Ecological Responses of Nannophya koreana (Odonata: Libellulidae) to Temperature: Following Converse Bergmann’s Rule
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
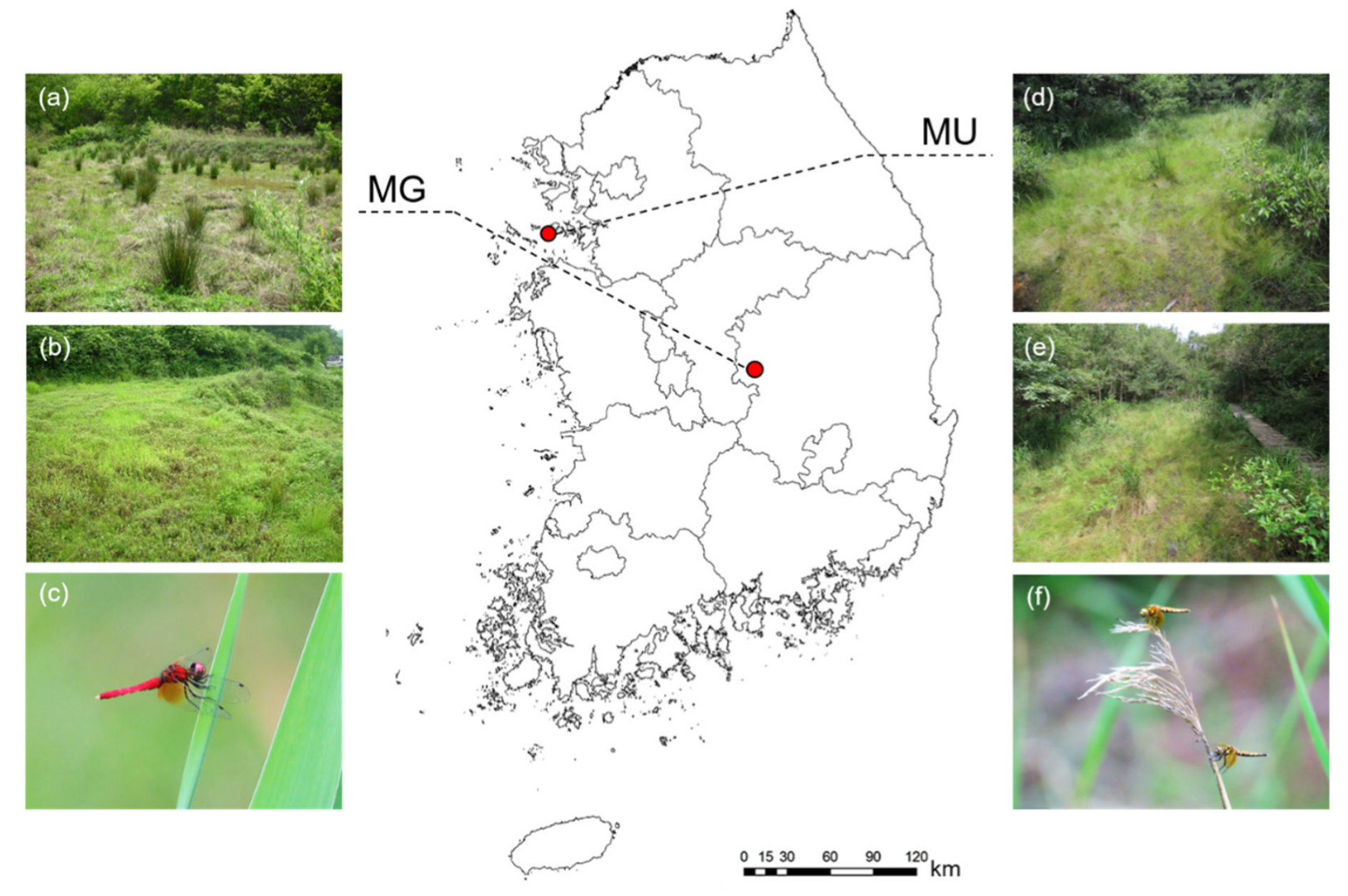
2.1. Study Sites
2.2. Temperature Measurement
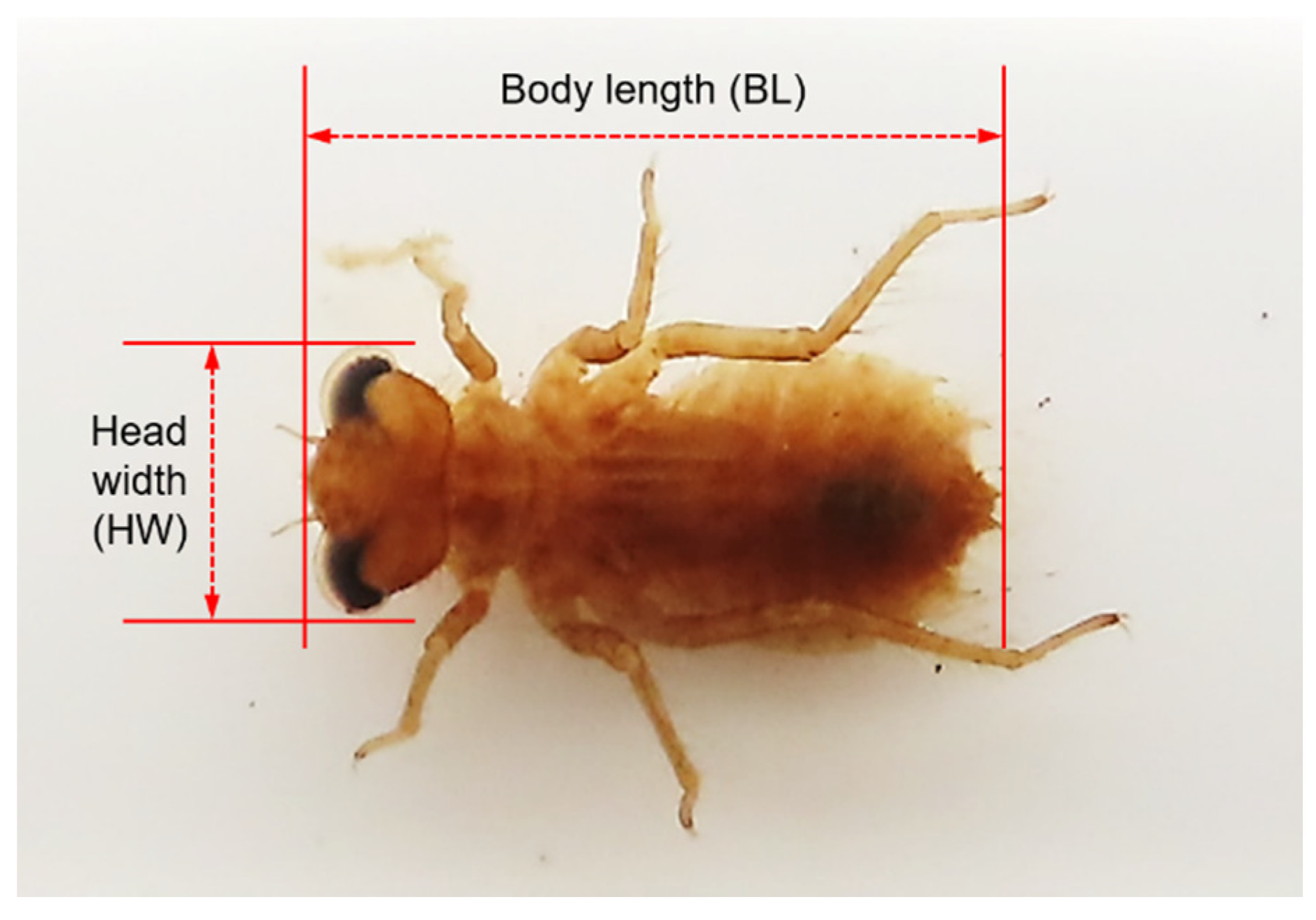
2.3. Sampling and Size Measurement
2.4. Data Analysis
3. Results
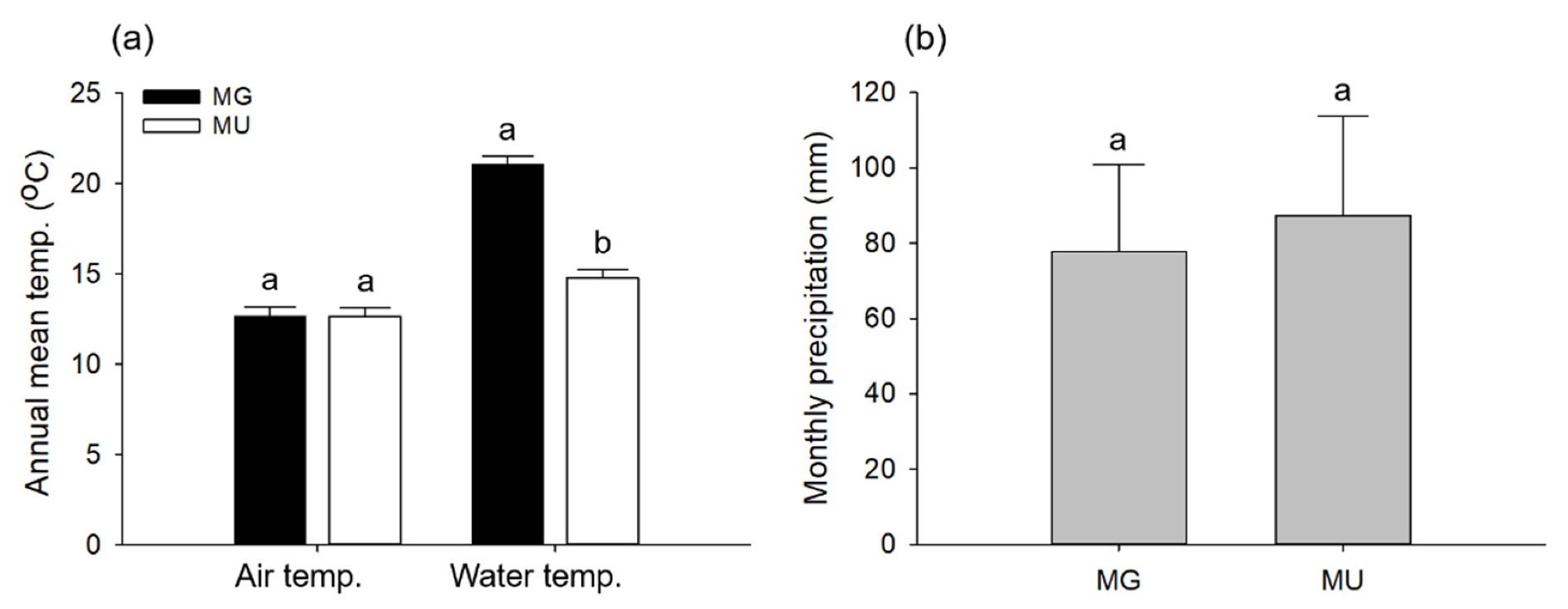
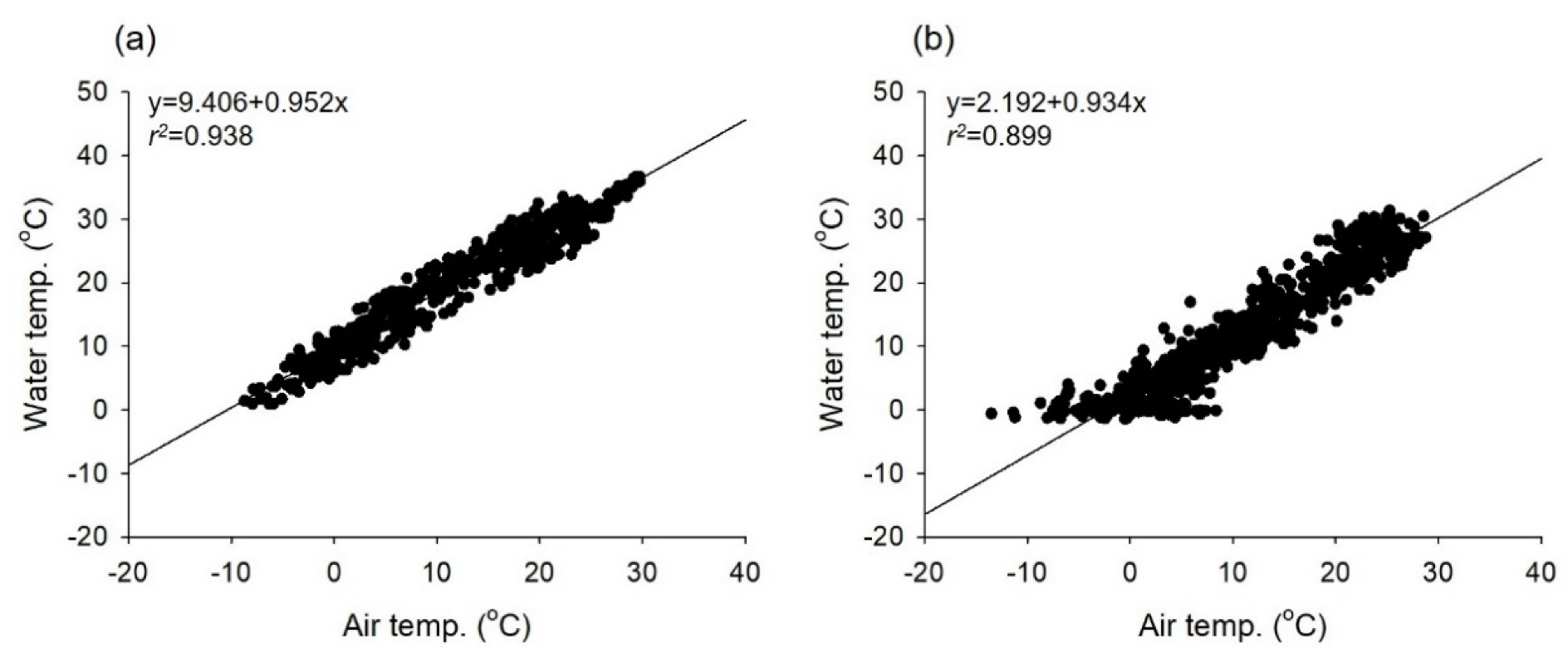
3.1. Environmental Conditions
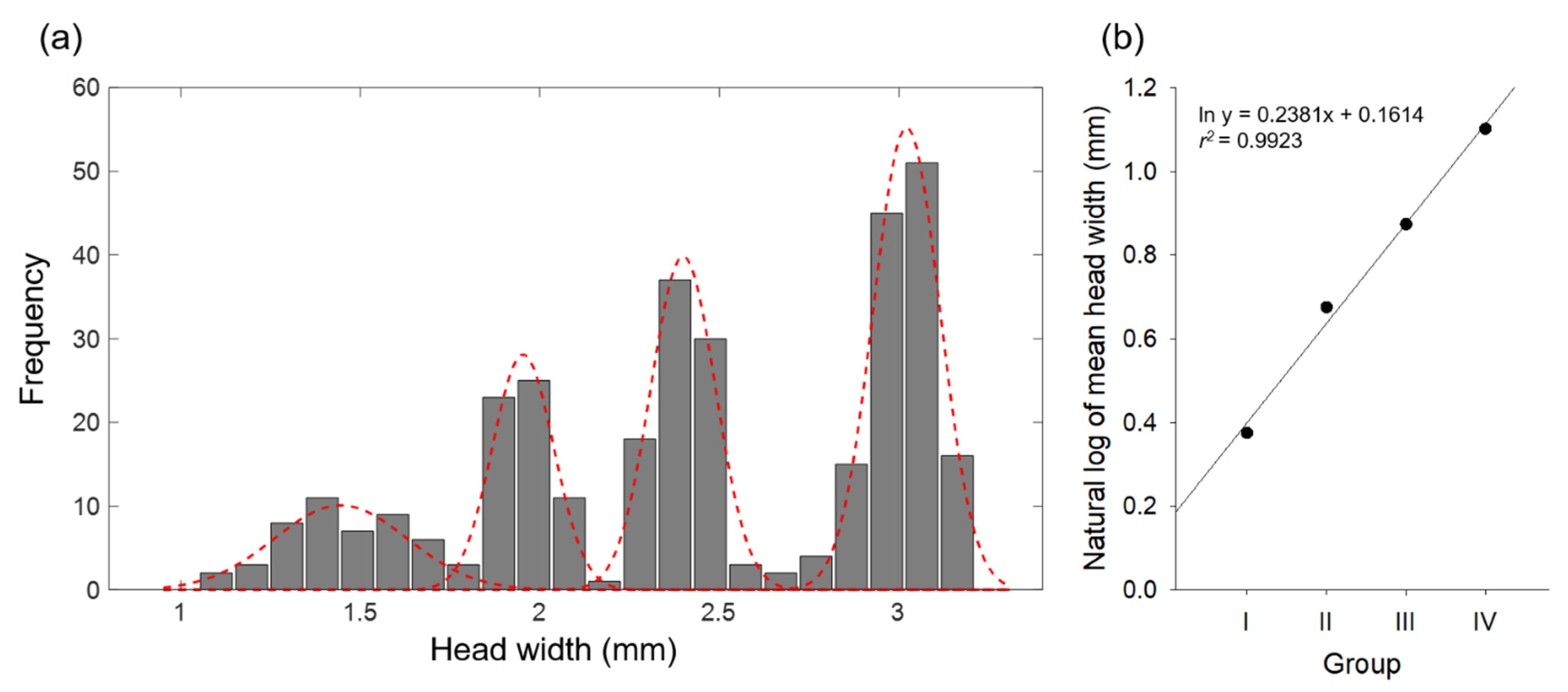
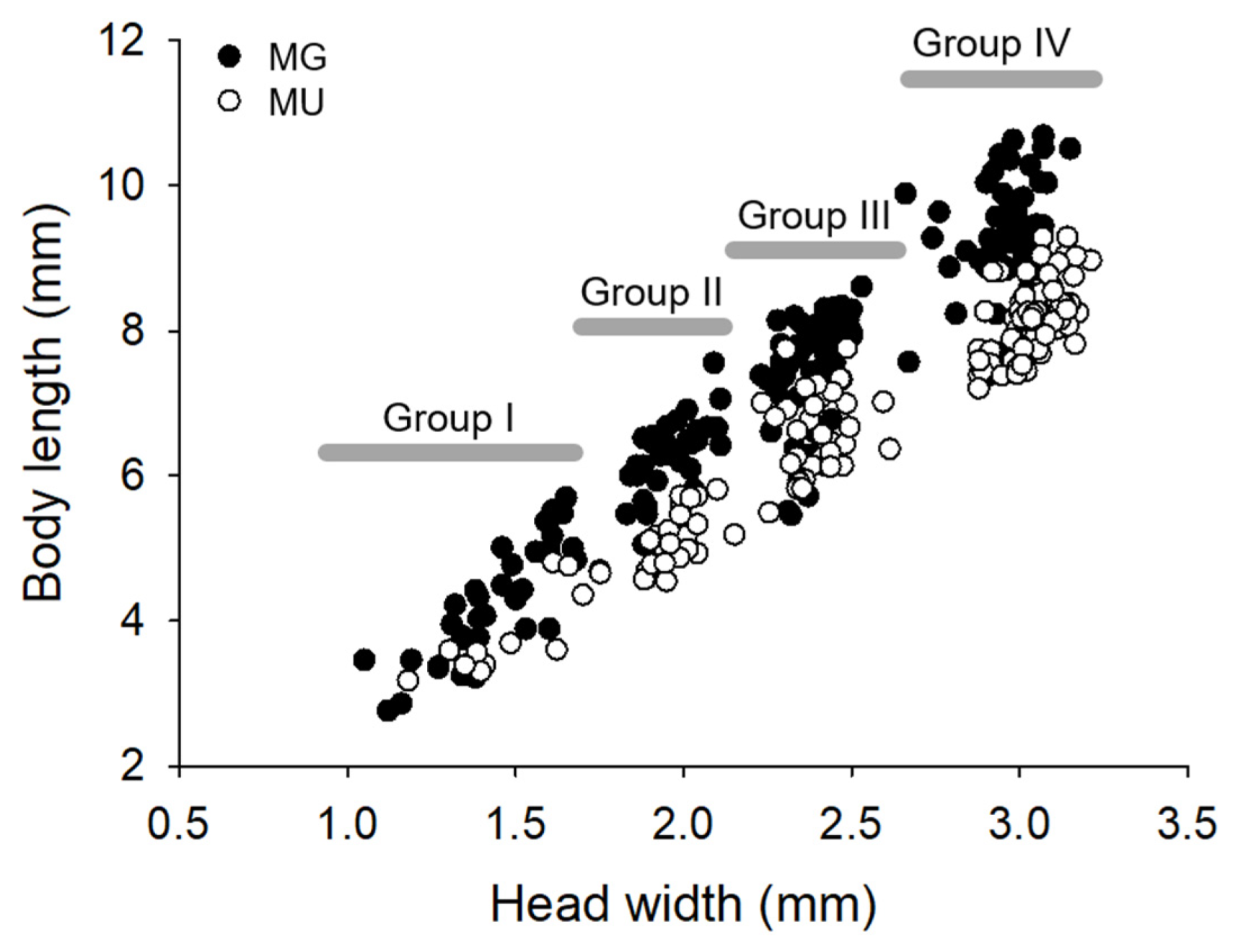
3.2. Grouping and Larval Size Distribution
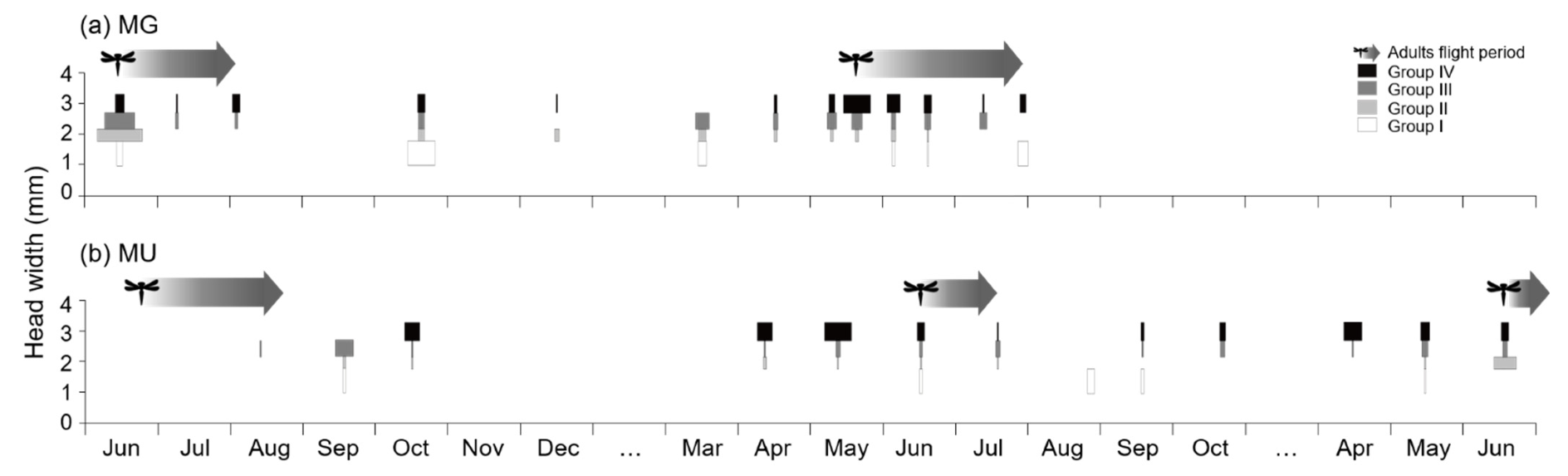
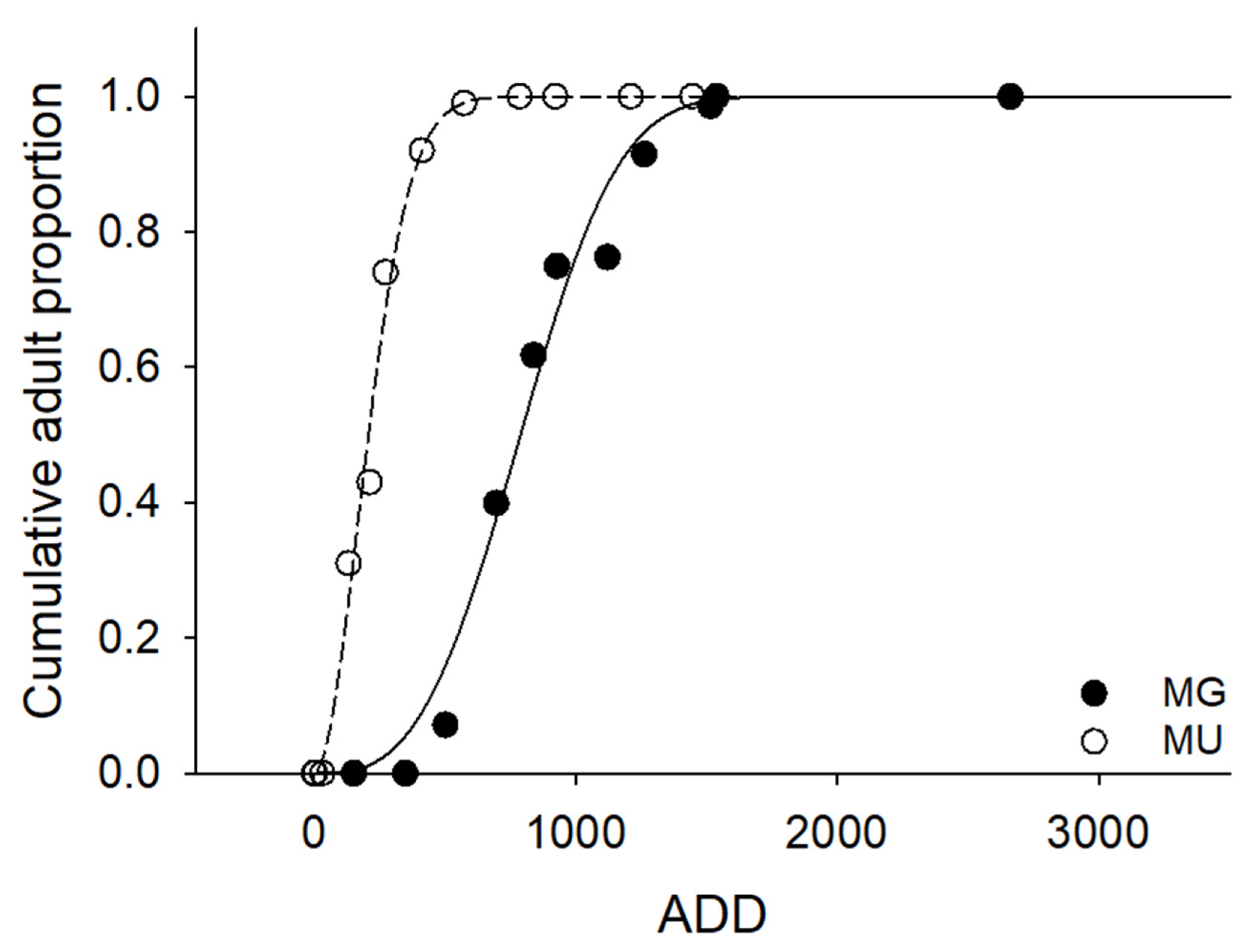
3.3. Life History
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peters, R.H. The Ecological Implications of Body Size; Cambridge University Press: Cambridge, UK, 1983; ISBN 9780521246842. [Google Scholar]
- Wonglersak, R.; Fenberg, P.B.; Langdon, P.G.; Brooks, S.J.; Price, B.W. Temperature-body size responses in insects: A case study of British Odonata. Ecol. Entomol. 2020, 45, 795–805. [Google Scholar] [CrossRef]
- Gibert, J.P.; DeLong, J.P. Temperature alters food web body-size structure. Biol. Lett. 2014, 10, 20140473. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, D. Temperature and organism size-a biological law for ectotherms? Adv. Ecol. Res. 1994, 25, 1–58. [Google Scholar] [CrossRef]
- Ashton, K.G.; Tracy, M.C.; de Queiroz, A. Is Bergmann’s rule valid for mammals? Am. Nat. 2000, 156, 390–415. [Google Scholar] [CrossRef] [PubMed]
- Mousseau, T.A. Ectotherms follow the converse to Bergmann’s Rule. Evolution 1997, 51, 630–632. [Google Scholar] [CrossRef] [Green Version]
- Johansson, F. Latitudinal shifts in body size of Enallagma cyathigerum (Odonata). J. Biogeogr. 2003, 30, 29–34. [Google Scholar] [CrossRef]
- van Voorhies, W.A. Bergmann size clines: A simple explanation for their occurrence in ectotherms. Evolution 1996, 50, 1259–1264. [Google Scholar] [CrossRef]
- Bergmann, C. Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse; Göttinger Studien: Göttingen, German, 1848; Volume 3, pp. 595–701. (In German) [Google Scholar]
