Environmental Factors Affecting Feather Taphonomy
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
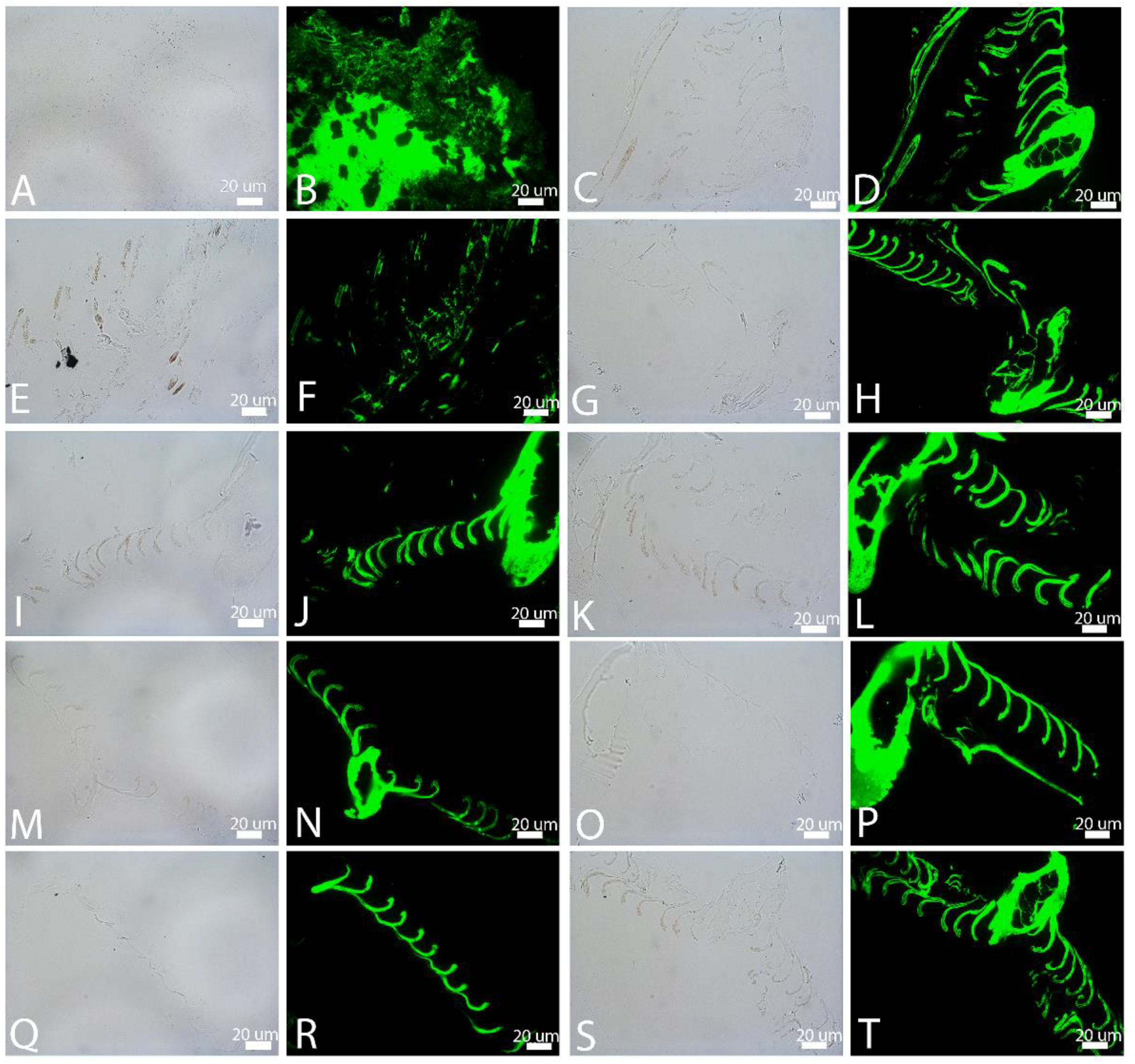
3.1. Transmitted Light Microscopy (LM)
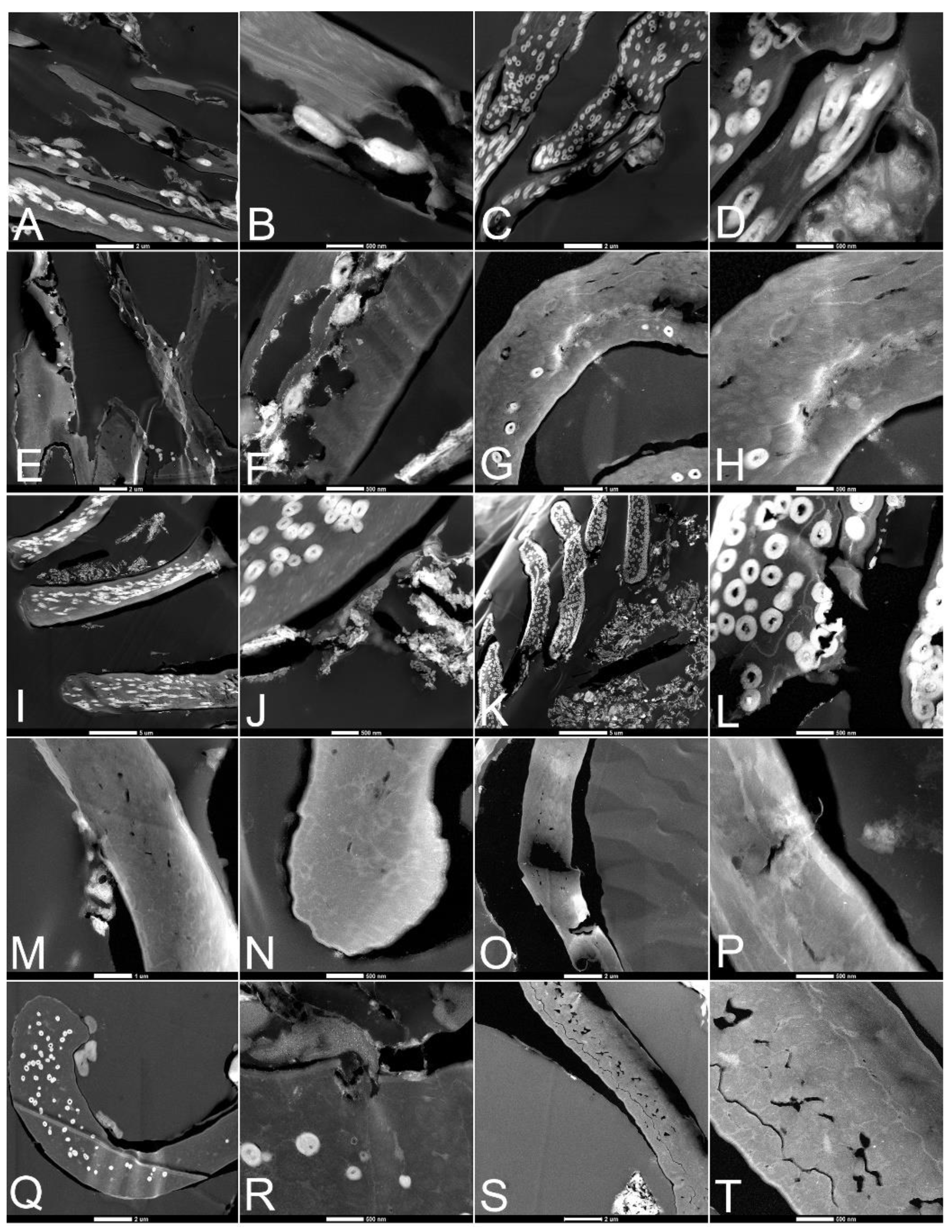
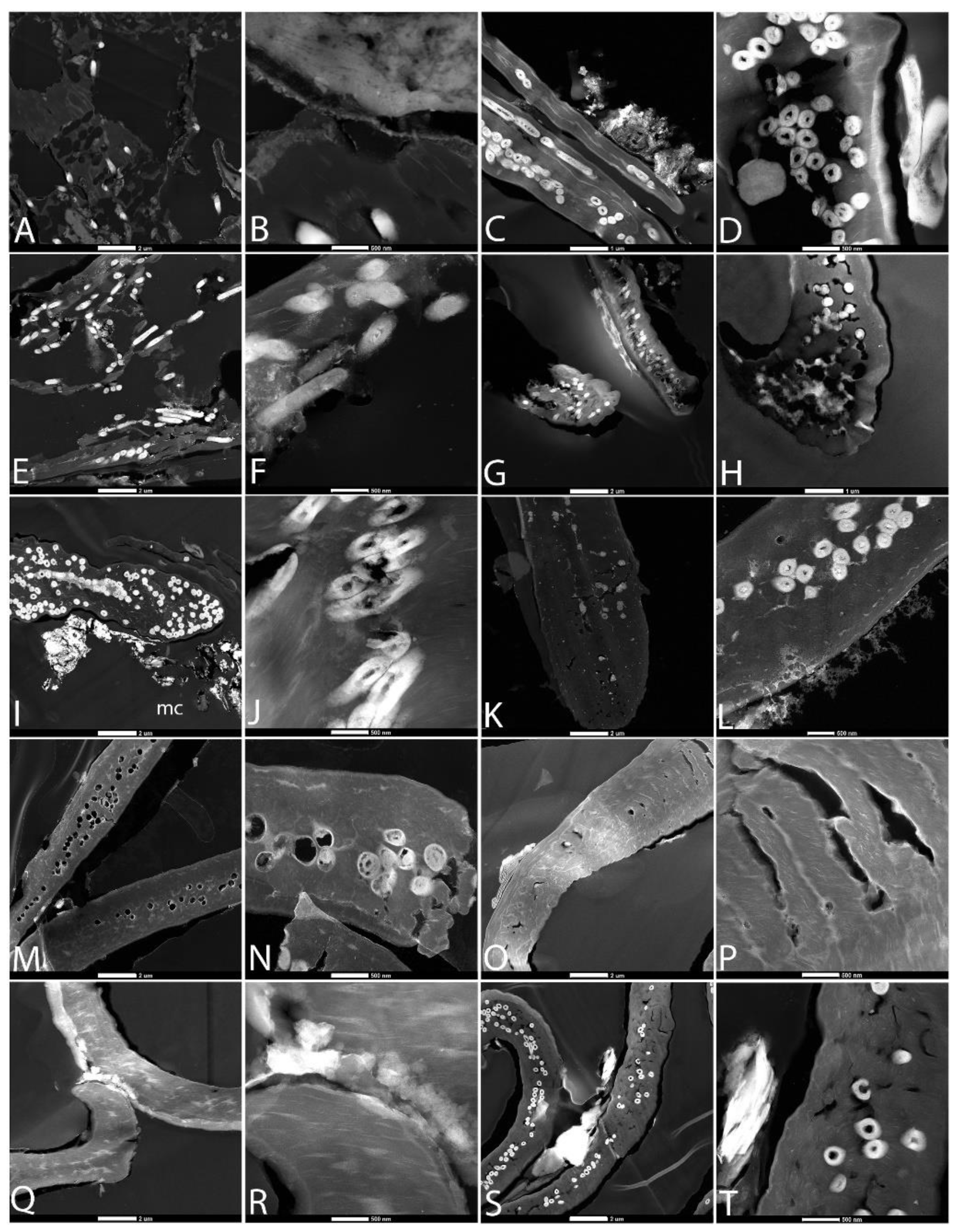
3.2. Scanning Electron Microscopy (SEM)
3.3. Transmission Electron Microscopy (TEM)
3.4. In Situ Immunohistochemistry (IHC)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, Y.; Zheng, W.; Moyer, A.E.; O’Connor, J.K.; Wang, M.; Zheng, X.; Wang, X.; Schroeter, E.R.; Zhou, Z.; Schweitzer, M.H. Molecular evidence of keratin and melanosomes in feathers of the Early Cretaceous bird Eoconfuciusornis. Proc. Natl. Acad. Sci. USA 2016, 113, E7900–E7907. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zheng, W.; Sawyer, R.H.; Pennington, M.W.; Zheng, X.; Wang, X.; Wang, M.; Hu, L.; O’Connor, J.; Zhao, T.; et al. The molecular evolution of feathers with direct evidence from fossils. Proc. Natl. Acad. Sci. USA 2019, 116, 3018–3023. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, M.H.; Watt, J.A.; Avci, R.; Knapp, L.; Chiappe, L.; Norell, M.; Marshall, M. Beta-keratin specific immunological reactivity in feather-like structures of the cretaceous alvarezsaurid, Shuvuuia deserti. J. Exp. Zool. 1999, 285, 146–157. [Google Scholar] [CrossRef]
- Lindgren, J.; Sjövall, P.; Carney, R.M.; Cincotta, A.; Uvdal, P.; Hutcheson, S.W.; Gustafsson, O.; Lefèvre, U.; Escuillié, F.; Heimdal, J.; et al. Molecular composition and ultrastructure of Jurassic paravian feathers. Sci. Rep. 2015, 5, 13520. [Google Scholar] [CrossRef]
- Alonso, J.; Arillo, A.; Barron, E.; Corral, J.C.; Grimalt, J.; Lopez, J.F.; Lopez, R.; Martinez-Delclos, X.; Ortuno, V.; Penalver, E.; et al. A new fossil resin with biological inclusions in Lower Cretaceous deposits from Alava (northern Spain, Basque-Cantabrian Basin). J. Paleontol. 2000, 74, 158–178. [Google Scholar] [CrossRef]
- Lindgren, J.; Sjövall, P.; Carney, R.M.; Cincotta, A.; Uvdal, P.; Hutcheson, S.W.; Gustafsson, O.; Lefèvre, U.; Escuillié, F.; Heimdal, J.; et al. Molecular composition and ultrastructure of jurassic paravian feathers. Lundqua Thesis 2018, 2018, 59–71. [Google Scholar]
- Gren, J.A.; Sjovall, P.; Eriksson, M.E.; Sylvestersen, R.L.; Marone, F.; Sigfridsson, C.K.G.V. Molecular and microstructural inventory of an isolated fossil bird feather from the Eocene Fur Formation of Denmark. Palaeontology 2016, 60, 73–90. [Google Scholar] [CrossRef]
- Manning, P.L.; Edwards, N.P.; Wogelius, R.A.; Bergmann, U.; Barden, H.P.E.; Larson, P.L.; Schwarz-Wings, D.; Egerton, V.M.; Sokaras, D.; Mori, R.A.; et al. Synchrotron-based chemical imaging reveals plumage patterns in a 150 million year old early bird. J. Anal. At. Spectrom. 2013, 28, 1024–1030. [Google Scholar] [CrossRef]
