Commercial Potential of the Cyanobacterium Arthrospira maxima: Physiological and Biochemical Traits and the Purification of Phycocyanin
Abstract
:Simple Summary
Abstract
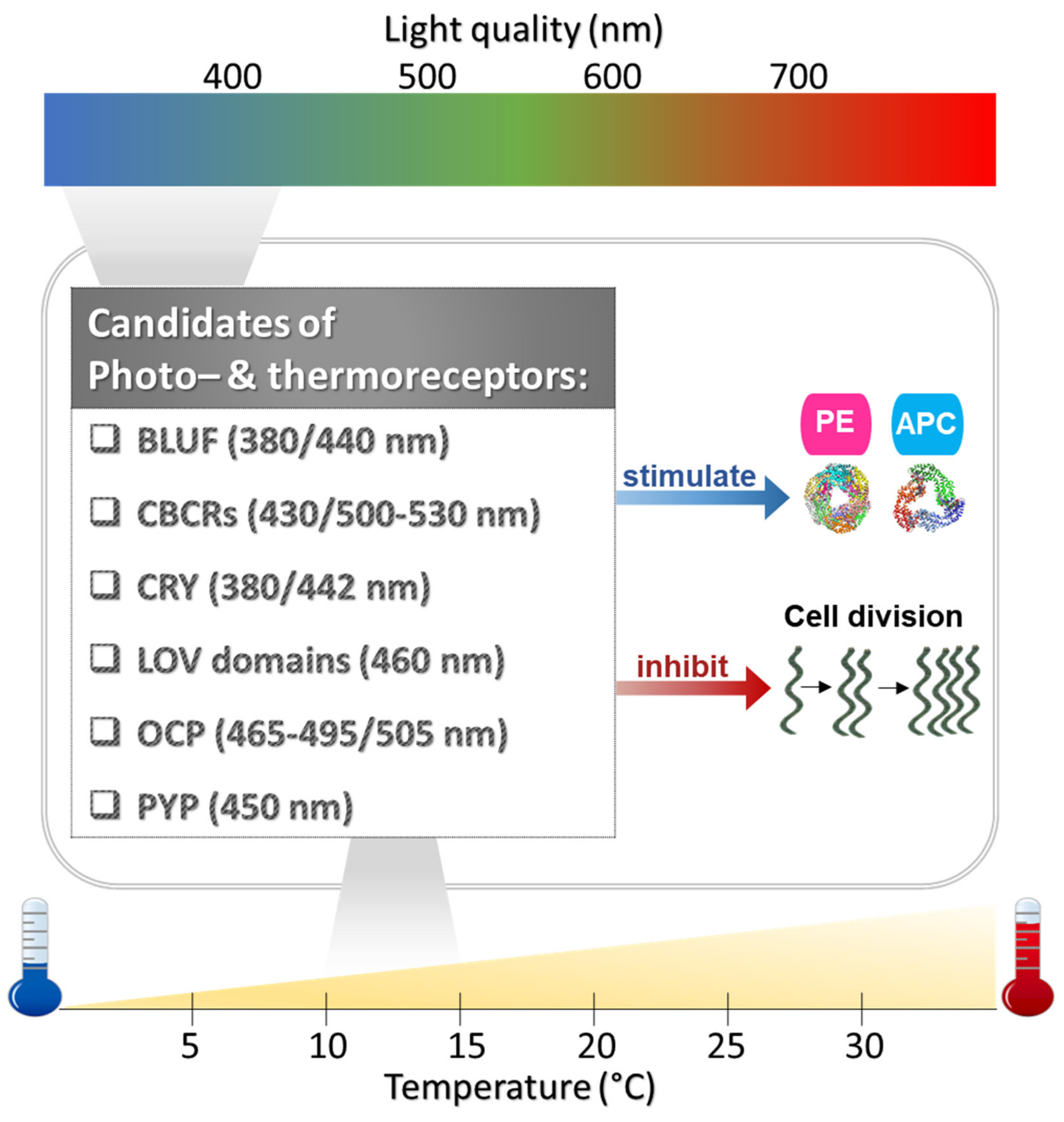
1. Introduction
2. Materials and Methods
2.1. A. maxima Culture
2.2. Treatments
2.3. Assessment
2.3.1. Growth Analysis
2.3.2. Pigment Analysis
2.3.3. Photosynthesis Analysis
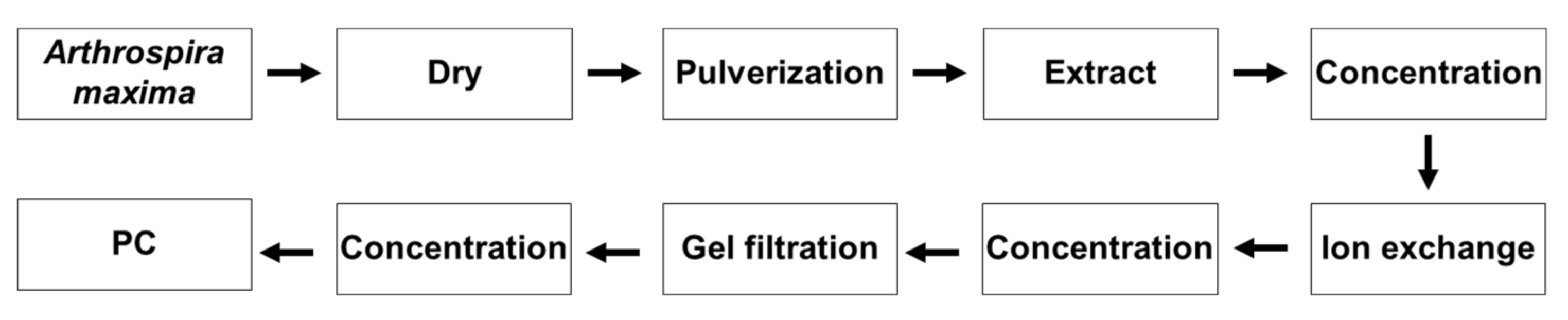
2.4. Purification of PC
2.4.1. Fractionation by Ultrafiltration
2.4.2. Separation by Ion Exchange
2.4.3. Further Separation by Gel Filtration
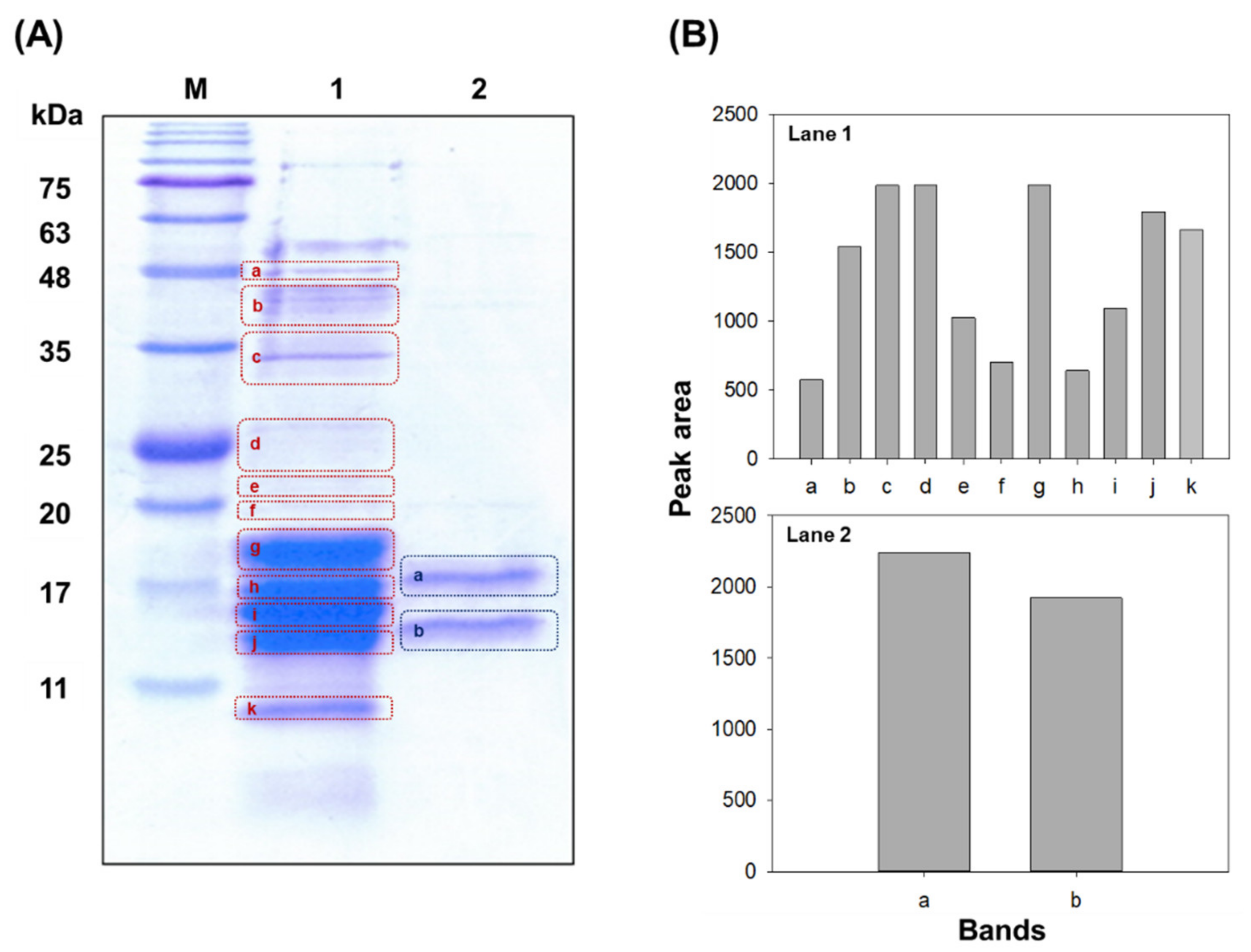
2.4.4. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS–PAGE)
2.5. Statistical Analysis
3. Results and Discussion
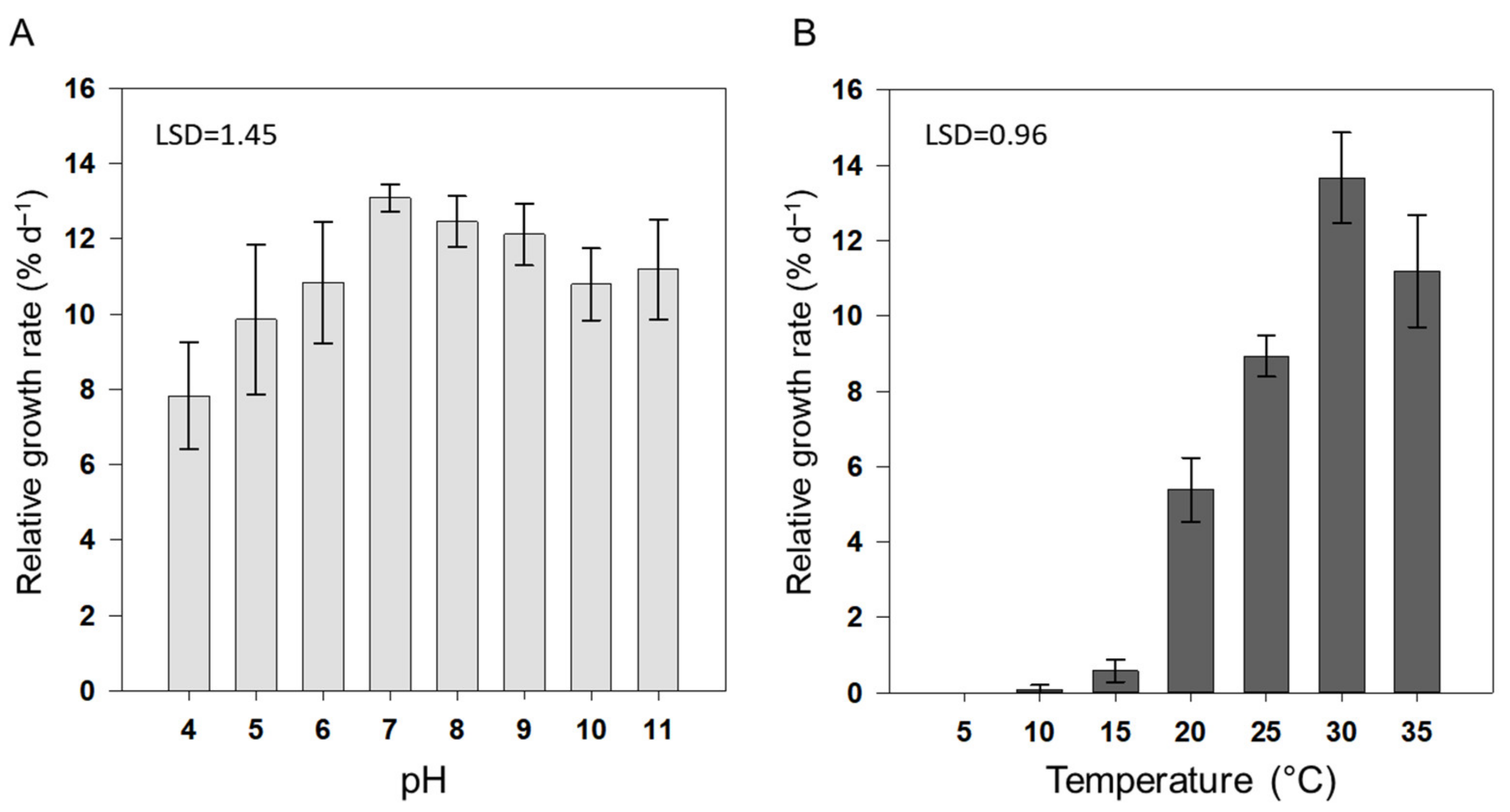
3.1. Growth
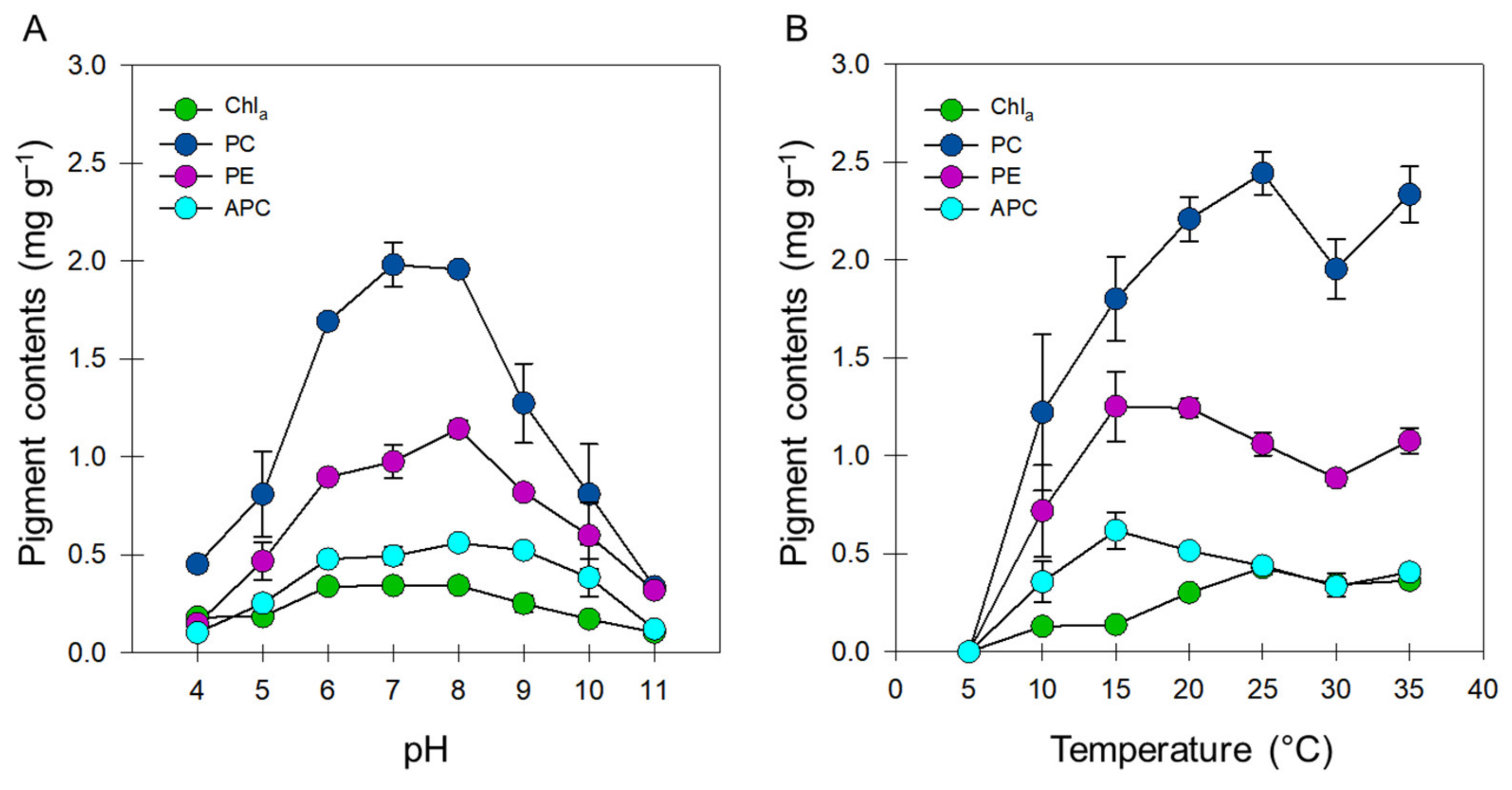
3.2. Pigment Biosynthesis
3.3. Photosynthetic Activity
3.4. Purification of PC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.-B.; Wu, J.-Y.; Wang, C.-F.; Fu, C.-C.; Shieh, C.-J.; Chen, C.-I.; Wang, C.-Y.; Liu, Y.-C. Modeling on chlorophyll a and phycocyanin production by Spirulina platensis under various light-emitting diodes. Biochem. Eng. J. 2010, 53, 52–56. [Google Scholar] [CrossRef]
- Zeng, X.; Danquah, M.K.; Zhang, S.; Zhang, X.; Wu, M.; Chen, X.D.; Ng, I.-S.; Jing, K.; Lu, Y. Autotrophic cultivation of Spirulina platensis for CO2 fixation and phycocyanin production. Chem. Eng. J. 2012, 183, 192–197. [Google Scholar] [CrossRef]
- Xie, Y.; Jin, Y.; Zeng, X.; Chen, J.; Lu, Y.; Jing, K. Fed-batch strategy for enhancing cell growth and C-phycocyanin production of Arthrospira (Spirulina) platensis under phototrophic cultivation. Bioresour. Technol. 2015, 180, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Da Fontoura Prates, D.; Radmann, E.M.; Duarte, J.H.; de Morais, M.G.; Costa, J.A.V. Spirulina cultivated under different light emitting diodes: Enhanced cell growth and phycocyanin production. Bioresour. Technol. 2018, 256, 38–43. [Google Scholar] [CrossRef]
- Wikfors, G.H.; Ohno, M. Impact of algal research in aquaculture. J. Phycol. 2001, 37, 968–974. [Google Scholar] [CrossRef]
- Pandey, J.; Pathak, N.; Tiwari, A. Standardization of pH and light intensity for the biomass production of Spirulina platensis. J. Algal Biomass Util. 2010, 1, 93–102. [Google Scholar]
- Sarkar, S.; Gayen, K.; Bhowmick, T.K. Green extraction of biomolecules from algae using subcritical and supercritical fluids. Biomass Convers. Biorefin. 2022, 1–23. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Kruse, O.; Hellingwerf, K.J. Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Curr. Opin. Biotechnol. 2013, 24, 405–413. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Lu, X. Engineering cyanobacteria to improve photosynthetic production of alka(e)nes. Biotechnol. Biofuels 2013, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Paliwal, C.; Mitra, M.; Bhayani, K.; Bharadwaj, S.V.; Ghosh, T.; Dubey, S.; Mishra, S. Abiotic stresses as tools for metabolites in microalgae. Bioresour. Technol. 2017, 244, 1216–1226. [Google Scholar] [CrossRef]
- Rafiqul, I.; Jalal, K.; Alam, M. Environmental factors for optimization of Spirulina biomass in laboratory culture. Biotechnology 2005, 4, 19–22. [Google Scholar]