- Roff, D. Optimizing development time in a seasonal environment: The “ups and downs” of clinal variation. Oecologia 1980, 45, 202–208. [Google Scholar] [CrossRef]
- Gardner, J.L.; Peters, A.; Kearney, M.R.; Joseph, L.; Heinsohn, R. Declining body size: A third universal response to warming? Trends Ecol. Evol. 2011, 26, 285–291. [Google Scholar] [CrossRef]
- Ohlberger, J. Climate warming and ectotherm body size—From individual physiology to community ecology. Funct. Ecol. 2013, 27, 991–1001. [Google Scholar] [CrossRef]
- Schutze, M.K.; Clarke, A.R. Converse Bergmann cline in a Eucalyptus herbivore, Paropsis atomaria Olivier (Coleoptera: Chrysomelidae): Phenotypic plasticity or local adaptation? Glob. Ecol. Biogeogr. 2008, 17, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Partridge, L.; Coyne, J.A. Bergmann’s rule in ectotherms: Is it adaptive? Evolution 1997, 51, 632–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-Delgado, V.H.; Sanabria-Urbán, S.; Serrano-Meneses, M.A.; Cueva del Castillo, R. The converse to Bergmann’s rule in bumblebees, a phylogenetic approach. Ecol. Evol. 2016, 6, 6160–6169. [Google Scholar] [CrossRef] [PubMed]
- Johansson, F.; Watts, P.C.; Sniegula, S.; Berger, D. Natural selection mediated by seasonal time constraints increases the alignment between evolvability and developmental plasticity. Evolution 2021, 75, 464–475. [Google Scholar] [CrossRef]
- Tseng, M.; Soleimani Pari, S. Body size explains interspecific variation in size—Latitude relationships in geographically widespread beetle species. Ecol. Entomol. 2019, 44, 151–156. [Google Scholar] [CrossRef]
- Pallarés, S.; Lai, M.; Abellán, P.; Ribera, I.; Sánchez-Fernández, D. An interspecific test of Bergmann’s rule reveals inconsistent body size patterns across several lineages of water beetles (Coleoptera: Dytiscidae). Ecol. Entomol. 2019, 44, 249–254. [Google Scholar] [CrossRef]
- Beerli, N.; Bärtschi, F.; Ballesteros-Mejia, L.; Kitching, I.J.; Beck, J. How has the environment shaped geographical patterns of insect body sizes? A test of hypotheses using sphingid Moths. J. Biogeogr. 2019, 46, 1687–1698. [Google Scholar] [CrossRef]
