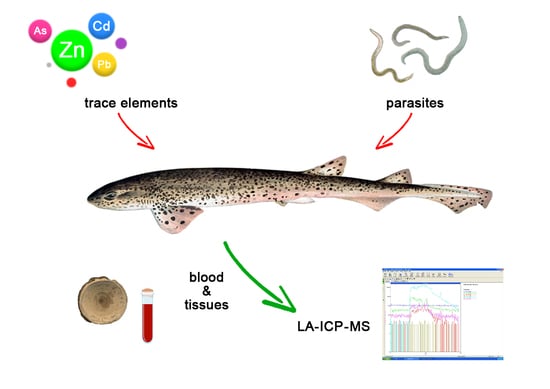
Parasitic Load, Hematological Parameters, and Trace Elements Accumulation in the Lesser Spotted Dogfish Scyliorhinus canicula from the Central Tyrrhenian Sea
Abstract
Simple Summary
Abstract
1. Introduction
- (1)
- the helminthic communities and epidemiological indices in the anatomical districts, including correlations between the parasitic load and sex of the sharks;
- (2)
- the hematological parameters in the blood and their correlations with parasitic load;
- (3)
- the concentrations of trace elements in target tissues (vertebrae, skin, and liver) and their differences in bioaccumulation related to sex, total length (TL), parasitic load and growth rates in vertebrae of these sharks.
2. Materials and Methods
2.1. Sampling Collection
2.2. Parasitological Analyses
2.3. Hematological Analyses
2.4. Ecotoxicological Analyses
2.5. Statistical Analyses
3. Results
3.1. Parasitology
3.2. Hematology
3.3. Ecotoxicology
- -
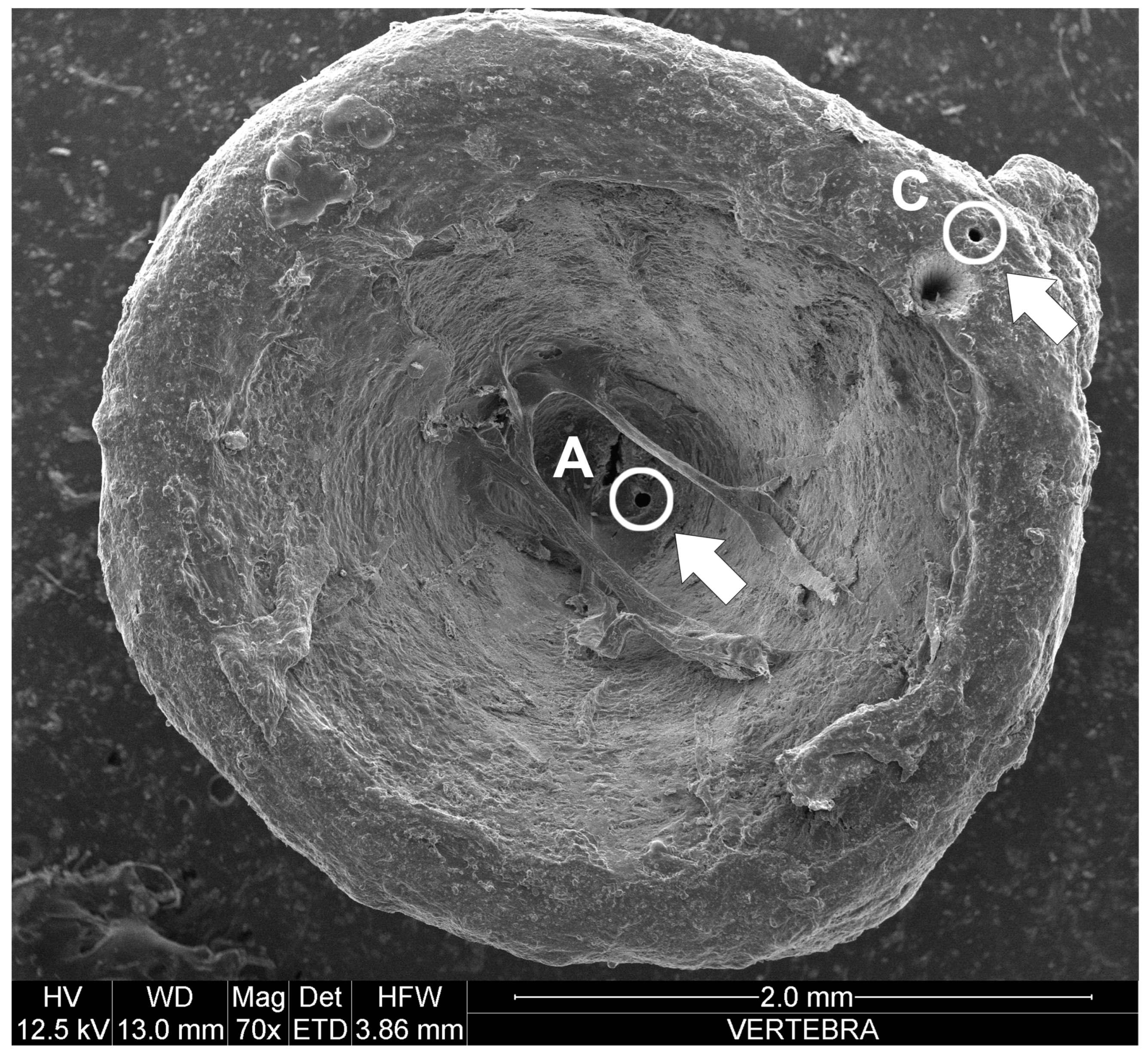
- in vertebrae, Pb decreased with the TL of the specimens (R = 0.8; p value = 0.02; n = 75);
- -
- in vertebrae, As decreased from the juvenile (Point A of the vertebrae) to the adult (Point C of the vertebrae) phase (U = 2083; U′ = 3542; p value = 0.006; n = 75).
