The Effect of Semen Cryopreservation Process on Metabolomic Profiles of Turkey Sperm as Assessed by NMR Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Semen Treatment
2.3. Cryopreservation Process
2.4. Semen Quality Evaluation
2.5. NMR Measurements
2.5.1. Sample Preparation
2.5.2. NMR Spectra
2.5.3. Measurement of the Metabolic Content in Aqueous Extract
2.5.4. Measurement of the Metabolic Content in Organic Extracts
2.6. Statistical Analysis
3. Results
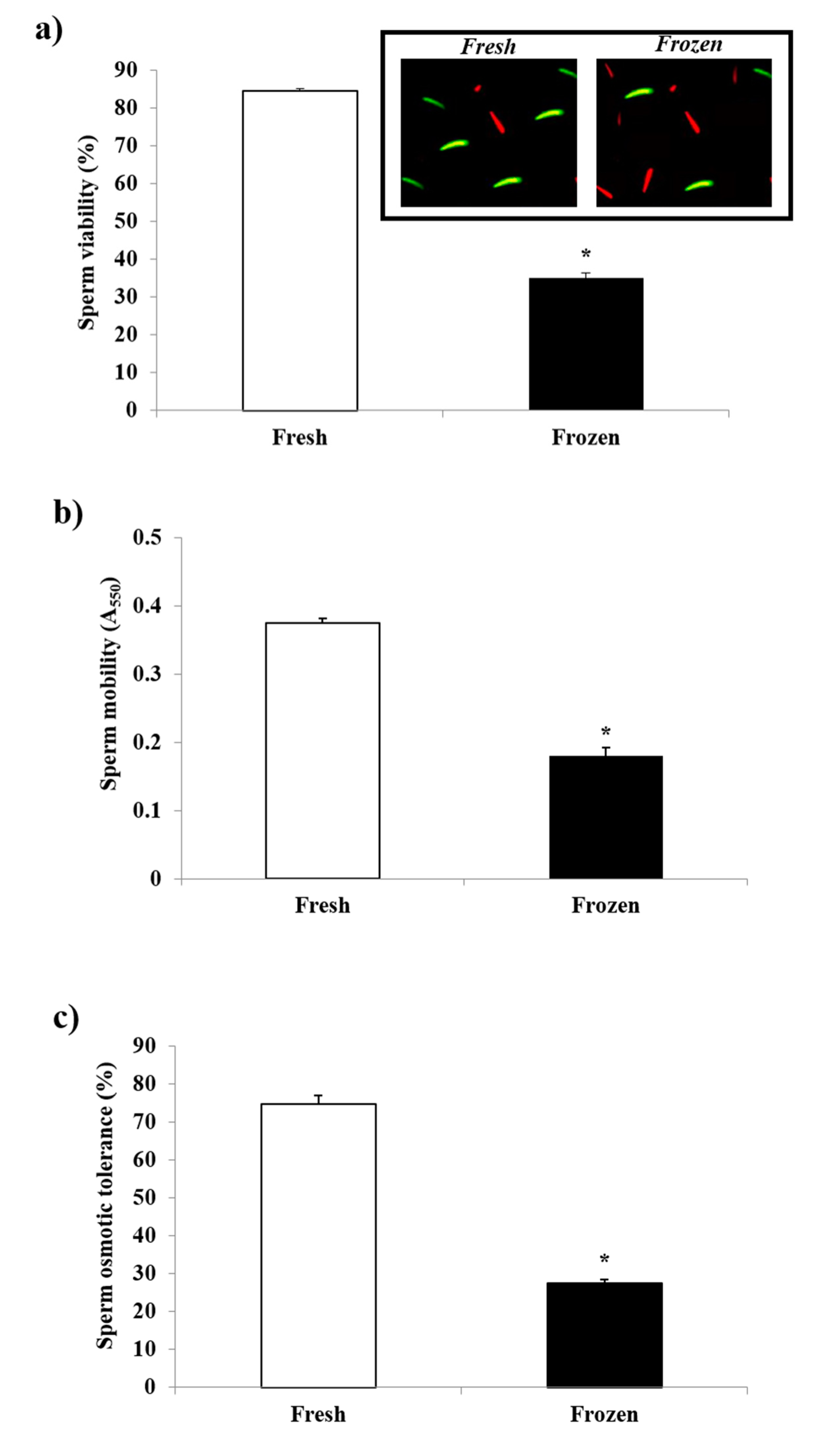
3.1. Sperm Quality
3.2. NMR Analysis
3.3. Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blesbois, E.; Seigneurin, F.; Grasseau, I.; Limouzin, C.; Besnard, J.; Gourichon, D.; Coquerelle, G.; Rault, P.; Tixier-Boichard, M. Semen Cryopreservation for Ex Situ Management of Genetic Diversity in Chicken: Creation of the French Avian Cryobank. Poult. Sci. 2007, 86, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Partyka, A.; Niżański, W. Advances in storage of poultry semen. Anim. Reprod. Sci. 2022, 106921. [Google Scholar] [CrossRef] [PubMed]
- Liptoi, K.; Buda, K.; Rohn, E.; Drobnyak, A.; Meleg, E.E.; Palinkas-Bodzsar, N.; Vegi, B.; Barna, J. Improvement of the application of gonadal tissue allotransplantation in the in vitro conservation of chicken genetic lines. Anim. Reprod. Sci. 2020, 213, 106280. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Moreno, J.; Blesbois, E. Animal board invited review: Germplasm technologies for use with poultry. Animal 2022, 16, 100475. [Google Scholar] [CrossRef]
- Blesbois, E. Freezing avian semen. Avian Biol. Res. 2011, 4, 52–58. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Łukaszewicz, E. Simple and Effective Methods of Freezing Capercaillie (Tetrao urogallus L.) Semen. PLoS ONE 2015, 10, e0116797. [Google Scholar] [CrossRef]
- Thélie, A.; Bailliard, A.; Seigneurin, F.; Zerjal, T.; Tixier-Boichard, M.; Blesbois, E. Chicken semen cryopreservation and use for the restoration of rare genetic resources. Poult. Sci. 2019, 98, 447–455. [Google Scholar] [CrossRef]
- Prentice, J.R.; Anzar, M. Cryopreservation of Mammalian Oocyte for Conservation of Animal Genetics. Vet. Med. Int. 2011, 2011, 146405. [Google Scholar] [CrossRef]
- Iaffaldano, N.; Di Iorio, M.; Rusco, G.; Antenucci, E.; Zaniboni, L.; Madeddu, M.; Marelli, S.; Schiavone, A.; Soglia, D.; Buccioni, A.; et al. Italian semen cryobank of autochthonous chicken and turkey breeds: A tool for preserving genetic biodiversity. Ital. J. Anim. Sci. 2021, 20, 2022–2033. [Google Scholar] [CrossRef]
- Iaffaldano, N.; Di Iorio, M.; Cerolini, S.; Manchisi, A. Overview of Turkey Semen Storage: Focus on Cryopreservation—A Review. Ann. Anim. Sci. 2016, 16, 961–974. [Google Scholar] [CrossRef]
- Blanco, J.M.; Gee, G.; Wildt, D.E.; Tselutin, K.; Donoghue, A.M. Semen cryopreservation in poultry and non-domestic species: A comparative approach to understanding the fundamentals of avian spermatozoa cryobiology. Br. Poult. Sci. 2000, 41, 3–5. [Google Scholar] [CrossRef]
- Blanco, J.M.; Long, J.A.; Gee, G.; Donoghue, A.M.; Wildt, D.E. Osmotic tolerance of avian spermatozoa: Influence of time, temperature, cryoprotectant and membrane ion pump function on sperm viability. Cryobiology 2008, 56, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Blesbois, E. Current status in avian semen cryopreservation. World’s Poult. Sci. J. 2007, 63, 213–222. [Google Scholar] [CrossRef]
- Iaffaldano, N.; Romagnoli, L.; Manchisi, A.; Rosato, M.P. Cryopreservation of turkey semen by the pellet method: Effects of variables such as the extender, cryoprotectant concentration, cooling time and warming temperature on sperm quality determined through principal components analysis. Theriogenology 2011, 76, 794–801. [Google Scholar] [CrossRef]
