Functional Remodeling of the Contractile Smooth Muscle Cell Cortex, a Provocative Concept, Supported by Direct Visualization of Cortical Remodeling
Abstract
Simple Summary
Abstract
1. Background
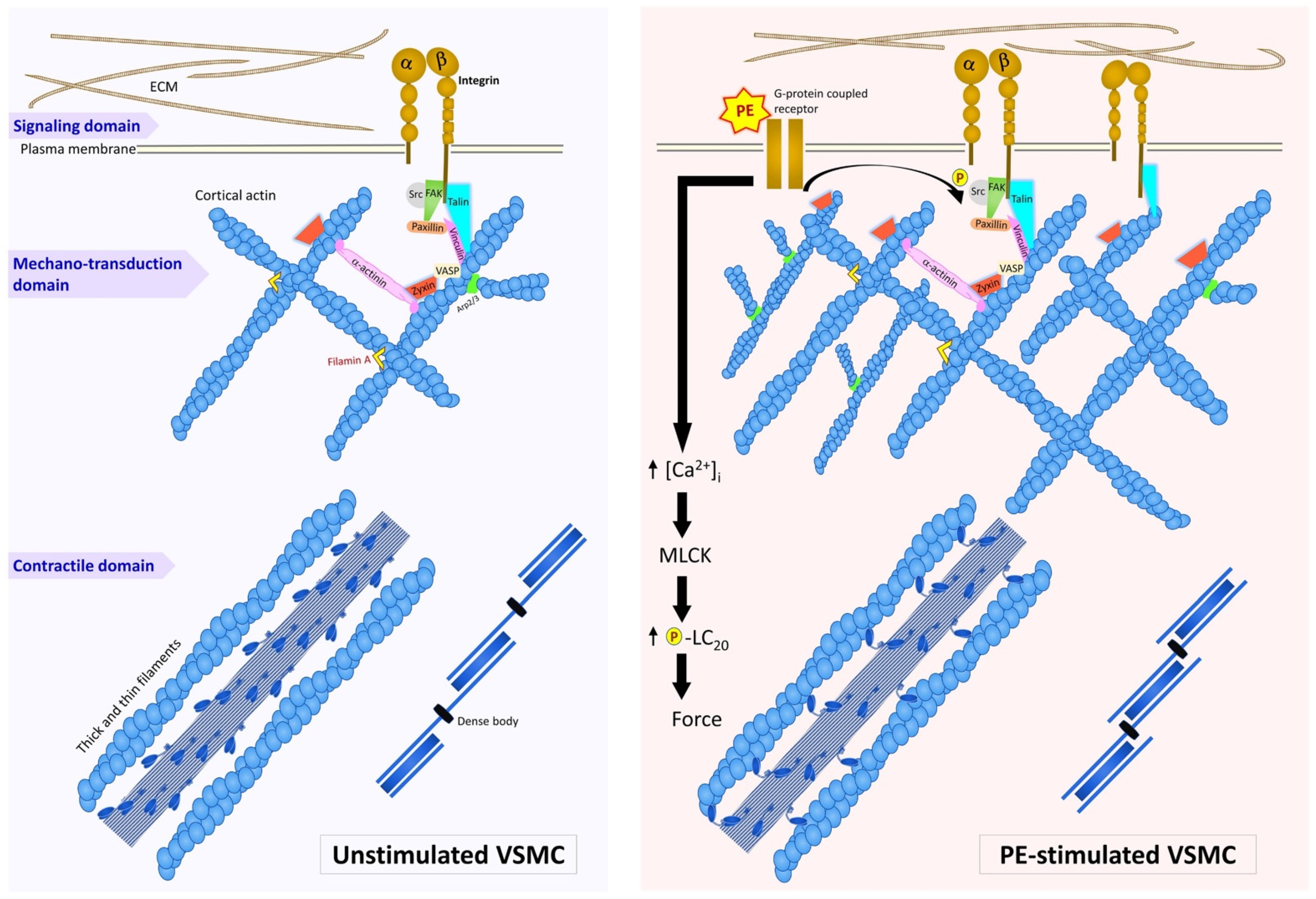
2. Organization of Actin Filaments in the Vascular Smooth Muscle Cell
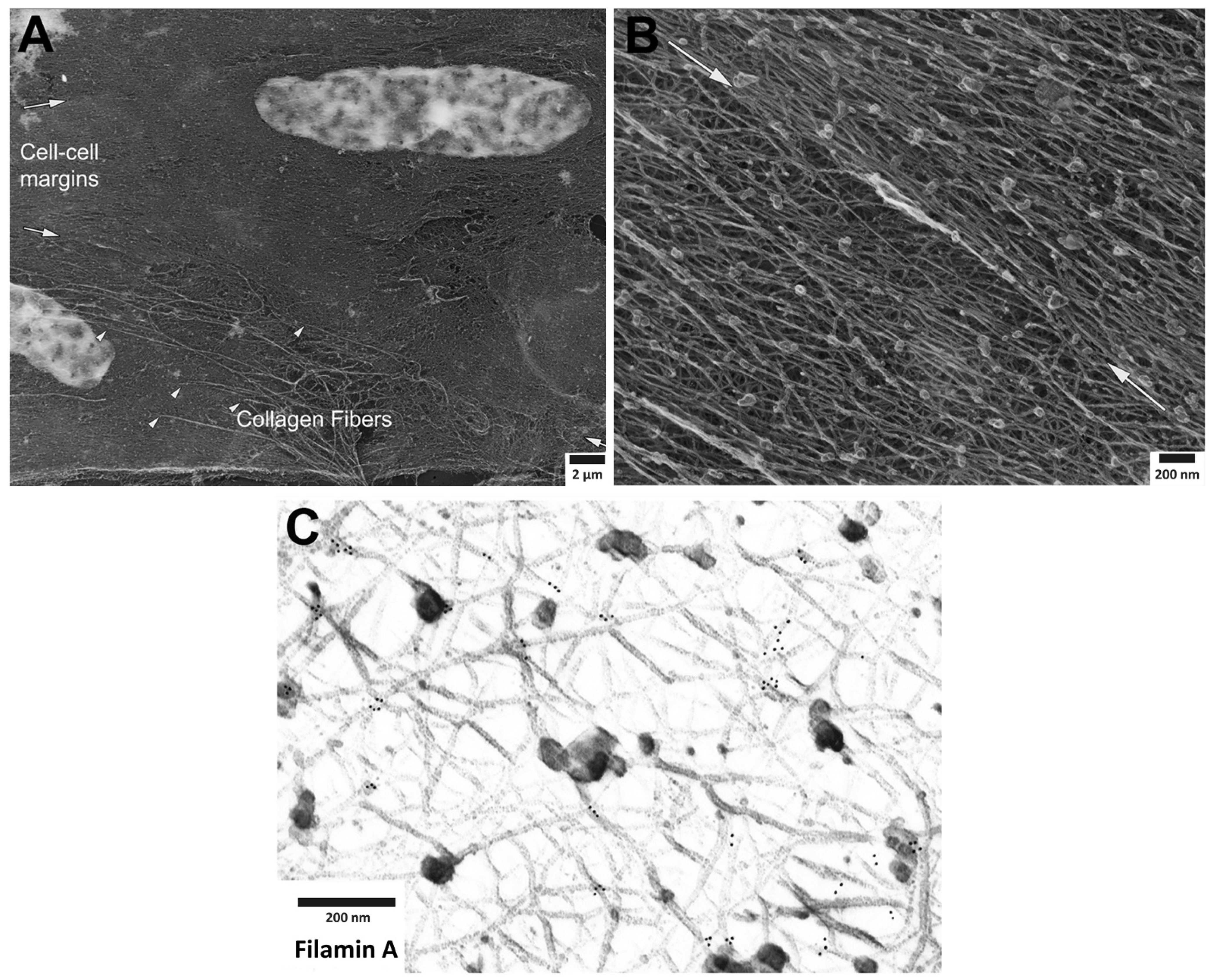
3. Roles of Adhesion Proteins on the Cortical Actin
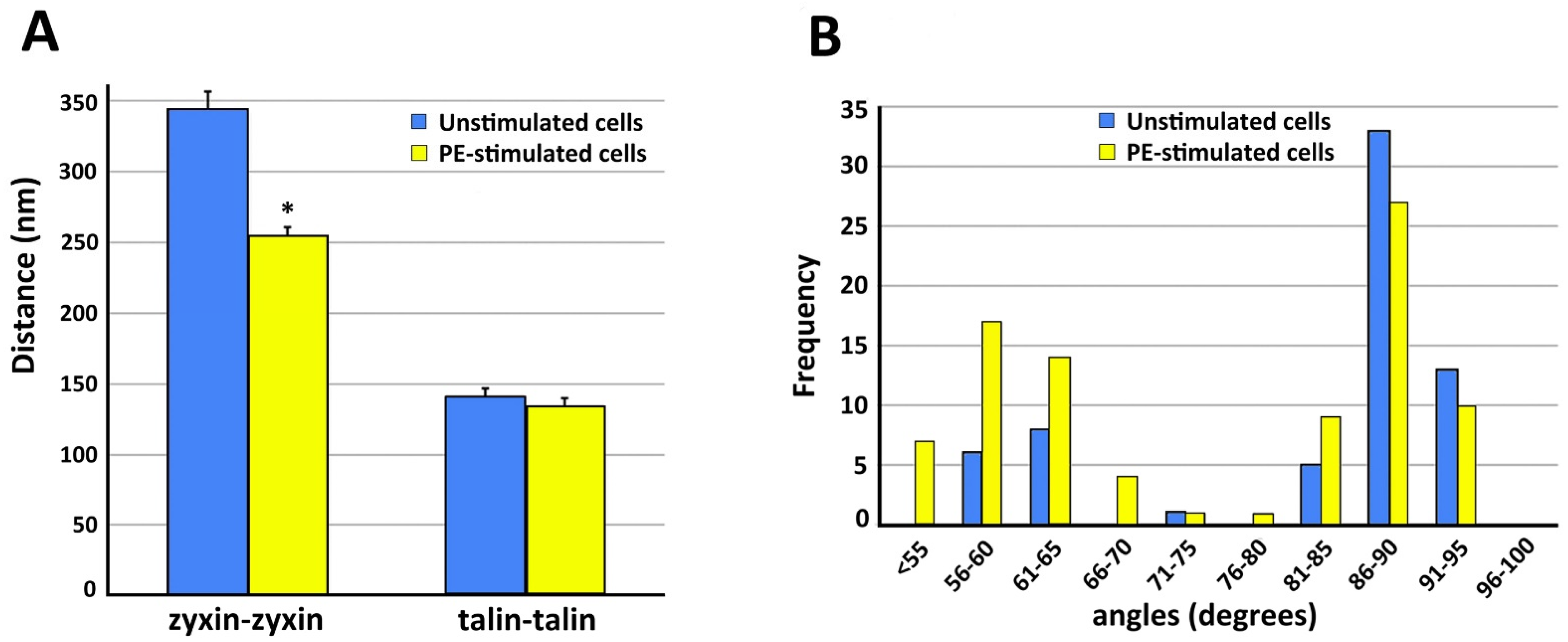
4. Dynamics of Smooth Muscle Cell Cytoskeleton
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Materials and Methods
Appendix A.1. Preparation of Smooth Muscle Cells
Appendix A.2. Immunoelectron Microscopy and Application of Metal Replica Methodology
Appendix A.3. Antibodies
References
- Guo, D.C.; Papke, C.L.; Tran-Fadulu, V.; Regalado, E.S.; Avidan, N.; Johnson, R.J.; Kim, D.H.; Pannu, H.; Wjlling, M.C.; Sparks, E.; et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am. J. Hum. Genet. 2009, 84, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Milewicz, D.M.; Guo, D.C.; Tran-Fadulu, V.; Lafont, A.L.; Papke, C.L.; Inamoto, S.; Kwartler, C.S.; Pannu, H. Genetic basis of thoracic aortic aneurysms and dissections: Focus on smooth muscle cell contractile dysfunction. Annu. Rev. Genom. Hum. Genet. 2008, 9, 283–302. [Google Scholar] [CrossRef]
- Gunning, P.W.; Ghoshdastider, U.; Whitaker, S.; Popp, D.; Robinson, R.C. The evolution of compositionally and functionally distinct actin filaments. J. Cell Sci. 2015, 128, 2009–2019. [Google Scholar] [CrossRef]
- Winder, S.J.; Ayscough, K.R. Actin-binding proteins. J. Cell Sci. 2005, 118, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D. Actin and actin-binding proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a018226. [Google Scholar] [CrossRef]
- Kadzik, R.S.; Homa, K.E.; Kovar, D.R. F-Actin cytoskeleton network self-organization through competition and cooperation. Annu. Rev. Cell Dev. Biol. 2020, 36, 35–60. [Google Scholar] [CrossRef] [PubMed]
