High-Efficiency Bovine Sperm Sexing Used Magnetic-Activated Cell Sorting by Coupling scFv Antibodies Specific to Y-Chromosome-Bearing Sperm on Magnetic Microbeads
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
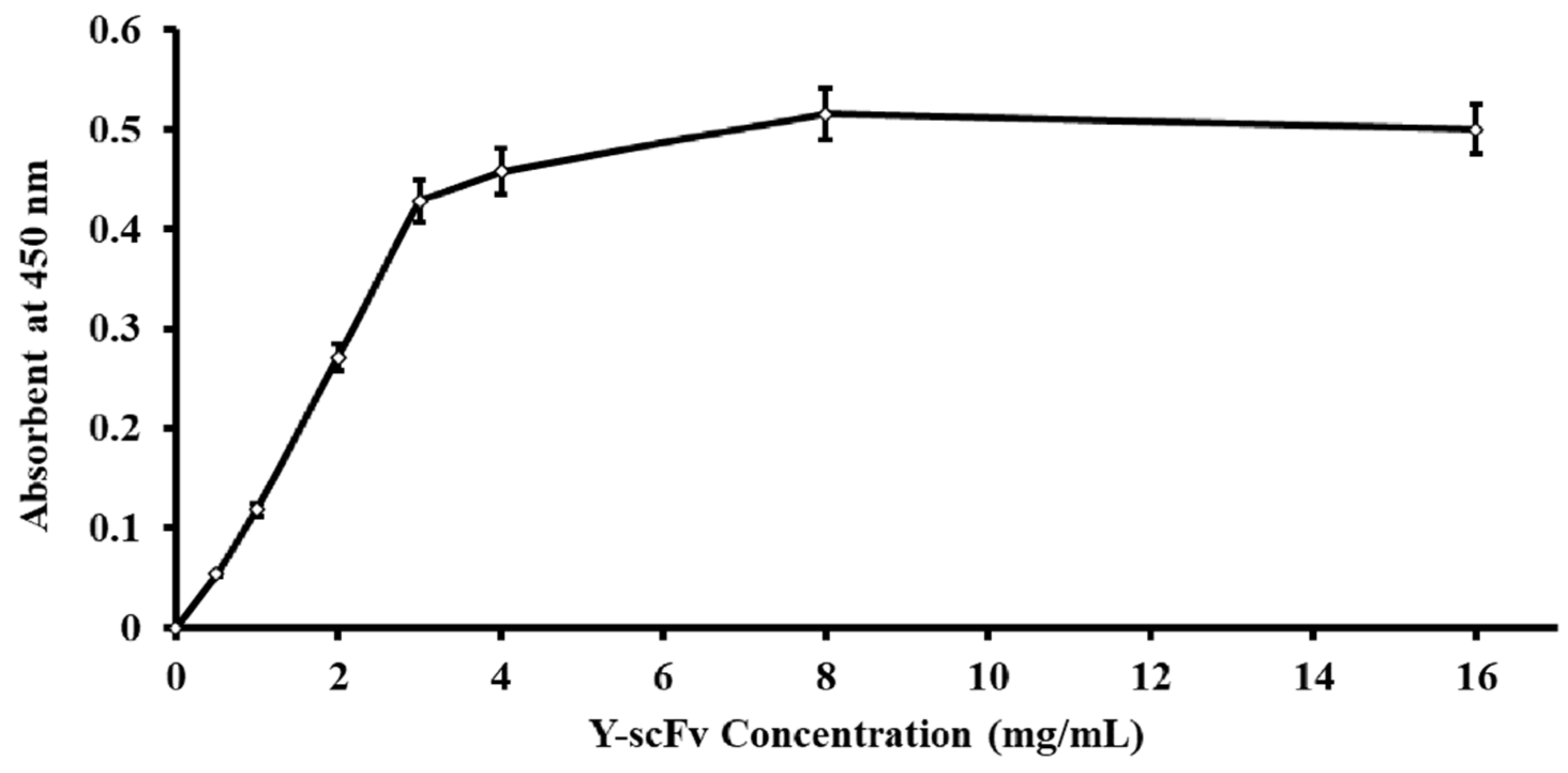
2.2. Estimation of the Amounts of Y-scFv Antibody Coupled to Magnetic Microbeads
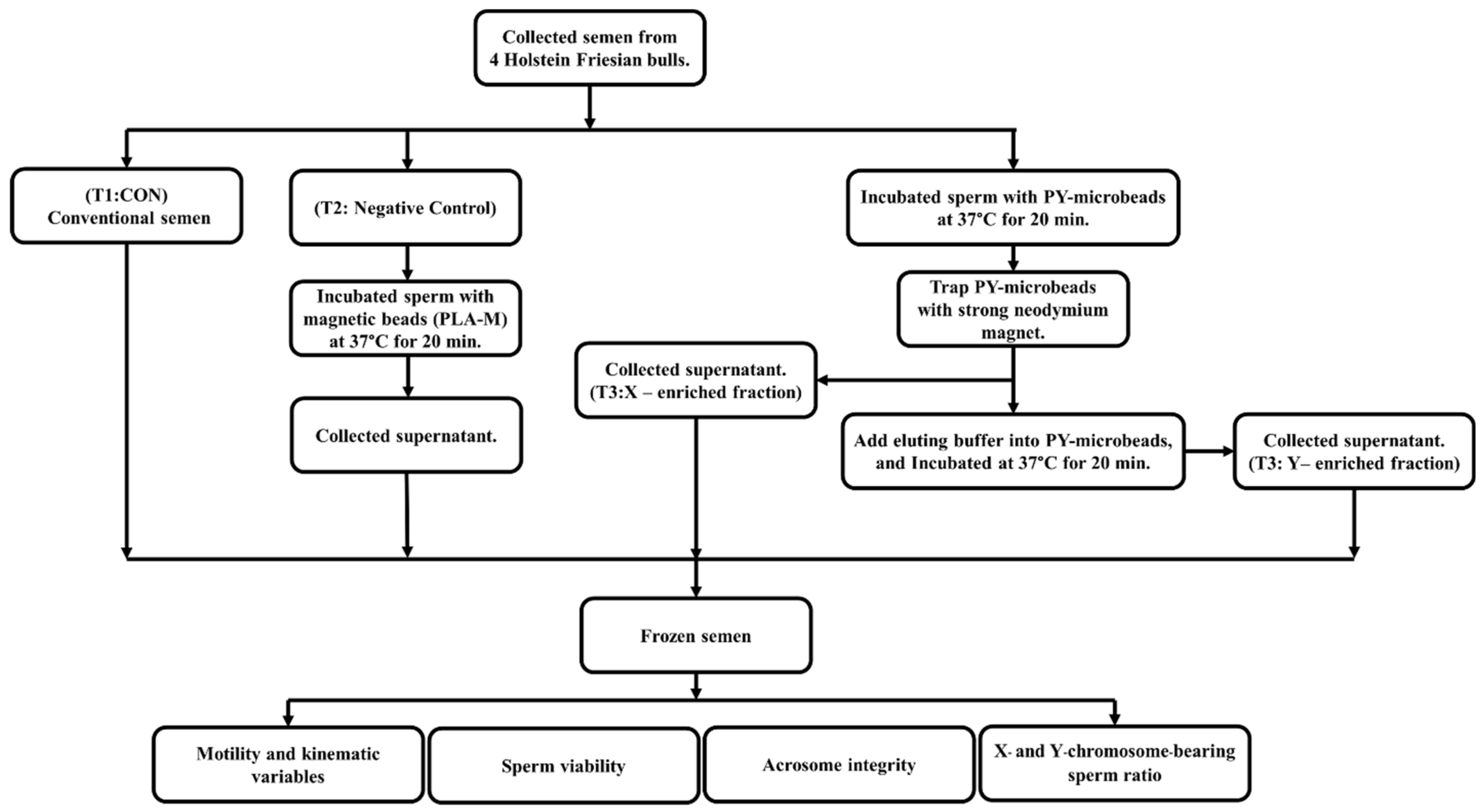
2.3. Semen Sample Collection
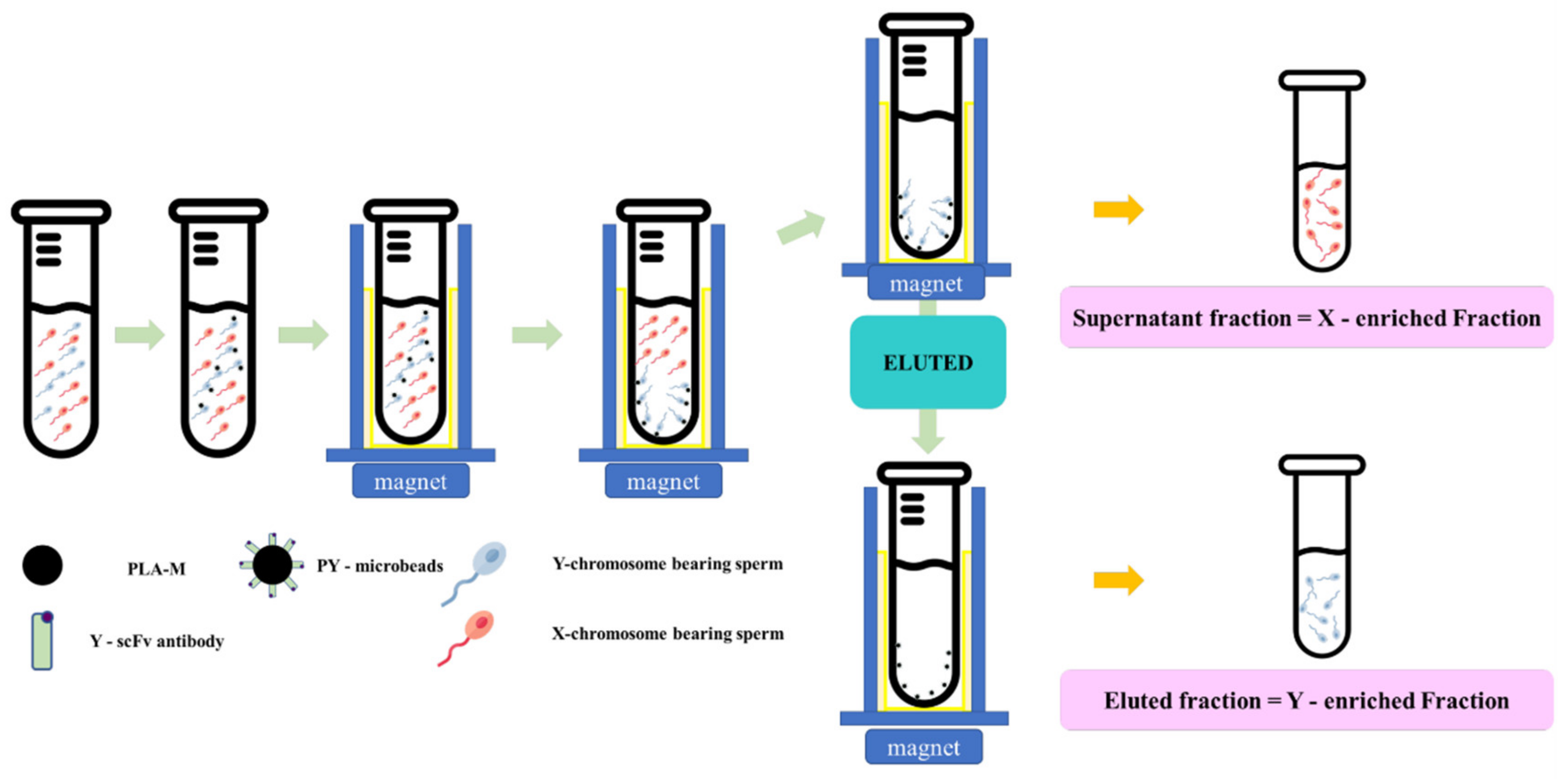
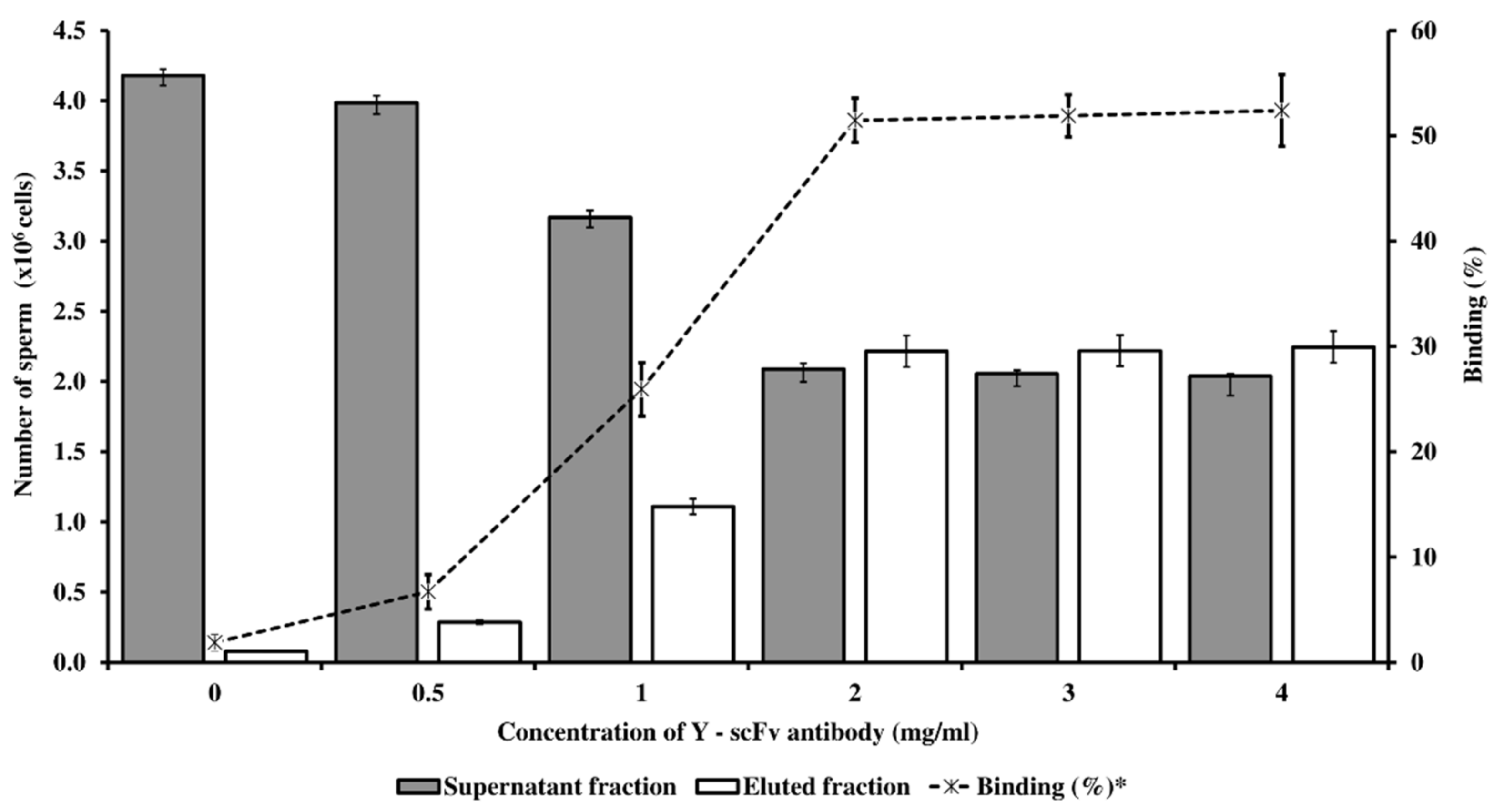
2.4. Optimization of Y-scFv Antibody Coupling on Magnetic Microbeads for Sorting Sperm
2.5. Production of Frozen Sexed Bovine Semen by PY-Microbeads
2.6. Evaluation of Frozen–Thawed Sperm Motility and Kinematic Variables Using Computer-Aided Sperm Analysis (CASA)
2.7. Evaluation of Frozen–Thawed Sperm Viability Using Imaging Flow Cytometry
2.8. Evaluation of Frozen–Thawed Sperm Acrosome Integrity Using Imaging Flow Cytometry
2.9. Discrimination of X/Y-Sperm by Imaging Flow Cytometry
2.10. Evaluation of Sexed Sperm by SYBR® Green RT–PCR
2.10.1. DNA Sample Preparation
2.10.2. Quantitative SYBR Green Real-Time PCR (qPCR) Analyses
2.11. Statistical Analysis
3. Results
3.1. Efficiency of Y-scFv Antibody Coupling to Magnetic Microbeads
3.2. Optimized Conditions for Sexed Sperm by PY-Magnetic Beads
3.3. Frozen–Thaw Sperm Motility and Kinematic Variables Evaluated by CASA
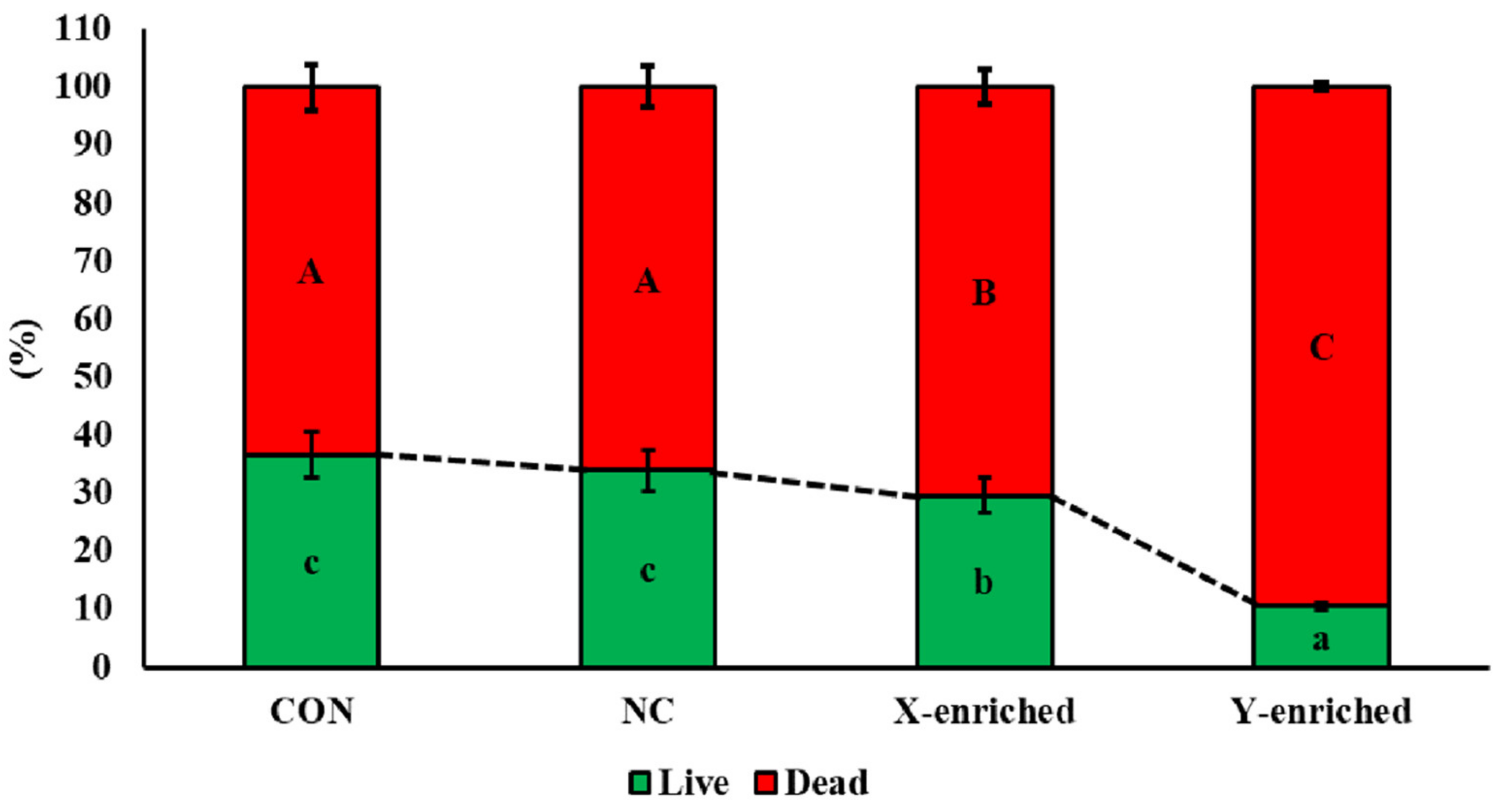
3.4. Viability of Frozen–Thawed Sexed Semen
3.5. Acrosome Integrity of Frozen–Thawed Sexed Semen
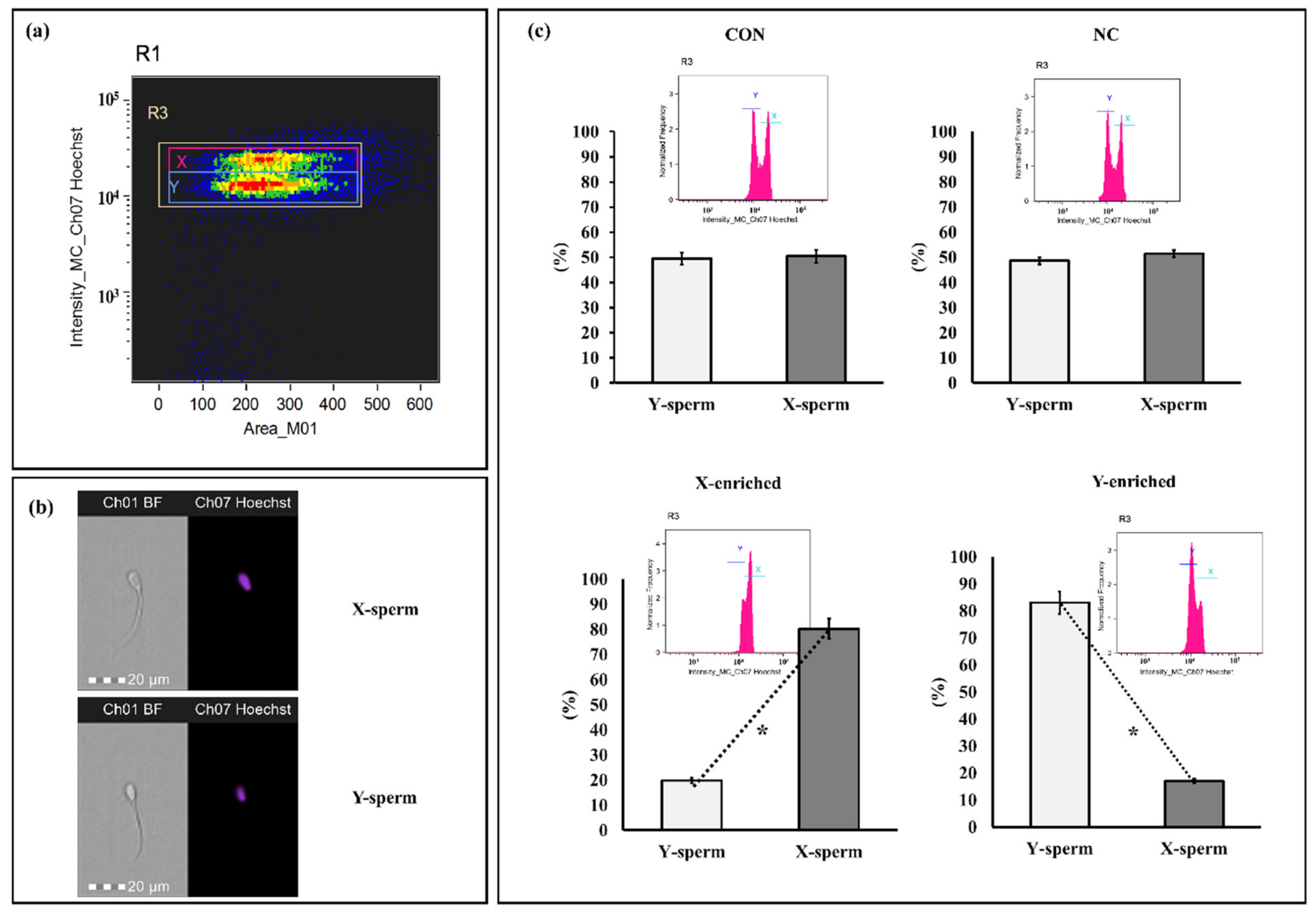
3.6. X/Y-Sperm Ratio in Frozen-Thaw Semen Evaluated by Imaging Flow Cytometry
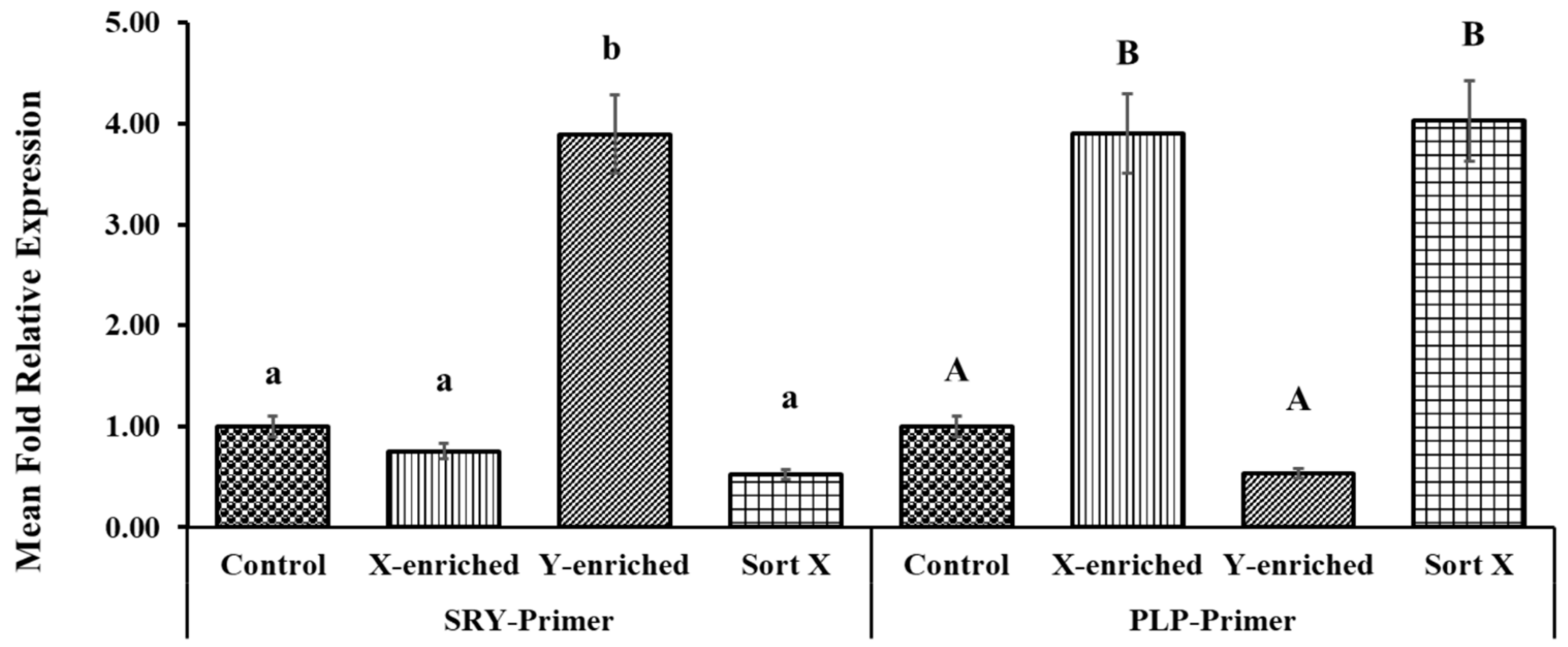
