Simple Summary
Analysis of sperm performance under in vitro conditions provides a good indication of fertilizing potential. Parameters such as motility, swimming kinetics, acrosome integrity, or ATP content are thus examined in efforts to characterize such potential. Hamster species are a good model to study sperm parameters that are key determinants of fertilizing capacity because these species are at the higher end of the diversity of mammalian sperm morphology and performance. In vitro functional studies demand that sperm remain viable during a long period of time under conditions that resemble those in the female tract. Sperm from certain species require supplementation of the incubation medium with factors that stimulate viability and swimming, or that promote acquisition of fertilizing capacity. Molecules important for sperm performance in hamsters have been identified, namely D-penicillamine, hypotaurine and epinephrine (PHE). In the present study, we investigated the effect of PHE on spermatozoa from five hamster species incubated for up to 4 h. Our results revealed that PHE maintains sperm performance in the golden hamster, whereas it improves sperm quality in the Chinese hamster. In contrast, it does not seem to have any effect on sperm from the Siberian (Djungarian), Roborovski and Campbell’s dwarf hamsters. These results are valuable to understand the different regulatory mechanisms of sperm motility and survival in different species.
Abstract
Assessments of sperm performance are valuable tools for the analysis of sperm fertilizing potential and to understand determinants of male fertility. Hamster species constitute important animal models because they produce sperm cells in high quantities and of high quality. Sexual selection over evolutionary time in these species seems to have resulted in the largest mammalian spermatozoa, and high swimming and bioenergetic performances. Earlier studies showed that golden hamster sperm requires motility factors such as D-penicillamine, hypotaurine and epinephrine (PHE) to sustain survival over time, but it is unknown how they affect swimming kinetics or ATP levels and if other hamster species also require them. The objective of the present study was to examine the effect of PHE on spermatozoa of five hamster species (Mesocricetus auratus, Cricetulus griseus, Phodopus campbelli, P. sungorus, P. roborovskii). In sperm incubated for up to 4 h without or with PHE, we assessed motility, viability, acrosome integrity, sperm velocity and trajectory, and ATP content. The results showed differences in the effect of PHE among species. They had a significant positive effect on the maintenance of sperm quality in M. auratus and C. griseus, whereas there was no consistent effect on spermatozoa of the Phodopus species. Differences between species may be the result of varying underlying regulatory mechanisms of sperm performance and may be important to understand how they relate to successful fertilization.
1. Introduction
Sperm performance, a major determinant of male fertility, can be dissected into a series of traits that are intricately connected to sperm fertilizing potential [1,2]. Therefore, a combination of various performance parameters is usually assessed to evaluate the quality of sperm samples and predict its fertilizing potential in man and other animals [2,3,4,5,6,7,8,9,10]. Sperm viability, motility and kinetics, as well as acrosome integrity, are all linked to sperm survival. The proportion of motile sperm and the velocity of spermatozoa are essential for sperm to swim along the cervix, uterus and the uterotubal junction, and cells that show higher values in these parameters also have higher chances to reach and fertilize the ovum [11]. The assessment of sperm motility may be carried out subjectively, estimating the percentage of motile sperm by microscopic visualization, and objectively through the quantification of sperm swimming parameters by computer aided sperm analysis (CASA) [1,2,10,12,13].
The sperm acrosome contains enzymes that are released during exocytosis, an essential step required for penetration of the oocyte’s vestments [14]. The timing of release is important, so the integrity of the acrosome during sperm transport in the female tract and, in particular, along the oviduct, has to be preserved in order to ensure fertilizing potential [15]. Controversy still exists as to which is the site where acrosomal exocytosis takes place or which is the physiological ligand (or ligands) responsible for initiating exocytosis [16,17,18,19]. Nevertheless, since this is a key event in the processes leading to completion of fertilization, acrosomal status has become a valuable assessment of fertilizing potential [20].
Other relevant sperm parameters relate to sperm bioenergetics, with sperm ATP content serving as an indicator of the balance between sperm ATP production and consumption [21]. High ATP levels are positively correlated to sperm swimming velocity in rodents [22] and mammals in general [23]. Moreover, assessments of the variation in sperm ATP content and sperm traits over time among rodent species revealed that maintenance of high performance in species with high competitive ability is associated with high concentrations of intracellular ATP over time [22,24,25].
Exogenous factors could influence sperm performance in vivo and under in vitro conditions [26]. Besides the provision of energetic substrates that are fundamental for the production of ATP for sperm motility and survival, conditions in the female tract promote the acquisition of sperm’s fertilizing ability, a process known as capacitation [27]. Among physical factors, extracellular pH, temperature, and viscosity are known to affect the survival and performance of sperm cells [28,29,30,31,32,33,34,35]. Several biological factors are also known to have important roles in sperm survival in vivo and in vitro. Early efforts to achieve in vitro fertilization had to rely on homologous or heterologous serum to ensure sperm survival and the acquisition of fertilizing ability [36,37]. Better definition of media was possible with replacement of serum by bovine serum albumin. However, these media were still not completely defined [38]. Components of the extracellular milieu may be required to sustain sperm motility, and variations between species may exist regarding the nature of such components. For golden hamster (Mesocricetus auratus) spermatozoa, one set of important motility factors are catecholamines, which maintain and stimulate sperm motility in vitro [39]. Within the group of catecholamines, epinephrine is an essential co-factor for golden hamster sperm as it activates motility as well as Na+/K+ ATPase and Ca2+-ATPase [40], and it is also involved in the acquisition of fertilizing ability [41]. However, epinephrine is not able to maintain golden hamster sperm motility on its own, and a second factor, hypotaurine, is also essential. Hypotaurine is a superoxide scavenger that functions inhibiting lipid peroxidation and superoxide dismutase [42], which prevents motility loss. Hypotaurine and epinephrine together also cause a mild increase of the acrosome reaction in hamster sperm [43]. Other factors have been shown to influence hamster sperm function [44,45,46].
Another molecule of interest, with regards to hamster sperm survival, is D-penicillamine. This is an α-amino acid which acts as a cation chelator, protecting sperm from oxidation in several species [40,47,48,49,50]. As a zinc chelator, D-penicillamine facilitates capacitation, acting in the early step of this process because it removes most of the zinc within the first ten minutes of its addition, but it is not enough to support full capacitation [51]. D-penicillamine also prolongs hyperactivated motility [52]. This amino acid together with epinephrine and hypotaurine (PHE: penicillamine + hypotaurine + epinephrine) act as motility factors, necessary for the maintenance of golden hamster sperm motility in vitro [51]. The addition of PHE to incubation media maintains golden hamster sperm motility within the first hour [53,54] and also promotes sperm capacitation in this [54] and other species [55]. The synergistic effect of the three components of PHE can reactivate immotile spermatozoa of golden hamsters [56].
