In Vitro Anthelmintic Activity of Sea Buckthorn (Hippophae rhamnoides) Berry Juice against Gastrointestinal Nematodes of Small Ruminants
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Determination of Total Polyphenol Content
2.4. HPLC and HPLC-MS Analyses
2.5. Recovery and Suspension of GIN Eggs and Third-Stage Larvae (L3), and Identification of GIN L3 at the Genus/Species Level
2.6. In Vitro Tests: Egg-Hatch Test (EHT) and Larval-Exsheathment Inhibition Assay (LEIA)
2.7. Addition of Polyvinylpolypyrrolidone (PVPP) to the Extracts
2.8. Statistical Analysis
3. Results
3.1. Chemical Analysis
3.1.1. Total Polyphenols Content (TPC)
3.1.2. HPLC and HPLC-MS Analyses
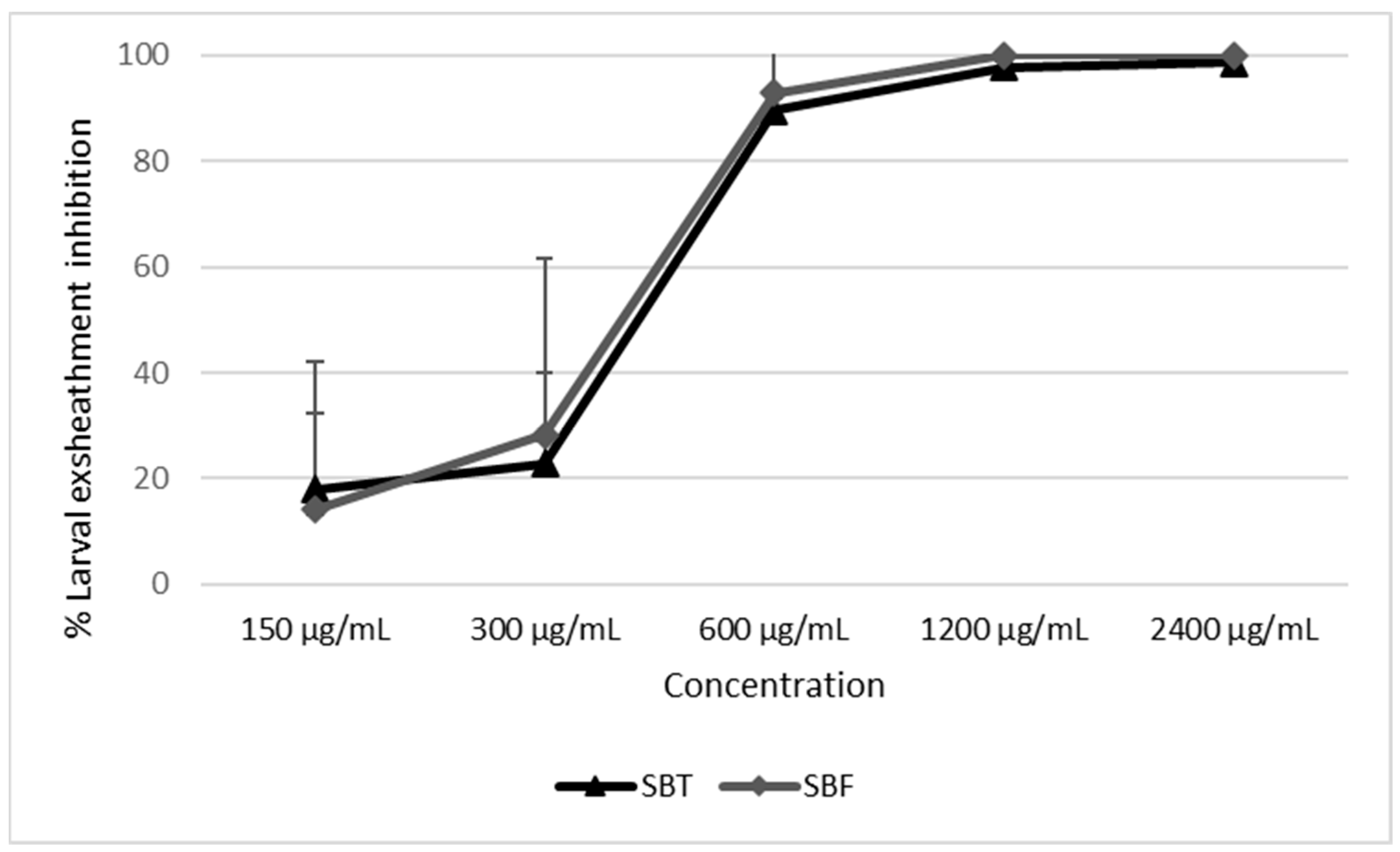
3.2. Egg-Hatch Test (EHT) and Larval-Exsheathment Inhibition Assay (LEIA)
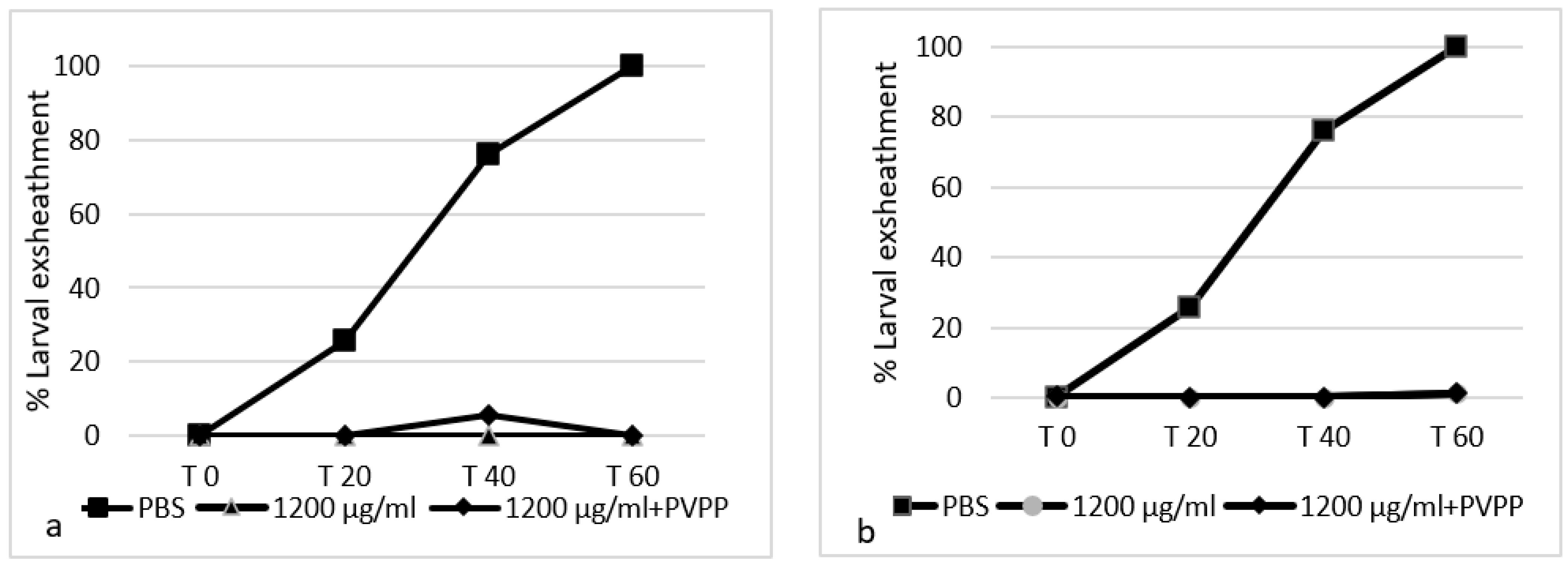
3.3. Effect of the Addition of Polyvinylpolypyrrolidone (PVPP) Treatment on Extracts’ Efficacy
3.4. GIN Third-Stage Larvae (L3) Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacobson, C.; Larsen, J.W.; Besier, R.B.; Lloyd, J.B.; Kahn, L.P. Diarrhoea associated with gastrointestinal parasites in grazing sheep. Vet. Parasitol. 2020, 282, 109–139. [Google Scholar] [CrossRef] [PubMed]
- Ikurior, S.J.; Pomroy, W.E.; Scott, I.; Corner-Thomas, R.; Marquetoux, N.; Leu, S.T. Gastrointestinal nematode infection affects overall activity in young sheep monitored with tri-axial accelerometers. Vet. Parasitol. 2020, 283, 109188. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Iqbal, Z.; Roohi, N. Ovine haemonchosis: A review. Trop. Anim. Health Prod. 2020, 53, 19. [Google Scholar] [CrossRef] [PubMed]
- Charlier, J.; Velde, F.V.; van der Voort, M.; Van Meensel, J.; Lauwers, L.; Cauberghe, V.; Vercruysse, J.; Claerebout, E. ECONOHEALTH: Placing helminth infections of livestock in an economic and social context. Vet. Parasitol. 2015, 212, 62–67. [Google Scholar] [CrossRef]
- Charlier, J.; Rinaldi, L.; Musella, V.; Ploeger, H.W.; Chartier, C.; Vineer, H.R.; Hinney, B.; von Samson-Himmelstjerna, G.; Băcescu, B.; Mickiewicz, M.; et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev. Vet. Med. 2020, 182, 105103. [Google Scholar] [CrossRef]
- Wolstenholme, A.J.; Martin, R.J. Anthelmintics–from discovery to resistance. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 218–219. [Google Scholar] [CrossRef][Green Version]
- Rose, H.; Wang, T.; van Dijk, J.; Morgan, E.R. GLOWORM-FL: A simulation model of the effects of climate and climate change on the free-living stages of gastro-intestinal nematode parasites of ruminants. Ecol. Modell. 2015, 297, 232–245. [Google Scholar] [CrossRef]
- Kaplan, R.M. Biology, Epidemiology, Diagnosis, and Management of Anthelmintic Resistance in Gastrointestinal Nematodes of Livestock. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 17–30. [Google Scholar] [CrossRef]