- Iaffaldano, N.; Di Iorio, M.; Miranda, M.; Zaniboni, L.; Manchisi, A.; Cerolini, S. Cryopreserving turkey semen in straws and nitrogen vapour using DMSO or DMA: Effects of cryoprotectant concentration, freezing rate and thawing rate on post-thaw semen quality. Br. Poult. Sci. 2016, 57, 264–270. [Google Scholar] [CrossRef]
- Iaffaldano, N.; Rosato, M.; Manchisi, A.; Centoducati, G.; Meluzzi, A. Comparison of different extenders on the quality characteristics of turkey semen during storage. Ital. J. Anim. Sci. 2016, 4, 513–515. [Google Scholar] [CrossRef]
- Izanloo, H.; Soleimanzadeh, A.; Bucak, M.N.; Imani, M.; Zhandi, M. The effects of varying concentrations of glutathione and trehalose in improving microscopic and oxidative stress parameters in Turkey semen during liquid storage at 5 °C. Cryobiology 2021, 101, 12–19. [Google Scholar] [CrossRef]
- Blesbois, E.; Grasseau, I.; Seigneurin, F. Membrane fluidity and the ability of domestic bird spermatozoa to survive cryopreservation. Reproduction 2005, 129, 371–378. [Google Scholar] [CrossRef]
- Di Iorio, M.; Rusco, G.; Iampietro, R.; Maiuro, L.; Schiavone, A.; Cerolini, S.; Iaffaldano, N. Validation of the Turkey Semen Cryopreservation by Evaluating the Effect of Two Diluents and the Inseminating Doses. Animals 2020, 10, 1329. [Google Scholar] [CrossRef]
- Di Iorio, M.; Rusco, G.; Iampietro, R.; Colonna, M.A.; Zaniboni, L.; Cerolini, S.; Iaffaldano, N. Finding an Effective Freezing Protocol for Turkey Semen: Benefits of Ficoll as Non-Permeant Cryoprotectant and 1:4 as Dilution Rate. Animals 2020, 10, 421. [Google Scholar] [CrossRef]
- Mosca, F.; Madeddu, M.; Abdel Sayed, A.; Zaniboni, L.; Iaffaldano, N.; Cerolini, S. Combined effect of permeant and non-permeant cryoprotectants on the quality of frozen/thawed chicken sperm. Cryobiology 2016, 73, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Zaniboni, L.; Rizzi, R.M.; Cerolini, S. Combined effect of DHA and α-tocopherol enrichment on sperm quality and fertility in the turkey. Theriogenology 2006, 65, 1813–1827. [Google Scholar] [CrossRef] [PubMed]
- Iaffaldano, N.; Manchisi, A.; Gambacorta, M.; Di Iorio, M.; Rosato, M.P. Effect of different sperm concentrations on the post-thaw viability and motility of turkey spermatozoa cryopreserved by the pellet method. Ital. J. Anim. Sci. 2009, 8, 760–762. [Google Scholar] [CrossRef]
- Mosca, F.; Zaniboni, L.; Abdel Sayed, A.; Madeddu, M.; Iaffaldano, N.; Cerolini, S. Effect of dimethylacetamide and N-methylacetamide on the quality and fertility of frozen/thawed chicken semen. Poult. Sci. 2019, 98, 6071–6077. [Google Scholar] [CrossRef]
- Mosca, F.; Zaniboni, L.; Sayed, A.A.; Iaffaldano, N.; Soglia, D.; Schiavone, A.; Cerolini, S. Effect of N-Methylacetamide Concentration and Thawing Rate on Chicken Sperm Quality after Cryopreservation. Animals 2020, 10, 824. [Google Scholar] [CrossRef]
- Parks, J.E.; Lynch, D.V. Lipid composition and thermotropic phase behavior of boar, bull, stallion, and rooster sperm membranes. Cryobiology 1992, 29, 255–266. [Google Scholar] [CrossRef]
- Cerolini, S.; Zaniboni, L.; Maldjian, A.; Gliozzi, T. Effect of docosahexaenoic acid and α-tocopherol enrichment in chicken sperm on semen quality, sperm lipid composition and susceptibility to peroxidation. Theriogenology 2006, 66, 877–886. [Google Scholar] [CrossRef]
