Predator-Prey Relationship between Urban Bats and Insects Impacted by Both Artificial Light at Night and Spatial Clutter
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Selection of Recording Sites
2.2. Acoustic Recording of Bats and Call Analysis
2.3. Aerial Insect Sampling
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russo, D.; Salinas-Ramos, V.B.; Cistrone, L.; Smeraldo, S.; Bosso, L.; Ancillotto, L. Do We Need to Use Bats as Bioindicators? Biology 2021, 10, 693. [Google Scholar] [CrossRef] [PubMed]
- Falchi, F.; Cinzano, P.; Duriscoe, D.; Kyba, C.C.M.; Elvidge, C.D.; Baugh, K.; Portnov, B.A.; Rybnikova, N.A.; Furgoni, R. The New World Atlas of Artificial Night Sky Brightness. Sci. Adv. 2016, 2, e1600377. [Google Scholar] [CrossRef] [PubMed]
- Longcore, T.; Rich, C. Ecological Light Pollution. Front. Ecol. Environ. 2004, 2, 191–198. [Google Scholar] [CrossRef]
- Sanders, D.; Frago, E.; Kehoe, R.; Patterson, C.; Gaston, K.J. A Meta-Analysis of Biological Impacts of Artificial Light at Night. Nat. Ecol. Evol. 2021, 5, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Rowse, E.G.; Lewanzik, D.; Stone, E.L.; Harris, S.; Jones, G. Dark Matters: The Effects of Artificial Lighting on Bats. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C.C., Kingston, T., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 187–213. ISBN 978-3-319-25220-9. [Google Scholar]
- Eisenbeis, G. Artificial Night Lighting and Insects: Attraction of Insects to Streetlamps in a Rural Setting in Germany. In Ecological Consequences of Artificial Night Lighting; Rich, C., Longcore, T., Eds.; Island Press: Washington, DC, USA, 2006; pp. 281–304. ISBN 978-1-59726-596-6. [Google Scholar]
- Van Langevelde, F.; Ettema, J.A.; Donners, M.; WallisDeVries, M.F.; Groenendijk, D. Effect of Spectral Composition of Artificial Light on the Attraction of Moths. Biol. Conserv. 2011, 144, 2274–2281. [Google Scholar] [CrossRef]
- Nabli, H.; Bailey, W.C.; Necibi, S. Beneficial Insect Attraction to Light Traps with Different Wavelengths. Biol. Control 1999, 16, 185–188. [Google Scholar] [CrossRef]
- van Grunsven, R.H.A.; Donners, M.; Boekee, K.; Tichelaar, I.; van Geffen, K.G.; Groenendijk, D.; Berendse, F.; Veenendaal, E.M. Spectral Composition of Light Sources and Insect Phototaxis, with an Evaluation of Existing Spectral Response Models. J. Insect Conserv. 2014, 18, 225–231. [Google Scholar] [CrossRef]
- Scanlon, A.T.; Petit, S. Biomass and Biodiversity of Nocturnal Aerial Insects in an Adelaide City Park and Implications for Bats (Microchiroptera). Urban Ecosyst. 2008, 11, 91–106. [Google Scholar] [CrossRef]
- Svensson, A.M.; Rydell, J. Mercury Vapour Lamps Interfere with the Bat Defence of Tympanate Moths (Operophtera spp.; Geometridae). Anim. Behav. 1998, 55, 223–226. [Google Scholar] [CrossRef][Green Version]