- Machado Fernandes, M.A.; Gilaverte, S.; Bianchi, M.D.; da Silva, C.J.A.; Molento, M.B.; Reyes, F.G.R.; Monteiro, A.L.G. Moxidectin residues in tissues of lambs submitted to three endoparasite control programs. Res. Vet. Sci. 2017, 114, 406–411. [Google Scholar] [CrossRef]
- Vokřál, I.; Michaela, Š.; Radka, P.; Jiří, L.; Lukáš, P.; Dominika, S.; Kateřina, L.; Barbora, S.; Lenka, S. Ivermectin environmental impact: Excretion profile in sheep and phytotoxic effect in Sinapis alba. Ecotoxicol. Environ. Saf. 2019, 169, 944–949. [Google Scholar] [CrossRef]
- Mooney, D.; Richards, K.G.; Danaher, M.; Grant, J.; Gill, L.; Mellander, P.E.; Coxon, C.E. An analysis of the spatio-temporal occurrence of anthelmintic veterinary drug residues in groundwater. Sci. Total Environ. 2021, 769, 144804. [Google Scholar] [CrossRef] [PubMed]
- Lazaroiu, G.; Andronie, M.; Uţă, C.; Hurloiu, I. Trust Management in Organic Agriculture: Sustainable Consumption Behavior, Environmentally Conscious Purchase Intention, and Healthy Food Choices. Front. Public Health 2019, 19, 340. [Google Scholar] [CrossRef] [PubMed]
- Śmiglak-Krajewska, M.; Wojciechowska-Solis, J. Consumers versus organic products in the COVID-19 pandemic: Opportunities and barriers to market development. Energies 2021, 14, 5566. [Google Scholar] [CrossRef]
- Hoste, H.; Martinez-Ortiz-De-Montellano, C.; Manolaraki, F.; Brunet, S.; Ojeda-Robertos, N.; Fourquaux, I.; Torres-Acosta, J.F.; Sandoval-Castro, C.A. Direct and indirect effects of bioactive tannin-rich tropical and temperate legumes against nematode infections. Vet. Parasitol. 2012, 186, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Romero-Benavides, J.C.; Ruano, A.L.; Silva-Rivas, R.; Castillo-Veintimilla, P.; Vivanco-Jaramillo, S.; Bailon-Moscoso, N. Medicinal plants used as anthelmintics: Ethnomedical, pharmacological, and phytochemical studies. Eur. J. Med. Chem. 2017, 129, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Spiegler, V.; Liebau, E.; Hensel, A. Medicinal plant extracts and plant-derived polyphenols with anthelmintic activity against intestinal nematodes. Nat. Prod. Rep. 2017, 34, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Borges, D.G.L.; Echeverria, J.T.; de Oliveira, T.L.; Heckler, R.P.; de Freitas, M.G.; Damasceno-Junior, G.A.; Carollo, C.A.; Borges, F.A. Discovery of potential ovicidal natural products using metabolomics. PLoS ONE 2019, 25, 14. [Google Scholar] [CrossRef]
- Ciesarová, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolková, B.; Koplík, R.; Belajová, E.; Kukurová, K.; Daško, Ľ.; Panovská, Z.; et al. Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef]
- Pundir, S.; Garg, P.; Dviwedi, A.; Ali, A.; Kapoor, V.K.; Kapoor, D.; Kulshrestha, S.; Lal, U.R.; Negi, P. Ethnomedicinal uses, phytochemistry and dermatological effects of Hippophae rhamnoides L.: A review. J. Ethnopharmacol. 2021, 266, 113434. [Google Scholar] [CrossRef]
- Azienda Agricola San Mario. Available online: https://olivello.it/2018/07/10/olivello-spinoso/ (accessed on 1 January 2021).