- Blesbois, E.; Grasseau, I.; Seigneurin, F.; Mignon-Grasteau, S.; Saint Jalme, M.; Mialon-Richard, M.M. Predictors of success of semen cryopreservation in chickens. Theriogenology 2008, 69, 252–261. [Google Scholar] [CrossRef]
- Long, J.A.; Purdy, P.H.; Zuidberg, K.; Hiemstra, S.-J.; Velleman, S.G.; Woelders, H. Cryopreservation of turkey semen: Effect of breeding line and freezing method on post-thaw sperm quality, fertilization, and hatching. Cryobiology 2014, 68, 371–378. [Google Scholar] [CrossRef]
- Mussa, N.J.; Ratchamak, R.; Ratsiri, T.; Vongpralub, T.; Boonkum, W.; Semaming, Y.; Chankitisakul, V. Lipid profile of sperm cells in Thai native and commercial roosters and its impact on cryopreserved semen quality. Trop. Anim. Health Prod. 2021, 53, 321. [Google Scholar] [CrossRef]
- Mandal, R.; Badyakar, D.; Chakrabarty, J. Role of Membrane Lipid Fatty Acids in Sperm Cryopreservation. Adv. Androl. 2014, 2014, 190542. [Google Scholar] [CrossRef]
- Zaniboni, L.; Cerolini, S. Liquid storage of turkey semen: Changes in quality parameters, lipid composition and susceptibility to induced in vitro peroxidation in control, n-3 fatty acids and alpha-tocopherol rich spermatozoa. Anim. Reprod. Sci. 2009, 112, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Long, J.A. Avian semen cryopreservation: What are the biological challenges? Poult. Sci. 2006, 85, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Shinitzky, M.; Yuli, I. Lipid fluidity at the submacroscopic level: Determination by fluorescence polarization. Chem. Phys. Lipids 1982, 30, 261–282. [Google Scholar] [CrossRef]
- Vickram, A.S.; Ramesh Pathy, M.; Sridharan, T.B. Effect of various biomolecules for normal functioning of human sperm for fertilization: A review. Int. J. Pharm. Pharm. Sci. 2012, 4, 18–24. [Google Scholar]
- Cheah, Y.; Yang, W. Functions of essential nutrition for high quality spermatogenesis. Adv. Biosci. Biotechnol. 2011, 02, 182–197. [Google Scholar] [CrossRef]
- Atessahin, A.; Bucak, M.N.; Tuncer, P.B.; Kızıl, M. Effects of anti-oxidant additives on microscopic and oxidative parameters of Angora goat semen following the freeze–thawing process. Small Rumin. Res. 2008, 77, 38–44. [Google Scholar] [CrossRef]
- Ugur, M.R.; Dinh, T.; Hitit, M.; Kaya, A.; Topper, E.; Didion, B.; Memili, E. Amino Acids of Seminal Plasma Associated with Freezability of Bull Sperm. Front. Cell Dev. Biol. 2020, 7, 347. [Google Scholar] [CrossRef]
- Santiago-Moreno, J.; Bernal, B.; Perez-Cerezales, S.; Castaño, C.; Toledano-Díaz, A.; Esteso, M.C.; Gutierrez-Adan, A.; López-Sebastián, A.; Garcia-Gil, M.; Woelders, H.; et al. Seminal plasma amino acid profile in different breeds of chicken: Role of seminal plasma on sperm cryoresistance. PLoS ONE 2019, 14, e0209910. [Google Scholar] [CrossRef]
- Renard, P.; Grizard, G.; Francoisgriveaua, J.; Sion, B.; Boucher, D.; Le Lannou, D. Improvement of Motility and Fertilization Potential of Postthaw Human Sperm Using Glutamine. Cryobiology 1996, 33, 311–319. [Google Scholar] [CrossRef]
- Li, Y.; Si, W.; Zhang, X.; Dinnyes, A.; Ji, W. Effect of amino acids on cryopreservation of cynomolgus monkey (macaca fascicularis) sperm. Am. J. Primatol. 2003, 59, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Khlifaoui, M.; Battut, I.; Bruyas, J.F.; Chatagnon, G.; Trimeche, A.; Tainturier, D. Effects of glutamine on post-thaw motility of stallion spermatozoa: An approach of the mechanism of action at spermatozoa level. Theriogenology 2005, 63, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Bucak, M.N.; Tuncer, P.B.; Sarıözkan, S.; Ulutaş, P.A. Comparison of the effects of glutamine and an amino acid solution on post-thawed ram sperm parameters, lipid peroxidation and anti-oxidant activities. Small Rumin. Res. 2009, 81, 13–17. [Google Scholar] [CrossRef]