- Acharya, L.; Fenton, M.B. Bat Attacks and Moth Defensive Behaviour around Street Lights. Can. J. Zool. 1999, 77, 27–33. [Google Scholar] [CrossRef]
- Minnaar, C.; Boyles, J.G.; Minnaar, I.A.; Sole, C.L.; McKechnie, A.E. Stacking the Odds: Light Pollution May Shift the Balance in an Ancient Predator-Prey Arms Race. J. Appl. Ecol. 2015, 52, 522–531. [Google Scholar] [CrossRef]
- Rydell, J. Exploitation of Insects around Streetlamps by Bats in Sweden. Funct. Ecol. 1992, 6, 744–750. [Google Scholar] [CrossRef]
- Blake, D.; Hutson, A.M.; Racey, P.A.; Rydell, J.; Speakman, J.R. Use of Lamplit Roads by Foraging Bats in Southern England. J. Zool. 1994, 234, 453–462. [Google Scholar] [CrossRef]
- Avila-Flores, R.; Fenton, M.B. Use of Spatial Features by Foraging Insectivorous Bats in a Large Urban Landscape. J. Mammal. 2005, 86, 1193–1204. [Google Scholar] [CrossRef]
- Jung, K.; Kalko, E.K.V. Where Forest Meets Urbanization: Foraging Plasticity of Aerial Insectivorous Bats in an Anthropogenically Altered Environment. J. Mammal. 2010, 91, 144–153. [Google Scholar] [CrossRef]
- Threlfall, C.G.; Law, B.; Banks, P.B. The Urban Matrix and Artificial Light Restricts the Nightly Ranging Behaviour of Gould’s Long-Eared Bat (Nyctophilus gouldi). Austral Ecol. 2013, 38, 921–930. [Google Scholar] [CrossRef]
- Voigt, C.C.; Scholl, J.M.; Bauer, J.; Teige, T.; Yovel, Y.; Kramer-Schadt, S.; Gras, P. Movement Responses of Common Noctule Bats to the Illuminated Urban Landscape. Landsc. Ecol. 2020, 35, 189–201. [Google Scholar] [CrossRef]
- Zeale, M.R.K.; Stone, E.L.; Zeale, E.; Browne, W.J.; Harris, S.; Jones, G. Experimentally Manipulating Light Spectra Reveals the Importance of Dark Corridors for Commuting Bats. Glob. Chang. Biol. 2018, 24, 5909–5918. [Google Scholar] [CrossRef]
- Polak, T.; Korine, C.; Yair, S.; Holderied, M.W. Differential Effects of Artificial Lighting on Flight and Foraging Behaviour of Two Sympatric Bat Species in a Desert. J. Zool. 2011, 285, 21–27. [Google Scholar] [CrossRef]
- Stone, E.L.; Jones, G.; Harris, S. Street Lighting Disturbs Commuting Bats. Curr. Biol. 2009, 19, 1123–1127. [Google Scholar] [CrossRef]
- Stone, E.L.; Jones, G.; Harris, S. Conserving Energy at a Cost to Biodiversity? Impacts of LED Lighting on Bats. Glob. Chang. Biol. 2012, 18, 2458–2465. [Google Scholar] [CrossRef]
- Seewagen, C.L.; Adams, A.M. Turning to the Dark Side: LED Light at Night Alters the Activity and Species Composition of a Foraging Bat Assemblage in the Northeastern United States. Ecol. Evol. 2021, 11, 5635–5645. [Google Scholar] [CrossRef] [PubMed]
- McGuire, L.P.; Fenton, M.B. Hitting the Wall: Light Affects the Obstacle Avoidance Ability of Free-Flying Little Brown Bats (Myotis lucifugus). Acta Chiropterologica 2010, 12, 247–250. [Google Scholar] [CrossRef]