- Keithellakpam, O.S.; Nath, T.O.; Oinam, A.S.; Thingujam, I.; Oinam, G.; Dutt, S.G. Effect of external pH on cyanobacterial phycobiliproteins production and ammonium excretion. J. Appl. Biol. Biotechnol. 2015, 3, 38–42. [Google Scholar]
- Ismaiel, M.M.S.; El-Ayouty, Y.M.; Piercey-Normore, M. Role of pH on antioxidants production by Spirulina (Arthrospira) platensis. Braz. J. Microbiol. 2016, 47, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Hinners, J.; Hofmeister, R.; Hense, I. Modeling the role of pH on Baltic Sea cyanobacteria. Life 2015, 5, 1204–1217. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, C.S. The Ecology of Freshwater Phytoplankton; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Johnson, E.M.; Kumar, K.; Das, D. Physicochemical parameters optimization, and purification of phycobiliproteins from the isolated Nostoc sp. Bioresour. Technol. 2014, 166, 541–547. [Google Scholar] [CrossRef]
- Robarts, R.D.; Zohary, T. Temperature effects on photosynthetic capacity, respiration, and growth rates of bloom-forming cyanobacteria. N. Z. J. Mar. Freshw. Res. 1987, 21, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Coles, J.F.; Jones, R.C. Effect of temperature on photosynthesis-light response and growth of four phytoplankton species isolated from a tidal freshwater river. J. Phycol. 2000, 36, 7–16. [Google Scholar] [CrossRef]
- Paerl, H.W. Mitigating harmful cyanobacterial blooms in a human-and climatically-impacted world. Life 2014, 4, 988–1012. [Google Scholar] [CrossRef] [Green Version]
- Udayan, A.; Arumugam, M.; Pandey, A. Nutraceuticals from algae and cyanobacteria. In Algal Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 65–89. [Google Scholar]
- Eriksen, N.T. Production of phycocyanin—A pigment with applications in biology, biotechnology, foods and medicine. Appl. Microbiol. Biotechnol. 2008, 80, 1–14. [Google Scholar] [CrossRef]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Khatoon, H.; Leong, L.K.; Rahman, N.A.; Mian, S.; Begum, H.; Banerjee, S.; Endut, A. Effects of different light source and media on growth and production of phycobiliprotein from freshwater cyanobacteria. Bioresour. Technol. 2018, 249, 652–658. [Google Scholar] [CrossRef]
- Ojit, S.; Indrama, T.; Gunapati, O.; Avijeet, S.; Subhalaxmi, S.; Silvia, C.; Indira, D.; Romi, K.; Minerva, S.; Thadoi, D. The response of phycobiliproteins to light qualities in Anabaena circinalis. J. Appl. Biol. Biotechnol. 2015, 3, 1–6. [Google Scholar]
- Research and Markets. Phycobiliprotein Global Market Insights 2021, Analysis and Forecast to 2026, by Manufacturers, Regions, Technology, Application. July 2021. Available online: https://www.researchandmarkets.com/reports/5358532/phycobiliprotein-global-market-insights-2021#src-pos-1 (accessed on 6 April 2022).
- De Oliveira, M.; Monteiro, M.; Robbs, P.; Leite, S. Growth and chemical composition of Spirulina maxima and Spirulina platensis biomass at different temperatures. Aquac. Int. 1999, 7, 261–275. [Google Scholar] [CrossRef]
- Chaneva, G.; Furnadzhieva, S.; Minkova, K.; Lukavsky, J. Effect of light and temperature on the cyanobacterium Arthronema africanum—A prospective phycobiliprotein-producing strain. J. Appl. Phycol. 2007, 19, 537–544. [Google Scholar] [CrossRef]