- Moradi, B.; Faramarzi, A.; Ghasemi-Esmailabad, S.; Aghaz, F.; Hashemian, A.H.; Khazaei, M. L-proline as a novel additive to cryopreservation media improved post-thaw quality of human spermatozoon via reducing oxidative stress. Andrologia 2022, 54, e14301. [Google Scholar] [CrossRef] [PubMed]
- Jeulin, C.; Lewin, L.M. Role of free L-carnitine and acetyl-L-carnitine in post-gonadal maturation of mammalian spermatozoa. Hum. Reprod. Update 1996, 2, 87–102. [Google Scholar] [CrossRef]
- Agarwal, A.; Said, T.M. Carnitines and male infertility. Reprod. Biomed. Online 2004, 8, 376–384. [Google Scholar] [CrossRef]
- Stradaioli, G.; Sylla, L.; Zelli, R.; Chiodi, P.; Monaci, M. Effect of L-carnitine administration on the seminal characteristics of oligoasthenospermic stallions. Theriogenology 2004, 62, 761–777. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Z.; An, X.; Liu, H.; Wang, F.; Ji, C.; Liu, G.; Wang, Y.; He, F.; Dang, R.; et al. Metabolomic profiling of Dezhou donkey sperm associated with freezability. Theriogenology 2022, 181, 131–139. [Google Scholar] [CrossRef]
- Iaffaldano, N.; Di Iorio, M.; Mannina, L.; Paventi, G.; Rosato, M.P.; Cerolini, S.; Sobolev, A.P. Age-dependent changes in metabolic profile of turkey spermatozoa as assessed by NMR analysis. PLoS ONE 2018, 13, e0194219. [Google Scholar] [CrossRef]
- Tselutin, K.; Narubina, L.; Mavrodina, T.; Tur, B. Cryopreservation of poultry semen. Br. Poult. Sci. 1995, 36, 805–811. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Ingallina, C.; Maccelli, A.; Spano, M.; Di Matteo, G.; Di Sotto, A.; Giusti, A.M.; Vinci, G.; Di Giacomo, S.; Rapa, M.; Ciano, S.; et al. Chemico-Biological Characterization of Torpedino Di Fondi® Tomato Fruits: A Comparison with San Marzano Cultivar at Two Ripeness Stages. Antioxidants 2020, 9, 1027. [Google Scholar] [CrossRef] [PubMed]
- Ingallina, C.; Spano, M.; Sobolev, A.P.; Esposito, C.; Santarcangelo, C.; Baldi, A.; Daglia, M.; Mannina, L. Characterization of Local Products for Their Industrial Use: The Case of Italian Potato Cultivars Analyzed by Untargeted and Targeted Methodologies. Foods 2020, 9, 1216. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, M.; Mignon-Grasteau, S.; Grasseau, I.; Magistrini, M.; Blesbois, E. Ability of chicken spermatozoa to undergo acrosome reaction after liquid storage or cryopreservation. Theriogenology 2011, 75, 122–130. [Google Scholar] [CrossRef]
- Long, J.A.; Guthrie, H.D. Validation of a rapid, large-scale assay to quantify ATP concentration in spermatozoa. Theriogenology 2006, 65, 1620–1630. [Google Scholar] [CrossRef]
- Słowińska, M.; Liszewska, E.; Judycka, S.; Konopka, M.; Ciereszko, A. Mitochondrial membrane potential and reactive oxygen species in liquid stored and cryopreserved turkey (Meleagris gallopavo) spermatozoa. Poult. Sci. 2018, 97, 3709–3717. [Google Scholar] [CrossRef]
- Kundu, C.N.; Das, K.; Majumder, G.C. Effect of Amino Acids on Goat Cauda Epididymal Sperm Cryopreservation Using a Chemically Defined Model System. Cryobiology 2001, 42, 21–27. [Google Scholar] [CrossRef]
- Sánchez-Partida, L.G.; Setchell, B.P.; Maxwell, W.M.C. Effect of compatible solutes and diluent composition on the post-thaw motility of ram sperm. Reprod. Fertil. Dev. 1998, 10, 347–358. [Google Scholar] [CrossRef]
- Koskinen, E.; Junnila, M.; Katila, T.; Soini, H. A Preliminary Study on the Use of Betaine as a Cryoprotective Agent in Deep Freezing of Stallion Semen. J. Veter. Med. Ser. A 1989, 36, 110–114. [Google Scholar] [CrossRef]