Many early studies of sperm function have used the golden hamster as a model, particularly because of the ease of examining acrosomal status in motile spermatozoa [57]. Studies of sperm behavior in a related species, the Chinese hamster (Cricetulus griseus), have been performed [58,59,60] and these studies have also identified the need to support sperm viability and motility over time to achieve fertilization. Similar requirements seem to exist for Siberian hamster (Phodopus sungorus) spermatozoa because low success was achieved in in vitro fertilization with a variety of media and supplements [61], but characterization of these requirements has not been undertaken. Hamsters are a valuable model for studies of sperm-oocyte recognition and interaction. Hybridization has been observed among hamsters (Mesocricetus species: [62,63,64,65,66]; Phodopus species: [67]; Allocricetulus species: [68]), and cross-fertilization in vitro between golden and Chinese hamsters has been reported [37]. Despite the potential of this group of species as a model for sperm biology, little is still known about the spermatozoa and fertilization of most species in this group. Comparative and evolutionary studies are needed to understand diversity in function and underlying mechanisms of sperm survival, capacitation and acrosomal exocytosis. Hamster species are attractive because they are at the higher end of the range of mammalian sperm dimensions [69,70], have high sperm swimming velocities [22] and exhibit high levels of sperm ATP [22,24]. Such traits, characterizing these species as high performers, may be the result of intense sperm competition [71]. Detailed comparative studies of sperm performance may therefore be rewarding in hamsters of the subfamily Cricetinae for which good background information of phylogenetic relations currently exists based on molecular studies [72] and chromosome evolution [73,74].
In the present study, we evaluated whether several biological molecules had a role in the performance of spermatozoa from five different species of hamster: Mesocricetus auratus, Cricetulus griseus, Phodopus campbelli, P. sungorus and P. roborovskii, focusing on sperm motility, viability, sperm kinematics, acrosome integrity, and bioenergetics. Spermatozoa from these species are at the higher end of the range of sperm quality parameters among rodent species [22,24]; thus, a detailed analysis of modulators of sperm function in these species could help understand determinants of male fertility.
2. Materials and Methods
2.1. Reagents
Unless stated otherwise, reagents were purchased from Sigma or Merck (both of Madrid, Spain).
2.2. Animals and Sperm Collection
Adult males (4–6 months old) of Cricetulus griseus (n = 5), Mesocricetus auratus (n = 6), Phodopus campbelli (n = 5), P. sungorus (n = 7), and P. roborovskii (n = 5) were kept in captivity in our animal facilities. Animals were maintained under standard conditions (14 h light–10 h darkness, 22–24 °C), with food and water provided ad libitum. Each male to be used in this study was housed alone (i.e., in individual cages) for at least one month before sperm collection. Males were sacrificed by cervical dislocation and weighed immediately. Testes were then removed and weighed. Relative testes size was calculated using the potential equation defined for rodents by Kenagy and Trombulak [75].
The caudae epididymides were excised after removing all blood vessels, fat, and surrounding connective tissues. Each cauda epididymis was placed in a Petri dish containing one of two variants of culture medium pre-warmed to 37 °C and spermatozoa were collected by performing three to five incisions in the distal region of the cauda, and allowing them to swim out for 5 min. As standard medium (“control” treatment), we used a Hepes-buffered modified Tyrode medium with albumin (see mT-H in [76]), with the addition of lactate and pyruvate (mTALP: 120.89 mM NaCl, 2.68 mM KCl, 0.49 mM MgCl2·6H2O, 0.36 mM NaH2PO4·2H2O, 20 mM Hepes, 1.80 mM CaCl2, 5.56 mM glucose, 1 mM sodium pyruvate, 10 mM sodium lactate, 4 mg mL−1 bovine serum albumin) under air (pH 7.4). A modified medium (PHE treatment) was mTALP supplemented with 20 µM D-penicillamine, 100 µM hypotaurine, and 1 µM epinephrine. Sperm suspensions were placed in plastic tubes, where sperm concentration was estimated by using a modified Neubauer chamber and adjusted to 20 × 106 sperm mL−1 by diluting with medium. Sperm parameters were assessed in the sperm suspensions corresponding to each treatment immediately after adjusting the concentration (hereafter referred as 0 h), and after 2, 3, and 4 h of incubation at 37 °C under air. Large-bore pipette tips were used to minimize damage to spermatozoa in all procedures.
2.3. Sperm Motility, Viability and Acrosomal Integrity
Sperm motility was evaluated by examining 10 µL of a previously diluted sperm suspension, placed between a pre-warmed slide and a coverslip, at 100× magnification under phase-contrast optics. The percentage of motile sperm was estimated by at least two independent, experienced observers, whose estimations were averaged and rounded to the nearest 5% value.
The assessment of sperm viability and acrosome integrity was performed by staining sperm first with eosin-nigrosin and subsequently with Giemsa [77]. Briefly, 5 µL sperm suspension and 10 µL eosin-nigrosin solution were mixed on a glass slide placed on a stage at 37 °C and 30 s later the mix was smeared and allowed to air-dry. Smears were fixed by immersion during 10 min in a solution of 4% formaldehyde in TPB buffer. After fixation, the smears were stained with Giemsa solution and mounted with DPX. Slides were examined at 1000× under bright field and 200 spermatozoa per male were examined to evaluate sperm viability and integrity of the acrosome. Viable spermatozoa were those excluding eosin (from the eosin-nigrosin stain). Acrosome integrity was reported as the percentage of sperm with intact acrosomes, excluding cells that showed damaged or missing acrosomes.
2.4. Sperm Velocity and Trajectory
To assess sperm swimming velocity and trajectory, an aliquot of sperm suspension was placed in a pre-warmed microscopy chamber with a depth of 20 µm (Leja, Nieuw-Vennep, The Netherlands) and filmed using a phase contrast microscope with pseudo-negative phase connected to a digital video camera (Basler A312fc, Vision Technologies, Glen Burnie, MD, USA). A 4× objective was used instead of the 10× objective traditionally used for CASA analysis on sperm from humans and domestic animals. This resulted in larger field of observation, which allowed for tracking of the unusually large and fast hamster sperm for longer, and a deeper focal plane to account for the depth of the observation chamber. Sperm trajectories were assessed using a computer aided sperm analyzer (Sperm Class Analyzer—SCA v.4.0, Microptic, Barcelona, Spain), and the following swimming parameters were estimated for each track: curvilinear velocity (VCL, µm s−1), straight-line velocity (VSL, µm s−1), average path velocity (VAP, µm s−1), linearity (LIN = VSL/VCL), straightness (STR = VSL/VAP), wobble (WOB = VAP/VCL), amplitude of lateral head displacement (ALH, µm), and beat-cross frequency (BCF, Hz). The software was set with frame rate 50 s−1, maximum particle size 500 μm, minimum particle size 50 μm, connectivity 30, contrast 400, and brightness 160. All video captures were compared to their overlaying analyzed tracks, and trajectories that did not belong to sperm were removed. In addition, trajectories with VAP values lower than 20 µm s−1, and LIN, STR and WOB values of 100 in the post-capture analysis were discarded as these are typical of drifting but immotile sperm or other non-sperm particles. Since spermatozoa of Cricetulus griseus are significantly larger than those of the other species studied (about 250 µm [69]), and their head has a slender falciform shape that makes it almost undistinguishable from the midpiece in the moving sperm, the SCA software was not able to obtain accurate sperm trajectories. Thus, data on sperm velocity and trajectory for C. griseus are not presented.
