Effect of Seed Traits and Waterbird Species on the Dispersal Effectiveness of Wetland Plants
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species and Seed Selection
2.2. Seed Trait Measurement
2.3. Feeding Experiments
2.4. Germination Experiment
2.5. Statistical Analysis
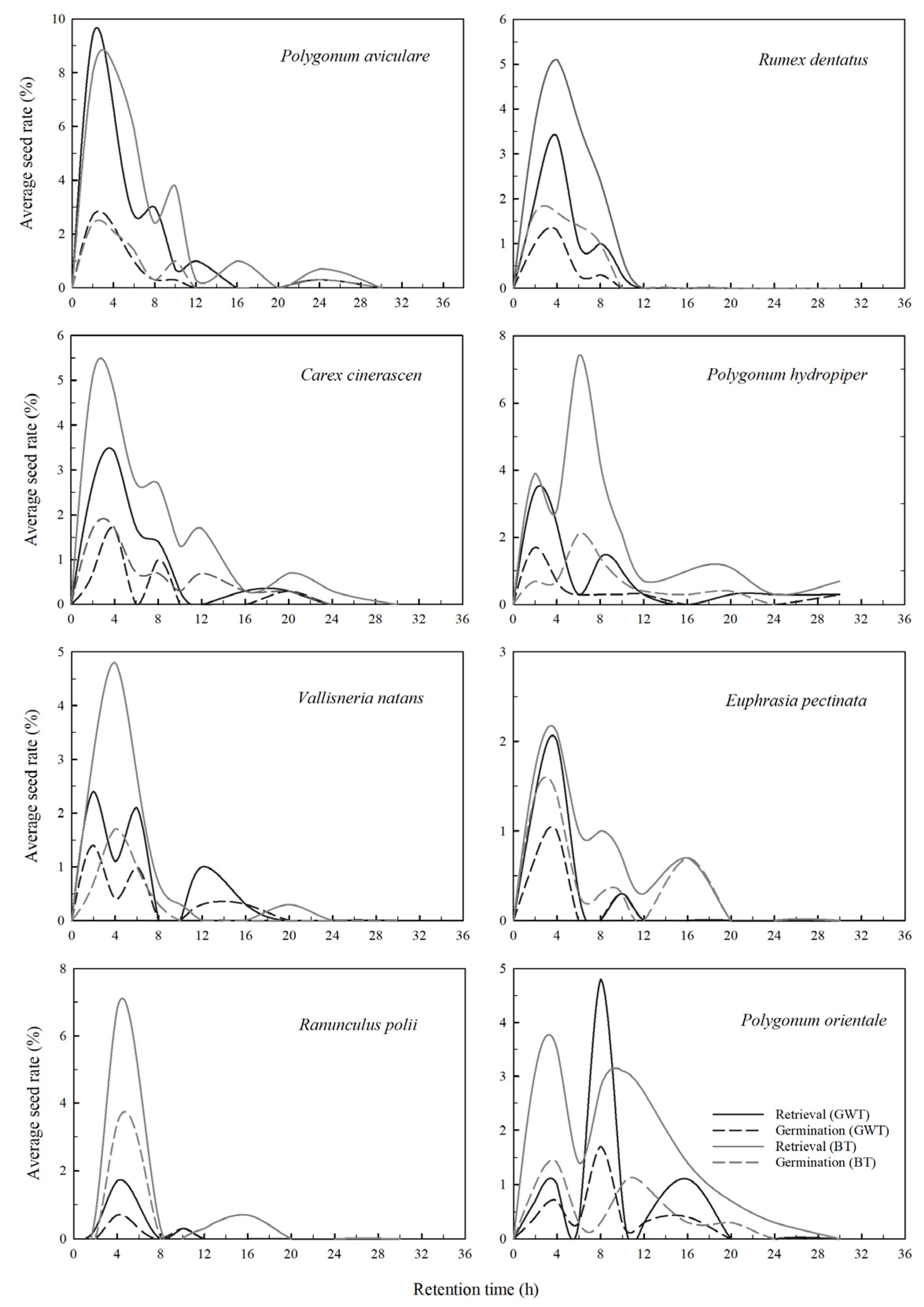
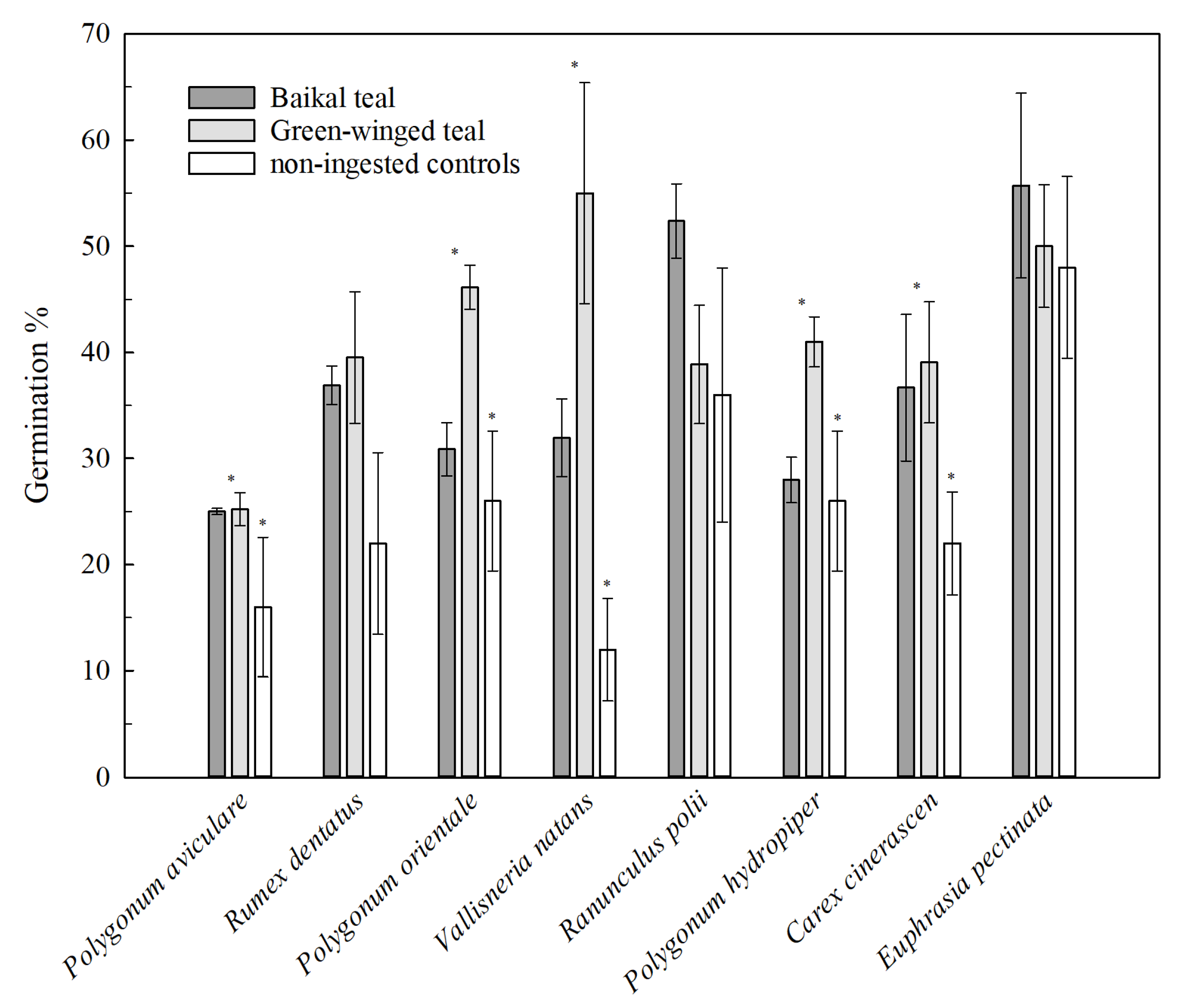
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nathan, R.; Muller-Landau, H.C. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evol. 2000, 15, 278–285. [Google Scholar] [CrossRef]
- Levine, J.M.; Murrell, D.J. The community-level consequences of seed dispersal patterns. Annu. Rev. Ecol. Syst. 2003, 34, 549–574. [Google Scholar] [CrossRef]
- Howe, H.F.; Miriti, M.N. When seed dispersal matters. BioScience 2004, 54, 651–660. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P. Plant-animal mutualistic networks: The architecture of biodiversity. Annu. Rev. Ecol. Syst. 2007, 38, 567–593. [Google Scholar] [CrossRef]
- Pollux, B.J.A.; Santamaria, L.; Ouborg, N.J. Differences in endozoochorous dispersal between aquatic plant species, with reference to plant population persistence in rivers. Freshw. Biol. 2005, 50, 232–242. [Google Scholar] [CrossRef]
- Wongsriphuek, C.; Dugger, B.D.; Bartuszevige, A.M. Dispersal of wetland plant seeds by mallards: Influence of gut passage on recovery, retention, and germination. Wetlands 2008, 28, 290. [Google Scholar] [CrossRef]
- Figuerola, J.; Green, A.J. Dispersal of aquatic organisms by waterbirds: A review of past research and priorities for future studies. Freshw. Biol. 2002, 47, 483–494. [Google Scholar] [CrossRef]
- Santamaria, L. Why are most aquatic plants widely distributed? Dispersal, clonal growth and small-scale heterogeneity in a stressful environment. Acta Oecol. 2002, 23, 137–154. [Google Scholar] [CrossRef]