- Sangeeta, S.; Arangasamy, A.; Kulkarni, S.; Selvaraju, S. Role of amino acids as additives on sperm motility, plasma membrane integrity and lipid peroxidation levels at pre-freeze and post-thawed ram semen. Anim. Reprod. Sci. 2015, 161, 82–88. [Google Scholar] [CrossRef]
- Ahmed, H.; Jahan, S.; Khan, A.; Khan, L.; Ullah, H.; Riaz, M.; Ullah, K.; Ullah, F. Supplementation of l-tryptophan (an aromatic amino acid) in tris citric acid extender enhances post-thaw progressive motility, plasmalemma, mitochondrial membrane potential, acrosome, and DNA integrities, and in vivo fertility rate of buffalo (Bubalus bubalis) bull spermatozoa. Cryobiology 2020, 92, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, R.; Asadi, A.; Eshratkhah, B.; Kandi, J.G.G.; Ghorbani, A. The effect of cysteine on post-thawed buffalo bull (Bubalus bubalis) sperm parameters. Adv. Environ. Biol. 2011, 5, 1260–1263. [Google Scholar]
- Thananurak, P.; Chuaychu-Noo, N.; Thélie, A.; Phasuk, Y.; Vongpralub, T.; Blesbois, E. Different concentrations of cysteamine, ergothioneine, and serine modulate quality and fertilizing ability of cryopreserved chicken sperm. Poult. Sci. 2020, 99, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Bernal, B.; Iglesias-Cabeza, N.; Sánchez-Rivera, U.; Toledano-Díaz, A.; Castaño, C.; Pérez-Cerezales, S.; Gutiérrez-Adán, A.; López-Sebastián, A.; García-Casado, P.; Gil, M.; et al. Effect of supplementation of valine to chicken extender on sperm cryoresistance and post-thaw fertilization capacity. Poult. Sci. 2020, 99, 7133–7141. [Google Scholar] [CrossRef] [PubMed]
- Khiabani, A.B.; Moghaddam, G.; Kia, H.D. Effects of adding different levels of Glutamine to modified Beltsville extender on the survival of frozen rooster semen. Anim. Reprod. Sci. 2017, 184, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Partyka, A.; Rodak, O.; Bajzert, J.; Kochan, J.; Niżański, W. The Effect of L-Carnitine, Hypotaurine, and Taurine Supplementation on the Quality of Cryopreserved Chicken Semen. BioMed Res. Int. 2017, 2017, 7279341. [Google Scholar] [CrossRef]
- Heber, U.; Tyankova, L.; Santarius, K.A. Stabilization and inactivation of biological membranes during freezing in the presence of amino acids. Biochim. Biophys. Acta Biomembr. 1971, 241, 578–592. [Google Scholar] [CrossRef]
- Kumar, A.; Kroetsch, T.; Blondin, P.; Anzar, M. Fertility-associated metabolites in bull seminal plasma and blood serum: 1H nuclear magnetic resonance analysis. Mol. Reprod. Dev. 2015, 82, 123–131. [Google Scholar] [CrossRef]
- Santiago-Moreno, J.; Blesbois, E. Functional Aspects of Seminal Plasma in Bird Reproduction. Int. J. Mol. Sci. 2020, 21, 5664. [Google Scholar] [CrossRef]
- Cerolini, S.; Zaniboni, L.; Mangiagalli, M.G.; Gliozzi, T.M. Effect of glycine on cryopreservation of chicken spermatozoa. Avian Poult. Biol. Rev. 2007, 18, 65. [Google Scholar]
- Iaffaldano, N.; Paventi, G.; Pizzuto, R.; Passarella, S.; Cerolini, S.; Zaniboni, L.; Marzoni, M.; Castillo, A.; Rosato, M.P. The post-thaw irradiation of avian spermatozoa with He-Ne laser differently affects chicken, pheasant and turkey sperm quality. Anim. Reprod. Sci. 2013, 142, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Iaffaldano, N.; Meluzzi, A.; Manchisi, A.; Passarella, S. Improvement of stored turkey semen quality as a result of He–Ne laser irradiation. Anim. Reprod. Sci. 2005, 85, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Vessey, W.; Saifi, S.; Sharma, A.; McDonald, C.; Almeida, P.; Figueiredo, M.; Minhas, S.; Virmani, A.; Dhillo, W.S.; Ramsay, J.W.; et al. Baseline levels of seminal reactive oxygen species predict improvements in sperm function following antioxidant therapy in men with infertility. Clin. Endocrinol. 