3.7. X/Y-Sperm Ratio in Frozen-Thaw Semen Evaluated by Real-Time PCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, Y.; Xu, Z.; Wu, Z.; Hong, L. Sex Manipulation Technologies Progress in Livestock: A Review. Front. Vet. Sci. 2020, 7, 481. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.L. Flow cytometric sexing of mammalian sperm. Theriogenology 2006, 65, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Pahmeyer, C.; Britz, W. Economic opportunities of using crossbreeding and sexing in Holstein dairy herds. J. Dairy Sci. 2020, 103, 8218–8230. [Google Scholar] [CrossRef] [PubMed]
- Rath, D.; Tiedemann, D.; Gamrad, L.; Johnson, L.; Klein, S.; Kues, W.; Mancini, R.; Rehbock, C.; Taylor, U.; Barcikowski, S. Sex Sorted Boar Sperm—An Update on Related Production Methods. Reprod. Domest. Anim. 2015, 50, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Li, X.X.; Yang, X.G.; Lu, Y.Q.; Lu, S.S.; Zhang, M.; Yao, H.L.; Meng, L.J.; Lu, K.H. Protective Effects of Melatonin against Oxidative Stress in Flow Cytometry-sorted Buffalo Sperm. Reprod. Domest. Anim. 2012, 47, 299–307. [Google Scholar] [CrossRef]
- Wheeler, M.B.; Rutledge, J.J.; Fischer-Brown, A.; VanEtten, T.; Malusky, S.; Beebe, D.J. Application of sexed semen technology to in vitro embryo production in cattle. Theriogenology 2006, 65, 219–227. [Google Scholar] [CrossRef]
- Thongkham, M.; Thaworn, W.; Pattanawong, W.; Teepatimakorn, S.; Mekchay, S.; Sringarm, K. Spermatological parameters of immunologically sexed bull semen assessed by imaging flow cytometry, and dairy farm trial. Reprod. Biol. 2021, 21, 100486. [Google Scholar] [CrossRef]
- Patthanawong, W.; Pongpiachan, P.; Mekchay, S.; Sumretprasong, J. Production of Monoclonal Antibody Against Male Specific Anti-gen on Cell Membrane of Bovine Spermatozoa. Indian J. Anim. Res. 2010, 44, 22–27. [Google Scholar]
- Ahmad, Z.A.; Yeap, S.K.; Ali, A.M.; Ho, W.Y.; Alitheen, N.B.M.; Hamid, M. scFv Antibody: Principles and Clinical Application. Clin. Dev. Immunol. 2012, 2012, 980250. [Google Scholar] [CrossRef]