- Russo, D.; Cosentino, F.; Festa, F.; De Benedetta, F.; Pejic, B.; Cerretti, P.; Ancillotto, L. Artificial Illumination near Rivers May Alter Bat-Insect Trophic Interactions. Environ. Pollut. 2019, 252, 1671–1677. [Google Scholar] [CrossRef]
- Barré, K.; Spoelstra, K.; Bas, Y.; Challéat, S.; Kiri Ing, R.; Azam, C.; Zissis, G.; Lapostolle, D.; Kerbiriou, C.; Le Viol, I. Artificial Light May Change Flight Patterns of Bats near Bridges along Urban Waterways. Anim. Conserv. 2021, 24, 259–267. [Google Scholar] [CrossRef]
- Straka, T.M.; Wolf, M.; Gras, P.; Buchholz, S.; Voigt, C.C. Tree Cover Mediates the Effect of Artificial Light on Urban Bats. Front. Ecol. Evol. 2019, 7, 91. [Google Scholar] [CrossRef]
- Lewanzik, D.; Voigt, C.C. Transition from Conventional to Light-Emitting Diode Street Lighting Changes Activity of Urban Bats. J. Appl. Ecol. 2017, 54, 264–271. [Google Scholar] [CrossRef]
- Santini, L.; González-Suárez, M.; Russo, D.; Gonzalez-Voyer, A.; von Hardenberg, A.; Ancillotto, L. One Strategy Does Not Fit All: Determinants of Urban Adaptation in Mammals. Ecol. Lett. 2019, 22, 365–376. [Google Scholar] [CrossRef]
- Li, H.; Crihfield, C.; Feng, Y.; Gaje, G.; Guzman, E.; Heckman, T.; Mellis, A.; Moore, L.; Romo Bechara, N.; Sanchez, S.; et al. The Weekend Effect on Urban Bat Activity Suggests Fine Scale Human-Induced Bat Movements. Animals 2020, 10, 1636. [Google Scholar] [CrossRef]
- Schimpp, S.A.; Li, H.; Kalcounis-Rueppell, M.C. Determining Species Specific Nightly Bat Activity in Sites with Varying Urban Intensity. Urban Ecosyst. 2018, 21, 541–550. [Google Scholar] [CrossRef]
- Voigt, C.C.; Dekker, J.; Fritze, M.; Gazaryan, S.; Hölker, F.; Jones, G.; Lewanzik, D.; Limpens, H.J.G.A.; Mathews, F.; Rydell, J.; et al. The Impact Of Light Pollution On Bats Varies According To Foraging Guild And Habitat Context. BioScience 2021, 71, 1103–1109. [Google Scholar] [CrossRef]
- Schoeman, M.C. Light Pollution at Stadiums Favors Urban Exploiter Bats. Anim. Conserv. 2016, 19, 120–130. [Google Scholar] [CrossRef]
- Scanlon, A.T.; Petit, S. Effects of Site, Time, Weather and Light on Urban Bat Activity and Richness: Considerations for Survey Effort. Wildl. Res. 2008, 35, 821–834. [Google Scholar] [CrossRef]
- Cravens, Z.M.; Boyles, J.G. Illuminating the Physiological Implications of Artificial Light on an Insectivorous Bat Community. Oecologia 2019, 189, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Cravens, Z.M.; Brown, V.A.; Divoll, T.J.; Boyles, J.G. Illuminating Prey Selection in an Insectivorous Bat Community Exposed to Artificial Light at Night. J. Appl. Ecol. 2018, 55, 705–713. [Google Scholar] [CrossRef]
- Bolliger, J.; Hennet, T.; Wermelinger, B.; Bösch, R.; Pazur, R.; Blum, S.; Haller, J.; Obrist, M.K. Effects of Traffic-Regulated Street Lighting on Nocturnal Insect Abundance and Bat Activity. Basic Appl. Ecol. 2020, 47, 44–56. [Google Scholar] [CrossRef]