- Hou, D.D.; Di, Z.H.; Qi, R.Q.; Wang, H.X.; Zheng, S.; Hong, Y.X.; Guo, H.; Chen, H.D.; Gao, X.H. Sea Buckthorn (Hippophaë rhamnoides L.) Oil Improves Atopic Dermatitis-Like Skin Lesions via Inhibition of NF-κB and STAT1 Activation. Skin Pharmacol. Physiol. 2017, 30, 268–276. [Google Scholar] [CrossRef]
- Olas, B.; Kontek, B.; Malinowska, P.; Żuchowski, J.; Stochmal, A. Hippophae rhamnoides L. Fruits Reduce the Oxidative Stress in Human Blood Platelets and Plasma. Oxid. Med. Cell. Longev. 2016, 2016, 4692486. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S.; Negi, P.S.; Ramteke, R.S. Antioxidant and antibacterial activities of aqueous extract of Seabuckthorn (Hippophae rhamnoides) seeds. Fitoterapia 2007, 78, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hao, Q.; Yuan, F.; Gao, Y. Nonenzymatic browning criteria to sea buckthorn juice during thermal processing. J. Food Process Eng. 2015, 38, 67–75. [Google Scholar] [CrossRef]
- Zielińska, A.; Nowak, I. Abundance of active ingredients in sea-buckthorn oil. Lipids Health Dis. 2017, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Doner, L.W.; Becard, G.; Irwin, P.L. Binding of flavonoids by polyvinylpolypyrrolidone. J. Agric. Food Chem. 1993, 41, 753–757. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Taylor, M.A.; Coop, R.L.; Wall, R.L. Veterinary Parasitology, 3rd ed.; Blackwell Publishing Ltd.: Oxford, UK, 2007. [Google Scholar]
- Hubert, J.; Kerboeuf, D. A microlarval development assay for the detection of anthelmintic resistance in sheep nematodes. Vet. Rec. 1992, 130, 442–446. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Fisheries and Food (MAFF). Manual of Veterinary Parasitological Laboratory Techniques; Her Majesty’s Stationary Office (HMSO): London, UK, 1986; pp. 1–152.
- van Wyk, J.A.; Mayhew, E. Morphological identification of parasitic nematode infective larvae of small ruminants and cattle: A practical lab guide. Onderstepoort J. Vet. Res. 2013, 80, 539. [Google Scholar] [CrossRef]
- Coles, G.C.; Jackson, F.; Pomroy, W.E.; Prichard, R.K.; von Samson-Himmelstjerna, G.; Silvestre, A.; Taylor, M.A.; Vercruysse, J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 2006, 136, 167–185. [Google Scholar] [CrossRef]
- Jackson, F.; Hoste, H. In Vitro methods for the primary screening of plant products for direct activity against ruminant gastrointestinal nematodes. In In vitro Screening of Plant Resources for Extra-Nutritional Attributes in Ruminants: Nuclear and Related Methodologies; Springer: Dordrecht, South Africa, 2010; pp. 25–45. [Google Scholar]
- Oliveira, M.; Lima, C.S.; Ketavong, S.; Llorent-Martínez, E.J.; Hoste, H.; Custódio, L. Disclosing the bioactive metabolites involved in the in vitro anthelmintic effects of salt-tolerant plants through a combined approach using PVPP and HPLC-ESI-MSn. Sci. Rep. 2021, 11, 24303. [Google Scholar] [CrossRef]