- Lamberski, J.A.; Thompson, N.E.; Burgess, R.R. Expression and purification of a single-chain variable fragment antibody derived from a polyol-responsive monoclonal antibody. Protein Expr. Purif. 2006, 47, 82–92. [Google Scholar] [CrossRef]
- Thaworn, W.; Hongsibsong, S.; Thongkham, M.; Mekchay, S.; Pattanawong, W.; Sringarm, K. Production of single-chain fragment variable (scFv) antibodies specific to plasma membrane epitopes on bull Y-bearing sperm. Anim. Biotechnol. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Gangwar, D.K.; Singh, J.; Tikadar, C.K.; Khanna, V.V.; Saini, S.; Dholpuria, S.; Palta, P.; Manik, R.S.; Singh, M.K.; et al. An immunological approach of sperm sexing and different methods for identification of X- and Y-chromosome bearing sperm. Vet. World 2017, 10, 498–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, S.K.; Gangwar, D.K.; Singh, J.; Tikadar, C.K.; Khanna, V.V.; Saini, S.; Dholpuria, S.; Palta, P.; Manik, R.S.; Singh, M.K.; et al. The untapped potential of magnetic nano-particles for forensic investigations: A comprehensive review. Talanta 2021, 230, 122297. [Google Scholar]
- Said, T.M.; Agarwal, A.; Zborowski, M.; Grunewald, S.; Glander, H.J.; Paasch, U. Utility of magnetic cell separation as a molecular sperm preparation technique. J. Androl. 2008, 29, 134–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil Juliá, M.; Hervás, I.; Navarro-Gómez Lechón, A.; Quintana, F.; Amorós, D.; Pacheco, A.; González-Ravina, C.; Rivera-Egea, R.; Garrido, N. Sperm Selection by Magnetic-Activated Cell Sorting before Microinjection of Autologous Oocytes Increases Cumulative Live Birth Rates with Limited Clinical Impact: A Retrospective Study in Unselected Males. Biology 2021, 10, 430. [Google Scholar] [CrossRef]