- Chuong, C.M.; Ping, W.; Zhang, F.C.; Xu, X.; Yu, M.; Widelitz, R.B.; Jiang, T.X.; Hou, L. Adaptation to the Sky: Defining the Feather with Integument Fossils from Mesozoic China and Experimental Evidence from Molecular Laboratories. J. Exp. Zool. Part B Mol. Dev. Evol. 2003, 298, 42–56. [Google Scholar] [CrossRef]
- Alibardi, L. Review: Cornification, morphogenesis and evolution of feathers. Protoplasma 2017, 254, 1259–1281. [Google Scholar] [CrossRef]
- Greenwold, M.J.; Bao, W.; Jarvis, E.D.; Hu, H.; Li, C.; Gilbert, M.T.P.; Zhang, G.; Sawyer, R.H. Dynamic evolution of the alpha (α) and beta (β) keratins has accompanied integument diversification and the adaptation of birds into novel lifestyles. BMC Evol. Biol. 2014, 14, 249. [Google Scholar] [CrossRef] [PubMed]
- Greenwold, M.J.; Sawyer, R.H. Linking the molecular evolution of avian beta (β) keratins to the evolution of feathers. J. Exp. Zool. Part B Mol. Dev. Evol. 2011, 316, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Dhouailly, D. A new scenario for the evolutionary origin of hair, feather, and avian scales. J. Anat. 2009, 214, 587–606. [Google Scholar] [CrossRef] [PubMed]
- Prum, R.O.; Brush, A.H. The Evolutionary Origin and Diversification of Feathers. Q. Rev. Biol. 2002, 77, 261–295. [Google Scholar] [CrossRef]
- Greenwold, M.J.; Sawyer, R.H. Genomic organization and molecular phylogenies of the beta keratin multigene family in the chicken (Gallus gallus) and zebra finch (Taeniopygia guttata): Implications for feather evolution. BMC Evol. Biol. 2010, 10, 148. [Google Scholar] [CrossRef]
- Sawyer, R.H.; Knapp, L.W. Avian Skin Development and the Evolutionary Origin of Feathers. J. Exp. Zool. 2003, 298, 57–72. [Google Scholar] [CrossRef]
- Sawyer, R.H.; Washington, L.D.; Salvatore, B.A.; Glenn, T.; Knapp, L.W. Origin of archosaurian integumentary appendages: The bristles of the wild turkey beard express feather-type b-keratins. J. Exp. Zool. B Mol. Devel. Evol. 2003, 279, 27–34. [Google Scholar] [CrossRef]
- Sawyer, R.H.; Salvatore, B.A.; Potylicki, T.T.F.; French, J.O.; Glenn, T.C.; Knapp, L.W. Origin of Feathers: Feather Beta (β) Keratins Are Expressed in Discrete Epidermal Cell Populations of Embryonic Scutate Scales. J. Exp. Zool. Part B Mol. Dev. Evol. 2003, 295, 12–24. [Google Scholar] [CrossRef]
- Hu, D.; Hou, L.; Zhang, L.; Xu, X.; Xing, X.; Xu, X. A pre-Archaeopteryx troodontid theropod from China with long feathers on the metatarsus. Nature 2009, 461, 640–643. [Google Scholar] [CrossRef]
- Qiang, J.; Currie, P.J.; Norell, M.A.; Shu-an, J. Two feathered dinosaurs from northeastern China. Nature 1998, 393, 753–761. [Google Scholar] [CrossRef]