2021, 94, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Vicari, E.S.D.; Calogero, A.E. Effects of treatment with carnitines in infertile patients with prostato-vesiculo-epididymitis. Hum. Reprod. 2001, 16, 2338–2342. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, S.; Aghaei, A. Effect of l-carnitine on sperm quality during liquid storage of chicken semen. Comp. Clin. Pathol. 2012, 21, 711–717. [Google Scholar] [CrossRef]
- Aldaraji, H.J.; Tahir, A.O. Effect of L-carnitine supplementation on drake semen quality. S. Afr. J. Anim. Sci. 2014, 44, 18. [Google Scholar] [CrossRef]
- Banihani, S.; Agarwal, A.; Sharma, R.; Bayachou, M. Cryoprotective effect of l-carnitine on motility, vitality and DNA oxidation of human spermatozoa. Andrologia 2014, 46, 637–641. [Google Scholar] [CrossRef]
- Wishart, G.J. Maintenance of ATP concentrations in and of fertilizing ability of fowl and turkey spermatozoa in vitro. Reproduction 1982, 66, 457–462. [Google Scholar] [CrossRef]
- Sasanami, T.; Izumi, S.; Sakurai, N.; Hirata, T.; Mizushima, S.; Matsuzaki, M.; Hiyama, G.; Yorinaga, E.; Yoshimura, T.; Ukena, K.; et al. A unique mechanism of successful fertilization in a domestic bird. Sci. Rep. 2015, 5, 7700. [Google Scholar] [CrossRef]
- Sancho, S.; Casas, I.; Ekwall, H.; Saravia, F.; Rodriguez-Martinez, H.; Rodriguez-Gil, J.E.; Flores, E.; Pinart, E.; Briz, M.D.; Garcia-Gil, N.; et al. Effects of cryopreservation on semen quality and the expression of sperm membrane hexose transporters in the spermatozoa of Iberian pigs. Reproduction 2007, 134, 111–121. [Google Scholar] [CrossRef][Green Version]
- Labas, V.; Grasseau, I.; Cahier, K.; Gargaros, A.; Harichaux, G.; Teixeira-Gomes, A.-P.; Alves, S.; Bourin, M.; Gérard, N.; Blesbois, E. Qualitative and quantitative peptidomic and proteomic approaches to phenotyping chicken semen. J. Proteom. 2015, 112, 313–335. [Google Scholar] [CrossRef]
- Paventi, G.; Lessard, C.; Bailey, J.L.; Passarella, S. In boar sperm capacitation l -lactate and succinate, but not pyruvate and citrate, contribute to the mitochondrial membrane potential increase as monitored via safranine O fluorescence. Biochem. Biophys. Res. Commun. 2015, 462, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Darr, C.R.; Varner, D.D.; Teague, S.; Cortopassi, G.A.; Datta, S.; Meyers, S.A. Lactate and Pyruvate Are Major Sources of Energy for Stallion Sperm with Dose Effects on Mitochondrial Function, Motility, and ROS Production. Biol. Reprod. 2016, 95, 34. [Google Scholar] [CrossRef] [PubMed]
- Passarella, S.; de Bari, L.; Valenti, D.; Pizzuto, R.; Paventi, G.; Atlante, A. Mitochondria and L-lactate metabolism. FEBS Lett. 2008, 582, 3569–3576. [Google Scholar] [CrossRef] [PubMed]
- Paventi, G.; Pizzuto, R.; Passarella, S. The occurrence of l-lactate dehydrogenase in the inner mitochondrial compartment of pig liver. Biochem. Biophys. Res. Commun. 2017, 489, 255–261. [Google Scholar] [CrossRef]
- Passarella, S.; Paventi, G.; Pizzuto, R. The mitochondrial L-lactate dehydrogenase affair. Front. Neurosci. 2014, 8, 407. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Bellanca, S.; Vicari, E.; Calogero, A.E. Myoinositol: Does it improve sperm mitochondrial function and sperm motility? Urology 2012, 79, 1290–1295. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Di Bari, F.; Unfer, V.; Calogero, A.E. Effects of myoinositol on sperm mitochondrial function in-vitro. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 129–134. [Google Scholar]