- Rowse, E.G.; Harris, S.; Jones, G. Effects of Dimming Light-Emitting Diode Street Lights on Light-Opportunistic and Light-Averse Bats in Suburban Habitats. R. Soc. Open Sci. 2018, 5, 180205. [Google Scholar] [CrossRef]
- Kennedy, C.E.J.; Southwood, T.R.E. The Number of Species of Insects Associated with British Trees: A Re-Analysis. J. Anim. Ecol. 1984, 53, 455–478. [Google Scholar] [CrossRef]
- Kunz, T.H.; Parsons, S. Ecological and Behavioral Methods for the Study of Bats; Johns Hopkins University Press: Baltimore, MD, USA, 2009; ISBN 978-0-8018-9147-2. [Google Scholar]
- Blakey, R.V.; Webb, E.B.; Kesler, D.C.; Siegel, R.B.; Corcoran, D.; Johnson, M. Bats in a Changing Landscape: Linking Occupancy and Traits of a Diverse Montane Bat Community to Fire Regime. Ecol. Evol. 2019, 9, 5324–5337. [Google Scholar] [CrossRef]
- Li, H.; Wilkins, K.T. Selection of Building Roosts by Mexican Free-Tailed Bats (Tadarida brasiliensis) in an Urban Area. Acta Chiropterologica 2015, 17, 321–330. [Google Scholar] [CrossRef]
- Owens, A.C.S.; Lewis, S.M. The Impact of Artificial Light at Night on Nocturnal Insects: A Review and Synthesis. Ecol. Evol. 2018, 8, 11337–11358. [Google Scholar] [CrossRef] [PubMed]
- Grubisic, M.; van Grunsven, R.H. Artificial Light at Night Disrupts Species Interactions and Changes Insect Communities. Curr. Opin. Insect Sci. 2021, 47, 136–141. [Google Scholar] [CrossRef] [PubMed]
- van Grunsven, R.H.A.; Becker, J.; Peter, S.; Heller, S.; Hölker, F. Long-Term Comparison of Attraction of Flying Insects to Streetlights after the Transition from Traditional Light Sources to Light-Emitting Diodes in Urban and Peri-Urban Settings. Sustainability 2019, 11, 6198. [Google Scholar] [CrossRef]
- Mathews, F.; Roche, N.; Aughney, T.; Jones, N.; Day, J.; Baker, J.; Langton, S. Barriers and Benefits: Implications of Artificial Night-Lighting for the Distribution of Common Bats in Britain and Ireland. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140124. [Google Scholar] [CrossRef]
- Spoelstra, K.; Ramakers, J.J.C.; van Dis, N.E.; Visser, M.E. No Effect of Artificial Light of Different Colors on Commuting Daubenton’s Bats (Myotis daubentonii) in a Choice Experiment. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2018, 329, 506–510. [Google Scholar] [CrossRef]
- Spoelstra, K.; van Grunsven, R.H.A.; Ramakers, J.J.C.; Ferguson, K.B.; Raap, T.; Donners, M.; Veenendaal, E.M.; Visser, M.E. Response of Bats to Light with Different Spectra: Light-Shy and Agile Bat Presence Is Affected by White and Green, but Not Red Light. Proc. R. Soc. B 2017, 284, 20170075. [Google Scholar] [CrossRef]