- Amado, T.F.; Bidau, C.J.; Olalla-Tárraga, M.Á. Geographic variation of body size in New World anurans: Energy and water in a balance. Ecography 2019, 42, 456–466. [Google Scholar] [CrossRef] [Green Version]
- Blanckenhorn, W.U.; Bauerfeind, S.S.; Berger, D.; Davidowitz, G.; Fox, C.W.; Guillaume, F.; Nakamura, S.; Nishimura, K.; Sasaki, H.; Stillwell, R.C.; et al. Life history traits, but not body size, vary systematically along latitudinal gradients on three continents in the widespread yellow dung fly. Ecography 2018, 41, 2080–2091. [Google Scholar] [CrossRef] [Green Version]
- Shelomi, M. Where are we now? Bergmann’s rule sensu lato in insects. Am. Nat. 2012, 180, 511–519. [Google Scholar] [CrossRef]
- Śniegula, S.; Johansson, F.; Nilsson-Örtman, V. Differentiation in developmental rate across geographic regions: A photoperiod driven latitude compensating mechanism? Oikos 2012, 121, 1073–1082. [Google Scholar] [CrossRef]
- Brehm, G.; Zeuss, D.; Colwell, R.K. Moth body size increases with elevation along a complete tropical elevational gradient for two hyperdiverse clades. Ecography 2019, 42, 632–642. [Google Scholar] [CrossRef] [Green Version]
- Berger, D.; Walters, R.; Gotthard, K. What keeps insects small?—Size dependent predation on two species of butterfly Larvae. Evol. Ecol. 2006, 20, 575–589. [Google Scholar] [CrossRef]
- Moore, M.P.; Martin, R.A. Trade-offs between larval survival and adult ornament development depend on predator regime in a territorial dragonfly. Oecologia 2018, 188, 97–106. [Google Scholar] [CrossRef]
- McCauley, S.J. Slow, Fast and in between: Habitat distribution and behaviour of larvae in nine species of libellulid dragonfly. Freshw. Biol. 2008, 53, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Bhowmik, A.K.; Schäfer, R.B. Large scale relationship between aquatic insect traits and climate. PLoS ONE 2015, 10, e0130025. [Google Scholar] [CrossRef] [Green Version]
- Starr, S.M.; McIntyre, N.E. Effects of water temperature under projected climate change on the development and survival of Enallagma civile (Odonata: Coenagrionidae). Environ. Entomol. 2020, 49, 230–237. [Google Scholar] [CrossRef]