- Ajayan, K.; Selvaraju, M.; Thirugnanamoorthy, K. Enrichment of chlorophyll and phycobiliproteins in Spirulina platensis by the use of reflector light and nitrogen sources: An in-vitro study. Biomass Bioenergy 2012, 47, 436–441. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, R.; Ortiz-Butrón, R.; Blas-Valdivia, V.; Hernández-García, A.; Cano-Europa, E. Phycobiliproteins or C-phycocyanin of Arthrospira (Spirulina) maxima protect against HgCl2-caused oxidative stress and renal damage. Food Chem. 2012, 135, 2359–2365. [Google Scholar] [CrossRef]
- Evans, L. The Effects of Spectral Composition and Irradiance Level on Pigment Levels in Seaweeds. In Experimental Phycology; Lobban, C.S., Chapman, D.J., Kremer, B.P., Eds.; Cambridge University Press: New York, NY, USA, 1988; pp. 123–133. [Google Scholar]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
- Schreiber, U. Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: An overview. Chlorophyll Fluoresc. 2004, 279–319. [Google Scholar] [CrossRef]
- Jassby, A.D.; Platt, T. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol. Oceanogr. 1976, 21, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Walsby, A. Cell-Water and Cell-Solute. In The Biology of Cyanobacteria; Carr, N.G., Whitton, B.A., Eds.; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 1982; Volume 19, p. 237. [Google Scholar]
- Fogg, G. The comparative physiology and biochemistry of the blue-green algae. Bacteriol. Rev. 1956, 20, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.P.; Tiwari, A. Optimization of biomass production of Spirulina maxima. J. Algal Biomass Util. 2010, 1, 20–32. [Google Scholar]
- Thirumala, M. Optimization of growth of Spirulina platensis LN1 for production of carotenoids. Int. J. Life Sci. Biotechnol. Pharma Res. 2012, 1, 152–157. [Google Scholar]
- Brock, T.D. Lower pH limit for the existence of blue-green algae: Evolutionary and ecological implications. Science 1973, 179, 480–483. [Google Scholar] [CrossRef]
- Kallas, T.; Castenholz, R.W. Internal pH and ATP-ADP pools in the cyanobacterium Synechococcus sp. during exposure to growth-inhibiting low pH. J. Bacteriol. 1982, 149, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Mangan, N.M.; Flamholz, A.; Hood, R.D.; Milo, R.; Savage, D.F. pH determines the energetic efficiency of the cyanobacterial CO2 concentrating mechanism. Proc. Natl. Acad. Sci. USA 2016, 113, E5354–E5362. [Google Scholar] [CrossRef] [Green Version]
- Boatman, T.G.; Oxborough, K.; Gledhill, M.; Lawson, T.; Geider, R.J. An integrated response of Trichodesmium erythraeum IMS101 growth and photo-physiology to iron, CO2, and light intensity. Front. Microbiol. 2018, 9, 624. [Google Scholar] [CrossRef] [Green Version]
- Manduzio, H.; Rocher, B.; Durand, F.; Galap, C.; Leboulenger, F. The point about oxidative stress in molluscs. Invertebr. Surviv. J. 2005, 2, 91–104. [Google Scholar]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Hinga, K.R. Effects of pH on coastal marine phytoplankton. Mar. Ecol. Prog. Ser. 2002, 238, 281–300. [Google Scholar] [CrossRef]
- Hossain, M.F.; Ratnayake, R.R.; Meerajini, K.; Wasantha Kumara, K. Antioxidant properties in some selected cyanobacteria isolated from fresh water bodies of Sri Lanka. Food Sci. Nutr. 2016, 4, 753–758. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, L.; Subramanian, G.; Nazeer, T.T.; Simpson, H.S.; Rahuman, S.T.; Raju, P. Cyanobacteria cultivation in industrial wastewaters and biodiesel production from their biomass: A review. Biotechnol. Appl. Biochem. 2011, 58, 220–225. [Google Scholar] [CrossRef]
