The Importance of Energy Theory in Shaping Elevational Species Richness Patterns in Plants
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
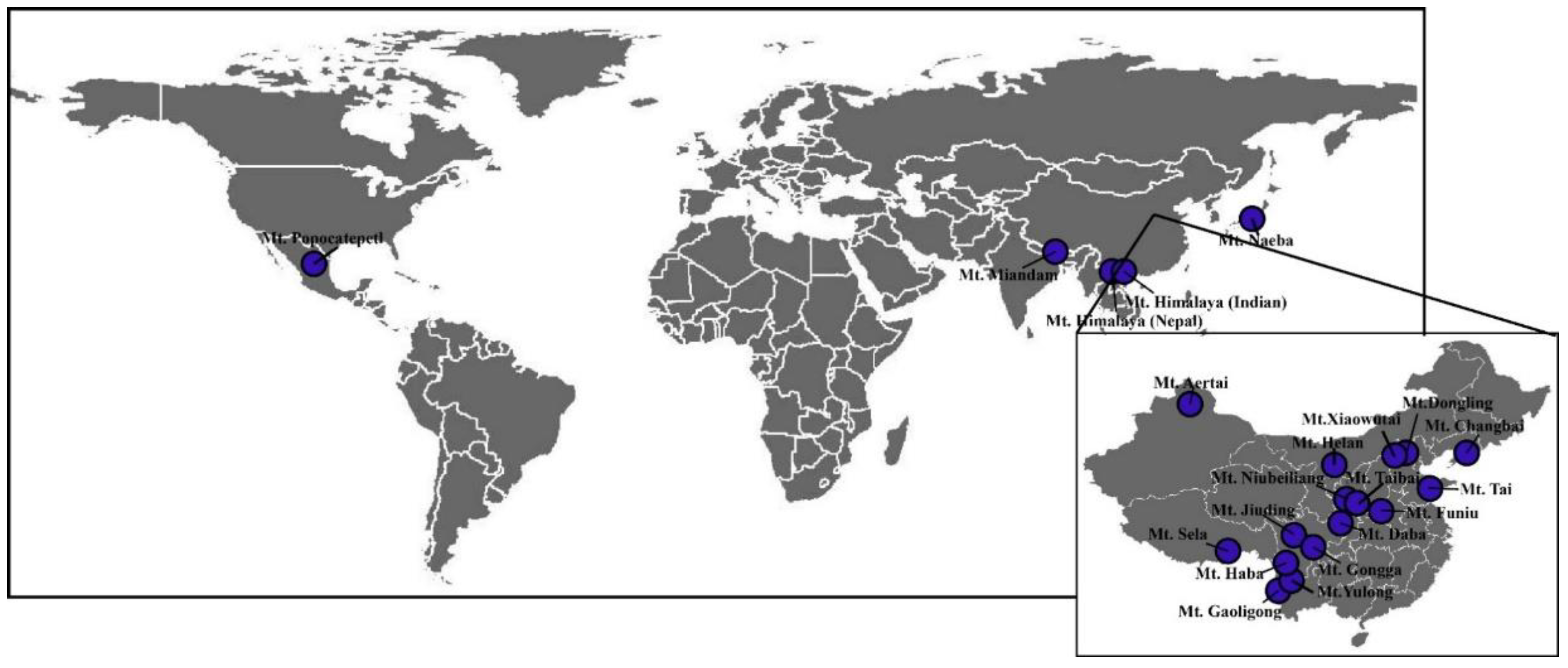
2.1. Data Collection
2.2. Analysis
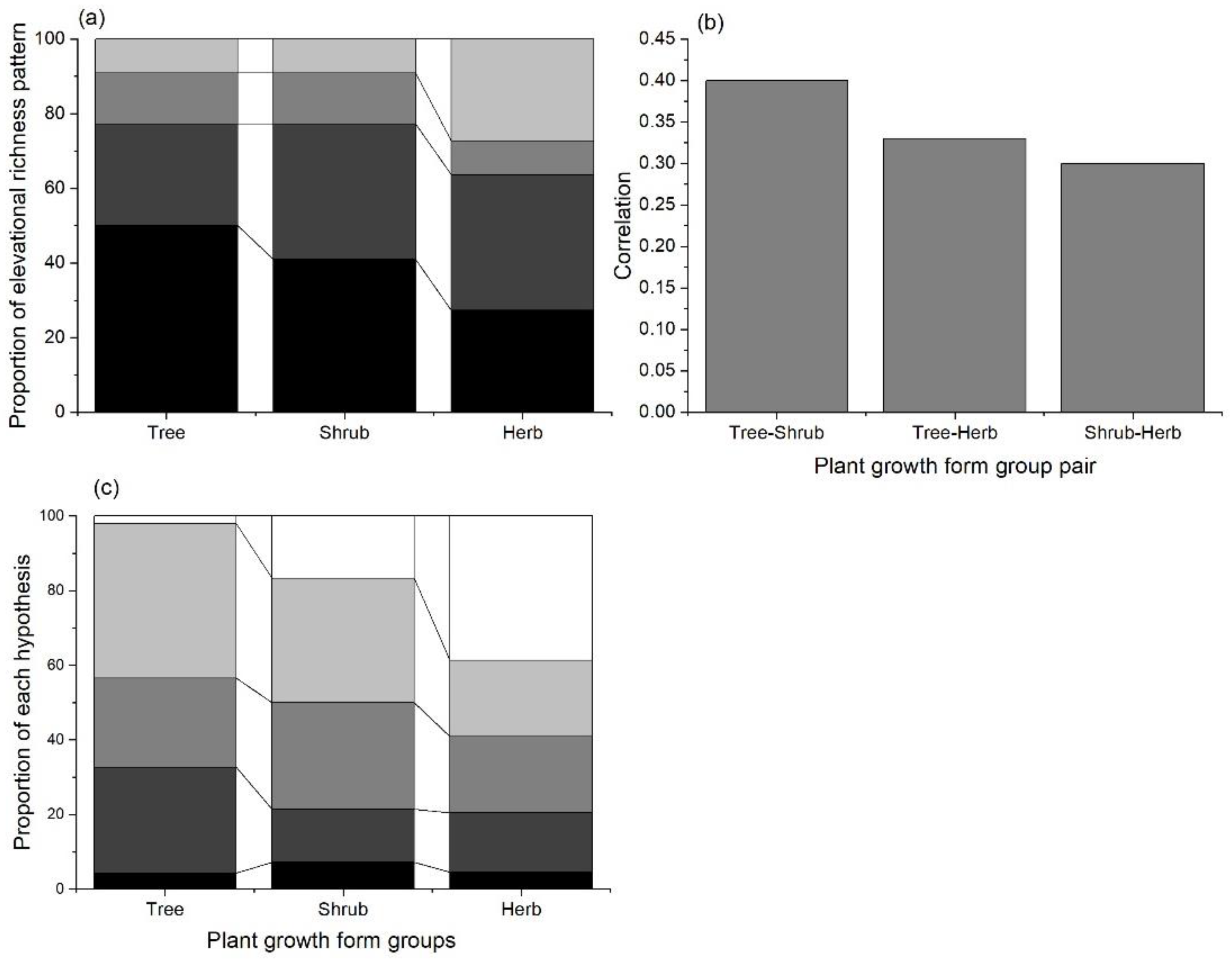
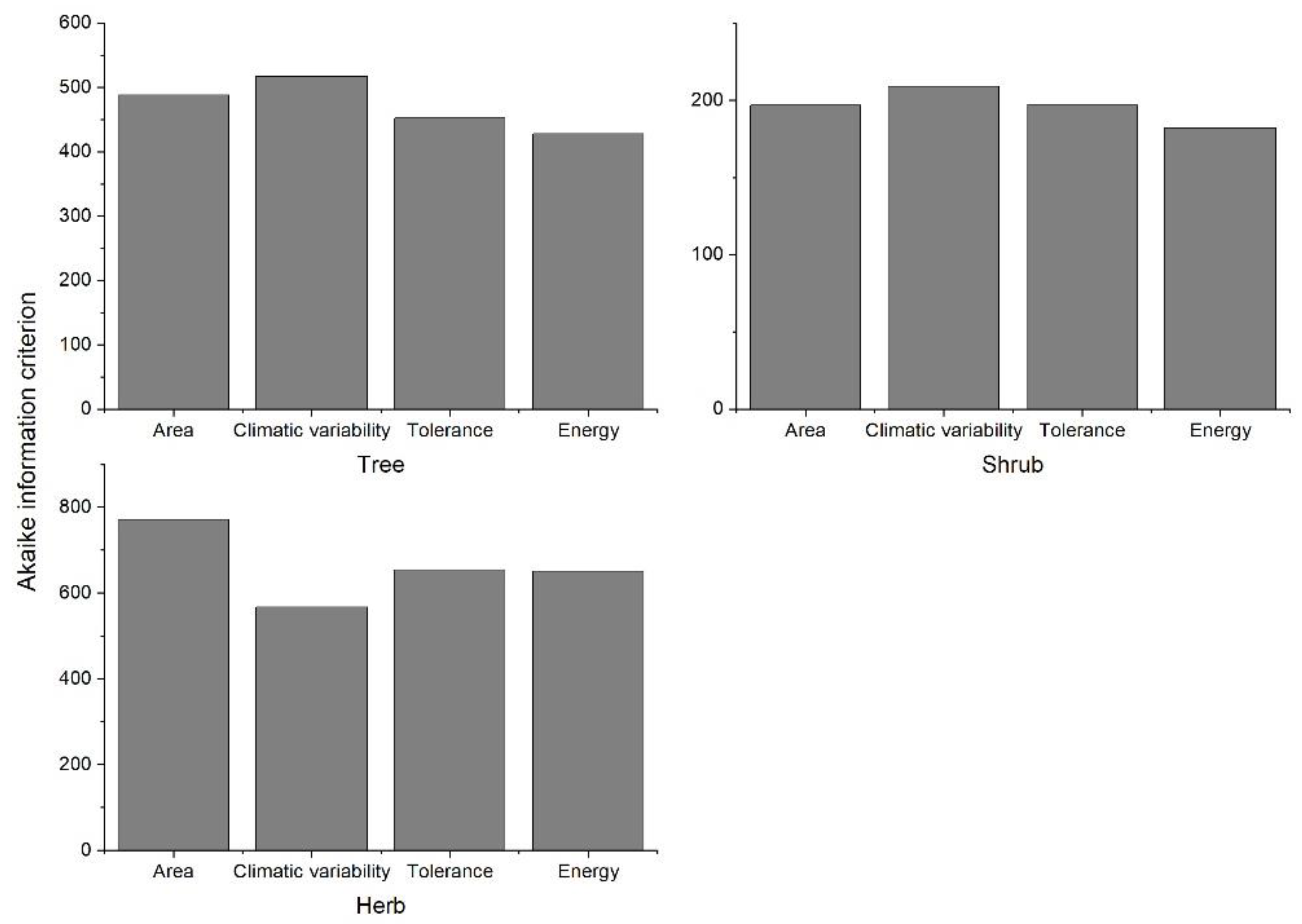
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shmida, A.; Wilson, M.V. Biological determinants of species diversity. J. Biogeogr. 1985, 12, 1–20. [Google Scholar] [CrossRef]
- Stevens, G.C. The elevational gradient in altitudinal range: An extension of Rapoport’s latitudinal rule to altitude. Am. Nat. 1992, 140, 893–911. [Google Scholar] [CrossRef] [PubMed]
- Rahbek, C. The elevational gradient of species richness: A uniform pattern? Ecography 1995, 18, 200–205. [Google Scholar] [CrossRef]
- Nogués-Bravo, D.; Araújo, M.B.; Romdal, T.; Rahbek, C. Scale effects and human impact on the elevational species richness gradients. Nature 2008, 453, 216–219. [Google Scholar] [CrossRef] [Green Version]
- Sanders, N.J.; Rahbek, C. The patterns and causes of elevational diversity gradients. Ecography 2012, 35, 1–3. [Google Scholar] [CrossRef]
- Sundqvist, M.K.; Sanders, N.J.; Wardle, D.A. Community and ecosystem responses to elevational gradients: Processes, mechanisms, and insights for global change. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 261–280. [Google Scholar] [CrossRef] [Green Version]
- Graham, C.H.; Carnaval, A.C.; Cadena, C.D.; Zamudio, K.R.; Roberts, T.E.; Parra, J.L.; McCain, C.M.; Bowie, R.C.K.; Moritz, C.; Baines, S.B.; et al. The origin and maintenance of montane diversity: Integrating evolutionary and ecological processes. Ecography 2014, 37, 711–719. [Google Scholar] [CrossRef]
- McCain, C.M.; Grytnes, J.A. Elevational gradients in species richness. Encyclopedia of Life Sciences 2010, 1–10. [Google Scholar]
- Colwell, R.K.; Rahbek, C.; Gotelli, N.J. The mid-domain effect and species richness patterns: What have we learned so far? Am. Nat. 2004, 163, E1–E23. [Google Scholar] [CrossRef] [Green Version]