- Chan-Pérez, J.I.; Torres-Acosta, J.F.; Sandoval-Castro, C.A.; Hoste, H.; Castañeda-Ramírez, G.S.; Vilarem, G.; Mathieu, C. In vitro susceptibility of ten Haemonchus contortus isolates from different geographical origins towards acetone:water extracts of two tannin rich plants. Vet. Parasitol. 2016, 217, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.L.; Russell, R.M.; Savin, N.E. POLO: A User’s Guide to Probit or Logit Analysis; General Technical Report PSW-038; Department of Agriculture, Forest Service, Pacific Southwest Research Station: Berkeley, CA, USA, 1980; p. 15. [Google Scholar] [CrossRef]
- Roditakis, E.; Roditakis, N.E.; Tsagkarakou, A. Insecticide resistance in Bemisia tabaci (Homoptera: Aleyrodidae) populations from Crete. Pest. Manag. Sci. 2005, 61, 577–582. [Google Scholar] [CrossRef]
- Jeske, D.R.; Xu, H.K.; Blessinger, T.; Jensen, P.; Trumble, J. Testing for the equality of EC50 values in the presence of unequal slopes with application to toxicity of selenium types. J. Agric. Biol. Environ. Stat. 2009, 14, 469–483. [Google Scholar] [CrossRef][Green Version]
- Guliyev, V.B.; Gul, M.; Yildirim, A. Hippophae rhamnoides L.: Chromatographic methods to determine chemical composition, use in traditional medicine and pharmacological effects. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 812, 291–307. [Google Scholar] [CrossRef]
- Khan, B.A.; Akhtar, N.; Mahmood, T. A comprehensive review of a magic plant, Hippophae rhamnoides. Pharmacogn. J. 2010, 2, 65–68. [Google Scholar] [CrossRef]
- Michel, T.; Destandau, E.; Le Floch, G.; Lucchesi, M.E.; Elfakir, C. Antimicrobial, antioxidant and phytochemical investigations of sea buckthorn (Hippophaë rhamnoides L.) leaf, stem, root and seed. Food Chem. 2012, 131, 754–760. [Google Scholar] [CrossRef]
- Barrau, E.; Fabre, N.; Fouraste, I.; Hoste, H. Effect of bioactive compounds from Sainfoin (Onobrychis viciifolia Scop.) on the in vitro larval migration of Haemonchus contortus: Role of tannins and flavonol glycosides. Parasitology 2005, 131, 531–538. [Google Scholar] [CrossRef]
- Delgado-Núñez, E.J.; Zamilpa, A.; González-Cortazar, M.; Olmedo-Juárez, A.; Cardoso-Taketa, A.; Sánchez-Mendoza, E.; Tapia-Maruri, D.; Salinas-Sánchez, D.O.; Mendoza-de Gives, P. Isorhamnetin: A Nematocidal Flavonoid from Prosopis laevigata Leaves Against Haemonchus contortus Eggs and Larvae. Biomolecules 2020, 10, 773. [Google Scholar] [CrossRef]
- Hertzberg, H.; Huwyler, U.; Kohle, L.; Rehbein, S.; Wanner, M. Kinetics of exsheathment of infective ovine and bovine strongylid larvae in vivo and in vitro. Parasitology 2002, 125, 65–70. [Google Scholar] [CrossRef]