- Markou, G.; Georgakakis, D. Cultivation of filamentous cyanobacteria (blue-green algae) in agro-industrial wastes and wastewaters: A review. Appl. Energy 2011, 88, 3389–3401. [Google Scholar] [CrossRef]
- Liotenberg, S.; Campbell, D.; Rippka, R.; Houmard, J.; de Marsac, N.T. Effect of the nitrogen source on phycobiliprotein synthesis and cell reserves in a chromatically adapting filamentous cyanobacterium. Microbiology 1996, 142, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Lürling, M.; Eshetu, F.; Faassen, E.J.; Kosten, S.; Huszar, V.L. Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshw. Biol. 2013, 58, 552–559. [Google Scholar] [CrossRef]
- Kumar, M.; Kulshreshtha, J.; Singh, G.P. Growth and biopigment accumulation of cyanobacterium Spirulina platensis at different light intensities and temperature. Braz. J. Microbiol. 2011, 42, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Ranjitha, K.; Kaushik, B. Purification of phycobiliproteins from Nostoc muscorum. NISCARI Online Period. Repos. 2005, 64, 372–375. [Google Scholar]
- Richmond, A.; Grobbelaar, J.U. Factors affecting the output rate of Spirulina platensis with reference to mass cultivation. Biomass 1986, 10, 253–264. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Algal physiology and large-scale outdoor cultures of microalgae. In The Physiology of Microalgae; Springer: Berlin/Heidelberg, Germany, 2016; pp. 601–652. [Google Scholar]
- Sharma, G.; Kumar, M.; Ali, M.I.; Jasuja, N.D. Effect of carbon content, salinity and pH on Spirulina platensis for phycocyanin, allophycocyanin and phycoerythrin accumulation. J. Microb. Biochem. Technol. 2014, 6, 202–206. [Google Scholar] [CrossRef] [Green Version]
- Soltani, N.; Khavari-Nejad, R.A.; Yazdi, M.T.; Shokravi, S.; Fernández-Valiente, E. Variation of nitrogenase activity, photosynthesis and pigmentation of the cyanobacterium Fischerella ambigua strain FS18 under different irradiance and pH values. World J. Microbiol. Biotechnol. 2006, 22, 571–576. [Google Scholar] [CrossRef]
- Poza-Carrión, C.; Fernández-Valiente, E.; Piñas, F.F.; Leganés, F. Acclimation of photosynthetic pigments and photosynthesis of the cyanobacterium Nostoc sp. strain UAM206 to combined fluctuations of irradiance, pH, and inorganic carbon availability. J. Plant Physiol. 2001, 158, 1455–1461. [Google Scholar] [CrossRef]
- Koca, N.; Karadeniz, F.; Burdurlu, H.S. Effect of pH on chlorophyll degradation and colour loss in blanched green peas. Food Chem. 2007, 100, 609–615. [Google Scholar] [CrossRef]
- Schlodder, E.; Meyer, B. pH dependence of oxygen evolution and reduction kinetics of photooxidized chlorophyll aII (P-680) in Photosystem II particles from Synechococcus sp. Biochim. Biophys. Acta Bioenerg. 1987, 890, 23–31. [Google Scholar] [CrossRef]
- Li, D.; Lijan, X.; Xu, Q.; Wang, K.; Gong, Y.; Kuang, T.; Zhang, X.; Zhao, N. pH dependence of chlorophyll states, protein structures and function of the PSII membranes. Tsinghua Sci. Technol. 2003, 8, 493–497. [Google Scholar]
- Guedes, A.C.; Amaro, H.M.; Gião, M.S.; Malcata, F.X. Optimization of ABTS radical cation assay specifically for determination of antioxidant capacity of intracellular extracts of microalgae and cyanobacteria. Food Chem. 2013, 138, 638–643. [Google Scholar] [CrossRef]
- Fatma, T. Screening of cyanobacteria for phycobiliproteins and effect of different environmental stress on its yield. Bull. Environ. Contam. Toxicol. 2009, 83, 509. [Google Scholar]