- Said, T.M.; Agarwal, A.; Grunewald, S.; Rasch, M.; Glander, H.; Paasch, U. Short communication Evaluation of sperm recovery following annexin V magnetic-activated cell sorting separation. Reprod. Biomed. Online 2006, 13, 336–339. [Google Scholar] [CrossRef]
- Ybarra, G.; Viale, D.L.; Vichera, G.; Farini, V.L.; Cama, C.V.; Yakisich, J.S.; Radrizzani, M. Improvement of bovine semen quality by re-moval of membrane-damaged sperm cells with DNA aptamers and magnetic nanoparticles. J. Biotechnol. 2016, 229, 33–41. [Google Scholar]
- Assumpção TI de Severo, N.C.; Zandonaide, J.P.B.; Macedo, G.G. Magnetic-activated cell sorting improves high-quality spermatozoa in bovine semen. J. Anim. Reprod. Biotechnol. 2021, 36, 91–98. [Google Scholar] [CrossRef]
- Malama, E.; Zeron, Y.; Janett, F.; Siuda, M.; Roth, Z.; Bollwein, H. Use of computer-assisted sperm analysis and flow cytometry to detect seasonal variations of bovine semen quality. Theriogenology 2017, 87, 79–90. [Google Scholar] [CrossRef]
- Parati, K.; Bongioni, G.; Aleandri, R.; Galli, A. Sex ratio determination in bovine semen: A new approach by quantitative real time PCR. Theriogenology 2006, 66, 2202–2209. [Google Scholar] [CrossRef]
- E Chandler, J.; Canal, A.M.; Paul, J.; Moser, E. Collection frequency affects percent Y-chromosome bearing sperm, sperm head area and quality of bovine ejaculates. Theriogenology 2002, 57, 1327–1346. [Google Scholar] [CrossRef]
- Ren, F.; Xi, H.; Ren, Y.; Li, Y.; Wen, F.; Xian, M.; Zhao, M.; Zhu, D.; Wang, L.; Lei, A.; et al. TLR7/8 signalling affects X-sperm motility via the GSK3 α/β-hexokinase pathway for the efficient production of sexed dairy goat embryos. J. Anim. Sci. Biotechnol. 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Seidel, G.E., Jr. Economics of selecting for sex: The most important genetic trait. Theriogenology 2003, 59, 585–598. [Google Scholar] [CrossRef]