- Letten, A.D.; Ashcroft, M.B.; Keith, D.A.; Gollan, J.R.; Ramp, D. The importance of temporal climate variability for spatial patterns in plant diversity. Ecography 2013, 36, 1341–1349. [Google Scholar] [CrossRef] [Green Version]
- Salas-Morales, S.H.; Meave, J.A.; Trejo, I. The relationship of meteorological patterns with changes in floristic richness along a large elevational gradient in a seasonally dry region of southern Mexico. Int. J. Biometeorol. 2015, 59, 1861–1874. [Google Scholar] [CrossRef] [PubMed]
- Spasojevic, M.J.; Grace, J.B.; Harrison, S.; Damschen, E.I. Functional diversity supports the physiological tolerance hypothesis for plant species richness along climatic gradients. J. Ecol. 2014, 102, 447–455. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Ma, K.M.; Anand, M. Can the physiological tolerance hypothesis explain herb richness patterns along an elevational gradient? A trait-based analysis. Community. Ecol. 2016, 17, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Binkenstein, J.; Klein, A.M.; Assmann, T.; Buscot, F.; Erfmeier, A.; Ma, K.; Pietsch, K.A.; Schmidt, K.; Scholten, T.; Wubet, T.; et al. Multi-trophic guilds respond differently to changing elevation in a subtropical forest. Ecography 2018, 41, 1013–1023. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Vetaas, O.R.; Grytnes, J.A. Fern species richness along a central Himalayan elevational gradient, Nepal. J. Biogeogr. 2004, 31, 389–400. [Google Scholar] [CrossRef]
- Ding, T.S.; Yuan, H.W.; Geng, S.; Lin, Y.S.; Lee, P.F. Energy flux, body size and density in relation to bird species richness along an elevational gradient in Taiwan. Glob. Ecol. Biogeogr. 2005, 14, 299–306. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y. Climate stability is more important than water–energy variables in shaping the elevational variation in species richness. Ecol. Evol. 2018, 8, 6872–6879. [Google Scholar] [CrossRef]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Sanders, N.J.; Dunn, R.R.; Fitzpatrick, M.C.; Carlton, C.E.; Pogue, M.R.; Parker, C.R.; Simons, T.R. Diverse elevational diversity gradients in Great Smoky Mountains National Park, U.S.A.: Chapter 10. In Data Mining for Global Trends in Mountain Biodiversity; CRC Press: Boca Raton, FL, USA, 2009; pp. 75–87. [Google Scholar] [CrossRef]
- Lomolino, M.V. Elevation gradients of species-density: Historical and prospective views. Glob. Ecol. Biogeogr. 2001, 10, 3–13. [Google Scholar] [CrossRef]
- Kluge, J.; Kessler, M.; Dunn, R.R. Elevational seed plants richness patterns in Bhutan, Eastern Himalaya. J. Biogeogr. 2017, 44, 1711–1722. [Google Scholar] [CrossRef]
- Kluge, J.; Kessler, M.; Dunn, R.R. What drives elevational patterns of diversity? A test of geometric constraints, climate and species pool effects for pteridophytes on an elevational gradient in Costa Rica. Glob. Ecol. Biogeogr. 2011, 15, 358–371. [Google Scholar] [CrossRef]
- Hurlbert, A.H.; Stegen, J.C. When should species richness be energy limited, and how would we know? Ecol. lett. 2014, 17, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Jarošík, V.; Kučera, T. Patterns of invasion in temperate nature reserves. Biol. Conserv. 2002, 104, 13–24. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Zeileis, A.; Grothendieck, G.; Ryan, J.A.; Andrews, F.; Zeileis, M.A. Package ‘zoo’. R Package Version 2014, 1, 7–12. [Google Scholar]
- Panda, R.M.; Behera, M.D.; Roy, P.S.; Biradar, C. Energy determines broad pattern of plant distribution in Western Himalaya. Ecol. Evol. 2017, 7, 10850–10860. [Google Scholar] [CrossRef]
- Wang, J.M.; Long, T.; Zhong, Y.M.; Li, J.W.; Zhang, T.H.; Feng, Y.M.; Lu, Q. Disentangling the influence of climate, soil and belowground microbes on local species richness in a dryland ecosystem of Northwest China. Sci. Rep. 2017, 7, 18029. [Google Scholar] [CrossRef] [Green Version]