- Gutzwiller, K.J.; Riffell, S.K. Using Statistical Models to Study Temporal Dynamics of Animal—Landscape Relations. In Temporal Dimensions of Landscape Ecology; Bissonette, J.A., Storch, I., Eds.; Springer: New York, NY, USA, 2007; pp. 93–118. ISBN 978-0-387-45445-0. [Google Scholar]
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with r.; Springer: New York, NY, USA, 2009; ISBN 1-4419-2764-6. [Google Scholar]
- Li, H.; Wilkins, K.T. Patch or Mosaic: Bat Activity Responds to Fine-Scale Urban Heterogeneity in a Medium-Sized City in the United States. Urban Ecosyst. 2014, 17, 1013–1031. [Google Scholar] [CrossRef]
- Li, H. Urban Bats: Distribution, Roost Selection, and Foraging Site Selection. Ph.D. Thesis, Baylor University, Waco, TX, USA, 2014. [Google Scholar]
- Senawi, J.; Kingston, T. Clutter Negotiating Ability in an Ensemble of Forest Interior Bats Is Driven by Body Mass. J. Exp. Biol. 2019, 222, jeb203950. [Google Scholar] [CrossRef]
- Ammerman, L.K.; Hice, C.L.; Schmidly, D.J. Bats of Texas; Texas A&M University Press: College Station, TX, USA, 2012; ISBN 1-60344-476-9. [Google Scholar]
- Li, H.; Kalcounis-Rueppell, M. Separating the Effects of Water Quality and Urbanization on Temperate Insectivorous Bats at the Landscape Scale. Ecol. Evol. 2018, 8, 667–678. [Google Scholar] [CrossRef]
- Griffin, D.R.; Webster, F.A.; Michael, C.R. The Echolocation of Flying Insects by Bats. Anim. Behav. 1960, 8, 141–154. [Google Scholar] [CrossRef]
- Kerbiriou, C.; Barré, K.; Mariton, L.; Pauwels, J.; Zissis, G.; Robert, A.; Le Viol, I. Switching LPS to LED Streetlight May Dramatically Reduce Activity and Foraging of Bats. Diversity 2020, 12, 165. [Google Scholar] [CrossRef]
- Anthony, E.L.P.; Kunz, T.H. Feeding Strategies of the Little Brown Bat, Myotis Lucifugus, in Southern New Hampshire. Ecology 1977, 58, 775–786. [Google Scholar] [CrossRef]
- Borror, D.J.; White, R.E. A Field Guide to Insects: America North of Mexico; Houghton Mifflin: Boston, MA, USA, 1987; ISBN 0-395-18523-8. [Google Scholar]
- Borror, D.J.; Johnson, N.F.; Triplehorn, C.A. Borror and DeLong’s Introduction to the Study of Insects; Thompson Brooks/Cole: Belmont, CA, USA, 2005; ISBN 0-03-096835-6. [Google Scholar]
- Rogers, L.E.; Hinds, W.T.; Buschbom, R.L. A General Weight vs. Length Relationship for Insects. Ann. Entomol. Soc. Am. 1976, 69, 387–389. [Google Scholar] [CrossRef]
- Sage, R.D. Wet and Dry-Weight Estimates of Insects and Spiders Based on Length. Am. Midl. Nat. 1982, 108, 407–411. [Google Scholar] [CrossRef]
- Sample, B.E.; Cooper, R.J.; Greer, R.D.; Whitmore, R.C. Estimation of Insect Biomass by Length and Width. Am. Midl. Nat. 1993, 129, 234–240. [Google Scholar] [CrossRef]