- Zajac, A.M.; Garza, J. Biology, Epidemiology, and Control of Gastrointestinal Nematodes of Small Ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 73–87. [Google Scholar] [CrossRef]
- Alonso-Díaz, M.A.; Torres-Acosta, J.F.; Sandoval-Castro, C.A.; Hoste, H. Comparing the sensitivity of two in vitro assays to evaluate the anthelmintic activity of tropical tannin rich plant extracts against Haemonchus contortus. Vet. Parasitol. 2011, 181, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Azando, E.V.; Hounzangbé-Adoté, M.S.; Olounladé, P.A.; Brunet, S.; Fabre, N.; Valentin, A.; Hoste, H. Involvement of tannins and flavonoids in the in vitro effects of Newbouldia laevis and Zanthoxylum zanthoxyloïdes extracts on the exsheathment of third-stage infective larvae of gastrointestinal nematodes. Vet. Parasitol. 2011, 180, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Mkandawire, T.T.; Grencis, R.K.; Berriman, M.; Duque-Correa, M.A. Hatching of parasitic nematode eggs: A crucial step determining infection. Trends Parasitol. 2022, 38, 174–187. [Google Scholar] [CrossRef]
- Vercruysse, J.; Holdsworth, P.; Letonja, T.; Barth, D.; Conder, G.; Hamamoto, K.; Okano, K. World Organization for Animal Health. International harmonisation of anthelmintic efficacy guidelines. Vet. Parasitol. 2001, 96, 171–193. [Google Scholar] [CrossRef]
- Sebai, E.; Serairi, R.; Saratsi, K.; Abidi, A.; Sendi, N.; Darghouth, M.A.; Wilson, M.S.; Sotiraki, S.; Akkari, H. Hydro-Ethanolic Extract of Mentha pulegium Exhibit Anthelmintic and Antioxidant Proprieties In Vitro and In Vivo. Acta Parasitol. 2020, 65, 375–387. [Google Scholar] [CrossRef]
- Akkari, H.; Rtibi, K.; B’chir, F.; Rekik, M.; Darghouth, M.A.; Gharbi, M. In vitro evidence that the pastoral Artemisia campestris species exerts an anthelmintic effect on Haemonchus contortus from sheep. Vet. Res. Commun. 2014, 38, 249–255. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, L.M.; Bevilaqua, C.M.; Macedo, I.T.; de Morais, S.M.; Machado, L.K.; Campello, C.C.; de Aquino Mesquita, M. Effects of Myracrodruon urundeuva extracts on egg hatching and larval exsheathment of Haemonchus contortus. Parasitol. Res. 2011, 109, 893–898. [Google Scholar] [CrossRef]
- Forzato, C.; Vida, V.; Berti, F. Biosensors and Sensing Systems for Rapid Analysis of Phenolic Compounds from Plants: A Comprehensive Review. Biosensors 2020, 10, 105. [Google Scholar] [CrossRef]
- Nowak, D.; Gośliński, M.; Wojtowicz, E.; Przygoński, K. Antioxidant Properties and Phenolic Compounds of Vitamin C-Rich Juices. J. Food Sci. 2018, 83, 2237–2246. [Google Scholar] [CrossRef]
- Pop, R.M.; Socaciu, C.; Pintea, A.; Buzoianu, A.D.; Sanders, M.G.; Gruppen, H.; Vincken, J.P. UHPLC/PDA-ESI/MS analysis of the main berry and leaf flavonol glycosides from different Carpathian Hippophaë rhamnoides L. varieties. Phytochem. Anal. 2013, 24, 484–492. [Google Scholar] [CrossRef]