- Kellner, A.W.A. A review of avian Mesozoic fossil feathers. In Mesozoic Birds: Above the Heads of Dinosaurs; Chiappe, L.M., Witmer, L.M., Eds.; UCal Press: Berkeley, CA, USA; Los Angeles, CA, USA; London, UK, 2002; p. 529. ISBN 0-520-20094-2. [Google Scholar]
- Kundrát, M.; Rich, T.H.; Lindgren, J.; Sjövall, P.; Vickers-Rich, P.; Chiappe, L.M.; Kear, B.P. A polar dinosaur feather assemblage from Australia. Gondwana Res. 2020, 80, 1–11. [Google Scholar] [CrossRef]
- Martin, L.D.; Czerkas, S.A. The fossil record of feather evolution in the mesozoic. Am. Zool. 2000, 40, 687–694. [Google Scholar] [CrossRef][Green Version]
- Xu, X.; Zhao, Q.; Norell, M.; Sullivan, C.; Hone, D.; Erickson, G.; Wang, X.; Han, F.; Guo, Y. A new feathered maniraptoran dinosaur fossil that fills a morphological gap in avian origin. Chin. Sci. Bull. 2009, 54, 430–435. [Google Scholar] [CrossRef]
- Wang, X.; Pittman, M.; Zheng, X.; Kaye, T.G.; Falk, A.R.; Hartman, S.A.; Xu, X. Basal paravian functional anatomy illuminated by high-detail body outline. Nat. Commun. 2017, 8, 14576. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, X.; Sullivan, C.; Wang, X.; Xing, L.; Wang, Y.; Zhang, X.; O’Connor, J.K.; Zhang, F.; Pan, Y. A bizarre Jurassic maniraptoran theropod with preserved evidence of membranous wings. Nature 2015, 521, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhou, Z.; Xing, X.; Wang, X.; Sullivan, C. A bizarre Jurassic maniraptoran from China with elongate ribbon-like feathers. Nature 2008, 455, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Lingham-Soliar, T.; Godefroit, P.; Sinitsa, S.M.; Dhouailly, D.; Bolotsky, Y.L.; Sizov, A.V.; McNamara, M.E.; Benton, M.J.; Spagna, P. A Jurassic ornithischian dinosaur from Siberia with both feathers and scales. Science 2014, 345, 451–455. [Google Scholar] [CrossRef][Green Version]
- Zheng, X.-T.; You, H.-L.; Xu, X.; Dong, Z.-M. An Early Cretaceous heterodontosaurid dinosaur with filamentous integumentary structures. Nature 2009, 458, 333–336. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, B.; McNamara, M.E.; Kearns, S.L.; Pittman, M.; Kaye, T.G.; Orr, P.J.; Xu, X.; Benton, M.J. Pterosaur integumentary structures with complex feather-like branching. Nat. Ecol. Evol. 2019, 3, 24–30. [Google Scholar] [CrossRef]
- Benton, M.J.; Dhouailly, D.; Jiang, B.; McNamara, M. The Early Origin of Feathers. Trends Ecol. Evol. 2019, 34, 856–869. [Google Scholar] [CrossRef]
- Saitta, E.T.; Rogers, C.S.; Brooker, R.A.; Vinther, J. Experimental taphonomy of keratin: A structural analysis of early taphonomic changes. Palaios 2017, 32, 647–657. [Google Scholar] [CrossRef]