- Moore, N.W. Dragonflies-Status Survey and Conservation Action Plan; IUCN/SSC Odonata Specialist Group: Gland, Switzerland, 1997; ISBN 2-8317-0420-0. [Google Scholar]
- Kim, D.G. Research history of Nannophya Rambur (Odonata: Libellulidae): A recently discovered species in addition to Nannophya koreana Bae in Korea. Korean J. Environ. Biol. 2020, 38, 308–314. [Google Scholar] [CrossRef]
- Bae, Y.J.; Yum, J.H.; Kim, D.G.; Suh, K.I.; Kang, J.H. Nannophya koreana Sp. Nov. (Odonata: Libellulidae): A new dragonfly species previously recognized in Korea as the endangered pygmy dragonfly Nannophya pygmaea Rambur. J. Species Res. 2020, 9, 1–10. [Google Scholar] [CrossRef]
- Kim, D.G.; Yum, J.W.; Yoon, T.J.; Bae, Y.J. Life history of an endangered dragonfly, Nannophya pygmaea Rambur, in Korea (Anisoptera: Libellulidae). Odonatologica 2010, 39, 39–46. [Google Scholar]
- Oh, K.C.; Ro, K.H.; Lee, H.G.; Kim, D.G. Suggestions for protecting and preserving the level II endangered species Nannophya pygmaea in Korea. Korean J. Environ. Biol. 2017, 35, 545–548. [Google Scholar] [CrossRef]
- Kim, D.G.; Yoon, T.J.; Oh, C.G.; Kim, J.G.; Lee, E.-H.; Bae, Y.J. Laval growth rate of Nannophya pygmaea (Odonata: Libellulidae), and endangered dragonfly in Korea. Korean J. Limnol. 2009, 42, 290–294. [Google Scholar]
- Bae, Y.; Yum, J.; Cha, J.; Yoon, I. Morphology, habitat, and distributional records of Nannophya pygmaea Rambur (Libellulidae, Odonata). Korean J. Entomol. 1999, 29, 287–290. [Google Scholar]
- Kim, D.G.; Hwang, J.M.; Yoon, T.J.; Bae, Y.J. Relationship between temperature and egg development of Nannophya pygmaea Rambur (Odonata: Libellulidae), an endangered dragonfly in Korea. Korean J. Environ. Biol. 2009, 27, 292–296. [Google Scholar]
- Kim, D.G.; Yum, J.W.; Yoon, T.J.; Bae, Y.J. Effect of temperature on hatching rate of Nannophya pygmaea eggs (Odonata: Libellulidae). Korean J. Appl. Entomol. 2006, 45, 381–383. [Google Scholar] [CrossRef]
- Yoon, J.; Nam, J.M.; Kim, H.; Bae, Y.J.; Kim, J.G. Nannophya pygmaea (Odonata: Libellulidae), an endangered dragonfly in Korea, prefers abandoned paddy fields in the early seral stage. Environ. Entomol. 2010, 39, 278–285. [Google Scholar] [CrossRef]