- Ganihar, S.R. Biomass Estimates of Terrestrial Arthropods Based on Body Length. J. Biosci. 1997, 22, 219–224. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; ISBN 3-900051-07-0. [Google Scholar]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press U.S.: New York, NY, USA, 2002; ISBN 0-521-00976-6. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Davies, T.W.; Bennie, J.; Gaston, K.J. Street Lighting Changes the Composition of Invertebrate Communities. Biol. Lett. 2012, 8, 764–767. [Google Scholar] [CrossRef]
- Manfrin, A.; Singer, G.; Larsen, S.; Weiß, N.; van Grunsven, R.H.A.; Weiß, N.-S.; Wohlfahrt, S.; Monaghan, M.T.; Hölker, F. Artificial Light at Night Affects Organism Flux across Ecosystem Boundaries and Drives Community Structure in the Recipient Ecosystem. Front. Environ. Sci. 2017, 5, 61. [Google Scholar] [CrossRef]
- Nankoo, S.; Raymond, S.; Galvez-Cloutier, R. The Impact of the Jacques Cartier Bridge Illumination on the Food Chain: From Insects to Predators. Community Ecol. 2019, 20, 172–180. [Google Scholar] [CrossRef]
- McMunn, M.S.; Yang, L.H.; Ansalmo, A.; Bucknam, K.; Claret, M.; Clay, C.; Cox, K.; Dungey, D.R.; Jones, A.; Kim, A.Y.; et al. Artificial Light Increases Local Predator Abundance, Predation Rates, and Herbivory. Environ. Entomol. 2019, 48, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.A.; Ginzburg, L.R. The Nature of Predation: Prey Dependent, Ratio Dependent or Neither? Trends Ecol. Evol. 2000, 15, 337–341. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, T.; Yuan, S. Dynamics of a Stochastic Predator-Prey Model with Habitat Complexity and Prey Aggregation. Ecol. Complex. 2021, 45, 100889. [Google Scholar] [CrossRef]
- Jung, K.; Threlfall, C.G. Trait-Dependent Tolerance of Bats to Urbanization: A Global Meta-Analysis. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181222. [Google Scholar] [CrossRef]
- Schnitzler, H.-U.; Moss, C.F.; Denzinger, A. From Spatial Orientation to Food Acquisition in Echolocating Bats. Trends Ecol. Evol. 2003, 18, 386–394. [Google Scholar] [CrossRef]
- Canion, C.R.; Heck, K. Effect of Habitat Complexity on Predation Success: Re-Evaluating the Current Paradigm in Seagrass Beds. Mar. Ecol. Prog. Ser. 2009, 393, 37–46. [Google Scholar] [CrossRef]
- Brigham, R.M.; Grindal, S.D.; Firman, M.C.; Morissette, J.L. The Influence of Structural Clutter on Activity Patterns of Insectivorous Bats. Can. J. Zool. 1997, 75, 131–136. [Google Scholar] [CrossRef]
- Lintott, P.R.; Fuentes-Montemayor, E.; Goulson, D.; Park, K.J. Testing the Effectiveness of Surveying Techniques in Determining Bat Community Composition within Woodland. Wildl. Res. 2013, 40, 675–684. [Google Scholar] [CrossRef]
- Reichert, B.E.; Bayless, M.; Cheng, T.L.; Coleman, J.T.H.; Francis, C.M.; Frick, W.F.; Gotthold, B.S.; Irvine, K.M.; Lausen, C.; Li, H.; et al. NABat: A Top-down, Bottom-up Solution to Collaborative Continental-Scale Monitoring. Ambio 2021, 50, 901–913. [Google Scholar] [CrossRef]