- Rassier, D.E.; MacIntosh, B.R.; Herzog, W. Length dependence of active force production in skeletal muscle. J. Appl. Physiol. 1999, 86, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- North, A.J.; Gimona, M.; Lando, Z.; Small, J.V. Actin isoform compartments in chicken gizzard smooth muscle cells. J. Cell Sci. 1994, 107, 445–455. [Google Scholar] [CrossRef]
- Kim, H.R.; Gallant, C.; Leavis, P.C.; Gunst, S.J.; Morgan, K.G. Cytoskeletal remodeling in differentiated vascular smooth muscle is actin isoform dependent and stimulus dependent. Am. J. Physiol. Cell Physiol. 2008, 295, C768–C778. [Google Scholar] [CrossRef]
- Exton, J.H. Mechanisms involved in alpha-adrenergic phenomena. Am. J. Physiol. 1985, 248, E633–E647. [Google Scholar] [CrossRef]
- Poythress, R.H.; Gallant, C.; Vetterkind, S.; Morgan, K.G. Vasoconstrictor-induced endocytic recycling regulates focal adhesion protein localization and function in vascular smooth muscle. Am. J. Physiol. Cell Physiol. 2013, 305, C215–C227. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Drew, J.S.; Moos, C.; Murphy, R.A. Localization of isoactins in isolated smooth muscle thin filaments by double gold immunolabeling. Am. J. Physiol. 1991, 260, C1332–C1340. [Google Scholar] [CrossRef]
- Perrin, B.J.; Ervasti, J.M. The actin gene family: Function follows isoform. Cytoskeleton 2010, 67, 630–634. [Google Scholar] [CrossRef]
- Vedula, P.; Kurosaka, S.; Leu, N.A.; Wolf, Y.I.; Shabalina, S.A.; Wang, J.; Sterling, S.; Dong, D.W.; Kashina, A. Diverse functions of homologous actin isoforms are defined by their nucleotide, rather than their amino acid sequence. eLife 2017, 6, e31661. [Google Scholar] [CrossRef]
- Gallant, C.; Appel, S.; Graceffa, P.; Leavis, P.; Lin, J.J.; Gunning, P.W.; Schevzov, G.; Chaponnier, C.; DeGnore, J.; Lehman, W.; et al. Tropomyosin variants describe distinct functional subcellular domains in differentiated vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2011, 300, C1356–C1365. [Google Scholar] [CrossRef]
- Ingber, D.E. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ. Res. 2002, 91, 877–887. [Google Scholar] [CrossRef]