- Kim, K.-G.; Jang, S.K.; Park, D.W.; Hong, M.Y.; Oh, K.-H.; Kim, K.Y.; Hwang, J.S.; Han, Y.S.; Kim, I. Mitochondrial DNA sequence variation of the tiny dragonfly, Nannophya pygmaea (Odonata: Libellulidae). Int. J. Indust. Entomol. 2007, 15, 47–58. [Google Scholar]
- Lee, S.J.; Bae, Y.J.; Yoon, I.B.; Watanabe, N.C. Comparisons of temperature-related life histories in Two ephemerid mayflies (Epemera separigata and E. strigata: Ephemeridae, Ephemeroptera, Insecta) from a Mountain Stream in Korea. Korean J. Limnol. 1999, 32, 253–260. [Google Scholar]
- Logan, J.A.; Bentz, B.J.; Vandygriff, J.C.; Turner, D.L. General program for determining instar distributions from headcapsule widths: Example analysis of mountain pine beetle (Coleoptera: Scolytide) data. Environ. Entomol. 1998, 27, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Seo, Y.J.; Kim, D.G.; Baek, M.J.; Bae, Y.J. Life history differences of Psilotreta locumtenens (Trichoptera: Odontoceridae) in Two Reaches of a Mountain Stream in Korea. Entomol. Res. 2014, 44, 293–301. [Google Scholar] [CrossRef]
- Cazado, L.E.; van Nieuwenhove, G.A.; O’brien, C.W.; Gastaminza, G.A.; Murúa, M.G. Determination of number of instars of Rhyssomatus subtilis (Coleoptera: Curculionidae) based on head capsule widths. Fla. Entomol. 2014, 97, 639–643. [Google Scholar] [CrossRef]
- Brunet, B.M.T.; Hundsdoerfer, A.E.; Sperling, F.A.H. Identification and ecological characterisation of Choristoneura occidentalis (Lepidoptera: Tortricidae) populations in Southwestern Alberta, Canada. Can. Entomol. 2013, 145, 521–528. [Google Scholar] [CrossRef]
- Panzavolta, T. Instar determination for Pissodes castaneus (Coleoptera: Curculionidae) using head capsule widths and lengths. Environ. Entomol. 2007, 36, 1054–1058. [Google Scholar] [CrossRef] [Green Version]
- Iano, D.M.; Araújo, B.L.; Rossi, M.N. Intraspecific competition and persistence in Acanthoscelides macrophthalmus (Coleoptera: Chrysomelidae): An experimental analysis in a stage-structured population. Austral Entomol. 2021, 61, 86–96. [Google Scholar] [CrossRef]