- Ashry, M.; Lee, K.B.; Folger, J.K.; Rajput, S.K.; Smith, G.W. Follistatin supplementation during in vitro embryo culture improves developmental competence of bovine embryos produced using sex-sorted semen. Reprod. Biol. 2018, 18, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, R.-X.; Chen, X.; He, X.-X.; Han, A.-L.; Fang, G.-Z.; Liu, J.-F.; Wang, S. Study of Efficiency of Coupling Peptides with Gold Nanoparticles. Chin. J. Anal. Chem. 2017, 45, 662–667. [Google Scholar] [CrossRef]
- Otieno, B.; Krause, C.; Rusling, J. Bioconjugation of Antibodies and Enzyme Labels onto Magnetic Beads. Methods Enzymol. 2016, 571, 135–150. [Google Scholar] [CrossRef]
- Contri, A.; Valorz, C.; Faustini, M.; Wegher, L.; Carluccio, A. Effect of semen preparation on casa motility results in cryopreserved bull spermatozoa. Theriogenology 2010, 74, 424–435. [Google Scholar] [CrossRef]
- Abaigar, T.; Holt, W.V.; Harrison, R.A.; del Barrio, G. Sperm subpopulations in boar (Sus scrofa) and gazelle (Gazella dama mhorr) semen as revealed by pattern analysis of computer-assisted motility assessments. Biol. Reprod. 1999, 60, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Alquézar-Baeta, C.; Gimeno-Martos, S.; Jiménez, S.M.; Santolaria, P.; Yániz, J.; Palacín, I.; Casao, A.; Cebrián-Pérez, J.; Muiño-Blanco, T.; Pérez-Pé, R. OpenCASA: A new open-source and scalable tool for sperm quality analysis. PLoS Comput. Biol. 2019, 15, e1006691. [Google Scholar] [CrossRef]
- Domínguez, E.; Moreno-irusta, A.; Ramírez, H.; Flores, A.; Ugaz, C.; Clemente, H.; Giojalas, L.; Losnno, L. Sexing Mediated by Magnetic Nanoparticles in Donkeys, a Preliminary In Vitro Study. J. Equine Vet. Sci. 2018, 65, 123–127. [Google Scholar] [CrossRef]
