Use of Eisenia fetida as a Biological Risk Marker in a Qualitative Eco Assessment Test of a Romanian Watercourse
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
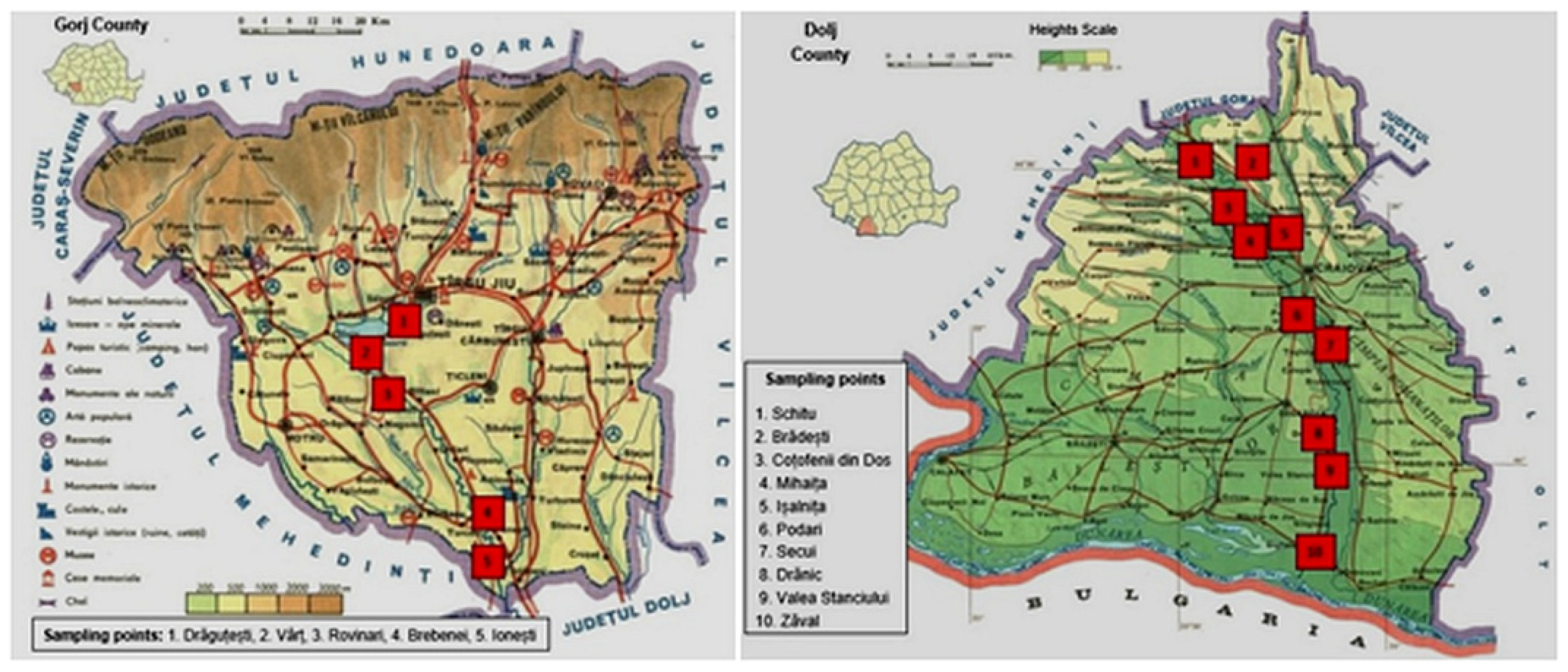
2.1. Locations Analyzed
2.2. Testing Methodology
2.3. The Statistic Analysis
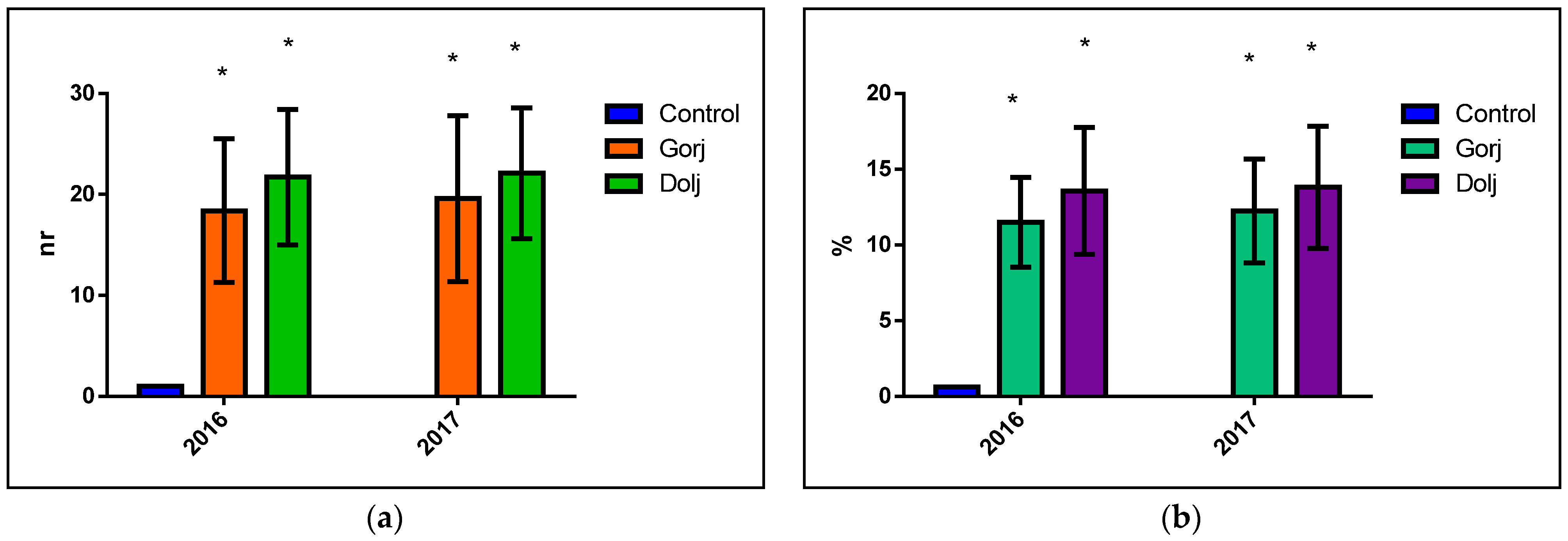
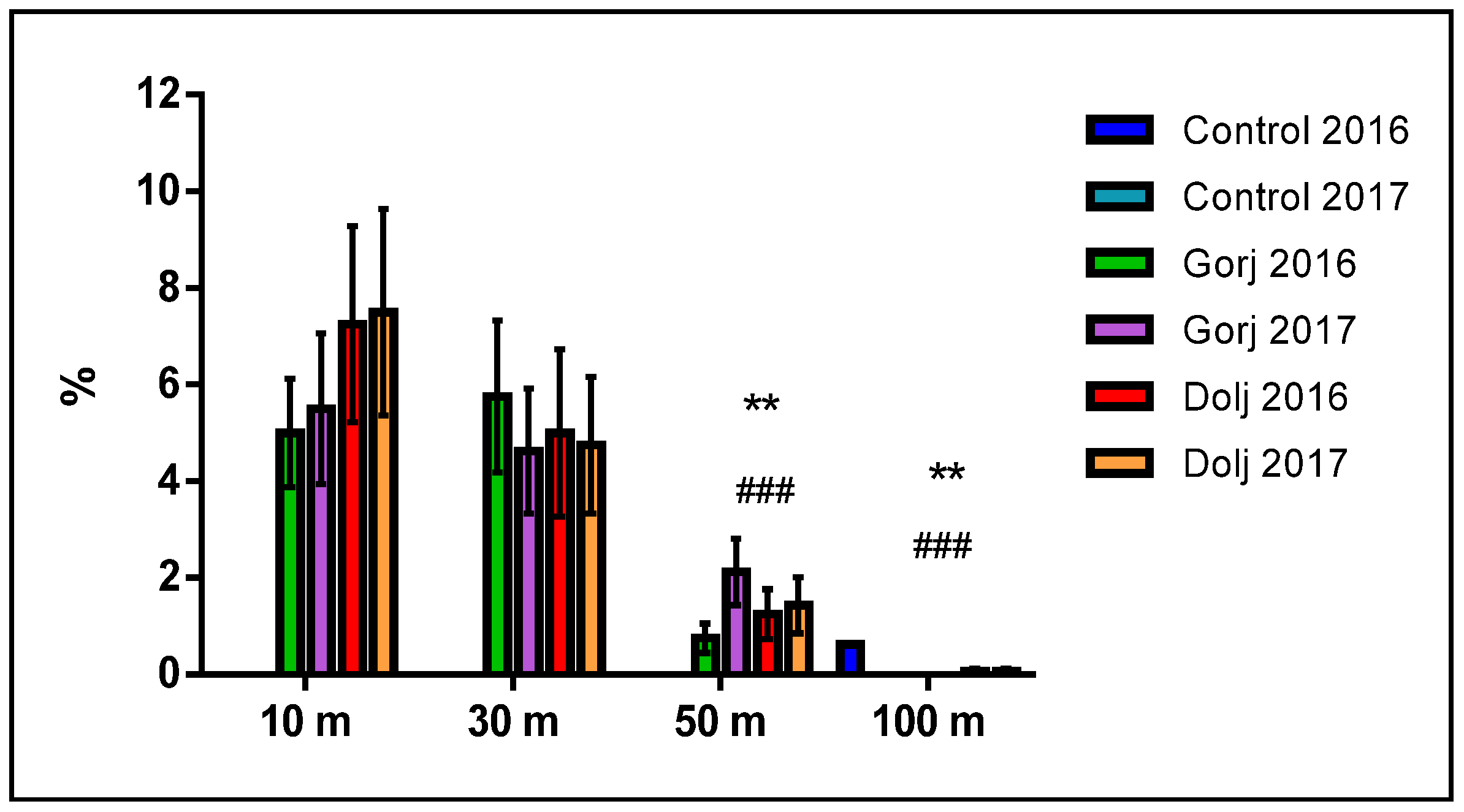
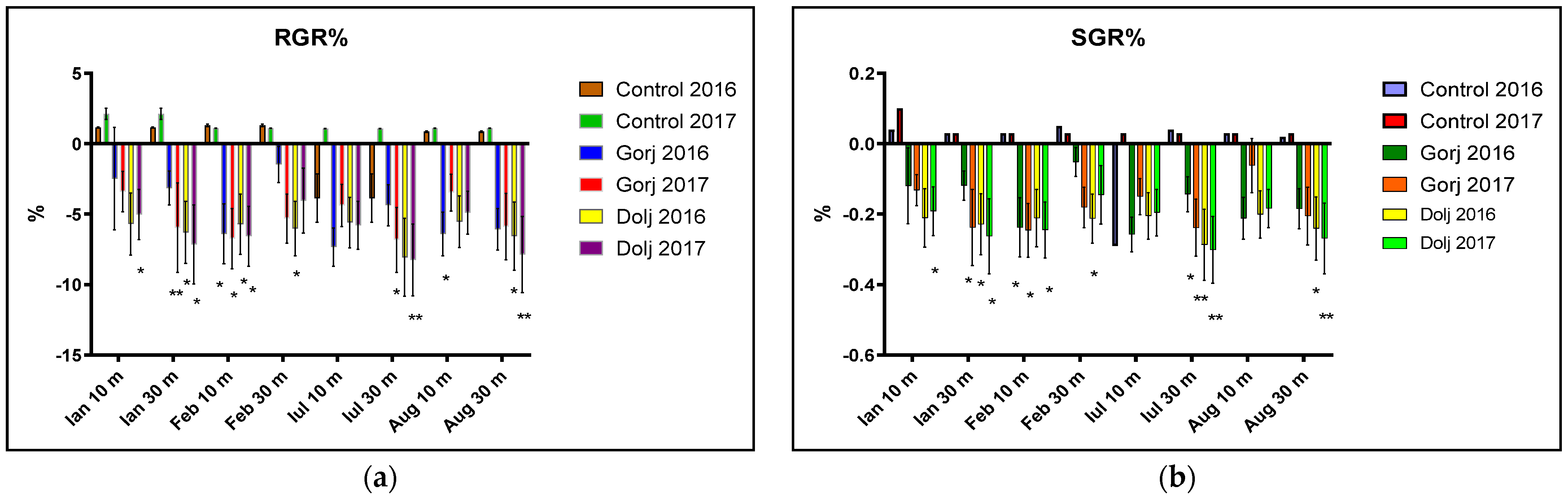
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Wang, X.; Sun, Z. Ecotoxicological effects of petroleum-contaminated soil on the earthworm Eisenia fetida. J. Hazard. Mater. 2020, 393, 122384. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Garbinski, L.D.; Rosen, B.; Zhang, J.; Xiang, P.; Ma, L.Q. Organoarsenical compounds: Occurrence, toxicology and biotransformation. Crit. Rev. Environ. Sci. Technol. 2019, 50, 217–243. [Google Scholar] [CrossRef]