- Gunst, S.J.; Zhang, W. Actin cytoskeletal dynamics in smooth muscle: A new paradigm for the regulation of smooth muscle contraction. Am. J. Physiol. Cell Physiol. 2008, 295, C576–C587. [Google Scholar] [CrossRef]
- Zaidel-Bar, R. Evolution of complexity in the integrin adhesome. J. Cell Biol. 2009, 186, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Rossier, O.; Octeau, V.; Sibarita, J.B.; Leduc, C.; Tessier, B.; Nair, D.; Gatterdam, V.; Destaing, O.; Albigès-Rizo, C.; Tampé, R.; et al. Integrins β1 and β3 exhibit distinct dynamic nanoscale organizations inside focal adhesions. Nat. Cell Biol. 2012, 14, 1057–1067. [Google Scholar] [CrossRef]
- Mullins, R.D.; Heuser, J.A.; Pollard, T.D. The interaction of Arp2/3 complex with actin: Nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl. Acad. Sci. USA 1998, 95, 6181–6186. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, F.; Stossel, T.P.; Hartwig, J.H. The filamins: Organizers of cell structure and function. Cell Adhes. Migr. 2011, 5, 160–169. [Google Scholar] [CrossRef]
- Lamsoul, I.; Dupré, L.; Lutz, P.G. Molecular tuning of filamin A activities in the context of adhesion and migration. Front. Cell Dev. Biol. 2020, 8, 591323. [Google Scholar] [CrossRef]
- Crawford, A.W.; Michelsen, J.W.; Beckerle, M.C. An interaction between zyxin and alpha-actinin. J. Cell Biol. 1992, 116, 1381–1393. [Google Scholar] [CrossRef]
- Svitkina, T.M.; Borisy, G.G. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 1999, 145, 1009–1026. [Google Scholar] [CrossRef] [PubMed]
- Drees, B.; Friederich, E.; Fradelizi, J.; Louvard, D.; Beckerle, M.C.; Golsteyn, R.M. Characterization of the interaction between zyxin and members of the Ena/vasodilator-stimulated phosphoprotein family of proteins. J. Biol. Chem. 2000, 275, 22503–22511. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Graceffa, P.; Ferron, F.; Gallant, C.; Boczkowska, M.; Dominguez, R.; Morgan, K.G. Actin polymerization in differentiated vascular smooth muscle cells requires vasodilator- stimulated phosphoprotein. Am. J. Physiol. Cell Physiol. 2010, 298, C559–C571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Wang, D.Y.; Guo, Y.C.; Guo, J. Zyxin: A mechanotransductor to regulate gene expression. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 413–425. [Google Scholar] [CrossRef]