- Adler, P.B.; Drake, J.M. Environmental variation, stochastic extinction, and competitive coexistence. Am. Nat. 2008, 172, E186–E195. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, J.; Zhang, Y. Effect of altitude and soil properties on biomass and plant richness in the grasslands of Tibet, China, and Manang District, Nepal. Ecosphere 2019, 10, e02915. [Google Scholar] [CrossRef]
- Zarfos, M.R.; Dovciak, M.; Lawrence, G.B.; McDonnell, T.C.; Sullivan, T.J. Plant richness and composition in hardwood forest understories vary along an acidic deposition and soil-chemical gradient in the northeastern United States. Plant Soil 2019, 438, 461–477. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.P.; Wang, J.Y.; Meng, Z.X.; Xu, R.; Chen, J.; Zhang, Y.J.; Hu, T.M. Fertility-related interplay between fungal guilds underlies plant richness–productivity relationships in natural grasslands. New Phytol. 2020, 226, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.H.; Ma, K.M.; Liu, H.Y.; Tang, Z.Y. A trait-based approach reveals the importance of biotic filter for elevational herb richness pattern. J. Biogeogr. 2018, 45, 2288–2298. [Google Scholar] [CrossRef]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, G.; Seastedt, T.R. Soil fauna and plant litter decomposition in tropical and subalpine forests. Ecology 2001, 82, 955–964. [Google Scholar] [CrossRef]
- Wardle, D.A. Communities and Ecosystems: Linking the Aboveground and Belowground Components, MPB 34; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Zhang, W.D.; Chao, L.; Yang, Q.P.; Wang, Q.K.; Fang, Y.T.; Wang, S.L. Litter quality mediated nitrogen effect on plant litter decomposition regardless of soil fauna presence. Ecology 2016, 97, 2834–2843. [Google Scholar] [CrossRef]
- Rahbek, C. The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Let. 2005, 8, 224–239. [Google Scholar] [CrossRef]
- Zhang, S.B.; Chen, W.Y.; Huang, J.L. Orchid species richness along elevational and environmental gradients in Yunnan, China. PLoS ONE 2015, 10, e0142621. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, Y.; Tian, Y. Distribution and conservation of orchid species richness in China. Biol. Conserv. 2015, 181, 64–72. [Google Scholar] [CrossRef]