- Deng, S.; Wu, Y.; Duan, H.; Cavanagh, J.-A.E.; Wang, X.; Qiu, J.; Li, Y. Toxicity assessment of earthworm exposed to arsenate using oxidative stress and burrowing behavior responses and an integrated biomarker index. Sci. Total Environ. 2021, 800, 149479. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, H.; Hu, Y.; Wang, X.; Ai, X.; Tang, L.; Qiu, J. Enrofloxacin at environmentally relevant concentrations enhances uptake and toxicity of cadmium in the earthworm Eisenia fetida in farm soils. J. Hazard. Mater. 2016, 308, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, Y.; Wang, Y.; Qiu, J.; Li, Y. Soil behaviour of the veterinary drugs lincomycin, monensin, and roxarsone and their toxicity on environmental organisms. Molecules 2019, 24, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wang, M.; Chen, W.; Jiang, R. Evaluation of combined toxicity of Siduron and cadmium on earthworm (Eisenia fetida) using Biomarker Response Index. Sci. Total Environ. 2019, 646, 893–901. [Google Scholar] [CrossRef]
- Tang, H.; Yan, Q.; Wang, X.; Ai, X.; Robin, P.; Matthew, C.; Li, Y. Earthworm (Eisenia fetida) behavioral and respiration responses to sublethal mercury concentrations in an artificial soil substrate. Appl. Soil Ecol. 2016, 104, 48–53. [Google Scholar] [CrossRef]
- Gu, H.; Yuan, Y.; Cai, M.; Wang, D.; Lv, W. Toxicity of isoprocarb to earthworms (Eisenia fetida): Oxidative stress, neurotoxicity, biochemical responses and detoxification mechanisms. Environ. Pollut. 2021, 290, 118038. [Google Scholar] [CrossRef]
- Gunadi, B.; Edwards, C.A. The effects of multiple applications of different organic wastes on the growth, fecundity and survival of Eisenia fetida (Savigny) (Lumbricidae). Pedobiologia 2003, 47, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Hund-Rinke, K.; Wiechering, H. Earthworm avoidance test for soil assessment. J. Soils Sedim. 2001, 1, 15–20. [Google Scholar] [CrossRef]
- Hund-Rinke, K.; Achazi, T.; Roembke, J.; Warnecke, D. Avoidance test with Eisenia fetida as indicator for the habitat function of soils: Soils of a laboratory comparison test. J. Soils Sedim. 2003, 3, 7–12. [Google Scholar] [CrossRef]
- Kiewa Catchment Landcare Group. Earthworm Project: Common Introduced Paddock Earthworms—Identification Guide. Available online: https://kclg.org.au/images/Worms/earthworm-resources/earthworm-identification-guide.pdf (accessed on 29 June 2021).
- Bailey, L.; Buckley, K. Land application of hog manure: Agronomic and environmental considerations, the Canadian perspective. In Proceedings of the HEMS Workshop, Toronto, ON, Canada, 27–28 April 2008; pp. 98–104. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.600.7761&rep=rep1&type=pdf (accessed on 29 June 2021).