- Vinther, J.; Nicholls, R.; Lautenschlager, S.; Pittman, M.; Kaye, T.G.; Rayfield, E.; Mayr, G.; Cuthill, I.C. 3D Camouflage in an Ornithischian Dinosaur. Curr. Biol. 2016, 26, 2456–2462. [Google Scholar] [CrossRef] [PubMed]
- Von Meyer, H. Archaeopteryx lithographica (Vogel-Feder) und Pterodactylus von Solnhofen. Geol. Palaeontol. 1861, 29, 678–679. [Google Scholar]
- Owen, R. On the Archaeopteryx of von Meyer, with a description of the fossil remains of a long-tailed species, from the lithographic limestone of Solenhofen. Philos. Trans. R. Soc. London 1863, 153, 33–47. [Google Scholar]
- Von Meyer, H. Vogel-Feder und Palpipes priscus von Solenhofen. Neues Jahrb. Miner. Geogn. Geol. Petrefakten-Kd. 1861, 561, 1861a. [Google Scholar]
- Briggs, D.E.G.G. The role of decay and mineralization in the preservation of soft-bodied fossils. Annu. Rev. Earth Planet. Sci. 2003, 31, 275–301. [Google Scholar] [CrossRef]
- Griffiths, P.J. The isolated Archaeopteryx feather. Archaeopteryx 1996, 14, 1–26. [Google Scholar]
- Moyer, A.E.; Zheng, W.; Schweitzer, M.H. Keratin durability has implications for the fossil record: Results from a 10 year feather degradation experiment. PLoS ONE 2016, 11, e157699. [Google Scholar] [CrossRef]
- McKellar, R.C.; Chatterton, B.D.E.; Wolfe, A.P.; Currie, P.J. A Diverse Assemblage of Late Cretaceous Dinosaur and Bird Feathers from Canadian Amber. Science 2011, 333, 1619–1622. [Google Scholar] [CrossRef]
- Amber, T.M.; Xing, L.; Mckellar, R.C.; Xu, X.; Tseng, K.; Ran, H.; Currie, P.J. Report A Feathered Dinosaur Tail with Primitive Plumage Trapped in Mid-Cretaceous Amber. Curr. Biol. 2016, 26, 3352–3360. [Google Scholar] [CrossRef]
- Davis, P.G.; Briggs, D.E.G. Fossilization of feathers. Geology 1995, 23, 783–786. [Google Scholar] [CrossRef]
- Briggs, D.E.G. The role of biofilms in the fossilization of non-mineralized tissues. In Fossil and Recent Biofilms; Krumbein, W.E., Ed.; Springer Science and Business Media: Dordrecht, The Netherlands, 2003; pp. 281–290. [Google Scholar]
- Parry, L.A.; Smithwick, F.; Nordén, K.K.; Saitta, E.T.; Lozano-Fernandez, J.; Tanner, A.R.; Caron, J.B.; Edgecombe, G.D.; Briggs, D.E.G.; Vinther, J. Soft-Bodied Fossils Are Not Simply Rotten Carcasses—Toward a Holistic Understanding of Exceptional Fossil Preservation: Exceptional Fossil Preservation Is Complex and Involves the Interplay of Numerous Biological and Geological Processes. BioEssays 2018, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Babcock, L.E.; Leslie, S.A.; Elliot, D.H.; Stigall, A.L.; Ford, L.A.; Briggs, D.E.G. The “Preservation Paradox”: Microbes as a Key to Exceptional Fossil Preservation in the Kirkpatrick Basalt (Jurassic), Antarctica. Sediment. Rec. 2006, 4, 4–8. [Google Scholar] [CrossRef]