- Vazquez-Levin, M.H.; Verón, G.L. Myo-inositol in health and disease: Its impact on semen parameters and male fertility. Andrology 2020, 8, 277–298. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785. [Google Scholar] [CrossRef]
- Nguyen, T.M.D.; Grasseau, I.; Blesbois, E. New insights in the AMPK regulation in chicken spermatozoa: Role of direct AMPK activator and relationship between AMPK and PKA pathways. Theriogenology 2019, 140, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vadnais, M.L.; Cao, W.; Aghajanian, H.K.; Haig-Ladewig, L.; Lin, A.M.; AlAlao, O.; Gerton, G.L. Adenine Nucleotide Metabolism and a Role for AMP in Modulating Flagellar Waveforms in Mouse Sperm1. Biol. Reprod. 2014, 90, 128. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhang, G.; Zhang, H.; Chen, F.; Chen, Y.; Zhuang, Y.; Huang, Z.; Zou, F.; Liu, M.; An, G.; et al. Adenylate kinase 1 deficiency disrupts mouse sperm motility under conditions of energy stress. Biol. Reprod. 2020, 103, 1121–1131. [Google Scholar] [CrossRef]
- Sanocka, D.; Kurpisz, M. Reactive oxygen species and sperm cells. Reprod. Biol. Endocrinol. 2004, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Shabani, S.; Mehri, M.; Shirmohammad, F.; Sharafi, M. Enhancement of sperm quality and fertility-related parameters in Hubbard grandparent rooster fed diets supplemented with soybean lecithin and vitamin E. Poult. Sci. 2022, 101, 101635. [Google Scholar] [CrossRef] [PubMed]
- Giraud, M.N.; Motta, C.; Boucher, D.; Grizard, G. Membrane fluidity predicts the outcome of cryopreservation of human spermatozoa. Hum. Reprod. 2000, 15, 2160–2164. [Google Scholar] [CrossRef] [PubMed]
- Partyka, A.; Niżański, W. Supplementation of avian semen extenders with antioxidants to improve semen quality—Is it an effective strategy? Antioxidants 2021, 10, 1927. [Google Scholar] [CrossRef]
- Ladha, S. Lipid heterogeneity and membrane fluidity in a highly polarized cell, the mammalian spermatozoon. J. Membr. Biol. 1998, 165, 1–10. [Google Scholar] [CrossRef]
- Douard, V.; Hermier, D.; Blesbois, E. Changes in Turkey Semen Lipids During Liquid In Vitro Storage1. Biol. Reprod. 2000, 63, 1450–1456. [Google Scholar] [CrossRef]
- Bongalhardo, D.C.; Leeson, S.; Buhr, M.M. Dietary lipids differentially affect membranes from different areas of rooster sperm. Poult. Sci. 2009, 88, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.S.; Rakha, B.A.; Akhter, S.; Akhter, A.; Blesbois, E.; Santiago-Moreno, J. Effect of glutathione on pre and post-freezing sperm quality of Indian red jungle fowl (Gallus gallus murghi). Theriogenology 2021, 172, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Y.; Wu, C.; Qiu, S.; Chen, X.; Cai, B.; Xie, H. Freeze-thawing impairs the motility, plasma membrane integrity and mitochondria function of boar spermatozoa through generating excessive ROS. BMC Vet. Res. 2021, 17, 127. [Google Scholar] [CrossRef] [PubMed]
| Metabolite, 1H Chemical Shift (ppm) | Fresh | Frozen | |
|---|---|---|---|
| Water Extract | p-Value | ||
| Amino acids | mol % (n = 5) | mol % (n = 5) | |
| Ala (1.48) | 0.178 ± 0.007 a | 0.113 ± 0.007 b | 0.001 |
| Asp (2.83) | 0.239 ± 0.022 a | 0.221 ± 0.005 a | 0.466 |
| Gln (2.45) | 1.614 ± 0.314 a | 1.468 ± 0.197 a | 0.412 |
| Glu (2.07) | 66.547 ± 0.391 a | 66.956 ± 0.761 a | 0.725 |
| Gly (3.57) | 4.648 ± 0.043 a | 4.951 ± 0.073 b | 0.001 |
| Ile (1.02) | 0.015 ± 0.001 b | 0.009 ± 0.001 b | 0.009 |
| Leu (0.96) | 0.054 ± 0.003 a | 0.026 ± 0.001 b | 0.003 |
| Phe (7.43) | 0.022 ± 0.001 a | 0.017 ± 0.001 b | 0.030 |