- -
- in vertebrae, Mn increased from the juvenile (Point A of the vertebrae) to the adult (Point C of the vertebrae) phase (U = 1168.5; U′ = 4456.5; p value < 0.0001; n = 75);
- -
- in vertebrae, Zn increased from the juvenile (Point A of the vertebrae) to the adult (Point C of the vertebrae) phase (U = 1447; U′ = 4178; p value < 0.0001; n = 75);
- -
- in vertebrae, Ni decreased from the juvenile (Point A of the vertebrae) to the adult (Point C of the vertebrae) phase (U = 2281; U′ = 3344; p value = 0.04; n = 75);
- -
- in vertebrae, Cu and Fe did not show significant correlations or differences with sex, TL, parasitic load, nor with the two stages of the biological cycle.
4. Discussion
4.1. The Role of Trace Elements in Weakening of the Immune System
4.2. Trace Elements Accumulation in S. canicula from Tyrrhenian Sea
4.2.1. Lead (PB)
4.2.2. Arsenic (As)
4.2.3. Cadmium (Cd)
4.2.4. Other Trace Elements (Mn, Zn, Ni, Cu, Fe)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbato, M.; Barría, C.; Bellodi, A.; Bonanomi, S.; Borme, D.; Ćetković, I.; Colloca, F.; Colmenero, A.; Crocetta, F.; De Carlo, F.; et al. The use of fishers’ Local Ecological Knowledge to reconstruct fish behavioural traits and fishers’ perception of conservation relevance of elasmobranchs in the Mediterranean Sea. Mediterr. Mar. Sci. 2021, 22, 603–622. [Google Scholar] [CrossRef]
- Leonetti, F.; Giglio, G.; Leone, A.; Coppola, F.; Romano, C.; Bottaro, M.; Reinero, F.; Milazzo, C.; Micarelli, P.; Tripepi, S.; et al. An updated checklist of chondrichthyans of Calabria (Central Mediterranean, southern Italy), with emphasis on rare species. Mediterr. Mar. Sci. 2020, 21, 794–807. [Google Scholar] [CrossRef]
- Coelho, J.P.; Santos, H.; Reis, A.T.; Falcao, J.; Rodrigues, E.T.; Pereira, M.E.; Duarte, A.C.; Pardal, M.A. Mercury bioaccumulation in the spotted dogfish (Scyliorhinus canicula) from the Atlantic Ocean. Mar. Pollut. Bull. 2010, 60, 1372–1375. [Google Scholar] [CrossRef] [PubMed]
- Dallarès, S.; Perez-Del-Olmo, A.; Montero, F.E.; Carasson, M. Composition and seasonal dynamics of the parasite communities of Scyliorhinus canicula (L., 1758) and Galeus melastomus Rafinesque, 1810 (Elasmobranchii) from the NW Mediterranean Sea in relation to host biology and ecological features. Hydrobiologia 2017, 799, 275–291. [Google Scholar] [CrossRef]
- Henderson, A.C.; Dunne, J. The metazoan parasites of the lesser-spotted dogfish Scyliorhinus canicula (L.) from the Galway Bay area. Ir. Nat. J. 1998, 26, 104–107. [Google Scholar]
- Moore, A.B.M. Metazoan parasites of the lesser-spotted dogfish Scyliorhinus canicula and their potential as stock discrimination tools. J. Mar. Biol. Assoc. U. K. 2001, 81, 1009–1013. [Google Scholar] [CrossRef]