- Soons, M.B.; Brochet, A.L.; Kleyheeg, E.; Green, A.J. Seed dispersal by dabbling ducks: An overlooked dispersal pathway for a broad spectrum of plant species. J. Ecol. 2016, 104, 443–455. [Google Scholar] [CrossRef]
- van Leeuwen, C.H.; van der Velde, G.; van Groenendael, J.M.; Klaassen, M. Gut travellers: Internal dispersal of aquatic organisms by waterfowl. J. Biogeogr. 2012, 39, 2031–2040. [Google Scholar] [CrossRef]
- Nathan, R.; Schurr, F.M.; Spiegel, O.; Steinitz, O.; Trakhtenbrot, A.; Tsoar, A. Mechanisms of long-distance seed dispersal. Trends Ecol. Evol. 2008, 23, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Green, A.J.; Figuerola, J.; Sánchez, M.I. Implications of waterbird ecology for the dispersal of aquatic organisms. Acta Oecol. 2002, 23, 177–189. [Google Scholar] [CrossRef]
- Charalambidou, I.; Santamaría, L. Field evidence for the potential of waterbirds as dispersers of aquatic organisms. Wetlands 2005, 25, 252. [Google Scholar] [CrossRef]
- Lovas-Kiss, Á.; Vincze, O.; Kleyheeg, E.; Sramkó, G.; Laczkó, L.; Fekete, R.; Molnár, A.; Green, A.J. Seed mass, hardness and phylogeny determine the potential for endozoochory by granivorous waterbirds. Ecol. Evol. 2020, 10, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Brochet, A.L.; Guillemain, M.; Fritz, H.; Gauthier-Clerc, M.; Green, A.J. Plant dispersal by teal (Anas crecca) in the Camargue: Duck guts are more important than their feet. Freshw. Biol. 2010, 55, 1262–1273. [Google Scholar] [CrossRef]
- Reynolds, C.; Cumming, G.S. Seed dispersal by waterbirds in southern Africa: Comparing the roles of ectozoochory and endozoochory. Freshw. Biol. 2016, 61, 349–361. [Google Scholar] [CrossRef]
- Amezaga, J.M.; Santamaría, L.; Green, A.J. Biotic wetland connectivity-supporting a new approach for wetland policy. Acta Oecol. 2002, 23, 213–222. [Google Scholar] [CrossRef]
- Kleyheeg, E.; Treep, J.; De Jager, M.; Nolet, B.A.; Soons, M.B. Seed dispersal distributions resulting from landscape-dependent daily movement behaviour of a key vector species, Anas platyrhynchos. J. Ecol. 2017, 105, 1279–1289. [Google Scholar] [CrossRef]