- Wilby, P.R.; Briggs, D.E.G.G.; Bernier, P.; Gaillard, C. Role of microbial mats in the fossilization of soft tissues. Geology 1996, 24, 787–790. [Google Scholar] [CrossRef]
- Slater, T.S.; McNamara, M.E.; Orr, P.J.; Foley, T.B.; Ito, S.; Wakamatsu, K. Taphonomic experiments resolve controls on the preservation of melanosomes and keratinous tissues in feathers. Palaeontology 2020, 63, 103–115. [Google Scholar] [CrossRef]
- Fletcher, B.J.; Brentnall, S.J.; Anderson, C.W.; Berner, R.A.; Beerling, D.J. Atmospheric carbon dioxide linked with Mesozoic and early Cenozoic climate change. Nat. Geosci. 2008, 1, 43–48. [Google Scholar] [CrossRef]
- Justyn, N.M.; Peteya, J.A.; D’Alba, L.; Shawkey, M.D. Preferential attachment and colonization of the keratinolytic bacterium Bacillus licheniformis on black- A nd white-striped feathers. Auk 2017, 134, 466–473. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Zhang, J.; Zhang, Y.; Han, Y.; Hu, J.; Li, Y. Synthesis and characterization of wool keratin/hydroxyapatite nanocomposite. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 896–902. [Google Scholar] [CrossRef]
- Doney, S.C.; Balch, W.M.; Fabry, V.J.; Feely, R.A. Ocean acidification: A critical emerging problem for the ocean sciences. Oceanography 2009, 22, 16–25. [Google Scholar] [CrossRef]
- Weiss, L.C.; Pötter, L.; Steiger, A.; Kruppert, S.; Frost, U.; Tollrian, R. Rising pCO2 in Freshwater Ecosystems Has the Potential to Negatively Affect Predator-Induced Defenses in Daphnia. Curr. Biol. 2018, 28, 327–332.e3. [Google Scholar] [CrossRef]
- Oh, N.H.; Richter, D.D. Soil acidification induced by elevated atmospheric CO2. Glob. Chang. Biol. 2004, 10, 1936–1946. [Google Scholar] [CrossRef]
- Zeebe, R.E. History of seawater carbonate chemistry, atmospheric CO2, and ocean acidification. Annu. Rev. Earth Planet. Sci. 2012, 40, 141–165. [Google Scholar] [CrossRef]
- Hasler, C.T.; Jeffrey, J.D.; Schneider, E.V.C.; Hannan, K.D.; Tix, J.A.; Suski, C.D. Biological consequences of weak acidification caused by elevated carbon dioxide in freshwater ecosystems. Hydrobiologia 2018, 806, 1–12. [Google Scholar] [CrossRef]
- Stock, S.C.; Köster, M.; Dippold, M.A.; Nájera, F.; Matus, F.; Merino, C.; Boy, J.; Spielvogel, S.; Gorbushina, A.; Kuzyakov, Y. Environmental drivers and stoichiometric constraints on enzyme activities in soils from rhizosphere to continental scale. Geoderma 2019, 337, 973–982. [Google Scholar] [CrossRef]
- Trȩbacz, H.; Wójtowicz, K. Thermal stabilization of collagen molecules in bone tissue. Int. J. Biol. Macromol. 2005, 37, 257–262. [Google Scholar] [CrossRef]