- Crouch, K.; Smith, L.E.; Williams, R.; Cao, W.; Lee, M.; Jensen, A.; Dooley, H. Humoral immune response of the small-spotted catshark, Scyliorhinus canicula. Fish Shellfish Immunol. 2013, 34, 1158–1169. [Google Scholar] [CrossRef]
- Valls, E.; Navarro, J.; Barria, C.; Coll, M.; Fernàndez-Borràs, J.; Rotlant, G. Seasonal, ontogenetic and sexual changes in lipid metabolism of the small-spotted catshark (Scyliorhinus canicula) in deep-sea free-living conditions. J. Exp. Mar. Biol. Ecol. 2016, 483, 59–63. [Google Scholar] [CrossRef]
- Barràgan-Mèndez, C.; Ruiz-Jarabo, I.; Fuentes, J.; Mancera, J.M.; Sobrino, I. Survival rates and physiological recovery response in the lesser spotted catshark (Scyliorhinus canicula) after bottom-trawling. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 223, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pegado, M.R.; Santos, C.P.; Raffoul, D.; Konieczna, M.; Sampaio, E.; Maulvault, L.; Diniz, M.; Rosa, R. Impact of a simulated marine heatwave in the hematological profile of a temperate shark (Scyliorhinus canicula). Ecol. Indic. 2020, 114, 106327. [Google Scholar] [CrossRef]
- Jeffree, R.A.; Warnau, M.; Teyssiè, J.L.; Markich, S.J. Comparison of the bioaccumulation from seawater and depuration of heavy metals and radionuclides in the spotted dogfish Scyliorhinus canicula (Chondrichthys) and the turbot Psetta maxima (Actinopterygii: Teleostei). Sci. Total Environ. 2006, 368, 839–852. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, G.; Eyckmans, M.; Lardon, I.; Bobbaers, R.; Sinha, A.K.; Blust, R. Metal accumulation and metallothionein induction in the spotted dogfish Scyliorhinus canicula. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 155, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.F.S.; Alves, L.M.F.; Moutinho, A.; Lemos, M.F.L.; Novais, S.C. Scyliorhinus canicula (Linnaeus, 1758) metal accumulation: A public health concern for Atlantic fish consumers? Mar. Pollut. Bull. 2021, 169, 112477. [Google Scholar] [CrossRef] [PubMed]
- Pourret, O.; Hursthouse, A. It’s Time to Replace the Term “Heavy Metals” with “Potentially Toxic Elements” When Reporting Environmental Research. Int. J. Environ. Res. Public Health 2019, 16, 4446. [Google Scholar] [CrossRef]
- Simpfendorfer, C.A. Predicting population recovery rates for endangered western Atlantic sawfishes using demographic analysis. Environ. Biol. Fishes 2000, 58, 371–377. [Google Scholar] [CrossRef]
- Perugini, D.; De Campos, C.P.; Dingwell, D.B.; Dorfman, A. Relaxation of concentration variance: A new tool to measure chemical element mobility during mixing of magmas. Chem. Geol. 2013, 335, 8–23.15. [Google Scholar] [CrossRef]