- Acharya, K.P.; Vetaas, O.R.; Birks, H.J.B. Orchid species richness along Himalayan elevational gradients. J. Biogeogr. 2011, 38, 1821–1833. [Google Scholar] [CrossRef]
- Jetz, W.; Sekercioglu, C.H.; Watson, J.E.M. Ecological correlates and conservation implications of overestimating species geographic ranges. Conserv. Biol. 2008, 22, 110–119. [Google Scholar] [CrossRef]
- Rahbek, C.; Graves, G.R. Detection of macro-ecological patterns in South American hummingbirds is affected by spatial scale. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2000, 267, 2259–2265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahbek, C.; Graves, G.R. Multiscale assessment of patterns of avian species richness. Proc. Natl. Acad. Sci. USA 2001, 98, 4534–4539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rensburg, B.J.; Chown, S.L.; Gaston, K.J. Species richness, environmental correlates, and spatial scale: A test using South African birds. Am. Nat. 2002, 159, 566–577. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Liu, Q.; Xu, W.; Peng, C. The Importance of Energy Theory in Shaping Elevational Species Richness Patterns in Plants. Biology 2022, 11, 819. https://doi.org/10.3390/biology11060819
Jiang Z, Liu Q, Xu W, Peng C. The Importance of Energy Theory in Shaping Elevational Species Richness Patterns in Plants. Biology. 2022; 11(6):819. https://doi.org/10.3390/biology11060819
Chicago/Turabian StyleJiang, Zihan, Qiuyu Liu, Wei Xu, and Changhui Peng. 2022. "The Importance of Energy Theory in Shaping Elevational Species Richness Patterns in Plants" Biology 11, no. 6: 819. https://doi.org/10.3390/biology11060819
APA StyleJiang, Z., Liu, Q., Xu, W., & Peng, C. (2022). The Importance of Energy Theory in Shaping Elevational Species Richness Patterns in Plants. Biology, 11(6), 819. https://doi.org/10.3390/biology11060819