- Gaines, J.C.; Campbell, F.L. Dyar’s rule as related to the number of instars of the corn ear worm, Heliothis obsoleta (Fab.), Collected in the Field. Ann. Entomol. Soc. Am. 1935, 28, 445–461. [Google Scholar] [CrossRef]
- Daly, H.V. Insect Morphometrics. Annu. Rev. Entomol. 1985, 30, 415–438. [Google Scholar] [CrossRef]
- Norling, U. Growth, Winter preparations and timing of emergence in temperate zone Odonata: Control by a succession of larval response patterns. Int. J. Odonatol. 2021, 24, 1–36. [Google Scholar] [CrossRef]
- Règniére, J. A method of describing and using variability in development rates for the simulation of insect phenology. Can. Entomol. 1984, 116, 1367–1376. [Google Scholar] [CrossRef]
- Pruess, K.P. Day-degree methods for pest management. Environ. Entomol. 1983, 12, 613–619. [Google Scholar] [CrossRef]
- Parr, M.J. The life histories of Ischnura elegans (van Der Linden) and Coenagrion puella (L.) (Odonata) in South Lancashire. Proc. R. Entomol. 1970, 45, 172–181. [Google Scholar] [CrossRef]
- Ferreras-Romero, M.; Atienzar, M.D.; Corbet, P.S. Voltinism of Calopteryx haemorrhoidalis, (Vander Linden) in the Sierra Morena Mountains, Southern Spain (Zygoptera: Calopterygidae): A preliminary study. Int. J. Odonatol. 2000, 3, 125–130. [Google Scholar] [CrossRef]
- Nijhout, H.F. A threshold size for metamorphosis in the tobacco hornworm, Manduca sexta (L.). Biol. Bull. 1975, 149, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Kojima, W.; Nakakura, T.; Fukuda, A.; Lin, C.P.; Harada, M.; Hashimoto, Y.; Kawachi, A.; Suhama, S.; Yamamoto, R. Latitudinal cline of larval growth rate and its proximate mechanisms in a rhinoceros beetle. Funct. Ecol. 2020, 34, 1577–1588. [Google Scholar] [CrossRef]
- Horne, C.R.; Hirst, A.G.; Atkinson, D. Temperature-size responses match latitudinal-size clines in arthropods, revealing critical differences between aquatic and terrestrial species. Ecol. Lett. 2015, 18, 327–335. [Google Scholar] [CrossRef]
- Blanckenhorn, W.U.; Demont, M. Bergmann and converse Bergmann latitudinal clines in arthropods: Two ends of a continuum? Integr. Comp. Biol. 2004, 44, 413–424. [Google Scholar] [CrossRef] [Green Version]