- Sperone, E.; Coppola, F.; Parise, G.; Bernabò, I.; Reinero, F.R.; Micarelli, P.; Giglio, G.; Milazzo, C. Confirmation of the presence of the bigeye thresher Alopias superciliosus in the Tyrrhenian Sea, with first parsitological notes for the Mediterranean Sea. Cah. Biol. Mar. 2018, 59, 181–185. [Google Scholar]
- Comas, M.; Ribas, A.; Milazzo, C.; Sperone, E.; Tripepi, S. High levels of prevalence related to age and body conditions: Host-parasite interactions in a water frog Pelophylax kl. hispanicus. Acta Herpetol. 2014, 9, 25–31. [Google Scholar]
- Bakopoulos, V.; Tsepa, E.; Diakou, A.; Kokkoris, G.; Kolygas, M.N.; Athanassopoulou, F. Parasites of Scyliorhinus canicula (Linnaeus, 1758) in the north-eastern Aegean Sea. J. Mar. Biol. Assoc. U. K. 2018, 98, 2133–2143. [Google Scholar] [CrossRef]
- Berland, B. Nematodes from some Norwegian marine fishes. Sarsia 1961, 2, 1–50. [Google Scholar] [CrossRef]
- Arnold, J.E. Hematology of the sandbar shark, Carcharhinus plumbeus: Standardization of complete blood count techniques for elasmobranchs. Vet. Clin. Pathol. 2005, 34, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Bevacqua, L.; Reinero, F.R.; Becerril-Garcia, E.E.; Elorriaga-Verplancken, F.R.; Juaristi-Videgaray, D.; Micarelli, P.; Galvan-Magaña, F.; Curiel-Godoy, P.; Giglio, G.; Tripepi, S.; et al. Trace elements and isotopes analyses on historical samples of white sharks from the Mediterranean Sea. Eur. Zool. J. 2021, 88, 132–141. [Google Scholar] [CrossRef]
- Barca, D.; De Francesco, A.M.; Crisci Mirocle, G. Application of Laser Ablation ICP-MS for characterization of obsidian fragments from peri-Tyrrhenian area. J. Cult. Herit. 2017, 8, 141–150. [Google Scholar] [CrossRef]
- De Donato, C.; Barca, D.; Milazzo, C.; Santoro, R.; Giglio, G.; Tripepi, S.; Sperone, E. Is trace element concentration correlated to parasite abundance? A case study in a population of the green frog Pelophylax synkl. hispanicus from the Neto River (Calabria, southern Italy). Parasitol. Res. 2017, 116, 1745–1753. [Google Scholar] [CrossRef]
- Pearce, N.J.G.; Perkins, W.T.; Westgate, J.A.; Gorton, M.P.; Jackson, S.E.; Neal, C.R.; Chenery, S.P. A Compilation of New and Published Major and Trace Element Data for NIST SRM 610 and NIST SRM 612 Glass Reference Materials. Geostand. Newsl. 1997, 21, 115–144. [Google Scholar] [CrossRef]
- Fryer, B.J.; Jackson, S.E.; Longerich, H.P. The design, operation and role of the laser-ablation microprobe coupled with an inductively coupled plasma; mass spectrometer (LAM-ICP-MS) in the earth sciences. Can. Mineral. 1995, 33, 303–312.25. [Google Scholar]