2.5. Sperm ATP Content
Sperm ATP content was measured using a luciferase-based ATP bioluminescent assay kit (Roche, ATP Bioluminescence Assay Kit HS II), as previously described [22,24]. A 100 µL-aliquot of diluted sperm suspension was mixed with 100 µL of Cell Lysis Reagent, vortexed and incubated at room temperature for 5 min. The resulting cell lysate was centrifuged at 12,000× g for 2 min, and the supernatant was recovered and frozen in liquid N2. Bioluminescence was measured in triplicate in 96-well plates using a luminometer (Varioskan Flash, Thermo Fisher Scientific Inc., Waltham, MA, USA). In each well, 50 µL of Luciferase reagent were added to 50 µL of sample (via auto-injection), and, following a 1 s delay, light emission was measured over a 10 s integration period. Standard curves were constructed using solutions containing known concentrations of ATP diluted in mTALP and Cell Lysis Reagent in a proportion equivalent to that of the samples. ATP content was expressed as amol sperm−1.
2.6. Data Analysis
2.6.1. Principal Component Analyses for Sperm Velocity Parameters
Since swimming parameters tend to be highly correlated [78], principal component analyses (PCA) were performed to construct variables that summarize the information obtained through CASA. The variables were divided in two groups defining sperm velocity (VCL, VSL, VAP) and sperm trajectory (LIN, STR, WOB, ALH, BCF) and one independent PCA was carried out for each group. The first principal component for the velocity group (VPC1) accounted for 91.8% of the variability in the three summarized variables (VCL, VSL, VAP), while the second principal component (VPC2) only accounted for 7.6% of the variability (Figure 1A). The three sperm velocity descriptors showed extremely high correlation coefficients with VPC1 and were weakly or non-significantly correlated with VPC2 (Table 1). For the trajectory, the first principal component (TPC1) accounted for 58.4% of the variability, while the second principal component (TPC2) represented 26.6% of the variability (Figure 1B). Although the five variables included in this group were correlated with TPC1, the strength of the correlation was higher for LIN, STR, and WOB (Table 1). On the other hand, ALH and BCF showed a stronger correlation with TPC2 (Table 1). Thus, VPC1, TPC1 and TPC2 values for each treatment and species were used as our integrated sperm velocity and trach shape measures.
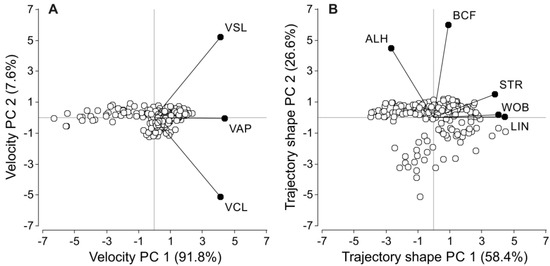
Figure 1.
Biplots detailing the relationships between principal components of sperm velocity (A) and trajectory shape (B) and their constituent variables. Variable values were log10 or arcsine transformed prior to analysis. VCL: curvilinear velocity. VSL: straight-line velocity. VAP: average path velocity. LIN: linearity. STR: straightness. WOB: wobble coefficient. ALH: amplitude of the lateral head displacement. BCF: beat-cross frequency.

Table 1.
Loadings and correlation of sperm traits with principal components of sperm velocity and trajectory shape in five hamster species. Values presented are Pearson’s correlation coefficients. Significant correlation coefficients (p < 0.05) are shown in bold. PC1: principal component 1. PC2: principal component 2. Variable values were log10 or arcsine transformed prior to analysis.
2.6.2. Statistical Analyses
The effect of incubation with PHE on sperm parameters over time was analyzed with a two-factor repeated-measures ANOVA for each species, using treatment (2 levels: control and PHE) and time (4 levels: 0, 2, 3, 4 h) as factors. Differences between conditions were analyzed through a post-hoc DGC multiple comparisons test [79]. All variables were log10-transformed for statistical purposes, with the exception of percentages (motility, viability, acrosome integrity, LIN, STR and WOB), which were arcsine-transformed. The statistical analyses were performed using SPSS Statistics (SPSS v.23.0.0.0; SPSS, IBM Corporation, Somers, NY, USA), and InfoStat v.2015p (Grupo Infostat, Universidad Nacional de Córdoba, Córdoba, Argentina) with α = 0.05.
3. Results
3.1. Relative Testes Size and Sperm Numbers
Body mass, testes mass, relative testes size and sperm numbers per individual are presented in Table 2. The mass of testes in relation to body mass was high in all species when compared with other species of muroid rodents (see [22,77]). Among the species examined here, Cricetulus griseus showed the highest relative testes size (sensu [75]), while Phodopus sungorus presented the smallest testes in relation to body mass.

Table 2.
Corporal measurements and sperm numbers in five hamster species. Values indicate mean ± standard error. RTS: relative testes size calculated according to Kenagy and Trombulak [75].
3.2. Sperm Motility, Viability, Acrosome Integrity and ATP Content
Initial sperm motility (~80–90%), viability (~95–99%), and acrosome integrity (~75–90%) were high for all species in both media (Table 3). In addition, sperm of the five species showed relatively high initial ATP content in media without and with PHE (Table 3) when compared with other muroid rodent species (see [24,80]). In general, sperm showed a significant gradual decline in their motility and acrosome integrity throughout the 4 h of incubation (Table 4, Figure 2) with the exception of Mesocricetus auratus spermatozoa which exhibited a more pronounced decrease in motility between 0 and 2 h of incubation, with a slight decrease afterwards (Figure 2D). Sperm ATP content also decreased significantly with time in the five species (Table 4), with a pronounced phase of decline between 0 and 2 h of incubation in four of the five species (Figure 2), and a more constant decrease over the 4 h of incubation in the case of C. griseus (Figure 2C). Sperm viability did not change significantly during incubation time in any of the five species, maintaining high values (>90%) in all species with both treatments (Table 4, Figure S1).

Table 3.
Sperm motility, acrosome integrity, viability and ATP content in five hamster species. Values correspond to mean and standard error (SE) for spermatozoa in the absence (control) or presence of penicillamine, hypotaurine and epinephrine (PHE) at the start of the incubation period (time zero).

Table 4.
Effect of penicillamine, hypotaurine, and epinephrine (PHE) on sperm motility, acrosome integrity, viability, and ATP content, in five hamster species. F and p values correspond to repeated measures ANOVA using time of incubation and treatment (control vs. PHE) as independent variables and sperm parameters as dependent variables. Significant differences between treatments and times of incubation (p < 0.05) are shown in boldface.
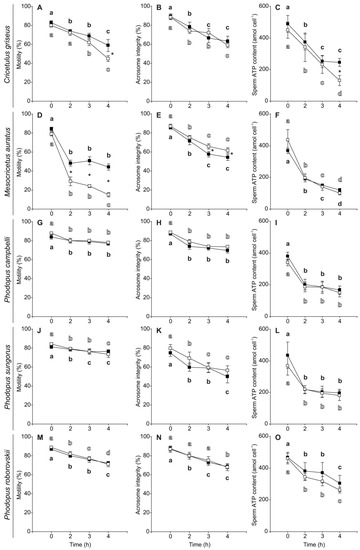
Figure 2.