- Maurya, S.; Maurya, J.; Pandey, V. Factors regulating phycobiliprotein production in cyanobacteria. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 764–771. [Google Scholar]
- Sakamoto, T.; Bryant, D.A. Growth at low temperature causes nitrogen limitation in the cyanobacterium Synechococcus sp. PCC 7002. Arch. Microbiol. 1997, 169, 10–19. [Google Scholar] [CrossRef]
- Tandeau de Marsac, N.; Houmard, J. Adaptation of cyanobacteria to environmental stimuli: New steps towards molecular mechanisms. FEMS Microbiol. Rev. 1993, 10, 119–189. [Google Scholar] [CrossRef]
- Boussiba, S.; Richmond, A.E. C-phycocyanin as a storage protein in the blue-green alga Spirulina platensis. Arch. Microbiol. 1980, 125, 143–147. [Google Scholar] [CrossRef]
- Wyman, M.; Gregory, R.; Carr, N. Novel role for phycoerythrin in a marine cyanobacterium, Synechococcus strain DC2. Science 1985, 230, 818–820. [Google Scholar] [CrossRef]
- Vonshak, A.; Torzillo, G.; Tomaseli, L. Use of chlorophyll fluorescence to estimate the effect of photoinhibition in outdoor cultures of Spirulina platensis. J. Appl. Phycol. 1994, 6, 31–34. [Google Scholar] [CrossRef]
- Gao, K.; Yu, H.; Brown, M.T. Solar PAR and UV radiation affects the physiology and morphology of the cyanobacterium Anabaena sp. PCC 7120. J. Photochem. Photobiol. B Biol. 2007, 89, 117–124. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Neelam, S.; Subramanyam, R. Alteration of photochemistry and protein degradation of photosystem II from Chlamydomonas reinhardtii under high salt grown cells. J. Photochem. Photobiol. B Biol. 2013, 124, 63–70. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, J.; Gong, H.; Wen, X.; Ren, H.; Lu, C. Effects of heat stress on PSII photochemistry in a cyanobacterium Spirulina platensis. Plant Sci. 2008, 175, 556–564. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J. Effects of heat stress on photosynthetic electron transport in a marine cyanobacterium Arthrospira sp. J. Appl. Phycol. 2016, 28, 757–763. [Google Scholar] [CrossRef]
- Saluri, M.; Kaldmäe, M.; Tuvikene, R. Reliable quantification of R-phycoerythrin from red algal crude extracts. J. Appl. Phycol. 2020, 32, 1421–1428. [Google Scholar] [CrossRef]
- Park, J.; Dinh, T.B. Contrasting effects of monochromatic LED lighting on growth, pigments and photosynthesis in the commercially important cyanobacterium Arthrospira maxima. Bioresour. Technol. 2019, 291, 121846. [Google Scholar] [CrossRef]
- Fujii, Y.; Tanaka, H.; Konno, N.; Ogasawara, Y.; Hamashima, N.; Tamura, S.; Hasegawa, S.; Hayasaki, Y.; Okajima, K.; Kodama, Y. Phototropin perceives temperature based on the lifetime of its photoactivated state. Proc. Natl. Acad. Sci. USA 2017, 114, 9206–9211. [Google Scholar] [CrossRef] [Green Version]
- Sachan, S.; Jangra, H.; Kumar, V. RFID Market by Product Type (Tags, Readers, and Software & Services), Frequency (Low Frequency, High Frequency, and Ultra High Frequency), and End Use (Commercial, Animal Tracking, Transportation, Agriculture, Security & Access Control, Healthcare, Retail, Logistics & Supply Chain, and Others): Global Opportunity Analysis and Industry Forecast, 2021–2030; Allied Market Research. November 2021. Available online: https://www.alliedmarketresearch.com/rfid-market-A14522 (accessed on 6 April 2022).