- Springall, B.T.; Li, H.; Kalcounis-Rueppell, M.C. The In-Flight Social Calls of Insectivorous Bats: Species Specific Behaviors and Contexts of Social Call Production. Front. Ecol. Evol. 2019, 7, 441. [Google Scholar] [CrossRef]
- Li, H.; Petric, R.; Alazzawi, Z.; Kauzlarich, J.; Mahmoud, R.H.; McFadden, R.; Perslow, N.; Flores, A.R.; Soufi, H.; Morales, K.; et al. Four Years Continuous Monitoring Reveals Different Effects of Urban Constructed Wetlands on Bats. Land 2021, 10, 1087. [Google Scholar] [CrossRef]
- Corcoran, A.J.; Conner, W.E. Bats Jamming Bats: Food Competition through Sonar Interference. Science 2014, 346, 745–747. [Google Scholar] [CrossRef]
- Salinas-Ramos, V.B.; Ancillotto, L.; Bosso, L.; Sánchez-Cordero, V.; Russo, D. Interspecific Competition in Bats: State of Knowledge and Research Challenges. Mammal Rev. 2020, 50, 68–81. [Google Scholar] [CrossRef]
- Orbach, D.N.; Fenton, B. Vision Impairs the Abilities of Bats to Avoid Colliding with Stationary Obstacles. PLoS ONE 2010, 5, e13912. [Google Scholar] [CrossRef]
- Chiu, C.; Xian, W.; Moss, C.F. Flying in Silence: Echolocating Bats Cease Vocalizing to Avoid Sonar Jamming. Proc. Natl. Acad. Sci. USA 2008, 105, 13116–13121. [Google Scholar] [CrossRef]
- Corcoran, A.J.; Weller, T.J.; Hopkins, A.; Yovel, Y. Silence and Reduced Echolocation during Flight Are Associated with Social Behaviors in Male Hoary Bats (Lasiurus cinereus). Sci. Rep. 2021, 11, 18637. [Google Scholar] [CrossRef]
- Whitby, M.D.; Kieran, T.J.; Glenn, T.C.; Allen, C. Agricultural Pests Consumed by Common Bat Species in the United States Corn Belt: The Importance of DNA Primer Choice. Agric. Ecosyst. Environ. 2020, 303, 107105. [Google Scholar] [CrossRef]
- Wray, A.K.; Peery, M.Z.; Jusino, M.A.; Kochanski, J.M.; Banik, M.T.; Palmer, J.M.; Lindner, D.L.; Gratton, C. Predator Preferences Shape the Diets of Arthropodivorous Bats More than Quantitative Local Prey Abundance. Mol. Ecol. 2021, 30, 855–873. [Google Scholar] [CrossRef]
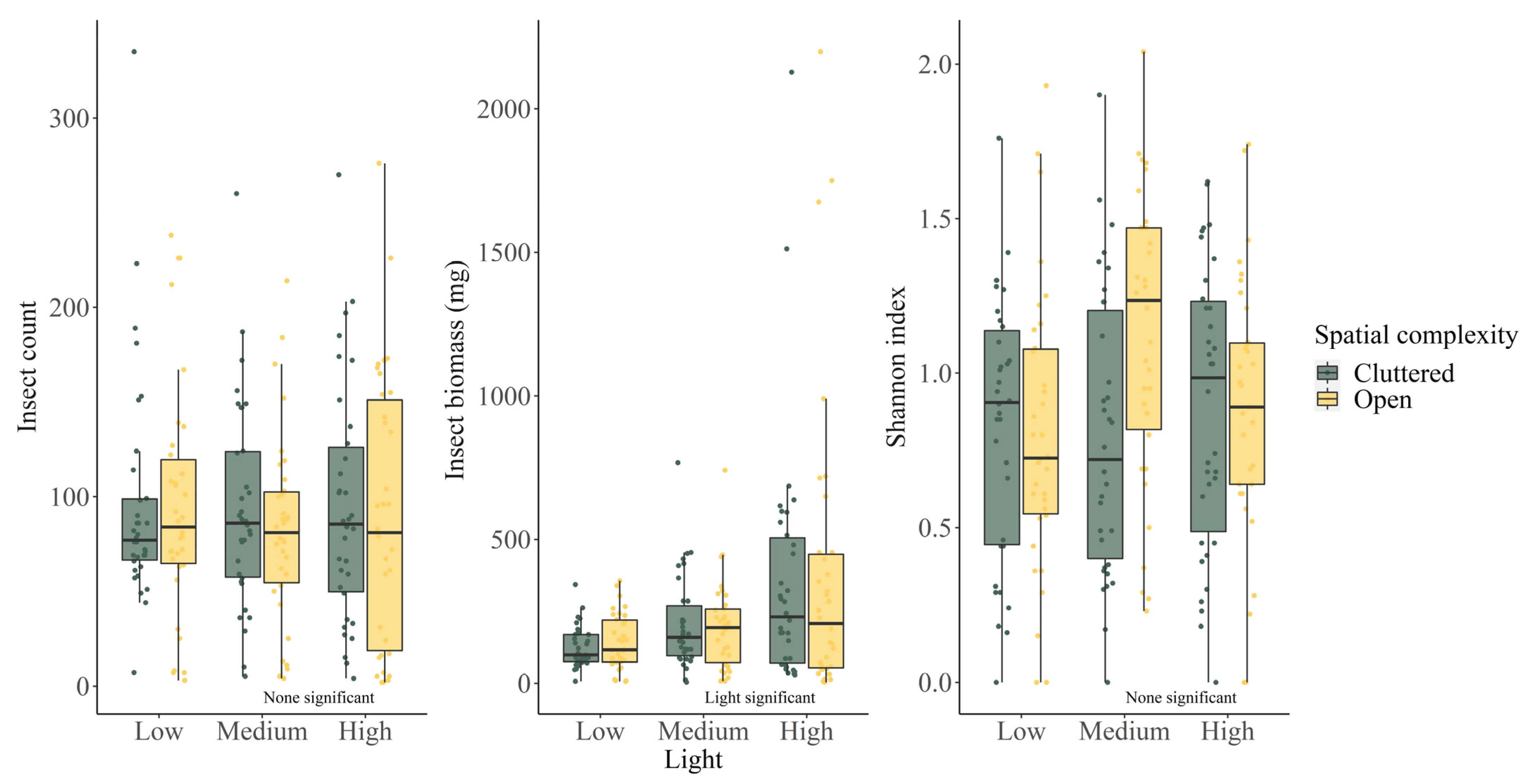
| Variable | Insect Count | Shannon Index | Insect Biomass |
|---|---|---|---|
| Total | 18,822 | N/A | 45,776.5 (mg) |
| ALAN medium | −0.061 ± 0.106 | 0.184 ± 0.097 | 0.440 ± 0.185 |
| 0.565 | 0.072 | 0.018 | |
| ALAN high | −0.000 ± 0.105 | 0.114 ± 0.009 | 1.084 ± 0.167 |
| 0.996 | 0.160 | <0.001 | |
| Open | 0.005 ± 0.087 | 0.166 ± 0.065 | 0.188 ± 0.125 |
| 0.953 | 0.115 | 0.135 | |
| ALAN medium × Open | N/A | N/A | N/A |
| ALAN high × Open | N/A | N/A | N/A |
| Temperature | 0.039 ± 0.006 | 0.031 ± 0.004 | 0.068 ± 0.009 |
| <0.001 | <0.001 | <0.001 |
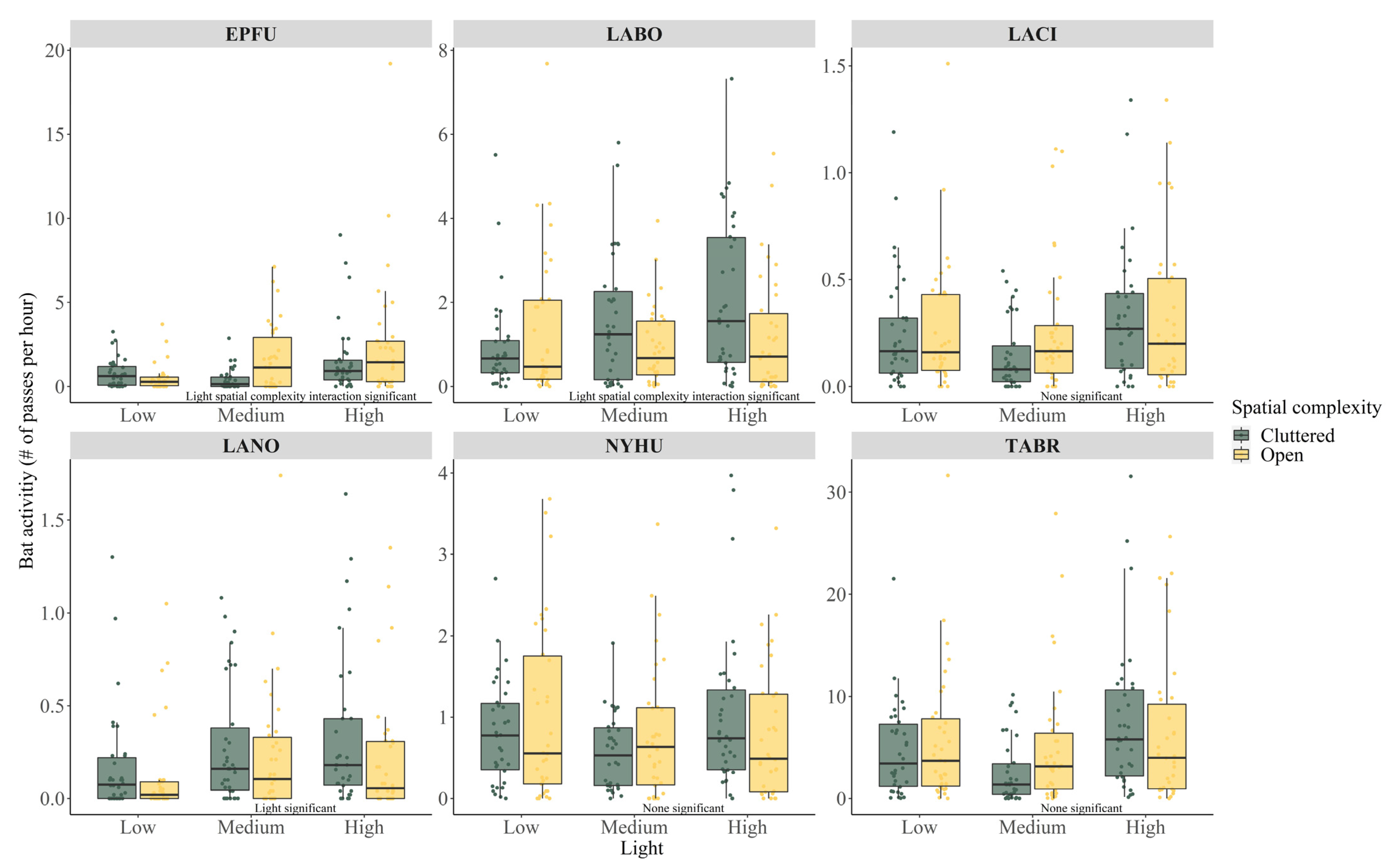
| Variable | EPFU | LABO | LACI | LANO | NYHU | TABR |
|---|---|---|---|---|---|---|
| Total passes identified | 11,608 | 18,223 | 3802 | 2473 | 12,229 | 70,318 |