- McMahon, S.; Anderson, R.P.; Saupe, E.E.; Briggs, D.E.G. Experimental evidence that clay inhibits bacterial decomposers: Implications for preservation of organic fossils. Geology 2016, 44, 867–870. [Google Scholar] [CrossRef]
- Collins, M.J.; Nielsen-Marsh, C.M.; Hiller, J.; Smith, C.I.; Roberts, J.P.; Prigodich, R.V.; Wess, T.J.; Csapò, J.; Millard, A.R.; Turner-Walker, G.; et al. The survival of organic matter in bone: A review. Archaeometry 2002, 44, 383–394. [Google Scholar] [CrossRef]
- Moyer, A.E.; Zheng, W.; Johnson, E.A.; Lamanna, M.C.; Li, D.D.Q.; Lacovara, K.J.; Schweitzer, M.H. Melanosomes or microbes: Testing an alternative hypothesis for the origin of microbodies in fossil feathers. Sci. Rep. 2014, 4, 4233. [Google Scholar] [CrossRef]
- Field, D.J.; D’Alba, L.; Vinther, J.; Webb, S.M.; Gearty, W.; Shawkey, M.D. Melanin Concentration Gradients in Modern and Fossil Feathers. PLoS ONE 2013, 8, e59451. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Moyer, A.E.; Zheng, W. Testing the hypothesis of biofilm as a source for soft tissue and cell-like structures preserved in dinosaur bone. PLoS ONE 2016, 11, e150238. [Google Scholar] [CrossRef]
- Chafetz, H.S.; Guidry, S.A. Bacterial shrubs, crystal shrubs, and ray-crystal shrubs: Bacterial vs. abiotic precipitation. Sediment. Geol. 1999, 126, 57–74. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R.; Shahin, M.A. Cementation of sand soil by microbially induced calcite precipitation at various degrees of saturation. Can. Geotech. J. 2013, 50, 81–90. [Google Scholar] [CrossRef]
- Mortensen, B.M.; Haber, M.J.; Dejong, J.T.; Caslake, L.F.; Nelson, D.C. Effects of environmental factors on microbial induced calcium carbonate precipitation. J. Appl. Microbiol. 2011, 111, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Darvell, B.W. Effect of carbonate on hydroxyapatite Solubility. Cryst. Growth Des. 2010, 10, 845–850. [Google Scholar] [CrossRef]
- Keenan, S.W. From bone to fossil: A review of the diagenesis of bioapatite. Am. Miner. 2016, 101, 1943–1951. [Google Scholar] [CrossRef]
- Sykes, G.A.; Collins, M.J.; Walton, D.I. The significance of a geochemically isolated intracrystalline organic fraction within biominerals. Org. Geochem. 1995, 23, 1059–1065. [Google Scholar] [CrossRef]
- Gunderson, A.R.; Frame, A.M.; Swaddle, J.P.; Forsyth, M.H. Resistance of melanized feathers to bacterial degradation: Is it really so black and white? J. Avian Biol. 2008, 39, 539–545. [Google Scholar] [CrossRef]
- Sadowsky, M.J.; Schortemeyer, M. Soil microbial responses to increased concentrations of atmospheric CO2. Glob. Chang. Biol. 1997, 3, 217–224. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Blagodatsky, S.; Dorodnikov, M.; Kuzyakov, Y. Elevated atmospheric CO2 increases microbial growth rates in soil: Results of three CO2 enrichment experiments. Glob. Chang. Biol. 2010, 16, 836–848. [Google Scholar] [CrossRef]