- Milledge, J.J.; Heaven, S. A review of the harvesting of micro-algae for biofuel production. Rev. Environ. Sci. Bio/Technol. 2013, 12, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Pragya, N.; Pandey, K.K.; Sahoo, P. A review on harvesting, oil extraction and biofuels production technologies from microalgae. Renew. Sustain. Energy Rev. 2013, 24, 159–171. [Google Scholar] [CrossRef]
- Yun, Y.J.; Hong, W.G.; Kim, W.J.; Jun, Y.; Kim, B.H. A novel method for applying reduced graphene oxide directly to electronic textiles from yarns to fabrics. Adv. Mater. 2013, 25, 5701–5705. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, B.-H.; Yang, K.S. TiO2 nanoparticles loaded on graphene/carbon composite nanofibers by electrospinning for increased photocatalysis. Carbon 2012, 50, 2472–2481. [Google Scholar] [CrossRef]
- Kim, J.K.; Kottuparambil, S.; Moh, S.H.; Lee, T.K.; Kim, Y.-J.; Rhee, J.-S.; Choi, E.-M.; Kim, B.H.; Yu, Y.J.; Yarish, C. Potential applications of nuisance microalgae blooms. J. Appl. Phycol. 2015, 27, 1223–1234. [Google Scholar] [CrossRef]
- Sonani, R.R.; Rastogi, R.P.; Patel, R.; Madamwar, D. Recent advances in production, purification and applications of phycobiliproteins. World J. Biol. Chem. 2016, 7, 100. [Google Scholar] [CrossRef]
- Hoff, W.D.; Van Stokkum, I.H.; Gural, J.; Hellingwerf, K.J. Comparison of acid denaturation and light activation in the eubacterial blue-light receptor photoactive yellow protein. Biochim. Biophys. Acta Bioenerg. 1997, 1322, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Enomoto, G.; Nomura, R.; Shimada, T.; Narikawa, R.; Ikeuchi, M. Cyanobacteriochrome SesA Is a Diguanylate Cyclase That Induces Cell Aggregation in Thermosynechococcus. J. Biol. Chem. 2014, 289, 24801–24809. [Google Scholar] [CrossRef] [Green Version]
- Brudler, R.; Hitomi, K.; Daiyasu, H.; Toh, H.; Kucho, K.-i.; Ishiura, M.; Kanehisa, M.; Roberts, V.A.; Todo, T.; Tainer, J.A. Identification of a new cryptochrome class: Structure, function, and evolution. Mol. Cell 2003, 11, 59–67. [Google Scholar] [CrossRef]
- Cao, Z.; Livoti, E.; Losi, A.; Gärtner, W. A blue light-inducible phosphodiesterase activity in the cyanobacterium Synechococcus elongatus. Photochem. Photobiol. 2010, 86, 606–611. [Google Scholar] [CrossRef]
- Boulay, C.; Abasova, L.; Six, C.; Vass, I.; Kirilovsky, D. Occurrence and function of the orange carotenoid protein in photoprotective mechanisms in various cyanobacteria. Biochim. Biophys. Acta Bioenerg. 2008, 1777, 1344–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, I.; Boyé-Péronne, S.; El Ghazaly, M.; Kristensen, M.B.; Nielsen, S.B.; Andersen, L. Absorption spectra of photoactive yellow protein chromophores in vacuum. Biophys. J. 2005, 89, 2597–2604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedetti, S.; Rinalducci, S.; Benvenuti, F.; Francogli, S.; Pagliarani, S.; Giorgi, L.; Micheloni, M.; D’Amici, G.M.; Zolla, L.; Canestrari, F. Purification and characterization of phycocyanin from the blue-green alga Aphanizomenon flos-aquae. J. Chromatogr. B 2006, 833, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Piron, R.; Bustamante, T.; Barriga, A.; Lagos, N. Phycobilisome isolation and C-phycocyanin purification from the cyanobacterium Aphanizomenon gracile. Photosynthetica 2019, 57, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Minkova, K.; Tchernov, A.; Tchorbadjieva, M.; Fournadjieva, S.; Antova, R.; Busheva, M.C. Purification of C-phycocyanin from Spirulina (Arthrospira) fusiformis. J. Biotechnol. 2003, 102, 55–59. [Google Scholar] [CrossRef]
- Munawaroh, H.; Darojatun, K.; Gumilar, G.; Aisyah, S.; Wulandari, A. Characterization of phycocyanin from Spirulina fusiformis and its thermal stability. In Proceedings of the Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2018; p. 12205. [Google Scholar]
- Rito-Palomares, M.; Nunez, L.; Amador, D. Practical application of aqueous two-phase systems for the development of a prototype process for c-phycocyanin recovery from Spirulina maxima. J. Chem. Technol. Biotechnol. 2001, 76, 1273–1280. [Google Scholar] [CrossRef]
- Bhaskar, S.U.; Gopalaswamy, G.; Raghu, R. A simple method for efficient extraction and purification of C-phycocyanin from Spirulina platensis Geitler. NISCARI Online Period. Repos. 2005, 43, 277–279. [Google Scholar]
- Patil, G.; Chethana, S.; Sridevi, A.; Raghavarao, K. Method to obtain C-phycocyanin of high purity. J. Chromatogr. A 2006, 1127, 76–81. [Google Scholar] [CrossRef]
- Jian-Feng, N.; Guang-Ce, W.; Lin, X.-z.; Zhou, B.-C. Large-scale recovery of C-phycocyanin from Spirulina platensis using expanded bed adsorption chromatography. J. Chromatogr. B 2007, 850, 267–276. [Google Scholar]
- Patil, G.; Raghavarao, K. Aqueous two phase extraction for purification of C-phycocyanin. Biochem. Eng. J. 2007, 34, 156–164. [Google Scholar] [CrossRef]