- Cattaruzza, M.; Lattrich, C.; Hecker, M. Focal adhesion protein zyxin is a mechanosensitive modulator of gene expression in vascular smooth muscle cells. Hypertension 2004, 43, 726–730. [Google Scholar] [CrossRef]
- Ghosh, S.; Kollar, B.; Nahar, T.; Babu, S.S.; Wojtowicz, A.; Sticht, C.; Gretz, N.; Wagner, A.H.; Korff, T.; Hecker, M. Loss of the mechanotransducer zyxin promotes a synthetic phenotype of vascular smooth muscle cells. J. Am. Heart Assoc. 2015, 4, e001712. [Google Scholar] [CrossRef] [PubMed]
- Yoshigi, M.; Hoffman, L.M.; Jensen, C.C.; Yost, H.J.; Beckerle, M.C. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J. Cell Biol. 2005, 171, 209–215. [Google Scholar] [CrossRef]
- Klapholz, B.; Brown, N.H. Talin—The master of integrin adhesions. J. Cell Sci. 2017, 130, 2435–2446. [Google Scholar] [CrossRef] [PubMed]
- Margadant, F.; Chew, L.L.; Hu, X.; Yu, H.; Bate, N.; Zhang, X.; Sheetz, M. Mechanotransduction in vivo by repeated talin stretch-relaxation events depends upon vinculin. PLoS Biol. 2011, 9, e1001223. [Google Scholar] [CrossRef]
- Peng, X.; Nelson, E.S.; Maiers, J.L.; DeMali, K.A. New insights into vinculin function and regulation. Int. Rev. Cell Mol. Biol. 2011, 287, 191–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, G.; Cai, Y.; Monkley, S.J.; Critchley, D.R.; Sheetz, M.P. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat. Cell Biol. 2008, 10, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Pasapera, A.M.; Schneider, I.C.; Rericha, E.; Schlaepfer, D.D.; Waterman, C.M. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J. Cell Biol. 2010, 188, 877–890. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Goh, W.I.; Goh, H.; Baird, M.A.; Ruehland, S.; Teo, S.; Bate, N.; Critchley, D.R.; Davidson, M.W.; et al. Talin determines the nanoscale architecture of focal adhesions. Proc. Natl. Acad. Sci. USA 2015, 112, E4864–E4873. [Google Scholar] [CrossRef] [PubMed]