| ALAN medium | −0.598 ± 0.499 | 0.509 ± 0.258 | −0.266 ± 0.199 | 0.562 ± 0.274 | −0.274 ± 0.163 | −0.296 ±0.209 |
| 0.232 | 0.047 | 0.183 | 0.042 | 0.095 | 0.159 | |
| ALAN high | 0.739 ± 0.361 | 0.749 ± 0.246 | 0.172 ± 0.178 | 0.640 ± 0.270 | 0.025 ± 0.151 | 0.284 ± 0.181 |
| 0.042 | 0.003 | 0.334 | 0.019 | 0.865 | 0.119 | |
| Open | −0.419 ± 0.478 | 0.375 ± 0265 | 0.182 ± 0.153 | −0.335 ± 0.205 | 0.159 ± 0.130 | 0.162 ± 0.156 |
| 0.376 | 0.158 | 0.235 | 0.105 | 0.222 | 0.302 | |
| ALAN medium × Open | 1.781 ± 0.651 | −0.819 ± 0.370 | N/A | N/A | N/A | N/A |
| 0.007 | 0.028 | |||||
| ALAN high × Open | 0.849 ± 0.541 | −0.771 ± 0.352 | N/A | N/A | N/A | N/A |
| 0.118 | 0.030 | |||||
| Temperature | 0.009 ± 0.014 | 0.052 ± 0.010 | −0.006 ± 0.010 | −0.013 ± 0.014 | 0.043 ± 0.009 | 0.005 ± 0.010 |
| 0.521 | <0.001 | 0.541 | 0.352 | <0.001 | 0.610 |
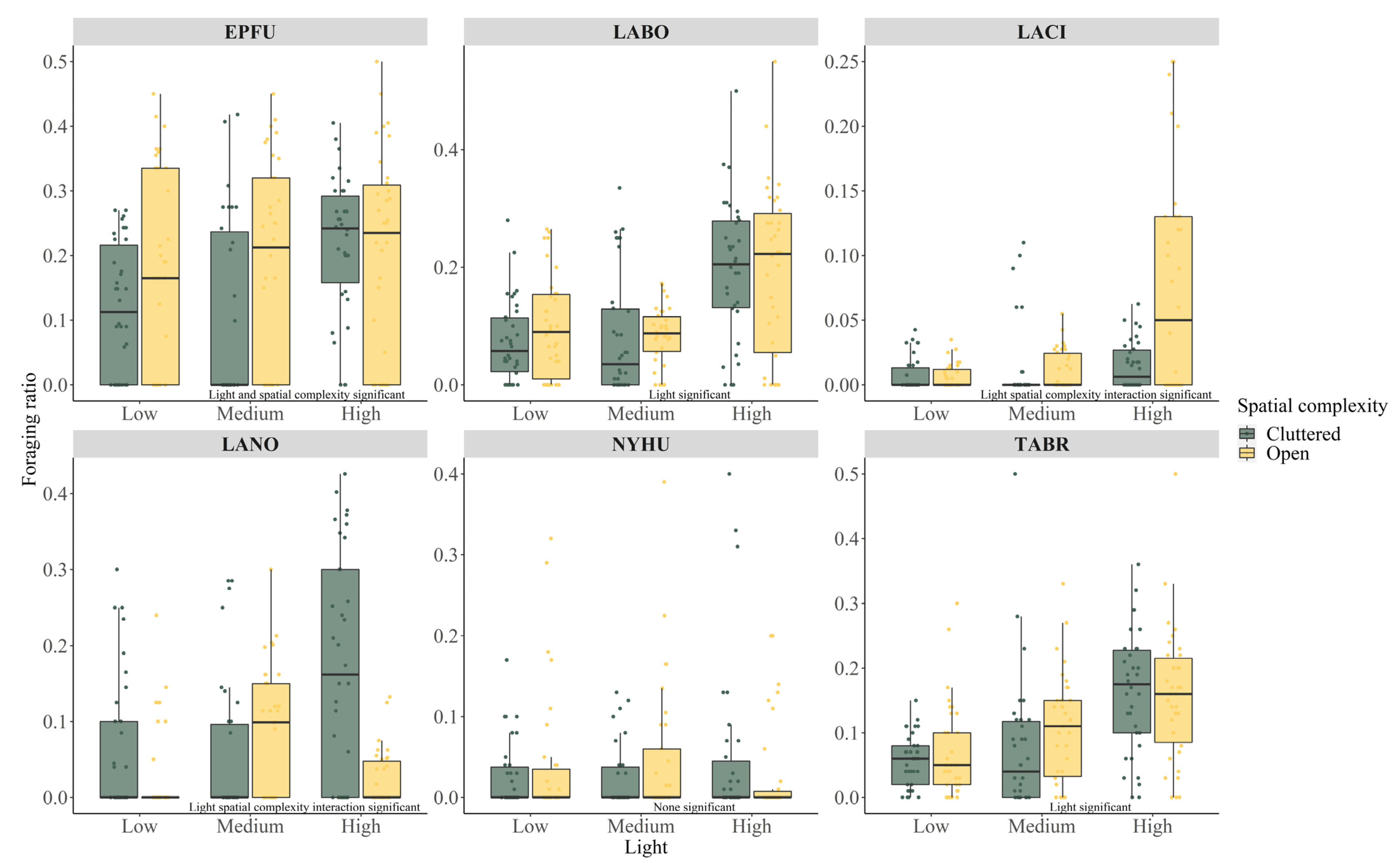
| Variable | EPFU | LABO | LACI | LANO | NYHU | TABR |
|---|---|---|---|---|---|---|
| Total foraing passes | 1920 | 2212 | 81 | 175 | 438 | 7352 |
| ALAN medium | −0.028 ± 0.187 | −0.037 ± 0.187 | 0.551 ±0.520 | −0.154 ±0.373 | −0.309 ± 0.377 | 0.382 ± 0.184 |
| 0.880 | 0.845 | 0.290 | 0.680 | 0.413 | 0.039 | |
| ALAN high | 0.445 ± 0.174 | 0.951 ± 0.160 | 0.701 ± 0.507 | 1.163 ± 0.302 | 0.063 ± 0.345 | 1.014 ± 0.168 |
| 0.012 | <0.001 | 0.168 | <0.001 | 0.855 | <0.001 | |
| Open | 0.296 ± 0.145 | 0.066 ± 0.131 | −0.149 ± 0.607 | −0.750 ± 0.440 | 0.263 ± 0.297 | 0.098 ± 0.131 |
| 0.043 | 0.617 | 0.807 | 0.099 | 0.377 | 0.457 | |
| ALAN medium × Open | N/A | N/A | 0.007 ± 0.763 | 1.263 ± 0.562 | N/A | N/A |
| 0.993 | 0.026 | |||||
| ALAN high × Open | N/A | N/A | 1.817 ± 0.687 | −1.403 ± 0.618 | N/A | N/A |
| 0.009 | 0.024 | |||||
| Temperature | 0.000 ± 0.009 | −0.007 ± 0.010 | −0.018 ± 0.013 | 0.009 ± 0.013 | 0.079 ± 0.022 | −0.025 ± 0.009 |
| 0.981 | 0.424 | 0.179 | 0.519 | <0.001 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wilkins, K.T. Predator-Prey Relationship between Urban Bats and Insects Impacted by Both Artificial Light at Night and Spatial Clutter. Biology 2022, 11, 829. https://doi.org/10.3390/biology11060829
Li H, Wilkins KT. Predator-Prey Relationship between Urban Bats and Insects Impacted by Both Artificial Light at Night and Spatial Clutter. Biology. 2022; 11(6):829. https://doi.org/10.3390/biology11060829
Chicago/Turabian StyleLi, Han, and Kenneth T. Wilkins. 2022. "Predator-Prey Relationship between Urban Bats and Insects Impacted by Both Artificial Light at Night and Spatial Clutter" Biology 11, no. 6: 829. https://doi.org/10.3390/biology11060829
APA StyleLi, H., & Wilkins, K. T. (2022). Predator-Prey Relationship between Urban Bats and Insects Impacted by Both Artificial Light at Night and Spatial Clutter. Biology, 11(6), 829. https://doi.org/10.3390/biology11060829