- Ma, X.; Laaksonen, O.; Zheng, J.; Yang, W.; Trépanier, M.; Kallio, H.; Yang, B. Flavonol glycosides in berries of two major subspecies of sea buckthorn (Hippophae rhamnoides L.) and influence of growth sites. Food Chem. 2016, 200, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Heinäaho, M.; Pusenius, J.; Julkunen-Tiitto, R. Effects of different organic farming methods on the concentration of phenolic compounds in sea buckthorn leaves. J. Agric. Food Chem. 2006, 54, 7678–7685. [Google Scholar] [CrossRef] [PubMed]
- Cosmulescu, S.; Trandafir, I.; Nour, V. Phenolic acids and flavonoids profiles of extracts from edible wild fruits and their antioxidant properties. Int. J. Food Prop. 2017, 20, 3124–3134. [Google Scholar] [CrossRef]
- Guo, R.; Guo, X.; Li, T.; Fu, X.; Liu, R.H. Comparative assessment of phytochemical profiles, antioxidant and antiproliferative activities of Sea buckthorn (Hippophaë rhamnoides L.) berries. Food Chem. 2017, 221, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Teleszko, M.; Wojdyło, A.; Rudzińska, M.; Oszmiański, J.; Golis, T. Analysis of Lipophilic and Hydrophilic Bioactive Compounds Content in Sea Buckthorn (Hippophaë rhamnoides L.) Berries. J. Agric. Food Chem. 2015, 63, 4120–4129. [Google Scholar] [CrossRef]
- Heinäaho, M.; Hagerman, A.E.; Julkunen-Tiitto, R. Effect of different organic farming methods on the phenolic composition of sea buckthorn berries. J. Agric. Food Chem. 2009, 57, 1940–1947. [Google Scholar] [CrossRef]
- Fatima, T.; Kesari, V.; Watt, I.; Wishart, D.; Todd, J.F.; Schroeder, W.R.; Paliyath, G.; Krishna, P. Metabolite profiling and expression analysis of flavonoid, vitamin C and tocopherol biosynthesis genes in the antioxidant-rich sea buckthorn (Hippophae rhamnoides L.). Phytochemistry 2015, 118, 181–191. [Google Scholar] [CrossRef]
- Giovanelli, F.; Mattellini, M.; Fichi, G.; Flamini, G.; Perrucci, S. In Vitro Anthelmintic Activity of Four Plant-Derived Compounds against Sheep Gastrointestinal Nematodes. Vet. Sci. 2018, 5, 78. [Google Scholar] [CrossRef]
- Kozan, E.; Anul, S.A.; Tatli, I.I. In vitro anthelmintic effect of Vicia pannonica var. purpurascens on trichostrongylosis in sheep. Exp. Parasitol. 2013, 134, 299–303. [Google Scholar] [CrossRef]
- Klongsiriwet, C.; Quijada, J.; Williams, A.R.; Mueller-Harvey, I.; Williamson, E.M.; Hoste, H. Synergistic inhibition of Haemonchus contortus exsheathment by flavonoid monomers and condensed tannins. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 127–134. [Google Scholar] [CrossRef]
- Verza, S.G.; Pavel, C.; Ortega, G.G. Study of the Specificity of Cross-Povidone (PVPP) as binding agent in the quantification of polyphenolic compounds. J. Braz. Chem. Soc. 2008, 19, 1627–1633. [Google Scholar] [CrossRef][Green Version]