| Tyr (6.92) | 0.048 ± 0.002 a | 0.020 ± 0.002 b | 0.001 |
| Val (0.99) | 0.038 ± 0.002 a | 0.019 ± 0.002 b | 0.002 |
| Organic acids | |||
| Acetate (1.93) | 0.486 ± 0.064 a | 0.353 ± 0.087 a | 0.403 |
| Citrate (2.57) | 0.089 ± 0.006 b | 0.125 ± 0.006 a | 0.039 |
| Formate (8.46) | 0.039 ± 0.003 a | 0.019 ± 0.004 b | 0.002 |
| Fumarate (6.53) | 0.032 ± 0.003 a | 0.040 ± 0.002 a | 0.072 |
| Lactate (1.33) | 1.362 ± 0.072 a | 0.884 ± 0.059 b | 0.001 |
| Other compounds | |||
| Ac-carnitine (3.20) | 0.037 ± 0.002 a | 0.045 ± 0.002 b | 0.006 |
| AMP (8.28) | 0.146 ± 0.007 a | 0.115 ± 0.008 b | 0.005 |
| Carnitine (3.24) | 0.080 ± 0.005 a | 0.037 ± 0.002 b | 0.002 |
| Creatine (3.94) | 1.828 ± 0.136 a | 1.431 ± 0.071 b | 0.028 |
| Glucose (3.26 and 5.25)* | 16.445 ± 0.437 a | 17.085 ± 0.592 a | 0.504 |
| Myo-inositol (3.65) | 6.054 ± 0.039 a | 6.067 ± 0.205 a | 0.949 |
| Lipid extract | |||
| mol % (n = 3) | mol % (n = 3) | ||
| CHO (0.74) | 9.587 ± 0.348 a | 6.944 ± 0.533 b | 0.036 |
| SFA | 38.001 ± 1.430 b | 43.088 ± 1.032 a | 0.006 |
| DUFA (2.81) | 4.200 ± 0.136 a | 3.857 ± 0.356 a | 0.293 |
| UFA (2.08) | 62.000 ± 1.430 a | 56.912 ± 1.032 b | 0.006 |
| PUFA (2.86) | 36.793 ± 0.561 a | 34.665 ± 0.633 b | 0.027 |
| PC (3.28) | 24.703 ± 0.760 a | 19.667 ± 0.612 a | 0.081 |
| PE (3.21) | 14.107 ± 0.152 a | 11.989 ± 0.611 a | 0.072 |
| SMN (5.76) | 6.990 ± 0.239 a | 6.200 ± 0.577 a | 0.167 |
| Metabolite | Sperm Variables | ||
|---|---|---|---|
| Mobility | Viability | Osmotic Tolerance | |
| Ala | 0.867 ** | 0.930 ** | 0.902 ** |
| Gly | −0.770 ** | −0.771 | −0.802 ** |
| Ile | 0.818 ** | 0.861 ** | 0.908 ** |
| Leu | 0.915 ** | 0.942 ** | 0.962 ** |
| Phe | 0.683 * | 0.784 ** | 0.781 ** |
| Tyr | 0.915 ** | 0.969 ** | 0.972 ** |
| Val | 0.871 ** | 0.937 ** | 0.947 ** |
| Citrate | −0.874 ** | −0.833 ** | −0.815 ** |
| Formate | 0.764 * | 0.854 ** | 0.811 ** |
| Fumarate | −0.723 * | −0.659 * | |
| Lactate | 0.806 ** | 0.887 ** | 0.830 ** |
| Ac-carnitine | −0.740 * | −0.695 * | −0.689 * |
| AMP | 0.653 * | 0.728 * | 0.708 * |
| Carnitine | 0.923 ** | 0.925 ** | 0.957 ** |
| Creatine | 0.673 * | 0.697 * | |
| CHO | 0.907 * | 0.884 * | 0.876 * |
| SFA | −0.865 * | −0.868 * | |
| UFA | 0.865 * | 0.868 * | |
| PC | 0.969 ** | 0.882 * | 0.884 * |
| PE | 0.851 * | 0.870 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paventi, G.; Di Iorio, M.; Rusco, G.; Sobolev, A.P.; Cerolini, S.; Antenucci, E.; Spano, M.; Mannina, L.; Iaffaldano, N. The Effect of Semen Cryopreservation Process on Metabolomic Profiles of Turkey Sperm as Assessed by NMR Analysis. Biology 2022, 11, 642. https://doi.org/10.3390/biology11050642
Paventi G, Di Iorio M, Rusco G, Sobolev AP, Cerolini S, Antenucci E, Spano M, Mannina L, Iaffaldano N. The Effect of Semen Cryopreservation Process on Metabolomic Profiles of Turkey Sperm as Assessed by NMR Analysis. Biology. 2022; 11(5):642. https://doi.org/10.3390/biology11050642
Chicago/Turabian StylePaventi, Gianluca, Michele Di Iorio, Giusy Rusco, Anatoly P. Sobolev, Silvia Cerolini, Emanuele Antenucci, Mattia Spano, Luisa Mannina, and Nicolaia Iaffaldano. 2022. "The Effect of Semen Cryopreservation Process on Metabolomic Profiles of Turkey Sperm as Assessed by NMR Analysis" Biology 11, no. 5: 642. https://doi.org/10.3390/biology11050642
APA StylePaventi, G., Di Iorio, M., Rusco, G., Sobolev, A. P., Cerolini, S., Antenucci, E., Spano, M., Mannina, L., & Iaffaldano, N. (2022). The Effect of Semen Cryopreservation Process on Metabolomic Profiles of Turkey Sperm as Assessed by NMR Analysis. Biology, 11(5), 642. https://doi.org/10.3390/biology11050642