Changes in motility, acrosome integrity, and ATP content in spermatozoa from five hamster species during incubation without or with penicillamine, hypotaurine and epinephrine (PHE). Spermatozoa were collected in medium with (black squares) or without PHE (white squares) and incubated at 37 °C under air for up to 4 h. (A,D,G,J,M) Percentage of motile sperm. (B,E,H,K,N) Percentage of spermatozoa with an intact acrosome. (C,F,I,L,O) Sperm ATP content (amol × cell−1). Values are means ± standard errors. Different letters between times of incubation for the same treatment indicate statistically significant differences (p < 0.05) in a DGC post-hoc test. Asterisks indicate statistical differences (p < 0.05) between treatments for the same time in a DGC post-hoc test.
The incubation in a medium with PHE showed a significantly positive effect on sperm motility in C. griseus and M. auratus (Table 4). The effect was stronger and appeared earlier in M. auratus (2 h of incubation, Figure 2D) than in C. griseus (4 h of incubation, Figure 2A). In C. griseus, sperm viability was significantly higher in the PHE treatment than in the control after 2 h of incubation (Table 4, Figure S1A). Conversely, M. auratus sperm showed differences favoring the control over the PHE treatment at 3 and 4 h (Table 4, Figure S1B). In the case of Phodopus species, the addition of PHE to the incubation medium showed no significant effects on sperm motility, viability, and acrosome integrity (Table 4, Figure 2 and Figure S1). Sperm ATP content was not affected by the addition of PHE to the incubation medium (Table 4, Figure 2) with the exception of C. griseus in which PHE promoted a less pronounced decline in ATP content (Table 4, Figure 2C).
3.3. Sperm Velocity and Trajectory
Regarding sperm velocity and trajectory parameters, all species presented similar values regardless of treatment at the beginning of the incubation period (0 h) (Table 5). As a general trend, velocity and trajectory parameters, as well as their estimated principal components (VPC1, TPC1, and TPC2) exhibited a time-related decline in both control and PHE treatment (Table 6, Figure 3). However, several variables did not show this time-related effect in some species. The sperm of M. auratus that were incubated in the presence of PHE maintained their linearity, straightness, and amplitude of lateral head displacement throughout the incubation period (Table 6, Figure 3B,C). In P. sungorus, straightness, amplitude of lateral head displacement, beat-cross frequency, and TPC1 were similar during the 4 h incubation regardless of treatment (Table 6, Figure 3H). This pattern was also observed in the values of linearity, and TPC2 but only for the control treatment (Table 6, Figure 3I). In P. roborovskii, sperm straight-line velocity, average path velocity, and VPC1 did not decrease over time (Table 6, Figure 3J). Notably, in this species, sperm linearity, straightness, wobble coefficient, and TPC1 registered a gradual increase during incubation time (Table 6, Figure 3K).

Table 5.
Sperm velocity and trajectory shape parameters in four hamster species. Values correspond to means and standard errors for spermatozoa in the absence (control) or with penicillamine, hypotaurine and epinephrine (PHE) at the start of the incubation period (time zero). VCL: curvilinear velocity. VSL: straight-line velocity. VAP: average path velocity. LIN: linearity. STR: straightness. WOB: wobble coefficient. ALH: amplitude of lateral head movement. BCF: beat-cross frequency.

Table 6.
Effect of penicillamine, hypotaurine and epinephrine (PHE) on sperm velocity and trajectory shape parameters in four hamster species. F and p values correspond to repeated measures ANOVA using time of incubation and treatment (control vs. PHE) as independent variables, and sperm parameters as dependent variables. VCL: curvilinear velocity. VSL: straight-line velocity. VAP: average path velocity. LIN: linearity. STR: straightness. WOB: wobble coefficient. ALH: amplitude of lateral head movement. BCF: beat-cross frequency. VPC1: velocity principal component 1. TPC1: trajectory shape principal component 1. TPC2: trajectory shape principal component 2. Significant differences between treatments and times of incubation (p < 0.05) are shown in boldface.
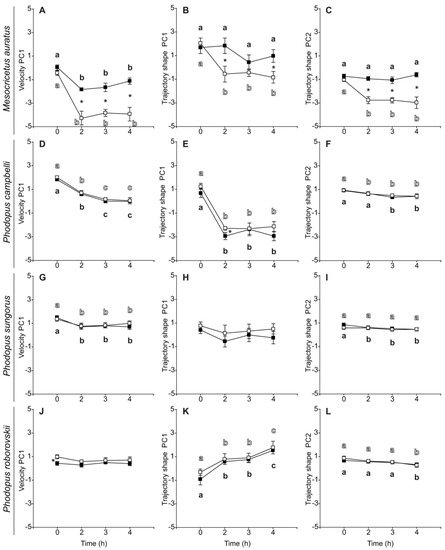
Figure 3.
Changes in velocity or trajectory of spermatozoa from five hamster species during incubation in the absence (control) or the presence of penicillamine, hypotaurine and epinephrine (PHE). Spermatozoa were collected in medium with (dark squares) or without PHE (white squares) and incubated at 37 °C under air for up to 4 h. Principal component analysis returned one main velocity component (velocity PC1) and two trajectory components (trajectory shape PC1, trajectory shape PC2). (A,D,G,J) Velocity PC1. (B,E,H,K) Trajectory shape PC1. (C,F,I,L) Trajectory shape PC2. Values are means ± standard errors. Different letters between times of incubation for the same treatment indicate statistically significant differences (p < 0.05) in a DGC post-hoc test. Asterisks indicate statistical differences (p < 0.05) between treatments for the same time in a DGC post-hoc test.
The sperm of M. auratus exhibited a strong significant response to the exposure to PHE during the incubation, in regard to their velocity and trajectory parameters. All velocity and trajectory variables, including their estimated principal components, showed significant higher values in the PHE treatment (Table 6, Figure 3A–C). The addition of PHE to the medium appears to prevent the time-related decline of sperm kinetics in this species. Conversely, the addition of PHE did not appear to have any positive effect on the kinetics of the sperm of any of the three species of the genus Phodopus. As a general trend, there were no significant differences in the values of most of the sperm velocity and trajectory variables in Phodopus species (Table 6, Figure 3D,F–I,K,L). In the few cases in which significant differences were observed (P. campbelli: VSL, LIN, WOB, ALH, and TPC1; P. sungorus: LIN, WOB; P. roborovskii: VCL, VSL, VAP, WOB, VPC1), these differences were of relatively low magnitude, were not maintained over time, and values were always higher in the control treatment (Table 6, Figure 3E,J).
4. Discussion
The results of this study show that the addition of D-penicillamine, hypotaurine and epinephrine (PHE) to the incubation medium seems to be necessary to sustain the performance (motility, sperm swimming velocity and trajectory) and, to a lesser extent, acrosome integrity and viability, of M. auratus sperm throughout incubation. Moreover, PHE appears to have a positive effect on the maintenance of sperm quality (motility, viability, and ATP content) in C. griseus. In contrast, the presence of PHE had no consistent effect on spermatozoa of the Phodopus species.