- Bartlett, M.D.; Briones, M.J.I.; Neilson, R.; Schmidt, O.; Spurgeon, D.; Creamer, R.E. A critical review of current methods in earthworm ecology: From individuals to populations. Eur. J. Soil Biol. 2010, 46, 67–73. [Google Scholar] [CrossRef]
- Cools, D. Manure-derived antibiotic resistant bacteria: Survival in soil and contamination of crop roots. Ph.D. Thesis, Katholieke Universiteit Leuven, Leuven, Belgium, 2001. [Google Scholar]
- Herrick, J.E. Soil quality: An indicator of sustainable land management. Appl. Soil Ecol. 2000, 15, 75–83. [Google Scholar] [CrossRef]
- Hoffman, D.J. Handbook of Ecotoxicology; CRC Press LLC: London, UK, 2003. [Google Scholar]
- De Sousa, A.P.; de Andrea, M.M. Earthworm (Eisenia andrei) avoidance of soils treated with cypermethrin. Sensors 2011, 11, 11056–11063. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Qiao, Y.; Li, H.; Huang, C. Use of integrated biomarker response for studying the resistance strategy of the earthworm Metaphire californica in Cd-contaminated field soils in Hunan Province, South China. Environ. Pollut. 2020, 260, 114056. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.A.; Bohlen, P.J. The effects of toxic chemicals on earthworms. Rev. Environ. Contam. Toxicol. 1992, 125, 23–99. [Google Scholar]
- Faheem, M.; Khan, M.F. Toxicity of imidacloprid (nicotinoid) against earthworm, Pheretima poshuma with reference to its effects on protein. J. Basic Appl. Sci. 2010, 6, 55–62. [Google Scholar]
- Wang, Y.; Cang, T.Z.; Yu, R.; Chen, L.; Wu, C.; Wang, Q. Comparative acute toxicity of twenty-four insecticides to earthworm Eisenia fetida. Ecotox. Environ. Saf. 2012, 79, 122–128. [Google Scholar] [CrossRef]
- Zhou, S.; Duan, C.; Fu, H.; Chen, Y.; Wang, X.; Yu, Z. Toxicity assessment for chlorpyrifos-contaminated soil with three different earthworm test methods. J. Environ. Sci. 2007, 19, 854–858. [Google Scholar] [CrossRef]
- Zorn, M.L.; van Gestel, C.A.M.; Eijsackers, H. Species-specific earthworms population responses in relation to flooding dynamics in a Dutch floodplain soil. Pedobiologia 2005, 49, 189–198. [Google Scholar] [CrossRef]
- EPS 1/RM/43; Biological Test Method. Tests for Toxicity of Contaminated soil to Earthworms (Eisenia andrei, Eisenia fetida, or Lumbricus terrestris). Environment Canada: Quebec, QC, Canada, 2004. Available online: http://publications.gc.ca/site/eng/437659/publication.html (accessed on 1 July 2021).
- ISO 17512-1:2008; Soil Quality-Avoidance Test for Determining the Quality of Soils and Effects of Chemicals on Behaviour-Part 1: Test with Earthworms (Eisenia fetida and Eisenia andrei). ISO: Geneva, Switzerland, 2008. Available online: https://www.iso.org/standard/38402.html (accessed on 1 July 2021).
- ISO 11268-2; Soil quality—Effects of Pollutants on Earthworms—Part 2: Determination of Effects on Reproduction of Eisenia fetida/Eisenia andrei. International Organization for Standardization (ISO): Geneva, Switzerland, 2012. Available online: https://cdn.standards.iteh.ai/samples/53528/4a2f9b0feb994027bf721dcc4f8bb47d/ISO-11268-2-2012.pdf (accessed on 3 July 2021).
- Dolj County Map. Available online: https://pe-harta.ro/judete/Dolj.jpg (accessed on 1 July 2021).
- Gorj County Map. Available online: https://pe-harta.ro/judete/Gorj.jpg (accessed on 1 July 2021).
- OECD. Test No. 207: Earthworm, Acute Toxicity Tests, OECD Guidelines for the Testing of Chemicals; Section 2; OECD Publishing: Paris, France, 1984. [Google Scholar]
- Fründ, H.C.; Wallrabenstein, H.; Leißner, S.; Blohm, R. Developing a soil quality test with 2D terraria and Aporrectodea caliginosa “Experimenting with Earthworms”. In Proceedings of the Workshop Kommission III der Deutschen Bodenkundlichen Gesellschaft, Trier, Germany, 20–21 March 2009; Available online: http://eprints.dbges.de/90/2/Fruend_TrierDBG_2009.pdf (accessed on 29 April 2021).
- Yeardley, R.B.; Lazorchak, J.M.; Gast, L.C. The potential of an earthworm avoidance test for evaluation of hazardous waste sites. Environ. Toxicol. Chem. 1996, 15, 1532–1537. [Google Scholar] [CrossRef]
- Sogbesan, O.A.; Ugwumba, A.A.A. Effect of different substrates on growth and productivity of Nigeria semi-arid zone earthworm (Hyperiodrilus euryaulos, Clausen 1842) (Oligochaeta: Eudrilinae). World J Zool. 2006, 1, 103–112. Available online: http://www.idosi.org/wjz/wjz1(2)2006/6.pdf (accessed on 18 July 2021).