- Zeuss, D.; Brunzel, S.; Brandl, R. Environmental drivers of voltinism and body size in insect assemblages across Europe. Glob. Ecol. Biogeogr. 2017, 26, 154–165. [Google Scholar] [CrossRef]
- de Block, M.; Stoks, R. Life history responses depend on timing of cannibalism in a damselfly. Freshw. Biol. 2004, 49, 775–786. [Google Scholar] [CrossRef]
- Śniegula, S.; Johansson, F. Photoperiod affects compensating developmental rate across latitudes in the damselfly Lestes sponsa. Ecol. Entomol. 2010, 35, 149–157. [Google Scholar] [CrossRef]
- Sniegula, S.; Golab, M.J.; Johansson, F. A large-scale latitudinal pattern of life-history traits in a strictly univoltine damselfly. Ecol. Entomol. 2016, 41, 459–472. [Google Scholar] [CrossRef] [Green Version]
- Woodward, G.; Perkins, D.M.; Brown, L.E. Climate change and freshwater ecosystems: Impacts across multiple levels of organization. Philos. Trans. R. Soc. 2010, 365, 2093–2106. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.Y.; Kim, D.G.; Baek, M.J.; Choe, L.J.; Bae, Y.J. Life history and emergence pattern of Cloeon dipterum (Ephemeroptera: Baetidae) in Korea. Environ. Entomol. 2013, 42, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Brodin, T.; Johansson, F. Conflicting selection pressures on the growth/predation-risk trade-off in a damselfly. Ecology 2004, 85, 2927–2932. [Google Scholar] [CrossRef]
- Anderson, N.H.; Cummins, K.W. Influences of diet on the life histories of aquatic insects. J. Fish. Res. Board Can. 1979, 36, 335–342. [Google Scholar] [CrossRef]
- Connolly, N.M.; Crossland, M.R.; Pearson, R.G. Effect of low dissolved oxygen on survival, emergence, and drift of tropical stream macroinvertebrates. J. N. Am. Benthol. Soc. 2004, 23, 251–270. [Google Scholar] [CrossRef]
- Fattorini, S.; lo Monaco, R.; di Giulio, A.; Ulrich, W. Climatic correlates of body size in European tenebrionid beetles (Coleoptera: Tenebrionidae). Org. Divers. Evol. 2014, 14, 215–224. [Google Scholar] [CrossRef]