Golden hamster sperm is known to be very susceptible to in vitro dilution for more than 15–30 min [81,82] unless the medium is supplemented with motility factors. The addition of epinephrine, taurine, hypotaurine, penicillamine, or their combination, to hamster sperm has been studied mainly in M. auratus [39,40,56,81,82,83,84,85]. In the present work, a combination of D-penicillamine (20 µM), hypotaurine (100 µM) and epinephrine (1 µM) was selected to analyze their effects on sperm performance. Our results showed an improvement in motility, integrity of the acrosome and velocity and trajectory parameters from 2 h onwards after addition of PHE in M. auratus. Several studies showed that epinephrine and hypotaurine have a synergistic effect in maintaining sperm motility and promoting the acrosome reaction [40,43]. These two molecules caused a slight enhancement in motility, acrosome reaction and fertility when added separately, and these effects were increased when used together [83]. Moreover, the effect of hypotaurine occurs within the first 2 h [83], in agreement with the present study, where sperm motility, velocity and trajectory showed better results 2 h after the addition of PHE in M. auratus.
Epinephrine is a catecholamine that stimulates Na+/K+ ATPase and Ca2+-ATPase, and inhibits certain phosphodiesterases [40,86,87]. The inhibition of Na+/K+ ATPase decreased the acrosome reaction, whereas the inhibition of phosphodiesterase increased it [85]. Hypotaurine is a β-amino acid present in ejaculated sperm and oviductal fluid of mammals [43]. It is an intracellular scavenger that protects from lipid peroxidation and inactivation of superoxide dismutase by superoxidation, preventing sperm motility loss [84]. D-penicillamine acts as a divalent cation chelator, increasing the effects of epinephrine by protecting it from oxidation [40]. It was found that this α-amino acid could maintain sperm motility in hamsters at lower concentrations (10 µM) and stimulate sperm capacitation at higher concentrations (50 µM) [51]. In the present study, the concentration of penicillamine was 20 µM, which seems to be adequate in M. auratus to maintain or improve sperm traits for 4 h, in combination with epinephrine and hypotaurine. Taken together, results lead to the conclusion that the addition of PHE to M. auratus spermatozoa improves sperm quality over time, probably through the interaction with ATPases and phosphodiesterases and by protecting sperm from oxidation.
Changes in sperm traits in C. griseus after the addition of PHE are different from those observed in M. auratus. In C. griseus sperm motility and ATP content are enhanced at a later time, i.e., after 4 h of incubation with PHE. Moreover, sperm viability improves from 2 h onwards. In an earlier study [58], it was found that only 20–30% of cauda epididymal C. griseus sperm exhibit motility, which is significantly lower than the results in our study. This discrepancy may be due to differences in media used to collect and incubate spermatozoa because in the earlier study [58] the medium contained epinephrine and taurine (instead of hypotaurine, as in our study), and lacked penicillamine. The improvement in sperm survival and performance observed in our work may be useful to improve the reduced in vitro fertilization success observed in this species [58,88]. Indeed, better fertilization rates were obtained when sperm were pre-exposed to PHE and to the Ca2+ ionophore A23187, while doubling the usual Ca2+ concentrations [60].
Different patterns of sperm motility have been found when comparing M. auratus with C. griseus [58]. Whereas M. auratus sperm flex the entire length of the tail when moving, C. griseus sperm move by vibrating the tails firmly. This difference in swimming patterns may explain the diverse results in both species. Despite these differences, the addition of PHE seems to promote an improvement of sperm performance in both species. There does not seem to be a relation between the presence or absence of PHE, ATP levels, and motility when comparing these two species. In M. auratus PHE sustains motility better and for longer times than in controls, but there does not seem to be an effect on ATP levels. In C. griseus, there seems to be a parallel improvement of PHE on both motility and ATP levels, but only at the end of incubation.
The results in Phodopus species are somewhat surprising because we did not find differences over time in variables assessed in sperm incubated in the absence or presence of PHE. To the best of our knowledge, this is the first report examining the effects of PHE in these three species of hamsters. Since previous studies found that epinephrine acts through α and β-adrenergic receptors [41] and hypotaurine activates ATPases and inhibits phosphodiesterases, differences between sperm from Phodopus, C. griseus and M. auratus may be explained by differences in receptors or signaling mechanisms. Thus, one possibility could be that targets of PHE action vary in concentration between species, and thus effects are not visible. Another possibility is that responses to each factor vary, since different concentrations of penicillamine, hypotaurine and epinephrine are known to have different effects in somatic and sperm cells [51,86,87]. More studies are necessary to elucidate if changes in concentrations of PHE may affect sperm traits in Phodopus species. In any case, sperm quality in these hamsters is extremely high when compared to other hamster species after 4 h of incubation without PHE, indicating that the addition of these molecules or other motility factors may not be required to sustain sperm performance.
5. Conclusions
The results of this study revealed that supplementation with a combination of D-penicillamine, hypotaurine and epinephrine maintains or improves the performance of spermatozoa from five hamster species in different manners, depending on the species. Further studies are needed to understand the different mechanisms underlying the stimulatory effects in some species and lack of stimulation in others, which suggest divergence in regulatory mechanisms of motility and survival.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biology11040526/s1, Figure S1. Changes in sperm viability in five hamster species during incubation without or with penicillamine, hypotaurine and epinephrine (PHE). (A) Cricetulus griseus. (B) Mesocricetus auratus. (C) Phodopus campbelli. (D) P. sungorus. (E) P. roborovskii. Spermatozoa were collected in medium with (black squares) or without PHE (white squares) and incubated at 37 °C under air for up to 4 h. Values are means ± standard errors. Different letters between times of incubation for the same treatment indicate statistically significant differences (p < 0.05) in a DGC post hoc test. Asterisks indicate statistical differences (p < 0.05) between treatments for the same time in a DGC post-hoc test.
Author Contributions
M.T. and E.R.S.R. conceived the study. M.T. performed experiments. M.T., A.S.-R. and E.R.S.R. analysed data. M.T., A.S.-R. and E.R.S.R. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Spanish Ministry of Science and Innovation (projects CGL2016-80577-P and PID2019-108649GB-I00). This research also received support from the SYNTHESYS+ Project which is financed by the European Commission via the H2020 Research Infrastructure programme ID (823827) at the Museo Nacional de Ciencias Naturales (CSIC). M.T and A.S.-R. held “Juan de la Cierva” postdoctoral fellowships from the Spanish Ministry of Science and Innovation.
Institutional Review Board Statement
This study was carried out in strict accordance with the recommendations of the Code of Good Scientific Practices of the Spanish Research Council (CSIC) and was approved by the Animal Experimentation Ethics Committee of the National Museum of Natural Sciences (CSIC) and Comunidad de Madrid, Spain (28079-47-A). Animal handling complied with Spanish Animal Protection Regulation RD53/2013, which conforms to European Union Regulation 2010/63.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are included in the article.