- Yu, T.; Chen, Y. Effects of elevated carbon dioxide on environmental microbes and its mechanisms: A review. Sci. Total Environ. 2019, 655, 865–879. [Google Scholar] [CrossRef]
- Jin, J.; Tang, C.; Robertson, A.; Franks, A.E.; Armstrong, R.; Sale, P. Increased microbial activity contributes to phosphorus immobilization in the rhizosphere of wheat under elevated CO2. Soil Biol. Biochem. 2014, 75, 292–299. [Google Scholar] [CrossRef]
- Crosby, C.H.; Bailey, J.V. The role of microbes in the formation of modern and ancient phosphatic mineral deposits. Front. Microbiol. 2012, 3, 241. [Google Scholar] [CrossRef] [PubMed]
- Plet, C.; Grice, K.; Pagès, A.; Ruebsam, W.; Coolen, M.J.L.; Schwark, L. Microbially-mediated fossil-bearing carbonate concretions and their significance for palaeoenvironmental reconstructions: A multi-proxy organic and inorganic geochemical appraisal. Chem. Geol. 2016, 426, 95–108. [Google Scholar] [CrossRef]
- Romanowski, G.; Lorenz, M.G.; Wackernagel, W. Adsorption of Plasmid DNA to Mineral Surfaces and Protection against Dnase-I. Appl. Environ. Microbiol. 1991, 57, 1057–1061. [Google Scholar] [CrossRef]
- Playter, T.; Konhauser, K.; Owttrim, G.; Hodgson, C.; Warchola, T.; Mloszewska, A.M.; Sutherland, B.; Bekker, A.; Zonneveld, J.P.; Pemberton, S.G.; et al. Microbe-clay interactions as a mechanism for the preservation of organic matter and trace metal biosignatures in black shales. Chem. Geol. 2017, 459, 75–90. [Google Scholar] [CrossRef]
- Muscente, A.D.; Schiffbauer, J.D.; Broce, J.; Laflamme, M.; O’Donnell, K.; Boag, T.H.; Meyer, M.; Hawkins, A.D.; Huntley, J.W.; McNamara, M.; et al. Exceptionally preserved fossil assemblages through geologic time and space. Gondwana Res. 2017, 48, 164–188. [Google Scholar] [CrossRef]
- Schiffbauer, J.D.; Laflamme, M. Lagersttten through time: A collection of exceptional preservational pathways from the terminal neoproterozoic through today. Palaios 2012, 27, 275–278. [Google Scholar] [CrossRef]
- Retallack, G.J. Permian and Triassic greenhouse crises. Gondwana Res. 2013, 24, 90–103. [Google Scholar] [CrossRef]
- Breecker, D.O.; Sharp, Z.D.; McFadden, L.D. Atmospheric CO2 concentrations during ancient greenhouse climates were similar to those predicted for A.D. 2100. Proc. Natl. Acad. Sci. USA 2010, 107, 576–580. [Google Scholar] [CrossRef]
- Gienapp, P.; Teplitsky, C.; Alho, J.S.; Mills, J.A.; Merilä, J. Climate change and evolution: Disentangling environmental and genetic responses. Mol. Ecol. 2008, 17, 167–178. [Google Scholar] [CrossRef]