- Bush, A.O.; Laffreti, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Painter, S.C.; Tsimplis, M.N. Temperature and salinity trends in the upper waters of the Mediterranean Sea as determined from the MEDATLAS dataset. Cont. Shelf Res. 2003, 23, 1507–1522. [Google Scholar] [CrossRef]
- Isbert, W.; Rodriguez-Cabello, C.; Frutos, I.; Preciado, I.; Montero, F.E.; Perez-Del-Olmo, A. Metazoan parasite communities and diet of the velvet belly lantern shark Etmopterus spinax (Squaliformes: Etmopteridae): A comparison of two deep-sea ecosystems. J. Fish Biol. 2015, 86, 687–706. [Google Scholar] [CrossRef]
- Henderson, A.C.; Flannery, K.; Dunne, J. Parasites of the blue shark (Prionace glauca L.), in the North-East Atlantic Ocean. J. Nat. Hist. 2002, 36, 1995–2004. [Google Scholar] [CrossRef]
- Palm, H.W.; Walter, T.; Schwerdtfeger, G.; Reimer, L.W. Nybelinia Poche, 1926 (Cestoda: Trypanorhyncha) from the Mozambique coast, with description of N. beveridgei sp. nov. and systematich consideration of the genus. S. Afr. J. Mar. Sci. 1997, 18, 273–285. [Google Scholar] [CrossRef]
- Constenla, M.; Montero, F.E.; Padrós, F.; Cartes, J.E.; Papiol, V.; Carrassón, M. Annual variation of parasite communities of deep-sea macrourid fishes from the western Mediterranean Sea and their relationship with fish diet and histopatological alterations. Deep-Sea Res. 2015, 104, 106–121. [Google Scholar] [CrossRef]
- Compagno, L.J.V. Sharks of the world. An annoted and illustrated catalogue of shark species known to date. Part 1, Hexanchiformes to Lamniformes. In FAO Species Catalogue; FAO: Rome, Italy, 1984; Volume 4. [Google Scholar]
- Carrasson, M.; Stefanescu, C.; Cartes, J.E. Diets and bathymetric distributions of two bathyal sharks of the Catalan deep sea (Western Mediterranean). Mar. Ecol. Prog. Ser. 1992, 82, 21–30. [Google Scholar] [CrossRef]
- Sasal, P.; Morand, S.; Guègan, J.F. Determinats of parasite species richness in Mediterranean marine fishes. Mar. Ecol. Prog. Ser. 1997, 149, 61–71. [Google Scholar] [CrossRef]
- Sardou, J.; Etienne, M.; Andersen, V. Seasonal abundance and vertical distributions of macroplankton and micronekton in the Northwestern Mediterranean Sea. Oceanol. Acta 1996, 19, 645–656.36. [Google Scholar]
- Centola, G.M.; Eberly, S. Seasonal variations and age-related changes in human sperm count, motility, motion parameters, morphology, and white blood cell concentration. Fertil. Steril. 1999, 72, 803–808. [Google Scholar] [CrossRef]
- Priyadarshani, S.; Madhushani, W.A.; Jayawardena, U.A.; Wickramasinghe, D.D.; Udagama, P.V. Heavy metal mediated immunomodulation of the Indian green frog, Euphlyctis hexadactylus (Anura:Ranidae) in urban wetlands. Ecotoxicol. Environ. Saf. 2015, 116, 40–49. [Google Scholar] [CrossRef]