Acknowledgments
We thank Juan Antonio Rielo for supervising animal facilities and Esperanza Navarro for animal care at the Museo Nacional de Ciencias Naturales in Madrid.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Roldan, E.R.S. Male fertility overview. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 1, pp. 408–415. [Google Scholar]
- Roldan, E.R.S. Assessments of sperm quality integrating morphology, swimming patterns, bioenergetics and cell signalling. Theriogenology 2020, 150, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, H. Laboratory semen assessment and prediction of fertility: Still utopia? Reprod. Domest. Anim. 2003, 38, 312–318. [Google Scholar] [CrossRef]
- Björndahl, L.; Mortimer, D.; Barratt, C.L.R.; Castilla, J.A.; Roelof, M.; Kvist, U.; Álvarez, J.G.; Haugen, T.B. A Practical Guide to Basic Laboratory Andrology; Cambridge University Press: Cambridge, UK, 2010; p. 336. [Google Scholar]
- Chenoweth, P.J.; Lorton, P.S. Animal Andrology: Theories and Applications; CABI: Wallingford, UK, 2014; p. 584. [Google Scholar]
- Srivastava, N.; Pande, M. Protocols in Semen Biology (Comparing Assays); Springer: Singapore, 2017; p. 288. [Google Scholar]
- Oehninger, S.; Ombelet, W. Limits of current male fertility testing. Fertil. Steril. 2019, 111, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Mortimer, D.; Kovacs, D. Male and Sperm Factors that Maximize IVF Success; Cambridge University Press: Cambridge, UK, 2020; p. 244. [Google Scholar]
- Turner, K.A.; Rambhatla, A.; Schon, S.; Agarwal, A.; Krawetz, S.A.; Dupree, J.M.; Avidor-Reiss, T. Male infertility is a women’s health issue-research and clinical evaluation of male infertility is needed. Cells 2020, 9, 990. [Google Scholar] [CrossRef] [Green Version]
- van der Horst, G. Status of sperm functionality assessment in wildlife species: From fish to primates. Animals 2021, 11, 1491. [Google Scholar] [CrossRef]
- Malo, A.F.; Garde, J.J.; Soler, A.J.; Garcia, A.J.; Gomendio, M.; Roldan, E.R.S. Male fertility in natural populations of red deer is determined by sperm velocity and the proportion of normal spermatozoa. Biol. Reprod. 2005, 72, 822–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaniz, J.L.; Silvestre, M.A.; Santolaria, P.; Soler, C. CASA-Mot in mammals: An update. Reprod. Fertil. Dev. 2018, 30, 799–809. [Google Scholar] [CrossRef]
- van der Horst, G. Computer aided sperm analysis (CASA) in domestic animals: Current status, three D tracking and flagellar analysis. Anim. Reprod. Sci. 2020, 220, 106350. [Google Scholar] [CrossRef]
- Buffone, M.G. Sperm Acrosome Biogenesis and Function during Fertilization; Springer: New York, NY, USA, 2016; p. 172. [Google Scholar]
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Hirohashi, N. Site of mammalian sperm acrosome reaction. Adv. Anat. Embryol. Cell Biol. 2016, 220, 145–158. [Google Scholar] [CrossRef]
- La Spina, F.A.; Puga Molina, L.C.; Romarowski, A.; Vitale, A.M.; Falzone, T.L.; Krapf, D.; Hirohashi, N.; Buffone, M.G. Mouse sperm begin to undergo acrosomal exocytosis in the upper isthmus of the oviduct. Dev. Biol. 2016, 411, 172–182. [Google Scholar] [CrossRef]
- Guidobaldi, H.A.; Hirohashi, N.; Cubilla, M.; Buffone, M.G.; Giojalas, L.C. An intact acrosome is required for the chemotactic response to progesterone in mouse spermatozoa. Mol. Reprod. Dev. 2017, 84, 310–315. [Google Scholar] [CrossRef]
- Hirohashi, N.; Yanagimachi, R. Sperm acrosome reaction: Its site and role in fertilization. Biol. Reprod. 2018, 99, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Balestrini, P.A.; Jablonski, M.; Schiavi-Ehrenhaus, L.J.; Marin-Briggiler, C.I.; Sanchez-Cardenas, C.; Darszon, A.; Krapf, D.; Buffone, M.G. Seeing is believing: Current methods to observe sperm acrosomal exocytosis in real time. Mol. Reprod. Dev. 2020, 87, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Tourmente, M.; Varea-Sanchez, M.; Roldan, E.R.S. Faster and more efficient swimming: Energy consumption of murine spermatozoa under sperm competitiondagger. Biol. Reprod. 2019, 100, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Tourmente, M.; Villar-Moya, P.; Varea-Sanchez, M.; Luque-Larena, J.J.; Rial, E.; Roldan, E.R.S. Performance of rodent spermatozoa over time is enhanced by increased ATP concentrations: The role of sperm competition. Biol. Reprod. 2015, 93, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tourmente, M.; Roldan, E.R.S. Mass-specific metabolic rate influences sperm performance through energy production in mammals. PLoS ONE 2015, 10, e0138185. [Google Scholar] [CrossRef] [Green Version]
- Tourmente, M.; Rowe, M.; Gonzalez-Barroso, M.M.; Rial, E.; Gomendio, M.; Roldan, E.R.S. Postcopulatory sexual selection increases ATP content in rodent spermatozoa. Evolution 2013, 67, 1838–1846. [Google Scholar] [CrossRef] [Green Version]
- Sansegundo, E.; Tourmente, M.; Roldan, E.R.S. Energy metabolism and hyperactivation of spermatozoa from three mouse species under capacitating conditions. Cells 2022, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Tvrda, E.; Benko, F.; Slanina, T.; du Plessis, S.S. The role of selected natural biomolecules in sperm production and functionality. Molecules 2021, 26, 5196. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Visconti, P.E. Chang’s meaning of capacitation: A molecular perspective. Mol. Reprod. Dev. 2016, 83, 860–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, M.; Ishikawa, A. Room temperature storage of mouse epididymal spermatozoa: Exploration of factors affecting sperm survival. Theriogenology 2004, 61, 1455–1469. [Google Scholar] [CrossRef] [PubMed]
- Contri, A.; Gloria, A.; Robbe, D.; Valorz, C.; Wegher, L.; Carluccio, A. Kinematic study on the effect of pH on bull sperm function. Anim. Reprod. Sci. 2013, 136, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, L.; Li, J.; Li, H.; Hong, Z.; Xie, M.; Chen, S.; Yao, B. The semen pH affects sperm motility and capacitation. PLoS ONE 2015, 10, e0132974. [Google Scholar] [CrossRef] [Green Version]
- Perez-Cerezales, S.; Lopez-Cardona, A.P.; Gutierrez-Adan, A. Progesterone effects on mouse sperm kinetics in conditions of viscosity. Reproduction 2016, 151, 501–507. [Google Scholar] [CrossRef] [Green Version]
- Mata-Martinez, E.; Darszon, A.; Trevino, C.L. pH-dependent Ca(+2) oscillations prevent untimely acrosome reaction in human sperm. Biochem. Biophys. Res. Commun. 2018, 497, 146–152. [Google Scholar] [CrossRef]
