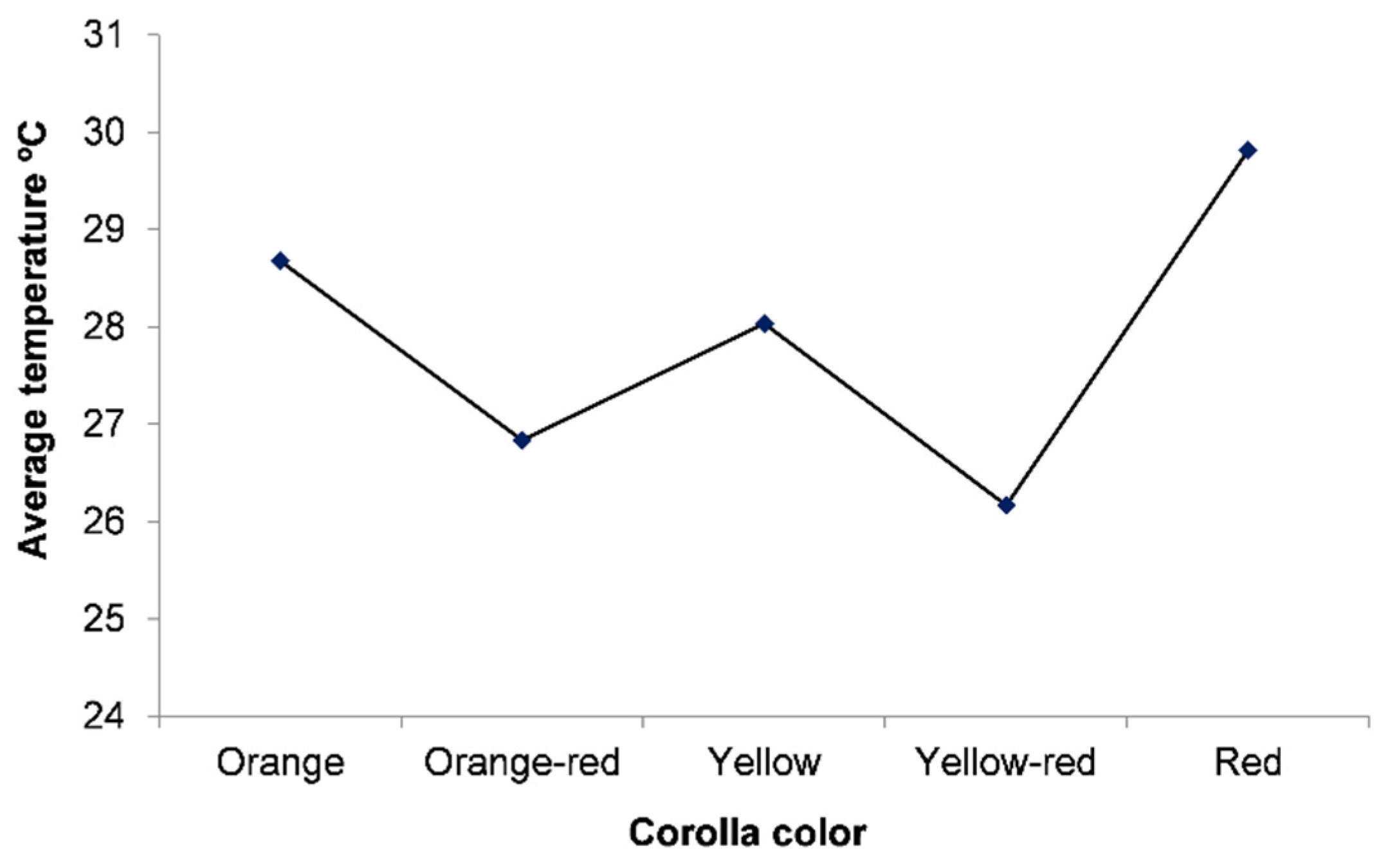

| Orange | Orange-Red | Yellow | Yellow-Red | Red | |
|---|---|---|---|---|---|
| Trigona spinipes | 19.33 ± 2.46 aA n = 406 | 2.76 ± 0.78 aC n = 58 | 9.76 ± 1.31 aB n = 205 | 2.76 ± 0.65 aC n = 58 | 2.62 ± 0.70 aC n = 55 |
| Apis mellifera | 5.67 ± 1.33 bA n = 119 | 0.29 ± 0.12 bB n = 6 | 1.71 ± 0.52 bB n = 36 | 0.67 ± 0.40 bB n = 14 | 1.10 ± 0.48 abB n = 23 |
| Paratrigona lineata | 2.00 ± 0.54 cA n = 42 | 0.05 ± 0.05 bB n = 1 | 0.24 ± 0.10 bAB n = 5 | 0.05 ± 0.05 bB n = 1 | 0.29 ± 0.12 bAB n = 6 |
| CV(%) | 50.57 |
| 9:00 a.m.–10:00 a.m. | 12:00 p.m.–1:00 p.m. | 2:00 p.m.–3:00 p.m. | |
|---|---|---|---|
| Trigona spinipes | 7.03 ± 1.89 aA n = 246 | 7.97 ± 1.35 aA n = 279 | 7.34 ± 1.28 aA n = 257 |
| Apis mellifera | 3.11 ± 0.76 bA n = 109 | 1.34 ± 0.57 bB n = 47 | 1.20 ± 0.48 bB n = 42 |
| Paratrigona lineata | 0.40 ± 0.11 cA n = 14 | 0.66 ± 0.25 bA n = 23 | 0.51 ± 0.30 bA n = 18 |
| CV (%) | 50.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fioratti, C.A.G.; Falcão, E.A.; da Silva, R.M.; do Carmo Vieira, M.; Caires, A.R.L.; Mussury, R.M. Application of Optical Fluorescence Spectroscopy for Studying Bee Abundance in Tropaeolum majus L. (Tropaeolaceae). Biology 2022, 11, 887. https://doi.org/10.3390/biology11060887
Fioratti CAG, Falcão EA, da Silva RM, do Carmo Vieira M, Caires ARL, Mussury RM. Application of Optical Fluorescence Spectroscopy for Studying Bee Abundance in Tropaeolum majus L. (Tropaeolaceae). Biology. 2022; 11(6):887. https://doi.org/10.3390/biology11060887
Chicago/Turabian StyleFioratti, Claudemir Antonio Garcia, Evaristo Alexandre Falcão, Rosicleia Matias da Silva, Maria do Carmo Vieira, Anderson Rodrigues Lima Caires, and Rosilda Mara Mussury. 2022. "Application of Optical Fluorescence Spectroscopy for Studying Bee Abundance in Tropaeolum majus L. (Tropaeolaceae)" Biology 11, no. 6: 887. https://doi.org/10.3390/biology11060887
APA StyleFioratti, C. A. G., Falcão, E. A., da Silva, R. M., do Carmo Vieira, M., Caires, A. R. L., & Mussury, R. M. (2022). Application of Optical Fluorescence Spectroscopy for Studying Bee Abundance in Tropaeolum majus L. (Tropaeolaceae). Biology, 11(6), 887. https://doi.org/10.3390/biology11060887