- Maleki, A.F.; Moussavi, A.R.H.; Nassiri, M.R.; Tahmoorespur, M.; Vakili, S.A. Introducing and validation of SYBR Green Real Time PCR method to determinate sex ratio in bovine semen. Anim. Reprod. Sci. 2013, 140, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Asma-ul-Husna Awan, M.A.; Mehmood, A.; Sultana, T.; Shahzad, Q.; Ansari, M.S.; Rakha, B.A.; Naqvi, S.M.S.; Akhter, S. Sperm sexing in Nili-Ravi buffalo through modified swim up: Validation using SYBR® green real-time PCR. Anim. Reprod. Sci. 2017, 182, 69–76. [Google Scholar] [CrossRef] [PubMed]
- A Habermann, F.; Winter, A.; Olsaker, I.; Reichert, P.; Fries, R. Validation of sperm sexing in the cattle (Bos taurus) by dual colour fluorescence in situ hybridization. J. Anim. Breed. Genet. 2005, 122, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Reinsalu, O.; Scheler, O.; Mikelsaar, R.; Mikelsaar, A.-V.; Hallap, T.; Jaakma, Ü.; Padrik, P.; Kavak, A.; Salumets, A.; Kurg, A. A dual colour FISH method for routine validation of sexed Bos taurus semen. BMC Veter. Res. 2019, 15, 104. [Google Scholar] [CrossRef] [PubMed]
| Gene | Sequence (5′ → 3′) | Length (bp) | Accession No. |
|---|---|---|---|
| Y chromosome specific | |||
| SRY-Forward | GAAAATAAGCACAAGAAAGTCCAGG | 124 | EU581861.1 |
| SRY-Reverse | CAAAAGGAGCATCACAGCAGC | ||
| X chromosome specific | |||
| PLP-Forward | GGTGTGTTAGTTTCTGCTGTACAATAAATGG | 96 | AJ009913.1 |
| PLP-Reverse | GATGGCAGGTGAGGGTAGGA | ||
| Housekeeping gene | |||
| GAPDH-Forward | GGCGCCAAGAGGGTCAT | 120 | NM_001034034.2 |
| GAPDH—Reverse | GGTGGTGCAGGAGGCATT | ||
| Concentration of Y-scFv Antibody (mg/mL) | Supernatant Fraction | Eluted Fraction | ||
|---|---|---|---|---|
| X-Sperm | Y-Sperm | X-Sperm | Y-Sperm | |
| 0 | 50.86 ± 0.58 a | 49.13 ± 0.58 d | 49.90 ± 0.60 b | 50.10 ± 0.60 a |
| 0.5 | 52.26 ± 1.15 ab | 47.73 ± 1.15 cd | 49.13 ± 0.66 b | 50.87 ± 0.66 a |