- Boroskova, Z.; Benkova, M.; Soltys, J.; Krupicer, I.; Simo, K. Effects of heavy metals imission on the cellular immunity of guinea pigs with experimental ascariosis. Vet. Parasitol. 1993, 47, 245–254. [Google Scholar] [CrossRef]
- Soltys, J.; Boroskova, Z.; Dvoroznakova, E. Effects of concurrently administered copper and mercury on phagocytic cell activity and antibody levels in guinea pigs with experimental ascariasis. J. Helminthol. 1997, 71, 339–344. [Google Scholar] [CrossRef]
- Schludermann, C.; Konecny, R.; Laimgruber, S.; Lewis, J.W.; Schiemer, F.; Chovanec, A.; Sures, B. Fish macroparasites as indicators of heavy metal pollution in river sites in Austria. Parasitology 2003, 126, S61–S69. [Google Scholar] [CrossRef]
- Soliman, M.F.M.; El-Shenawy, N.S.; Ghobashy, M.A. Parasitological aspects and biochemical changes of infected cultured tilapia (Oreochromis hybrid). Acta Ichthyol. Piscat. 2004, 34, 21–32. [Google Scholar] [CrossRef][Green Version]
- Sagerup, K.; Helgason, L.B.; Polder, A.; Strom, H.; Josefsen, T.D.; Skare, J.U.; Gabrielsen, G.W. Persistent organic pollutants and mercury in dead and dying glaucous gulls (Larus hyperboreus) at Bjornoya (Svalbard). Sci. Total Environ. 2009, 407, 6009–6016. [Google Scholar] [CrossRef] [PubMed]
- ARPAT. Relazione Sullo Stato Dell’ambiente in Toscana: Acque Marine ed Erosione Costiera; ARPAT: Regione Toscana, Italy, 2014; pp. 65–71. [Google Scholar]
- Storelli, M.M.; Busco, V.P.; Marcotrigiano, G.O. Mercury and arsenic speciation in the muscle tissue of Scyliorhinus canicula from the Mediterranean sea. Bull. Environ. Contam. Toxicol. 2005, 75, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Canli, M.; Atli, G. The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ. Pollut. 2003, 121, 129–136. [Google Scholar] [CrossRef]
- Nussey, G.; van Vuren, J.H.J.; du Preez, H.H. Bioaccumulation of chromium, manganese, nickel and lead in the tissues of the moggel, Labeo umbratus (Cyprinidae), from Witbank Dam, Mpumalanga. Water SA 2000, 26, 269–284. [Google Scholar] [CrossRef]
- Jeffree, R.A.; Oberhansli, F.; Teyssie, J.L. Phylogenetic consistencies among chondrichthyan and teleost fishes in their bioaccumulation of multiple trace elements from seawater. Sci. Total Environ. 2010, 408, 3200–3210. [Google Scholar] [CrossRef]
- Storelli, M.M.; Storelli, A.; Marcotrigiano, G.O. Polychlorinated biphenyls, hexachlorobenzene, hexachlorocyclohexane isomers, and pesticide organochlorine residues in cod-liver oil dietary supplements. J. Food Prot. 2004, 67, 1787–1791. [Google Scholar] [CrossRef]