- Manolaraki, F.; Sotiraki, S.; Stefanakis, A.; Skampardonis, V.; Volanis, M.; Hoste, H. Anthelmintic activity of some Mediterranean browse plants against parasitic nematodes. Parasitology 2010, 137, 685–696. [Google Scholar] [CrossRef]
- Grünz, G. Structure-Activity Relationship of Selected Flavonoids on Aging and Stress-Resistance in Caenorhabditis elegans. Ph.D. Thesis, Technische Universität München, Munich, Germany, 2012. [Google Scholar]
- Santos, A.C.V.; Santos, F.O.; Lima, H.G.; Silva, G.D.D.; Uzêda, R.S.; Dias, Ê.R.; Branco, A.; Cardoso, K.V.; David, J.M.; Botura, M.B.; et al. In vitro ovicidal and larvicidal activities of some saponins and flavonoids against parasitic nematodes of goats. Parasitology 2018, 145, 1884–1889. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Surco-Laos, F.; González-Manzano, S.; González-Paramás, A.M.; Gómez-Orte, E.; Cabello, J.; Santos-Buelga, C. Deglycosylation is a key step in biotransformation and lifespan effects of quercetin-3-O-glucoside in Caenorhabditis elegans. Pharmacol. Res. 2013, 76, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Van Binh, D.; Ørskov, E.R. Effect of foliages containing condensed tannins and on gastrointestinal parasites. Anim. Feed Sci. Technol. 2005, 121, 77–87. [Google Scholar] [CrossRef]
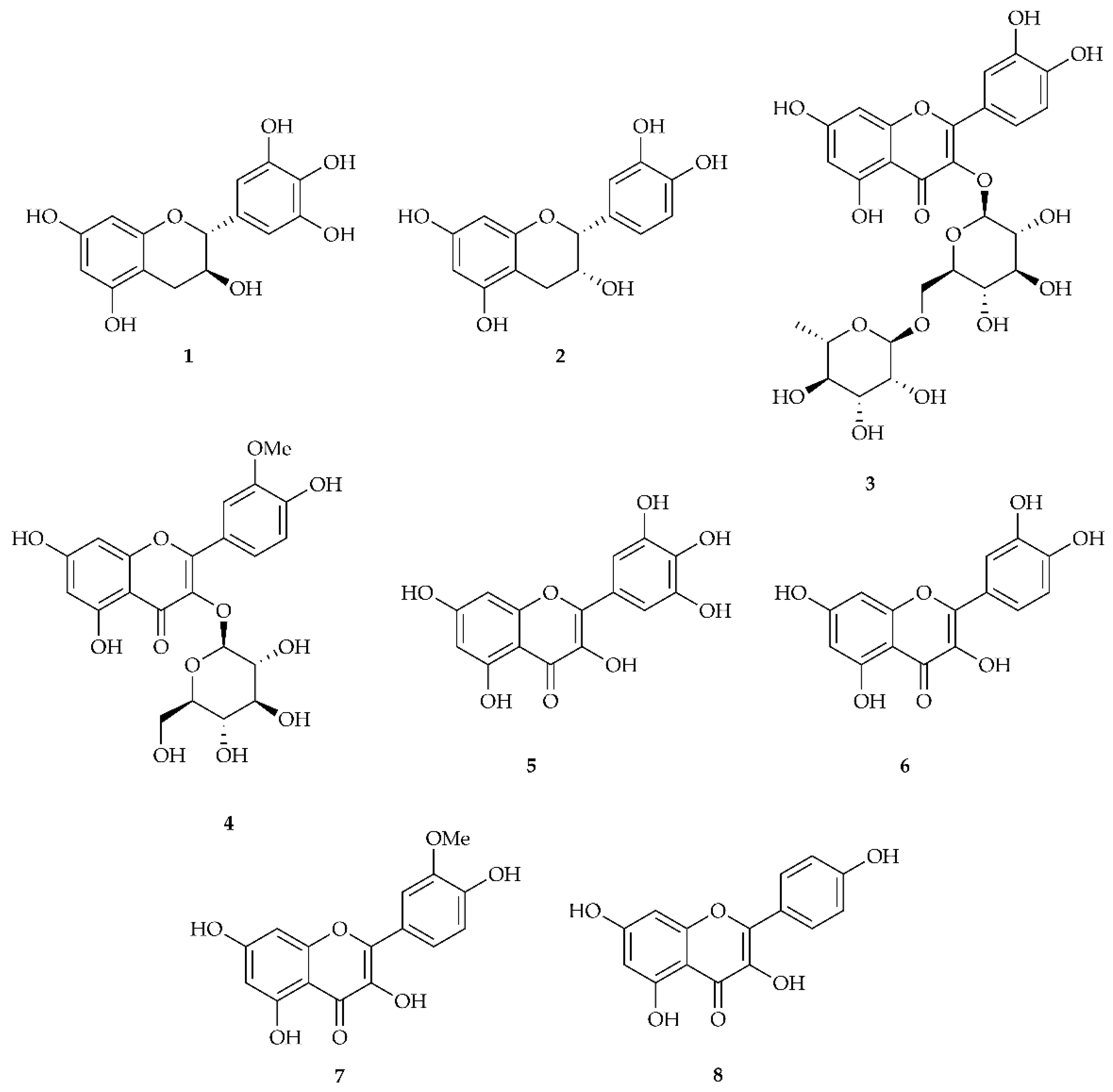
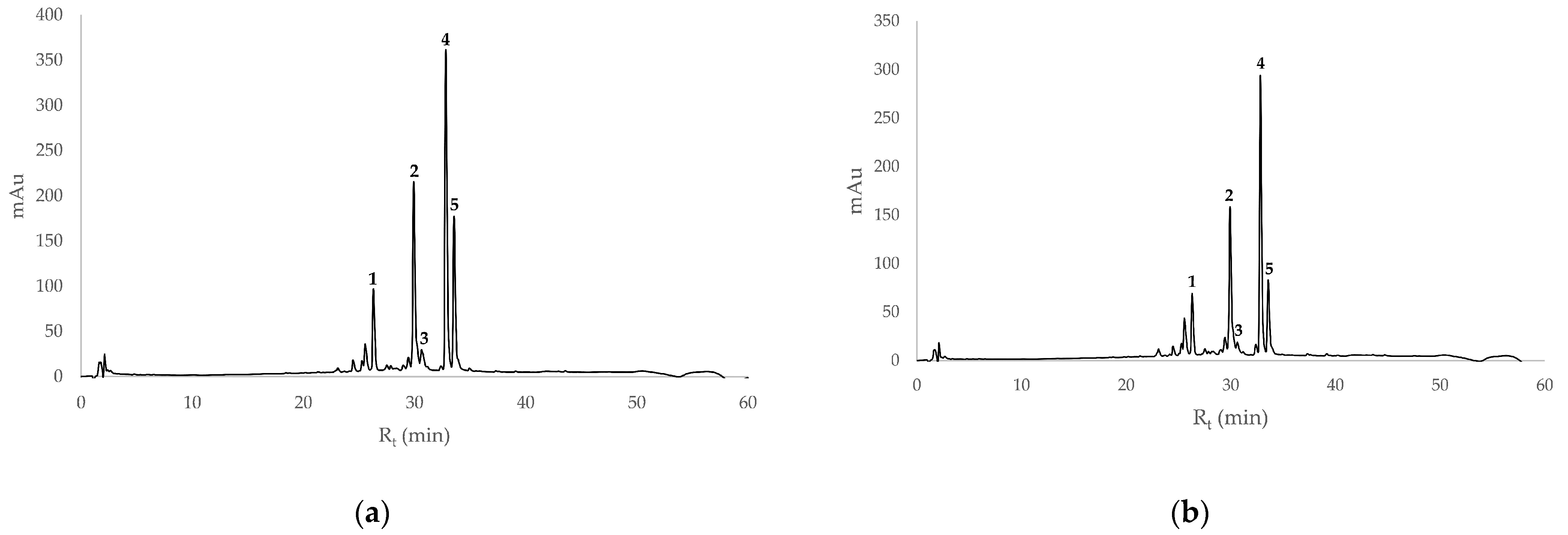
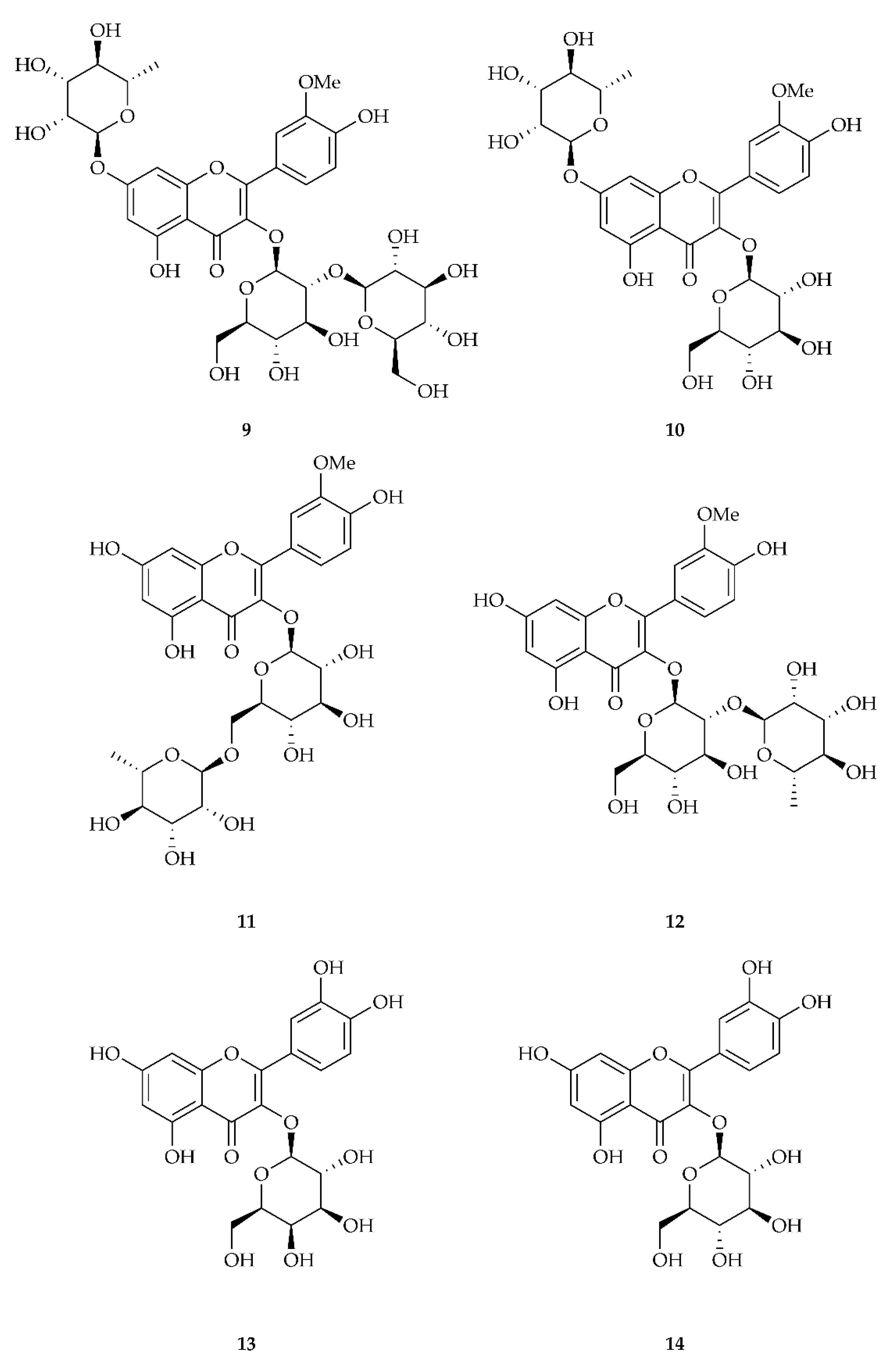
| Compound | M | M + H | M + Na | Rt |
|---|---|---|---|---|
| Gallocatechin 1 | C15H14O7 306.0740 | 307.0818 | 329.0637 | 5.98 min |
| Epicatechin 2 | C15H14O6 290.0790 | 291.0869 | 313.0688 | 22.94 min |
| Rutin 3 | C27H30O16 610.1534 | 611.1612 | 633.1432 | 29.97 min |
| isorhamnetin-3-O-glucoside 4 | C22H22O12 478.1111 | 479.1190 | 501.1009 | 33.45 min |
| Myricetin 5 | C15H10O8 318.0376 | 319.0454 | 341.0273 | 33.81 min |
| Quercetin 6 | C15H10O7 302.0427 | 303.0505 | 325.0324 | 39.11 min |
| Isorhamnetin 7 | C16H12O7 316.0583 | 317.0661 | 339.0481 | 39.95 min |
| Kaempferol 8 | C15H10O6 286.0477 | 287.0556 | 309.0375 | 44.4 min |
| Peak | Compound | Accurate Mass m/z | Exact Mass | Concentration (µg/mL) SBT | Concentration (µg/mL) SBF |
|---|---|---|---|---|---|
| 1 | isorhamnetin-3-O-sophoroside-7-rha 9 | 787.2327 463.1246 | 787.2297, M + H | 79.8 ± 0.1 | 45.7 ± 0.1 |
| 2 | isorhamnetin-3-O-glu-7-rha 10 or isorhamentin-3-O-rutinoside 11 or isorhamentin-3-O-neohesperoside 12 | 625.1782 | 625.1769, M + H | 269.7 ± 0.7 | 199.5 ± 0.4 |
| 3 | quercetin-3-O-galactoside 13 or quercetin-3-O-glucoside 14 | 465.1051 303.0511 | 465.1033, M + H | 35.8 ± 1.5 | 5.0 ± 0.5 |
| 4 | isorhamnetin-3-O-glu-7-rha 10 or isorhamentin-3-O-rutinoside 11 or isorhamentin-3-O-neohesperoside 12 | 625.1776 479.1198 317.0654 | 625.1769, M + H | 434.4 ± 0.7 | 340.8 ± 5.4 |