| 1 | 54.93 ± 0.64 b | 45.06 ± 0.64 c | 46.50 ± 0.60 b | 53.50 ± 0.60 a |
| 2 | 79.04 ± 0.15 c | 20.95 ± 0.15 b | 21.90 ± 0.95 a | 78.01 ± 0.95 b |
| 3 | 80.54 ± 0.61 cd | 19.46 ± 0.61 ab | 21.37 ± 3.06 a | 78.63 ± 3.06 b |
| 4 | 82.65 ± 0.87 d | 17.35 ± 0.87 a | 18.57 ± 1.00 a | 81.43 ± 1.00 b |
| Parameter | Treatment | p-Value | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | |||
| CON | NC | (X-Enriched) | (Y-Enriched) | ||
| TM (%) | 68.79 ± 7.54 b | 64.90 ± 14.30 b | 62.90 ± 12.30 b | 23.55 ± 6.82 a | 0.030 |
| PM (%) | 57.11 ± 9.49 b | 54.12 ± 16.13 b | 52.14 ± 10.16 b | 15.17 ± 6.61 a | 0.021 |
| VCL (µm/s) | 89.52 ± 9.63 b | 79.22 ± 12.85 b | 74.36 ± 10.87 b | 26.04 ± 6.77 a | 0.040 |
| VSL (µm/s) | 35.69 ± 6.07 b | 33.55 ± 10.11 b | 31.25 ± 11.29 b | 11.97 ± 5.66 a | 0.001 |
| VAP (µm/s) | 45.25 ± 6.51 b | 42.01 ± 10.67 b | 40.24 ± 12.55 b | 14.89 ± 5.41 a | 0.001 |
| DCL (µm) | 30.03 ± 5.64 b | 27.25 ± 9.39 b | 24.11 ± 10.48 b | 12.25 ± 5.30 a | 0.010 |
| DSL (µm) | 10.53 ± 3.95 b | 9.09 ± 3.12 b | 8.02 ± 3.42 b | 5.00 ± 2.89 a | 0.024 |
| DAP (µm) | 12.37 ± 2.87 b | 11.34 ± 4.12 b | 10.42 ± 3.12 b | 6.47 ± 3.12 a | 0.041 |
| ALH (µm) | 0.90 ± 0.25 b | 0.81 ± 0.12 b | 0.79 ± 0.41 b | 0.43 ± 0.25 a | 0.025 |
| BCF (Hz) | 9.55 ± 5.45 b | 9.02 ± 0.44 b | 8.34 ± 3.69 b | 4.33 ± 2.27 a | 0.024 |
| HAC (rad) | 0.24 ± 0.15 b | 0.23 ± 0.11 b | 0.21 ± 0.07 b | 0.11 ± 0.08 a | 0.010 |
| WOB (%) | 0.52 ± 0.11 b | 0.53 ± 0.15 b | 0.51 ± 0.06 b | 0.56 ± 0.09 a | 0.008 |
| Parameter | Treatment | p-Value | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | |||
| CON | NC | (X-Enriched) | (Y-Enriched) | ||
| LI | 42.40 ± 7.25 b | 40.20 ± 6.25 b | 38.90 ± 7.77 b | 12.44 ± 3.25 a | 0.001 |
| DI | 30.77 ± 4.42 a | 35.72 ± 5.52 a | 40.20 ± 7.25 a | 60.72 ± 9.01 b | 0.004 |
| LR | 0.77 ± 0.04 b | 0.82 ± 0.24 b | 0.44 ± 0.05 a | 0.42 ± 0.12 a | 0.032 |