- Pires, M.M.; Ely-Junior, G.L.; Dalzochio, M.S.; Sahlén, G.; Périco, E. Intraspecific morphological variation in the dragonfly Erythrodiplax media (Odonata: Libellulidae) among South American grassland physiognomies. Neotrop. Entomol. 2021, 50, 736–747. [Google Scholar] [CrossRef]
| Group | n | Head Width (mm) | Probability of Misclassification | Dyar’s Ratio | |||
|---|---|---|---|---|---|---|---|
| Mean ± SE | Range | i as i − 1 | i as i + 1 | Total | |||
| I | 48 | 1.45 ± 0.17 | 1.05−1.75 | 0.0000 | 0.0433 | 0.0433 | - |
| II | 61 | 1.97 ± 0.08 | 1.83−2.15 | 0.0129 | 0.0068 | 0.0197 | 1.35 |
| III | 90 | 2.40 ± 0.09 | 2.23−2.67 | 0.0046 | 0.0004 | 0.0050 | 1.22 |
| IV | 131 | 3.01 ± 0.09 | 2.74−3.21 | 0.0003 | 0.0000 | 0.0003 | 1.26 |
| Standard | Site | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|---|
| HW | MG | 1.44 ± 0.17 | 1.95 ± 0.08 | 2.40 ± 0.09 | 2.97 ± 0.08 * |
| MU | 1.49 ± 0.18 | 1.98 ± 0.06 | 2.40 ± 0.08 | 3.04 ± 0.08 | |
| BL | MG | 4.28 ± 0.78 | 6.18 ± 0.60 * | 7.62 ± 0.75 * | 9.26 ± 0.74 * |
| MU | 3.86 ± 0.58 | 5.17 ± 0.37 | 6.57 ± 0.55 | 8.21 ± 0.48 |
| Site | Growth Rate (mm/100 DD) | Maximum BL (mm) | Required ADD from Hatching to Maximum BL | ADD from Hatching until Winter |
|---|---|---|---|---|
| MG | 0.75 | 10.69 | 1425.3 | 1882.5 |
| MU | 1.16 | 9.29 | 800.9 | 1056.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.Y.; Kim, M.K.; Kim, D.-G. Ecological Responses of Nannophya koreana (Odonata: Libellulidae) to Temperature: Following Converse Bergmann’s Rule. Biology 2022, 11, 830. https://doi.org/10.3390/biology11060830
Lee CY, Kim MK, Kim D-G. Ecological Responses of Nannophya koreana (Odonata: Libellulidae) to Temperature: Following Converse Bergmann’s Rule. Biology. 2022; 11(6):830. https://doi.org/10.3390/biology11060830
Chicago/Turabian StyleLee, Cha Young, Min Kyung Kim, and Dong-Gun Kim. 2022. "Ecological Responses of Nannophya koreana (Odonata: Libellulidae) to Temperature: Following Converse Bergmann’s Rule" Biology 11, no. 6: 830. https://doi.org/10.3390/biology11060830
APA StyleLee, C. Y., Kim, M. K., & Kim, D.-G. (2022). Ecological Responses of Nannophya koreana (Odonata: Libellulidae) to Temperature: Following Converse Bergmann’s Rule. Biology, 11(6), 830. https://doi.org/10.3390/biology11060830