- Kaise, T.; Fukui, S. The chemical form and acute toxicity of arsenic compounds in marine organisms. Appl. Organomet. Chem. 1992, 6, 155–160. [Google Scholar] [CrossRef]
- Marcovecchio, J.E.; Moreno, V.J.; Pérez, A. Metal accumulation in tissues of sharks from the Bahía Blanca estuary, Argentina. Mar. Environ. Res. 1991, 31, 263–274. [Google Scholar] [CrossRef]
- Mathews, T.; Fisher, N.S.; Jeffree, R.A.; Teyssie, J.L. Assimilation and redention of metals in teleost and elasmobranch fishes following dietary exposure. Mar. Ecol. Prog. Ser. 2008, 360, 1–12. [Google Scholar] [CrossRef]
- Pentreath, R.J. Some further studies on the accumulation and retention of 65Zn and 54Mn by the plaice, Pleuronectes platessa L. J. Exp. Mar. Biol. Ecol. 1976, 21, 179–189. [Google Scholar] [CrossRef]
- Mathews, T.; Fisher, N.S. Dominance of dietary intake of metals in marine elasmobranch and teleost fish. Sci. Total Environ. 2009, 407, 5156–5161. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Collins, L.S.; Hayek, L.A.C. Biogeographic effects of the closing Central American seaway on benthic foraminifera of Venezuela. Bull. Mar. Sci. 2013, 89, 921–936. [Google Scholar]
- Wang, G.; Zhang, Q. Adsorption behavior of the bamboo-charcoal for Zn (2+). Biomass Chem. Eng. 2006, 3, 17–20. [Google Scholar]
- Vas, P. Trace metal levels in sharks from British and Atlantic waters. Mar. Pollut. Bull. 1991, 22, 67–72. [Google Scholar] [CrossRef]
- Reboa, A.; Mandich, A.; Cutroneo, L.; Carbone, C.; Malatesta, A.; Capello, M. Baseline evaluation of metal contamination in teleost fishes of the Gulf of Tigullio (north-western Italy): Histopathology and chemical analysis. Mar. Pollut. Bull. 2019, 141, 16–23. [Google Scholar] [CrossRef]
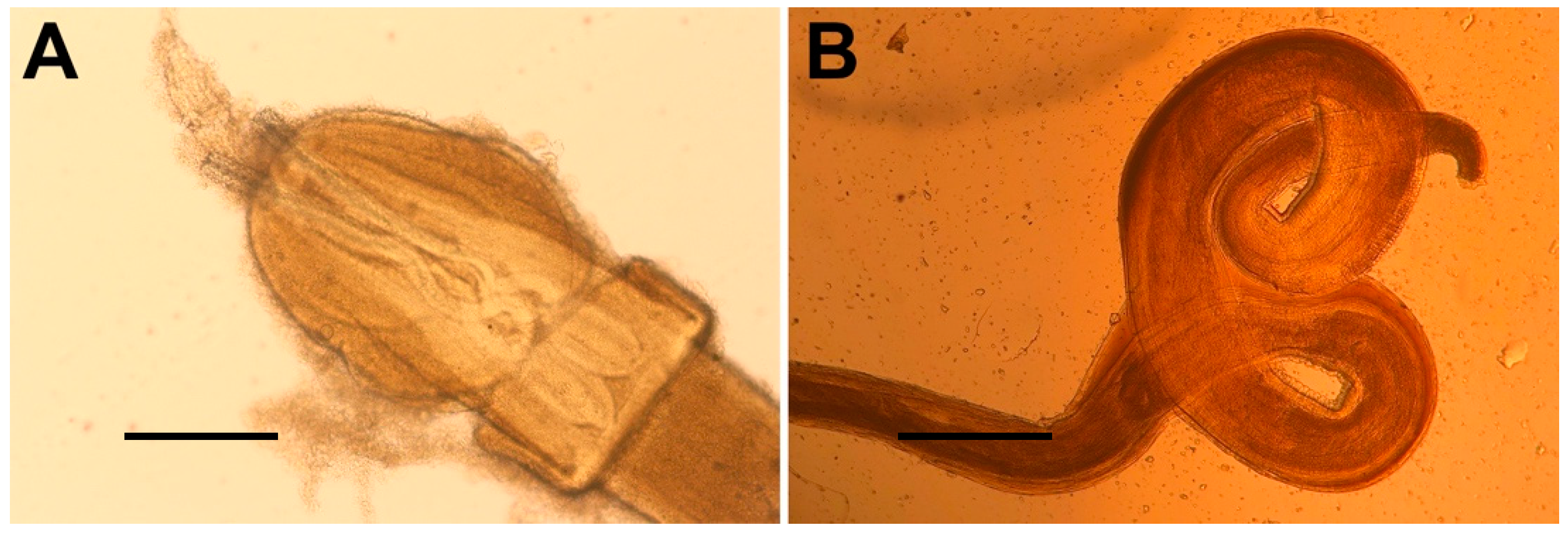
| Total ± sd | Females ± sd | Males ± sd | |
|---|---|---|---|
| TL (cm) | 39.65 ± 3.01 | 39.77 ± 2.80 | 39.34 ± 3.40 |
| W (g) | 234.51 ± 74.10 | 239.49 ± 77.20 | 219.8 ± 6.74 |
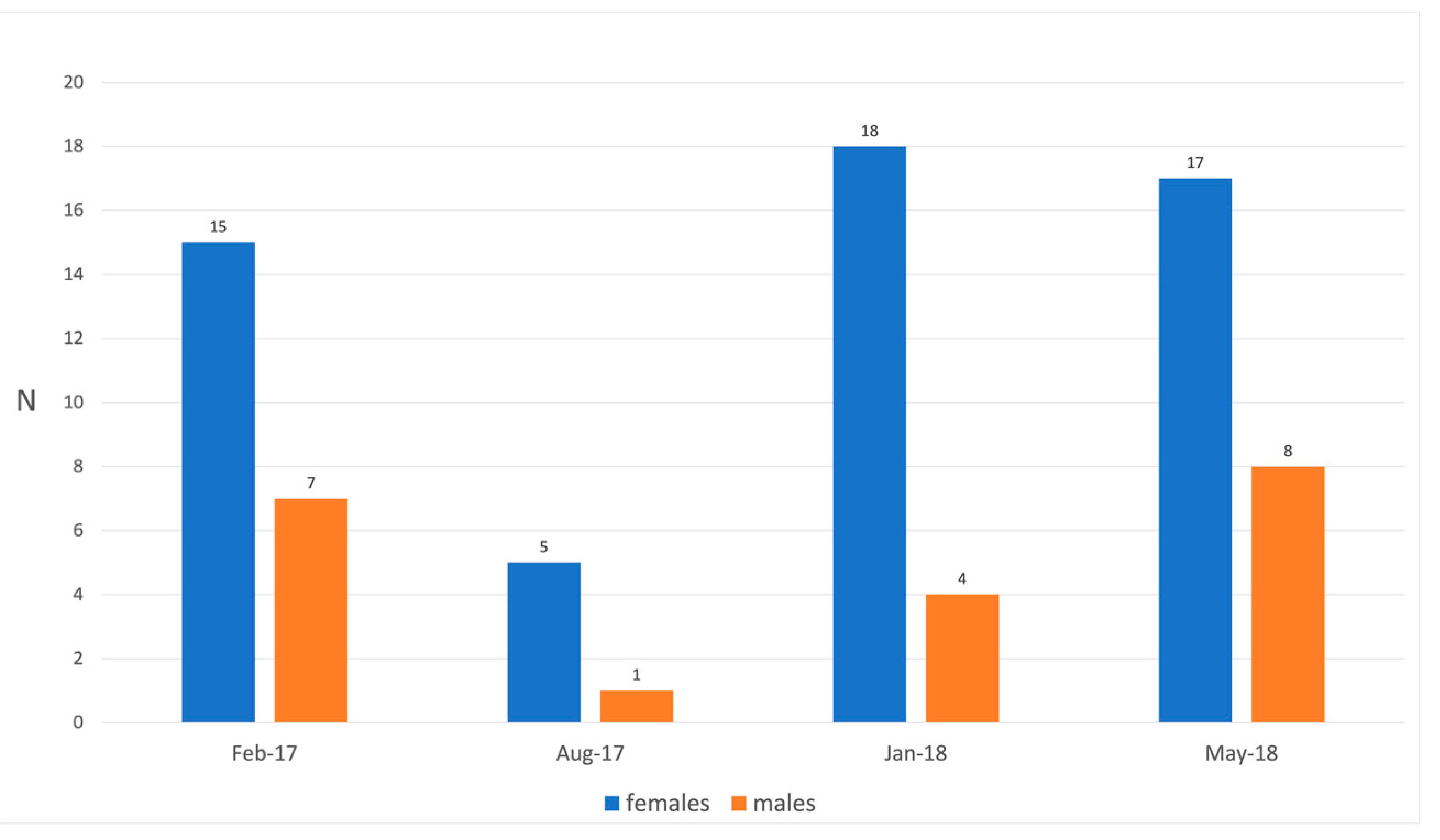
| Months | N° of Examined Hosts | N° of Parasitized Hosts | P% Males | P% Females | ||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||
| Feb 17 | 7 | 15 | 1 | 1 | 14.28 | 6.66 |
| Aug 17 | 1 | 5 | 0 | 0 | 0.00 | 0.00 |
| Jan 18 | 4 | 18 | 0 | 7 | 0.00 | 38.80 |
| May 18 | 8 | 17 | 2 | 7 | 25.00 | 41.10 |
| Total | 20 | 55 | 3 | 15 | 15.00 | 27.70 |
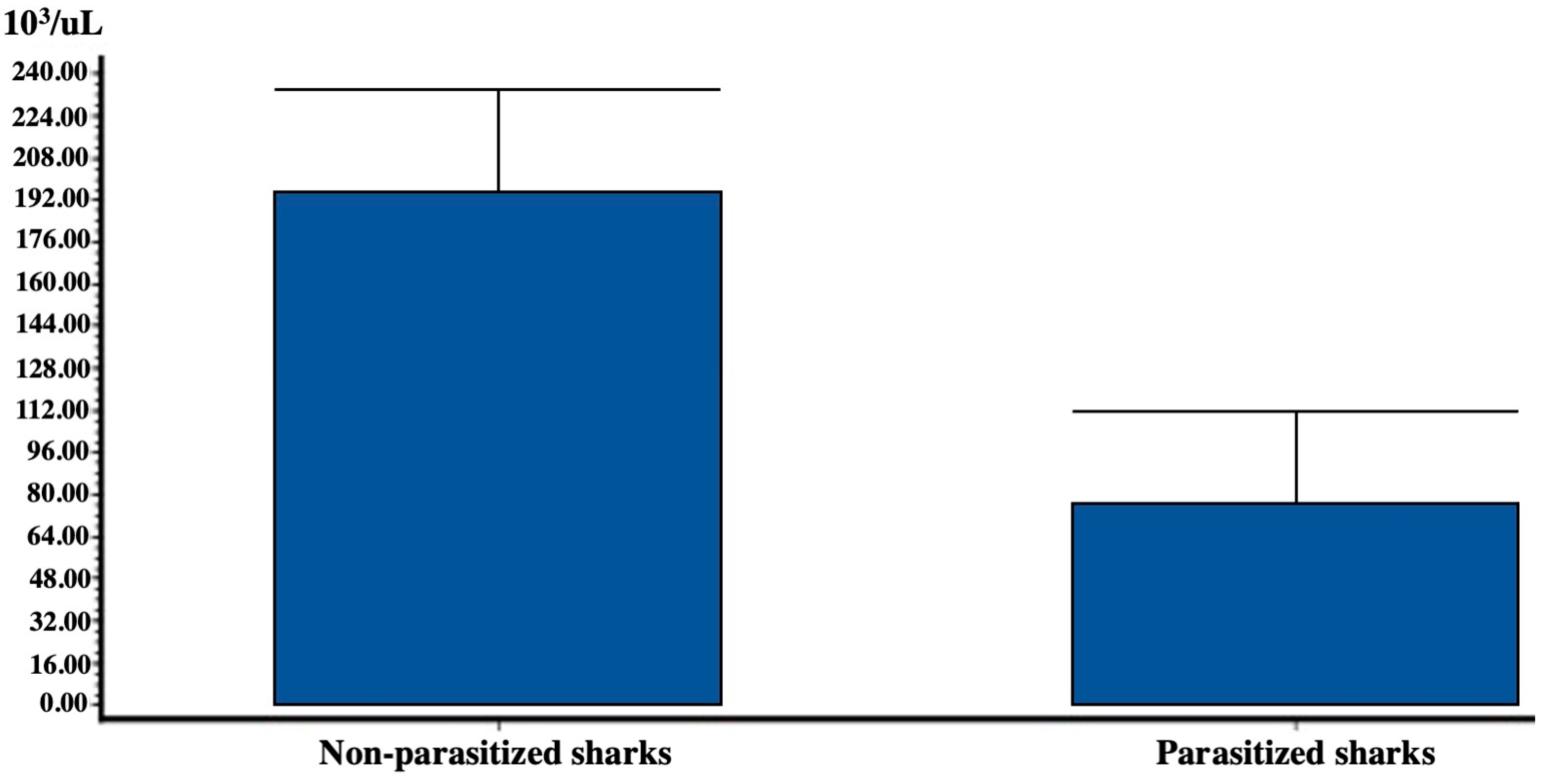
| RBC (106/μL) n = 10 | HGB (g/dL) n = 10 | MCV (fL) n = 10 | PLT (103/μL) n = 10 | WBC (103/μL) n = 32 | |
|---|---|---|---|---|---|
| Average value | 0.028 | 4.680 | 127.330 | 50.900 | 213.790 |
| SD (standard deviation) | 0.012 | 0.590 | 24.128 | 13.634 | 26.460 |
| n (number of samples) | 10 | 10 | 10 | 10 | 10 |
| SEM (standard error) | 0.004 | 0.180 | 7.630 | 4.310 | 8.360 |