- Charalambidou, I.; Santamaría, L. Waterbirds as endozoochorous dispersers of aquatic organisms: A review of experimental evidence. Acta Oecol. 2002, 23, 165–176. [Google Scholar] [CrossRef]
- Brochet, A.L.; Dessborn, L.; Legagneux, P.; Elmberg, J.; Gauthier-Clerc, M.; Fritz, H.; Guillemain, M. Is diet segregation between dabbling ducks due to food partitioning? A review of seasonal patterns in the Western Palearctic. J. Zool. 2012, 286, 171–178. [Google Scholar] [CrossRef]
- Thomas, G.J. Autumn and winter feeding ecology of waterfowl at the Ouse Washes, England. J. Zool. 1982, 197, 131–172. [Google Scholar] [CrossRef]
- Mueller, M.H.; van der Valk, A.G. The potential role of ducks in wetland seed dispersal. Wetlands 2002, 22, 170–178. [Google Scholar] [CrossRef]
- Cousens, R.D.; Hill, J.; French, K.; Bishop, I.D. Towards better prediction of seed dispersal by animals. Funct. Ecol. 2010, 24, 1163–1170. [Google Scholar] [CrossRef]
- Kleyheeg, E.; Claessens, M.; Soons, M.B. Interactions between seed traits and digestive processes determine the germinability of bird-dispersed seeds. PLoS ONE 2018, 13, e0195026. [Google Scholar] [CrossRef] [PubMed]
- Renne, I.J.; Gauthreaus, S.A., Jr.; Gresham, C.A. Seed dispersal of the Chinese tallow tree (Sapium sebiferum (L.) Roxb.) by birds in coastal South Carolina. Am. Midl. Nat. 2000, 144, 202–215. [Google Scholar] [CrossRef]
- Figuerola, J.; Green, A.J.; Santamaría, L. Comparative dispersal effectiveness of wigeongrass seeds by waterfowl wintering in south-west Spain: Quantitative and qualitative aspects. J. Ecol. 2002, 90, 989–1001. [Google Scholar] [CrossRef]
- Kleyheeg, E.; Klaassen, M.; Soons, M.B. Seed dispersal potential by wild ducks as estimated from digestive tract analysis. Freshw. Biol. 2016, 61, 1746–1758. [Google Scholar] [CrossRef]