- Saphirstein, R.J.; Gao, Y.Z.; Lin, Q.Q.; Morgan, K.G. Cortical actin regulation modulates vascular contractility and compliance in veins. J. Physiol. 2015, 593, 3929–3941. [Google Scholar] [CrossRef]
- Kanchanawong, P.; Shtengel, G.; Pasapera, A.M.; Ramko, E.B.; Davidson, M.W.; Hess, H.F.; Waterman, C.M. Nanoscale architecture of integrin-based cell adhesions. Nature 2010, 468, 580–584. [Google Scholar] [CrossRef]
- Burridge, K.; Fath, K.; Kelly, T.; Nuckolls, G.; Turner, C. Focal adhesions: Transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu. Rev. Cell Biol. 1988, 4, 487–525. [Google Scholar] [CrossRef]
- Zhang, W.; Gunst, S.J. Interactions of airway smooth muscle cells with their tissue matrix: Implications for contraction. Proc. Am. Thorac. Soc. 2008, 5, 32–39. [Google Scholar] [CrossRef]
- Ohanian, J.; Pieri, M.; Ohanian, V. Non-receptor tyrosine kinases and the actin cytoskeleton in contractile vascular smooth muscle. J. Physiol. 2015, 593, 3807–3814. [Google Scholar] [CrossRef] [PubMed]
- Brozovich, F.V.; Nicholson, C.J.; Degen, C.V.; Gao, Y.Z.; Aggarwal, M.; Morgan, K.G. Mechanisms of vascular smooth muscle contraction and the basis for pharmacologic treatment of smooth muscle disorders. Pharmacol. Rev. 2016, 68, 476–532. [Google Scholar] [CrossRef] [PubMed]
- Saphirstein, R.J.; Gao, Y.Z.; Jensen, M.H.; Gallant, C.M.; Vetterkind, S.; Moore, J.R.; Morgan, K.G. The focal adhesion: A regulated component of aortic stiffness. PLoS ONE 2013, 8, e62461. [Google Scholar] [CrossRef] [PubMed]
- Turk, M.; Baumeister, W. The promise and the challenges of cryo-electron tomography. FEBS Lett. 2020, 594, 3243–3261. [Google Scholar] [CrossRef]
- Burbaum, L.; Schneider, J.; Scholze, S.; Böttcher, R.T.; Baumeister, W.; Schwille, P.; Plitzko, J.M.; Jasnin, M. Molecular-scale visualization of sarcomere contraction within native cardiomyocytes. Nat. Commun. 2021, 12, 4086. [Google Scholar] [CrossRef] [PubMed]