| DR | 26.06 ± 2.94 | 23.26 ± 4.35 | 20.46 ± 8.88 | 26.42 ± 1.24 | 0.125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sringarm, K.; Thongkham, M.; Mekchay, S.; Lumsangkul, C.; Thaworn, W.; Pattanawong, W.; Rangabpit, E.; Rachtanapun, P.; Jantanasakulwong, K.; Sathanawongs, A.; et al. High-Efficiency Bovine Sperm Sexing Used Magnetic-Activated Cell Sorting by Coupling scFv Antibodies Specific to Y-Chromosome-Bearing Sperm on Magnetic Microbeads. Biology 2022, 11, 715. https://doi.org/10.3390/biology11050715
Sringarm K, Thongkham M, Mekchay S, Lumsangkul C, Thaworn W, Pattanawong W, Rangabpit E, Rachtanapun P, Jantanasakulwong K, Sathanawongs A, et al. High-Efficiency Bovine Sperm Sexing Used Magnetic-Activated Cell Sorting by Coupling scFv Antibodies Specific to Y-Chromosome-Bearing Sperm on Magnetic Microbeads. Biology. 2022; 11(5):715. https://doi.org/10.3390/biology11050715
Chicago/Turabian StyleSringarm, Korawan, Marninphan Thongkham, Supamit Mekchay, Chompunut Lumsangkul, Wannaluk Thaworn, Wiwat Pattanawong, Ekaphot Rangabpit, Pornchai Rachtanapun, Kittisak Jantanasakulwong, Anucha Sathanawongs, and et al. 2022. "High-Efficiency Bovine Sperm Sexing Used Magnetic-Activated Cell Sorting by Coupling scFv Antibodies Specific to Y-Chromosome-Bearing Sperm on Magnetic Microbeads" Biology 11, no. 5: 715. https://doi.org/10.3390/biology11050715
APA StyleSringarm, K., Thongkham, M., Mekchay, S., Lumsangkul, C., Thaworn, W., Pattanawong, W., Rangabpit, E., Rachtanapun, P., Jantanasakulwong, K., Sathanawongs, A., & Hongsibsong, S. (2022). High-Efficiency Bovine Sperm Sexing Used Magnetic-Activated Cell Sorting by Coupling scFv Antibodies Specific to Y-Chromosome-Bearing Sperm on Magnetic Microbeads. Biology, 11(5), 715. https://doi.org/10.3390/biology11050715