- Giojalas, L.C.; Guidobaldi, H.A. Getting to and away from the egg, an interplay between several sperm transport mechanisms and a complex oviduct physiology. Mol. Cell. Endocrinol. 2020, 518, 110954. [Google Scholar] [CrossRef]
- Ruiz-Diaz, S.; Luongo, C.; Fuentes-Albero, M.C.; Abril-Sanchez, S.; Sanchez-Calabuig, M.J.; Barros-Garcia, C.; De la Fe, C.; Garcia-Galan, A.; Ros-Santaella, J.L.; Pintus, E.; et al. Effect of temperature and cell concentration on dolphin (Tursiops truncatus) spermatozoa quality evaluated at different days of refrigeration. Anim. Reprod. Sci. 2020, 212, 106248. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, B.; Fu, Q.; Wu, J.; Liu, R. A fully integrated biomimetic microfluidic device for evaluation of sperm response to thermotaxis and chemotaxis. Lab Chip 2021, 21, 310–318. [Google Scholar] [CrossRef]
- Yanagimachi, R.; Chang, M.C. In vitro fertilization of golden hamster ova. J. Exp. Zool. 1964, 156, 361–375. [Google Scholar] [CrossRef]
- Roldan, E.R.S.; Yanagimachi, R. Cross-fertilization between Syrian and Chinese hamsters. J. Exp. Zool. 1989, 250, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Bavister, B.D. A consistently successful procedure for in vitro fertilization of golden hamster eggs. Gamete Res. 1989, 23, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Bavister, B.D.; Chen, A.F.; Fu, P.C. Catecholamine requirement for hamster sperm motility in vitro. J. Reprod. Fertil. 1979, 56, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Meizel, S.; Working, P.K. Further evidence suggesting the hormonal stimulation of hamster sperm acrosome reactionsby catecholamines in vitro. Biol. Reprod. 1980, 22, 211–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornett, L.E.; Meizel, S. Stimulation of in vitro activation and the acrosome reaction of hamster spermatozoa by catecholamines. Proc. Natl. Acad. Sci. USA 1978, 75, 4954–4958. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, J.G.; Storey, B.T. Spontaneous lipid peroxidation in rabbit and mouse epididymal spermatozoa: Dependence of rate on temperature and oxygen concentration. Biol. Reprod. 1985, 32, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Meizel, S.; Lui, C.W.; Working, P.K.; Mrsny, R.J. Taurine and Hypotaurine: Their effects on motility, capacitation and the acrosome reaction of hamster sperm in vitro and their presence in sperm and reproductive tract fluids of several mammals. Dev. Growth Differ. 1980, 22, 483–494. [Google Scholar] [CrossRef]
- Fujinoki, M. Regulation and disruption of hamster sperm hyperactivation by progesterone, 17beta-estradiol and diethylstilbestrol. Reprod. Med. Biol. 2014, 13, 143–152. [Google Scholar] [CrossRef]
- Fujinoki, M.; Takei, G.L. gamma-Aminobutyric acid suppresses enhancement of hamster sperm hyperactivation by 5-hydroxytryptamine. J. Reprod. Dev. 2017, 63, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, C.; Fujinoki, M.; Kitazawa, M.; Obayashi, S. Serotonergic signals enhanced hamster sperm hyperactivation. J. Reprod. Dev. 2021, 67, 241–250. [Google Scholar] [CrossRef]
- Brogan, P.T.; Beitsma, M.; Henning, H.; Gadella, B.M.; Stout, T.A. Liquid storage of equine semen: Assessing the effect of d-penicillamine on longevity of ejaculated and epididymal stallion sperm. Anim. Reprod. Sci. 2015, 159, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Leahy, T.; Rickard, J.P.; Aitken, R.J.; de Graaf, S.P. Penicillamine prevents ram sperm agglutination in media that support capacitation. Reproduction 2016, 151, 167–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, M.C.; Yu, A.; Moawad, A.R.; O’Flaherty, C. Peroxiredoxin 6 regulates the phosphoinositide 3-kinase/AKT pathway to maintain human sperm viability. Mol. Hum. Reprod. 2019, 25, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Uribe, P.; Merino, J.; Bravo, A.; Zambrano, F.; Schulz, M.; Villegas, J.V.; Sanchez, R. Antioxidant effects of penicillamine against in vitro-induced oxidative stress in human spermatozoa. Andrologia 2020, 52, e13553. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.C.; Nolan, J.P.; Hammerstedt, R.H.; Bavister, B.D. Role of zinc during hamster sperm capacitation. Biol. Reprod. 1994, 51, 1238–1247. [Google Scholar] [CrossRef]
- Ruiz-Diaz, S.; Oseguera-Lopez, I.; De La Cuesta-Diaz, D.; Garcia-Lopez, B.; Serres, C.; Sanchez-Calabuig, M.J.; Gutierrez-Adan, A.; Perez-Cerezales, S. The presence of D-penicillamine during the in vitro capacitation of stallion spermatozoa prolongs hyperactive-like motility and allows for sperm selection by thermotaxis. Animals 2020, 10, 1467. [Google Scholar] [CrossRef]
- Cornwall, G.A.; Smyth, T.B.; Vindivich, D.; Harter, C.; Robinson, J.; Chang, T.S. Induction and enhancement of progressive motility in hamster caput epididymal spermatozoa. Biol. Reprod. 1986, 35, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Andrews, J.C.; Bavister, B.D. Capacitation of hamster spermatozoa with the divalent cation chelators D-penicilamine, L-histidine, and L-cysteine in a protein-free culture medium. Gamete Res. 1989, 23, 159–170. [Google Scholar] [CrossRef]
- Kang, S.S.; Koyama, K.; Huang, W.; Yang, Y.; Yanagawa, Y.; Takahashi, Y.; Nagano, M. Addition of D-penicillamine, hypotaurine, and epinephrine (PHE) mixture to IVF medium maintains motility and longevity of bovine sperm and enhances stable production of blastocysts in vitro. J. Reprod. Dev. 2015, 61, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Boatman, D.E.; Bavister, B.D.; Cruz, E. Addition of hypotaurine can reactivate immotile golden hamster spermatozoa. J. Androl. 1990, 11, 67–72. [Google Scholar]
- Hirose, M.; Ogura, A. The golden (Syrian) hamster as a model for the study of reproductive biology: Past, present, and future. Reprod. Med. Biol. 2019, 18, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R.; Kamiguchi, Y.; Sugawara, S.; Mikamo, K. Gametes and fertilization in the Chinese hamster. Gamete Res. 1983, 8, 97–117. [Google Scholar] [CrossRef]
- Tateno, H.; Kamiguchi, Y. In vitro fertilisation of Chinese hamster oocytes by spermatozoa that have undergone ionophore A23187-induced acrosome reaction, and their subsequent development into blastocysts. Zygote 1996, 4, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Tateno, H.; Tamura-Nakano, M.; Kusakabe, H.; Hirohashi, N.; Kawano, N.; Yanagimachi, R. Sperm acrosome status before and during fertilization in the Chinese hamster (Cricetulus griseus), and observation of oviductal vesicles and globules. Mol. Reprod. Dev. 2021, 88, 793–804. [Google Scholar] [CrossRef]