- Tacke, S.; Erdmann, P.; Wang, Z.; Klumpe, S.; Grange, M.; Plitzko, J.; Raunser, S. A streamlined workflow for automated cryo focused ion beam milling. J. Struct. Biol. 2021, 213, 107743. [Google Scholar] [CrossRef]
- Wang, Z.; Grange, M.; Wagner, T.; Kho, A.L.; Gautel, M.; Raunser, S. The molecular basis for sarcomere organization in vertebrate skeletal muscle. Cell 2021, 184, 2135–2150.e13. [Google Scholar] [CrossRef]
- Fox, J.G.; Schultz, C.S.; Boler, B.M.V. Nutrition of the Ferret. In Biology and Diseases of the Ferret, 3rd ed.; Fox, J.G., Marini, R.P., Eds.; John Wiley & Sons: Ames, IA, USA, 2014; pp. 123–143. [Google Scholar] [CrossRef]
- Flanagan, L.A.; Chou, J.; Falet, H.; Neujahr, R.; Hartwig, J.H.; Stossel, T.P. Filamin A, the Arp2/3 complex, and the morphology and function of cortical actin filaments in human melanoma cells. J. Cell Biol. 2001, 155, 511–517. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suphamungmee, W.; Lehman, W.; Morgan, K.G. Functional Remodeling of the Contractile Smooth Muscle Cell Cortex, a Provocative Concept, Supported by Direct Visualization of Cortical Remodeling. Biology 2022, 11, 662. https://doi.org/10.3390/biology11050662
Suphamungmee W, Lehman W, Morgan KG. Functional Remodeling of the Contractile Smooth Muscle Cell Cortex, a Provocative Concept, Supported by Direct Visualization of Cortical Remodeling. Biology. 2022; 11(5):662. https://doi.org/10.3390/biology11050662
Chicago/Turabian StyleSuphamungmee, Worawit, William Lehman, and Kathleen G. Morgan. 2022. "Functional Remodeling of the Contractile Smooth Muscle Cell Cortex, a Provocative Concept, Supported by Direct Visualization of Cortical Remodeling" Biology 11, no. 5: 662. https://doi.org/10.3390/biology11050662
APA StyleSuphamungmee, W., Lehman, W., & Morgan, K. G. (2022). Functional Remodeling of the Contractile Smooth Muscle Cell Cortex, a Provocative Concept, Supported by Direct Visualization of Cortical Remodeling. Biology, 11(5), 662. https://doi.org/10.3390/biology11050662