| 5 | isorhamnetin-3-O-glucoside 4 | 317.0646 479.1191 501.1024 | 479.1190, M + H | 210.5 ± 0.7 | 78.8 ± 3.8 |
| Plant | Concentration (µL/mL) | Assay | |
|---|---|---|---|
| EHT (%) | LEIA (%) | ||
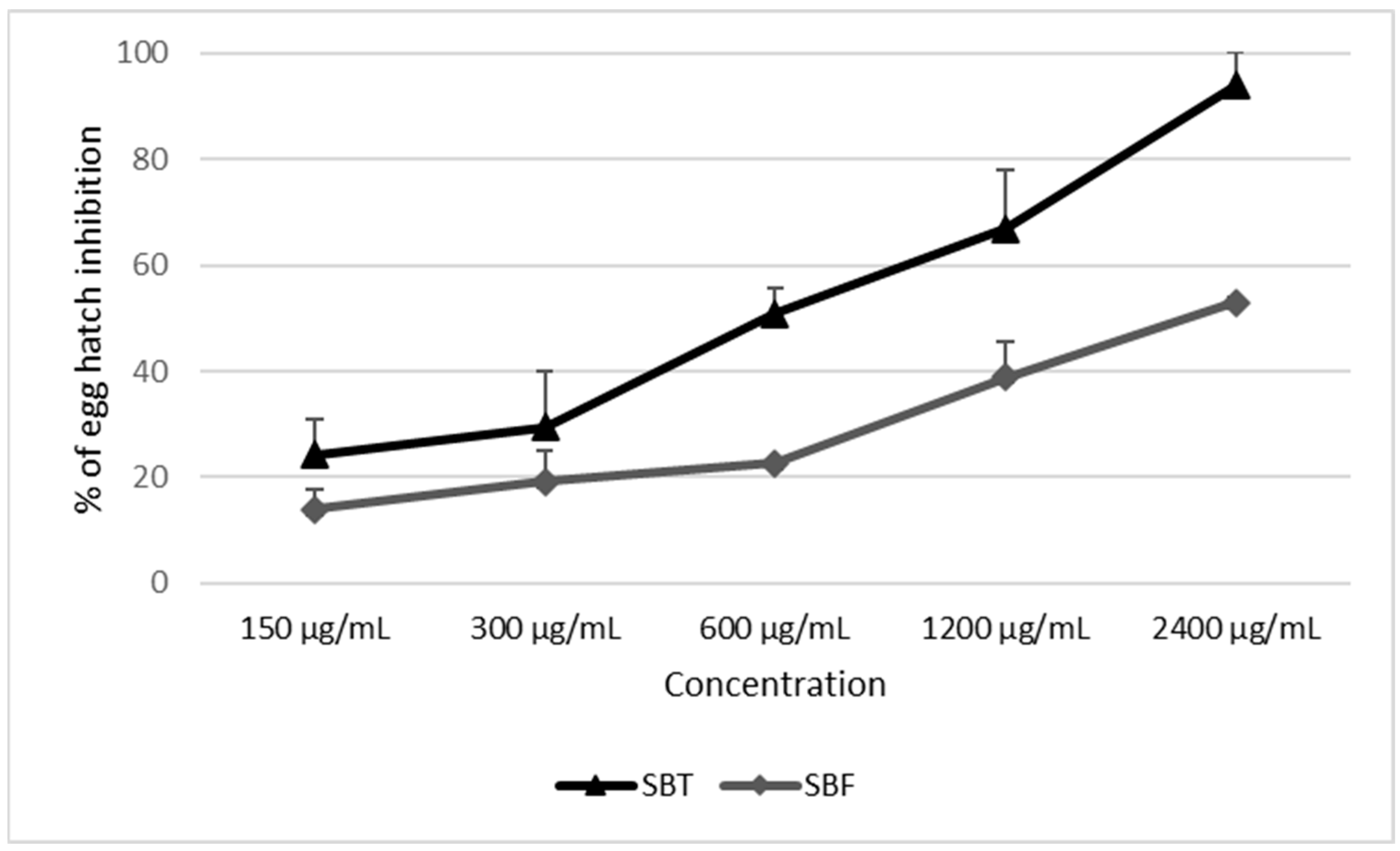
| Hippophae rhamnoides SBT | PBS | 4.44 ± 1.54 a | 10.61 ± 10.73 a |
| 2400 | 94.00 ± 6.77 d | 98.60 ± 1.66 b | |
| 1200 | 66.89 ± 10.34 c | 97.72 ± 2.84 b | |
| 600 | 50.89 ± 4.68 c | 89.61 ± 3.66 b | |
| 300 | 29.56 ± 11.20 b | 22.92 ± 17.01 a | |
| 150 | 24.22 ± 6.34 b | 17.94 ± 14.32 a | |
| TBZ | 99.78 ± 0.38 d | N.A. | |
| Hippophae rhamnoides SBF | PBS | 9.63 ± 1.24 a | 2.61 ± 3.21 a |
| 2400 | 52.99 ± 3.94 d | 100 ± 0.00 b | |
| 1200 | 38.95 ± 5.78 c | 100 ± 0.00 b | |
| 600 | 22.6 ± 0.85 b | 92.86 ± 14.29 b | |
| 300 | 19.16 ± 6.73 ab | 28.29 ± 33.38 a | |
| 150 | 13.94 ± 0.88 ab | 14.06 ± 28.13 a | |
| TBZ | 100 ± 0.00 e | N.A. | |
| Substance | Assay | |
|---|---|---|
| EHT (95% CI) | LEIA (95% CI) | |
| Hippophae rhamnoides SBT | 519 µg/mL (135–814 µg/mL) | 402 µg/mL (196–557 µg/mL) |
| Hippophae rhamnoides SBF | >2400 µg/mL (2178–3728 µg/mL) | 280 µg/mL (187–362 µg/mL) |
| Plant | Concentration (µL/mL) | EHI (%) ± S.D. | Concentration (µL/mL) | LEI (%) ± S.D. |
|---|---|---|---|---|
| Hippophae rhamnoides SBT | 2400 | 94.00 ± 6.77 | 1200 | 100 ± 0.00 |
| 2400 + PVPP | 62.63 ± 8.37 | 1200 + PVPP | 100 ± 0.00 | |
| Hippophae rhamnoides SBF | 2400 | 52.64 ± 4.51 | 1200 | 99.66 ± 0.68 |
| 2400 + PVPP | 49.24 ± 20.60 | 1200 + PVPP | 96.88 ± 6.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maestrini, M.; Forzato, C.; Mancini, S.; Pieracci, Y.; Perrucci, S. In Vitro Anthelmintic Activity of Sea Buckthorn (Hippophae rhamnoides) Berry Juice against Gastrointestinal Nematodes of Small Ruminants. Biology 2022, 11, 825. https://doi.org/10.3390/biology11060825
Maestrini M, Forzato C, Mancini S, Pieracci Y, Perrucci S. In Vitro Anthelmintic Activity of Sea Buckthorn (Hippophae rhamnoides) Berry Juice against Gastrointestinal Nematodes of Small Ruminants. Biology. 2022; 11(6):825. https://doi.org/10.3390/biology11060825
Chicago/Turabian StyleMaestrini, Michela, Cristina Forzato, Simone Mancini, Ylenia Pieracci, and Stefania Perrucci. 2022. "In Vitro Anthelmintic Activity of Sea Buckthorn (Hippophae rhamnoides) Berry Juice against Gastrointestinal Nematodes of Small Ruminants" Biology 11, no. 6: 825. https://doi.org/10.3390/biology11060825
APA StyleMaestrini, M., Forzato, C., Mancini, S., Pieracci, Y., & Perrucci, S. (2022). In Vitro Anthelmintic Activity of Sea Buckthorn (Hippophae rhamnoides) Berry Juice against Gastrointestinal Nematodes of Small Ruminants. Biology, 11(6), 825. https://doi.org/10.3390/biology11060825