- Mills, B.J.W.; Krause, A.J.; Scotese, C.R.; Hill, D.J.; Shields, G.A.; Lenton, T.M. Modelling the long-term carbon cycle, atmospheric CO2, and Earth surface temperature from late Neoproterozoic to present day. Gondwana Res. 2019, 67, 172–186. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Pagani, M.; Liu, Z.; Bohaty, S.M.; Deconto, R. A 40-million-year history of atmospheric CO 2 A -million-year history of atmospheric CO. Phil. Trans. R. Soc. B 2013, 271, 20130096. [Google Scholar] [CrossRef] [PubMed]
- Wilby, P.R. The Role of Organic Matrices in Post-Mortem Phosphatization of Soft-Tissues. Kaupla Darm. Beitr. Naturg. 1993, 2, 99–113. [Google Scholar]
- Hubert, B.; Álvaro, J.J.; Chen, J.Y. Microbially mediated phosphatization in the Neoproterozoic Doushantuo Lagerstätte, South China. Bull. Soc. Geol. Fr. 2005, 176, 355–361. [Google Scholar] [CrossRef]
- Dornbos, S.Q. Phosphatization through the Phanerozoic. In Taphonomy; Springer: Dordrecht, The Netherlands, 2010; Volume 32, pp. 435–456. [Google Scholar] [CrossRef]
- Martill, D.M. The Medusa effect: Instantaneous fossilization. Geol. Today 1989, 5, 201–205. [Google Scholar] [CrossRef]





| Ambient Atmosphere | Elevated CO2 Atmosphere |
|---|---|
| Pondwater, buried (PB) | Pondwater, buried (CPB) |
| Pondwater, exposed (PE) | Pondwater, exposed (CPE) |
| Pondwater + Bacillus licheniformis, buried (PBB) | Pondwater + B.licheniformis, buried (CPBB) |
| Pondwater + B.licheniformis, exposed (PBE) | Pondwater + B.licheniformis, exposed (CPBE) |
| Pondwater + Hydroxylapatite, buried (PMB) | Pondwater + HA, buried (CPMB) |
| Pondwater + HA, exposed (PME) | Pondwater + HA, exposed (CPME) |
| E-Pure water, sand + HA, buried (ESMB) | E-Pure water, sand + HA, buried (CESMB) |
| E-Pure water, sand + HA, exposed (ESME) | E-Pure water, sand + HA, exposed (CESME) |
| E-Pure water, sand, buried (ESB) | E-Pure water, sand, buried (CESB) |
| E-Pure water, sand, exposed (ESE) | E-Pure water, sand, exposed (CESE) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schweitzer, M.H.; Zheng, W.; Equall, N. Environmental Factors Affecting Feather Taphonomy. Biology 2022, 11, 703. https://doi.org/10.3390/biology11050703
Schweitzer MH, Zheng W, Equall N. Environmental Factors Affecting Feather Taphonomy. Biology. 2022; 11(5):703. https://doi.org/10.3390/biology11050703
Chicago/Turabian StyleSchweitzer, Mary Higby, Wenxia Zheng, and Nancy Equall. 2022. "Environmental Factors Affecting Feather Taphonomy" Biology 11, no. 5: 703. https://doi.org/10.3390/biology11050703
APA StyleSchweitzer, M. H., Zheng, W., & Equall, N. (2022). Environmental Factors Affecting Feather Taphonomy. Biology, 11(5), 703. https://doi.org/10.3390/biology11050703