- Parkening, T.A. In vitro fertilization of Siberian hamster oocytes. J. Exp. Zool. 1990, 254, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Raicu, P.; Bratosin, S. Interspecific reciprocal hybrids between Mesocricetus auratus and M. newtoni. Genet Res. 1968, 11, 113–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raicu, P.; Ionescu-Varo, M.; Duma, D. Interspecific crosses between the Rumanian and Syrian hamster. Cytogenetic and histological studies. J. Hered. 1969, 60, 149–152. [Google Scholar] [CrossRef]
- Raicu, P.; Ionescu-Varo, M.; Nicolaescu, M.; Kirillova, M. Interspecific hybrids between Romanian and Kurdistan hamsters. Genetica 1972, 43, 223–230. [Google Scholar] [CrossRef]
- Raicu, P.; Nicolaescu, M.; Kirillova, M. The interspecific hybrids between Kurdistan hamster (Mesocricetus brandti) and golden hamster (Mesocricetus auratus). Rev. Roum. Biol. 1973, 18, 451–455. [Google Scholar]
- Todd, N.B.; Nixon, C.W.; Mulvaney, D.A.; Connelly, M.E. Karyotypes of Mesocricetus brandti and hybridization within the genus. J. Hered. 1972, 63, 73–77. [Google Scholar] [CrossRef]
- Ishishita, S.; Matsuda, Y. Interspecific hybrids of dwarf hamsters and Phasianidae birds as animal models for studying the genetic and developmental basis of hybrid incompatibility. Genes Genet. Syst. 2016, 91, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gureeva, A.V.; Feoktistova, N.Y.; Matveevsky, S.N.; Kolomiets, O.L.; Surov, A.V. Speciation of Eversmann and Mongolian hamsters (Allocricetulus, Cricetinae): Experimental hybridization. Biol. Bull. 2017, 43, 736–742. [Google Scholar] [CrossRef]
- Cummins, J.M.; Woodall, P.F. On mammalian sperm dimensions. J. Reprod. Fertil. 1985, 75, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Tourmente, M.; Gomendio, M.; Roldan, E.R.S. Sperm competition and the evolution of sperm design in mammals. BMC Evol. Biol. 2011, 11, 12. [Google Scholar] [CrossRef] [Green Version]
- Teves, M.E.; Roldan, E.R.S. Sperm bauplan and function and underlying processes of sperm formation and selection. Physiol. Rev. 2022, 102, 7–60. [Google Scholar] [CrossRef]
- Lebedev, V.S.; Bannikova, A.A.; Neumann, K.; Ushakova, M.V.; Ivanova, N.V.; Surov, A.V. Molecular phylogenetics and taxonomy of dwarf hamsters Cricetulus Milne-Edwards, 1867 (Cricetidae, Rodentia): Description of a new genus and reinstatement of another. Zootaxa 2018, 4387, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Romanenko, S.A.; Volobouev, V.T.; Perelman, P.L.; Lebedev, V.S.; Serdukova, N.A.; Trifonov, V.A.; Biltueva, L.S.; Nie, W.; O’Brien, P.C.; Bulatova, N.; et al. Karyotype evolution and phylogenetic relationships of hamsters (Cricetidae, Muroidea, Rodentia) inferred from chromosomal painting and banding comparison. Chromosome Res. 2007, 15, 283–297. [Google Scholar] [CrossRef]
- Romanenko, S.A.; Lebedev, V.S.; Bannikova, A.A.; Pavlova, S.V.; Serdyukova, N.A.; Feoktistova, N.Y.; Jiapeng, Q.; Yuehua, S.; Surov, A.V.; Graphodatsky, A.S. Karyotypic and molecular evidence supports the endemic Tibetan hamsters as a separate divergent lineage of Cricetinae. Sci. Rep. 2021, 11, 10557. [Google Scholar] [CrossRef]
- Kenagy, G.J.; Trombulak, S.C. Size and function of mammalian testes in relation to body size. J. Mammal. 1986, 67, 1–22. [Google Scholar] [CrossRef]
- Shi, Q.X.; Roldan, E.R.S. Bicarbonate/CO2 is not required for zona pellucida- or progesterone-induced acrosomal exocytosis of mouse spermatozoa but is essential for capacitation. Biol. Reprod. 1995, 52, 540–546. [Google Scholar] [CrossRef]
- Gomez Montoto, L.; Magana, C.; Tourmente, M.; Martin-Coello, J.; Crespo, C.; Luque-Larena, J.J.; Gomendio, M.; Roldan, E.R.S. Sperm competition, sperm numbers and sperm quality in muroid rodents. PLoS ONE 2011, 6, e18173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez Montoto, L.; Varea Sanchez, M.; Tourmente, M.; Martin-Coello, J.; Luque-Larena, J.J.; Gomendio, M.; Roldan, E.R.S. Sperm competition differentially affects swimming velocity and size of spermatozoa from closely related muroid rodents: Head first. Reproduction 2011, 142, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, J.A.; Guzmán, A.W.; Casanoves, F. A multiple comparisons method based on the distribution of the root node distance of a binary tree. J. Agric. Biol. Environ. Stat. 2002, 7, 129–142. [Google Scholar] [CrossRef]
- Tourmente, M.; Villar-Moya, P.; Rial, E.; Roldan, E.R.S. Differences in ATP generation via glycolysis and oxidative phosphorylation and relationships with sperm motility in mouse species. J. Biol. Chem. 2015, 290, 20613–20626. [Google Scholar] [CrossRef] [Green Version]
- Bavister, B.D. The effect of variations in culture conditions on the motility of hamster spermatozoa. J. Reprod. Fertil. 1974, 38, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Bavister, B.D.; Yanagimachi, R. The effects of sperm extracts and energy sources on the motility and acrosome reaction of hamster spermatozoa in vitro. Biol. Reprod. 1977, 16, 228–237. [Google Scholar] [CrossRef]
- Leibfried, M.L.; Bavister, B.D. Effects of epinephrine and hypotaurine on in-vitro fertilization in the golden hamster. J. Reprod. Fertil. 1982, 66, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, J.G.; Storey, B.T. Taurine, hypotaurine, epinephrine and albumin inhibit lipid peroxidation in rabbit spermatozoa and protect against loss of motility. Biol. Reprod. 1983, 29, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Mrsny, R.J.; Meizel, S. Inhibition of hamster sperm Na+, K+-ATPase activity by taurine and hypotaurine. Life Sci. 1985, 36, 271–275. [Google Scholar] [CrossRef]
- Hexum, T.D. The effect of catecholamines on transport (Na,K) adenosine triphosphatase. Biochem. Pharmacol. 1977, 26, 1221–1227. [Google Scholar] [CrossRef]
- Fagan, J.B.; Racker, E. Reversible inhibition of (Na+, K+) ATPase by Mg2+, adenosine triphosphate, and K+. Biochemistry 1977, 16, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Pickworth, S.; Chang, M.C. Fertilization of Chinese hamster eggs in vitro. J. Reprod. Fertil. 1969, 19, 371–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).