- Stephenson, G.L.; Kaushik, A.; Kaushik, N.K.; Solomon, K.R.; Steele, T.; Scroggins, R.P. Use of An Avoidance-Response Test to Assess the Toxicity of Contaminated Soils to Earthworms. In Advances in Earthworm Ecotoxicology; Sheppard, S.C., Bembridge, J.D., Holmstrup, M., Posthuma, L., Eds.; SETAC: Pensalocola, FL, USA, 1998; pp. 67–81. [Google Scholar]
- Natal Da Luz, T.; Ribeiro, R.; Sousa, J.P. Avoidance tests with collembola and earthworms as early screening tools for site-specific assessment of polluted soils. Environ. Toxicol. Chem. 2004, 23, 2188–2193. [Google Scholar] [CrossRef] [PubMed]
- Feisthauer, N. Using earthworm behavior to assess contaminated soil, ESG International. Environmental Science & Engineering Magazine. May 2003. Available online: http://www.esemag.com/archive/0503/earthworm.html (accessed on 1 August 2020).
- Loureiro, S.; Soares, A.M.V.M.; Nogueira, J.A. Terrestrial avoidance bahaviour tests as screening tool to assess soil contamination. Environ. Pollut. 2005, 13, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Thiruketheeswaran, P.; Huch, R.; D’Haese, J. Soluble calcium-binding proteins (SCBPs) of the earthworm Lumbricus terrestris: Possible role as relaxation factors in muscle. J. Comp. Physiol. B 2018, 188, 919–927. [Google Scholar] [CrossRef]
- Hoang, D.T.T.; Razavi, B.S.; Kuzyakov, Y.; Blagodatskaya, E. Earthworm burrows: Kinetics and spatial distribution of enzymes of C.-, N- and P-cycles. Soil Biol. Biochem. 2016, 99, 94–103. [Google Scholar] [CrossRef]
- Tang, J.; Duan, W.; Deng, P.; Li, H.; Liu, C.; Duan, Y.; Xu, S. Cadmium disrupts mitochondrial distribution and activates excessive mitochondrial fission by elevating cytosolic calcium independent of MCU-mediated mitochondrial calcium uptake in its neurotoxicity. Toxicology 2021, 453, 152726. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, L.; Li, Q.; Yan, X.; Li, Z.; Liu, B.; Li, G. Use of integrated biomarker response for evaluating antioxidant stress and DNA damage of earthworms (Eisenia fetida) in decabromodiphenyl ethane-contaminated soil. Environ. Pollut. 2020, 264, 114706. [Google Scholar] [CrossRef]
- Romeu, J.L. Anderson-Darling: A goodness of fit test for small samples assumptions. START 2000, 10, 1–6. Available online: https://src.alionscience.com/pdf/A_DTest.pdf (accessed on 12 December 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristina, R.T.; Baroga, M.; Dumitrescu, E.; Muselin, F.; Doma, A.O.; Manea, D.; Banatean-Dunea, I. Use of Eisenia fetida as a Biological Risk Marker in a Qualitative Eco Assessment Test of a Romanian Watercourse. Biology 2022, 11, 820. https://doi.org/10.3390/biology11060820
Cristina RT, Baroga M, Dumitrescu E, Muselin F, Doma AO, Manea D, Banatean-Dunea I. Use of Eisenia fetida as a Biological Risk Marker in a Qualitative Eco Assessment Test of a Romanian Watercourse. Biology. 2022; 11(6):820. https://doi.org/10.3390/biology11060820
Chicago/Turabian StyleCristina, Romeo T., Mihai Baroga, Eugenia Dumitrescu, Florin Muselin, Alexandru O. Doma, Dan Manea, and Ioan Banatean-Dunea. 2022. "Use of Eisenia fetida as a Biological Risk Marker in a Qualitative Eco Assessment Test of a Romanian Watercourse" Biology 11, no. 6: 820. https://doi.org/10.3390/biology11060820
APA StyleCristina, R. T., Baroga, M., Dumitrescu, E., Muselin, F., Doma, A. O., Manea, D., & Banatean-Dunea, I. (2022). Use of Eisenia fetida as a Biological Risk Marker in a Qualitative Eco Assessment Test of a Romanian Watercourse. Biology, 11(6), 820. https://doi.org/10.3390/biology11060820