| Trace Element | Vertebrae (Point A) ± sd n = 75 | Vertebrae (Point C) ± sd n = 75 |
|---|---|---|
| Pb | 8.03 ± 0.40 | 5.20 ± 0.80 |
| As | 384 ± 37.00 | 192 ± 62.00 |
| Mn | 69.70 ± 5.01 | 136 ± 75.00 |
| Ni | 12.03 ± 1.25 | 9.50 ± 1.40 |
| Fe | 140 ± 7.00 | 96 ± 12.00 |
| Cu | 18 ± 0.50 | 7 ± 1.50 |
| Cd | 0.06 ± 0.02 | 0.09 ± 0.01 |
| Zn | 60.90 ± 4.30 | 125.70 ± 34.00 |
| Trace Element | Vertebrae (Point C) ± sd n = 12 | Skin ± sd n = 12 | Liver ± sd n = 12 | KW ± sd n = 12 | p Value |
|---|---|---|---|---|---|
| Pb | 5.20 ± 0.80 | 0.60 ± 0.08 | 0.50 ± 0.01 | 1.15 | 0.5 n.s. |
| As | 192 ± 62 | 48 ± 12.20 | 104 ± 34 | 6.03 | 0.049 * |
| Mn | 136 ± 75 | 12 ± 1.40 | 6 ± 2.90 | 30.50 | <0.0001 *** |
| Ni | 9.50 ± 1.40 | 11 ± 0.70 | 1.50 ± 0.09 | 13.51 | <0.0001 *** |
| Fe | 96 ± 12 | 592 ± 110 | 288 ± 99.90 | 21.55 | <0.0001 *** |
| Cu | 7 ± 1.50 | 3.40 ± 2.70 | 11 ± 2.30 | 1.63 | 0.44 n.s. |
| Cd | 0.09 ± 0.01 | 0.14 ± 0.02 | 0.81 ± 0.10 | 22.12 | <0.0001 *** |
| Zn | 125.70 ± 34 | 72 ± 13 | 138.30 ± 27 | 15.53 | 0.5 n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinero, F.R.; Milazzo, C.; Minervino, M.; Marchio, C.; Filice, M.; Bevacqua, L.; Giglio, G.; Leonetti, F.L.; Micarelli, P.; Tripepi, S.; et al. Parasitic Load, Hematological Parameters, and Trace Elements Accumulation in the Lesser Spotted Dogfish Scyliorhinus canicula from the Central Tyrrhenian Sea. Biology 2022, 11, 663. https://doi.org/10.3390/biology11050663
Reinero FR, Milazzo C, Minervino M, Marchio C, Filice M, Bevacqua L, Giglio G, Leonetti FL, Micarelli P, Tripepi S, et al. Parasitic Load, Hematological Parameters, and Trace Elements Accumulation in the Lesser Spotted Dogfish Scyliorhinus canicula from the Central Tyrrhenian Sea. Biology. 2022; 11(5):663. https://doi.org/10.3390/biology11050663
Chicago/Turabian StyleReinero, Francesca Romana, Concetta Milazzo, Marco Minervino, Cristian Marchio, Mariacristina Filice, Laura Bevacqua, Gianni Giglio, Francesco Luigi Leonetti, Primo Micarelli, Sandro Tripepi, and et al. 2022. "Parasitic Load, Hematological Parameters, and Trace Elements Accumulation in the Lesser Spotted Dogfish Scyliorhinus canicula from the Central Tyrrhenian Sea" Biology 11, no. 5: 663. https://doi.org/10.3390/biology11050663
APA StyleReinero, F. R., Milazzo, C., Minervino, M., Marchio, C., Filice, M., Bevacqua, L., Giglio, G., Leonetti, F. L., Micarelli, P., Tripepi, S., Barca, D., & Sperone, E. (2022). Parasitic Load, Hematological Parameters, and Trace Elements Accumulation in the Lesser Spotted Dogfish Scyliorhinus canicula from the Central Tyrrhenian Sea. Biology, 11(5), 663. https://doi.org/10.3390/biology11050663