- Silveira, S.T.; Burkert, J.d.M.; Costa, J.A.V.; Burkert, C.A.V.; Kalil, S.J. Optimization of phycocyanin extraction from Spirulina platensis using factorial design. Bioresour. Technol. 2007, 98, 1629–1634. [Google Scholar] [CrossRef]
- Patil, G.; Chethana, S.; Madhusudhan, M.; Raghavarao, K. Fractionation and purification of the phycobiliproteins from Spirulina platensis. Bioresour. Technol. 2008, 99, 7393–7396. [Google Scholar] [CrossRef]
- Moraes, C.C.; Sala, L.; Ores, J.d.C.; Braga, A.R.C.; Costa, J.A.V.; Kalil, S.J. Expanded and fixed bed ion exchange chromatography for the recovery of C-phycocyanin in a single step by using lysed cells. Can. J. Chem. Eng. 2015, 93, 111–115. [Google Scholar] [CrossRef]
- Yan, S.-G.; Zhu, L.-P.; Su, H.-N.; Zhang, X.-Y.; Chen, X.-L.; Zhou, B.-C.; Zhang, Y.-Z. Single-step chromatography for simultaneous purification of C-phycocyanin and allophycocyanin with high purity and recovery from Spirulina (Arthrospira) platensis. J. Appl. Phycol. 2011, 23, 1–6. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, B.; Wang, X.; Yan, H.; Zhang, X. Purification of C-phycocyanin from Spirulina platensis by single-step ion-exchange chromatography. Chromatographia 2011, 73, 291–296. [Google Scholar] [CrossRef]
- Bermejo, R.; Ramos, A. Pilot scale recovery of phycocyanin from Spirulina platensis using expanded bed adsorption chromatography. Chromatographia 2012, 75, 195–204. [Google Scholar] [CrossRef]
- Kumar, D.; Dhar, D.W.; Pabbi, S.; Kumar, N.; Walia, S. Extraction and purification of C-phycocyanin from Spirulina platensis (CCC540). Indian J. Plant Physiol. 2014, 19, 184–188. [Google Scholar] [CrossRef] [Green Version]
- Su, C.-H.; Liu, C.-S.; Yang, P.-C.; Syu, K.-S.; Chiuh, C.-C. Solid–liquid extraction of phycocyanin from Spirulina platensis: Kinetic modeling of influential factors. Sep. Purif. Technol. 2014, 123, 64–68. [Google Scholar] [CrossRef]
- Suman, S.; Puri, N.; Jasuja, N.; Kumar, M.; Sharma, G. Optimization, purification and characterization of phycocyanin from Spirulina platensis. Int. J. Appl. Pure Sci. Agric. 2016, 2, 15–20. [Google Scholar]
- Chen, C.-Y.; Kao, P.-C.; Tan, C.H.; Show, P.L.; Cheah, W.Y.; Lee, W.-L.; Ling, T.C.; Chang, J.-S. Using an innovative pH-stat CO2 feeding strategy to enhance cell growth and C-phycocyanin production from Spirulina platensis. Biochem. Eng. J. 2016, 112, 78–85. [Google Scholar] [CrossRef]
- Martínez, J.M.; Luengo, E.; Saldaña, G.; Álvarez, I.; Raso, J. C-phycocyanin extraction assisted by pulsed electric field from Artrosphira platensis. Food Res. Int. 2017, 99, 1042–1047. [Google Scholar] [CrossRef]
- Lauceri, R.; Zittelli, G.C.; Maserti, B.; Torzillo, G. Purification of phycocyanin from Arthrospira platensis by hydrophobic interaction membrane chromatography. Algal Res. 2018, 35, 333–340. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Mittal, R.; Chandrasekhar, J.; Raghavarao, K. Simple and efficient method for extraction of C-Phycocyanin from dry biomass of Arthospira platensis. Algal Res. 2018, 31, 239–251. [Google Scholar] [CrossRef]
- Figueira, F.d.S.; Moraes, C.C.; Kalil, S.J. C-phycocyanin purification: Multiple processes for different applications. Braz. J. Chem. Eng. 2018, 35, 1117–1128. [Google Scholar] [CrossRef]
- Izadi, M.; Fazilati, M. Extraction and purification of phycocyanin from spirulina platensis and evaluating its antioxidant and anti-inflammatory activity. Asian J. Green Chem. 2018, 2, 364–379. [Google Scholar]
- Nagar, S.; Sharma, N.; Kumar, S. C-phycocyanin extraction and purification from Spirulina platensis. Res. Inventy Int. J. Eng. Sci. 2018, 8, 60–63. [Google Scholar]
- Chew, K.W.; Chia, S.R.; Krishnamoorthy, R.; Tao, Y.; Chu, D.-T.; Show, P.L. Liquid biphasic flotation for the purification of C-phycocyanin from Spirulina platensis microalga. Bioresour. Technol. 2019, 288, 121519. [Google Scholar] [CrossRef]
- Kaewdam, S.; Jaturonglumlert, S.; Varith, J.; Nitatwichit, C.; Narkprasom, K. Effect of isothermal and thermal diffusion on aqueous two-phase extraction for the purification of C-phycocyanin from Spirulina platensis. Int. Food Res. J. 2020, 27, 280–286. [Google Scholar]
- Aoki, J.; Sasaki, D.; Asayama, M. Development of a method for phycocyanin recovery from filamentous cyanobacteria and evaluation of its stability and antioxidant capacity. BMC Biotechnol. 2021, 21, 40. [Google Scholar] [CrossRef]
- Patel, A.; Mishra, S.; Pawar, R.; Ghosh, P. Purification and characterization of C-Phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expr. Purif. 2005, 40, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Chaiklahan, R.; Chirasuwan, N.; Loha, V.; Tia, S.; Bunnag, B. Separation and purification of phycocyanin from Spirulina sp. using a membrane process. Bioresour. Technol. 2011, 102, 7159–7164. [Google Scholar] [CrossRef]
- Gorgich, M.; Passos, M.L.; Mata, T.M.; Martins, A.A.; Saraiva, M.L.M.; Caetano, N.S. Enhancing extraction and purification of phycocyanin from Arthrospira sp. with lower energy consumption. Energy Rep. 2020, 6, 312–318. [Google Scholar] [CrossRef]
- Santiago-Santos, M.C.; Ponce-Noyola, T.; Olvera-Ramírez, R.; Ortega-López, J.; Cañizares-Villanueva, R.O. Extraction and purification of phycocyanin from Calothrix sp. Process Biochem. 2004, 39, 2047–2052. [Google Scholar] [CrossRef]
- Demirel, Z.; Sukatar, A. Purification of phycocyanin from isolated and identified hot spring cyanobacteria. Indian J. Exp. Biol. 2019, 57, 338–345. [Google Scholar]
- Safaei, M.; Maleki, H.; Soleimanpour, H.; Norouzy, A.; Zahiri, H.S.; Vali, H.; Noghabi, K.A. Development of a novel method for the purification of C-phycocyanin pigment from a local cyanobacterial strain Limnothrix sp. NS01 and evaluation of its anticancer properties. Sci. Rep. 2019, 9, 9474. [Google Scholar] [CrossRef] [Green Version]
- Sonani, R.R.; Singh, N.K.; Kumar, J.; Thakar, D.; Madamwar, D. Concurrent purification and antioxidant activity of phycobiliproteins from Lyngbya sp. A09DM: An antioxidant and anti-aging potential of phycoerythrin in Caenorhabditis elegans. Process Biochem. 2014, 49, 1757–1766. [Google Scholar] [CrossRef]
- Chittapun, S.; Jonjaroen, V.; Khumrangsee, K.; Charoenrat, T. C-phycocyanin extraction from two freshwater cyanobacteria by freeze thaw and pulsed electric field techniques to improve extraction efficiency and purity. Algal Res. 2020, 46, 101789. [Google Scholar] [CrossRef]
- Soni, B.; Kalavadia, B.; Trivedi, U.; Madamwar, D. Extraction, purification and characterization of phycocyanin from Oscillatoria quadripunctulata—Isolated from the rocky shores of Bet-Dwarka, Gujarat, India. Process Biochem. 2006, 41, 2017–2023. [Google Scholar] [CrossRef]
- Soni, B.; Trivedi, U.; Madamwar, D. A novel method of single step hydrophobic interaction chromatography for the purification of phycocyanin from Phormidium fragile and its characterization for antioxidant property. Bioresour. Technol. 2008, 99, 188–194. [Google Scholar] [CrossRef]
- Sonani, R.R.; Patel, S.; Bhastana, B.; Jakharia, K.; Chaubey, M.G.; Singh, N.K.; Madamwar, D. Purification and antioxidant activity of phycocyanin from Synechococcus sp. R42DM isolated from industrially polluted site. Bioresour. Technol. 2017, 245, 325–331. [Google Scholar] [CrossRef]
- El-Mohsnawy, E.; Abu-Khudir, R. A highly purified C-phycocyanin from thermophilic cyanobacterium Thermosynechococcus elongatus and its cytotoxic activity assessment using an in vitro cell-based approach. J. Taibah Univ. Sci. 2020, 14, 1218–1225. [Google Scholar] [CrossRef]
| Treatments | Fluorescence Parameters † | ||
|---|---|---|---|
| Fv/Fm | rETRmax | ΦPSII | |
| pH 4 | 0.32 ± 0.019 a | 65.2 ± 8.54 a | 0.21 ± 0.008 ab |
| pH 5 | 0.31 ± 0.020 a | 77.7 ± 9.40 a | 0.22 ± 0.006 abc |
| pH 6 | 0.31 ± 0.016 a | 85.9 ± 9.53 a | 0.26 ± 0.017 c |
| pH 7 | 0.43 ± 0.013 c | 118.2 ± 15.32 b | 0.35 ± 0.021 de |
| pH 8 | 0.44 ± 0.011 c | 128.4 ± 16.64 b | 0.38 ± 0.012 e |
| pH 9 | 0.35 ± 0.017 ab | 78.3 ± 9.43 a | 0.31 ± 0.021 d |
| pH 10 | 0.37 ± 0.009 b | 74.4 ± 13.40 a | 0.25 ± 0.009 bc |
| pH 11 | 0.32 ± 0.014 a | 58.54 ± 15.28 a | 0.18 ± 0.010 a |
| 5 °C | NA § | NA § | NA § |
| 10 °C | 0.22 ± 0.021 ab | 40.9 ± 12.53 a | 0.16 ± 0.033 a |
| 15 °C | 0.20 ± 0.020 a | 37.0 ± 13.89 a | 0.21 ± 0.019 b |
| 20 °C | 0.30 ± 0.009 bc | 57.3 ± 9.28 a | 0.22 ± 0.034 bc |
| 25 °C | 0.31 ± 0.039 c | 99.9 ± 6.79 b | 0.27 ± 0.035 d |
| 30 °C | 0.33 ± 0.039 c | 87.4 ± 3.60 b | 0.25 ± 0.033 cd |
| 35 °C | 0.31 ± 0.039 c | 88.75 ± 6.68 b | 0.21 ± 0.022 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Lee, H.; Dinh, T.B.; Choi, S.; De Saeger, J.; Depuydt, S.; Brown, M.T.; Han, T. Commercial Potential of the Cyanobacterium Arthrospira maxima: Physiological and Biochemical Traits and the Purification of Phycocyanin. Biology 2022, 11, 628. https://doi.org/10.3390/biology11050628
Park J, Lee H, Dinh TB, Choi S, De Saeger J, Depuydt S, Brown MT, Han T. Commercial Potential of the Cyanobacterium Arthrospira maxima: Physiological and Biochemical Traits and the Purification of Phycocyanin. Biology. 2022; 11(5):628. https://doi.org/10.3390/biology11050628
Chicago/Turabian StylePark, Jihae, Hojun Lee, Thai Binh Dinh, Soyeon Choi, Jonas De Saeger, Stephen Depuydt, Murray T. Brown, and Taejun Han. 2022. "Commercial Potential of the Cyanobacterium Arthrospira maxima: Physiological and Biochemical Traits and the Purification of Phycocyanin" Biology 11, no. 5: 628. https://doi.org/10.3390/biology11050628
APA StylePark, J., Lee, H., Dinh, T. B., Choi, S., De Saeger, J., Depuydt, S., Brown, M. T., & Han, T. (2022). Commercial Potential of the Cyanobacterium Arthrospira maxima: Physiological and Biochemical Traits and the Purification of Phycocyanin. Biology, 11(5), 628. https://doi.org/10.3390/biology11050628