- Brochet, A.L.; Guillemain, M.; Gauthier-Clerc, M.; Fritz, H.; Green, A.J. Endozoochory of Mediterranean aquatic plant seeds by teal after a period of desiccation: Determinants of seed survival and influence of retention time on germinability and viability. Aquat. Bot. 2010, 93, 99–106. [Google Scholar] [CrossRef]
- Soons, M.B.; van der Vlugt, C.; van Lith, B.; Heil, G.W.; Klaassen, M. Small seed size increases the potential for dispersal of wetland plants by ducks. J. Ecol. 2008, 96, 619–627. [Google Scholar] [CrossRef]
- Reynolds, C.; Cumming, G.S. Seed traits and bird species influence the dispersal parameters of wetland plants. Freshw. Biol. 2016, 61, 1157–1170. [Google Scholar] [CrossRef]
- van Leeuwen, C.H.A.; Tollenaar, M.L.; Klaassen, M. Vector activity and propagule size affect dispersal potential by vertebrates. Oecologia 2012, 170, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Kleyheeg, E.; van Leeuwen, C.H.A.; Morison, M.A.; Nolet, B.A.; Soons, M.B. Bird-mediated seed dispersal: Reduced digestive efficiency in active birds modulates the dispersal capacity of plant seeds. Oikos 2015, 124, 899–907. [Google Scholar] [CrossRef][Green Version]
- Guillemain, M.; Fritz, H.; Guillon, N.; Simon, G. Ecomorphology and coexistence in dabbling ducks: The role of lamellar density and body length in winter. Oikos 2002, 98, 547–551. [Google Scholar] [CrossRef]
- Xu, L.; Xu, W.; Sun, Q.; Zhou, Z.; Shen, J.; Zhao, X. Flora and Vegetation in Shengjin Lake. Plant Sci. J. 2008, 26, 264–270. (In Chinese) [Google Scholar]
- Konkle, A.T.M.; Baker, S.L.; Kentner, A.C.; Barbagallo, L.S.M.; Merali, Z.; Bielajew, C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: Sex and strain compared. Brain Res. 2003, 992, 227–238. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.r-project.org (accessed on 22 January 2022).
- Euliss, N.H.; Harris, S.W. Feeding ecology of northern pintails and green-winged teal wintering in California. J. Wildl. Manag. 1987, 51, 724–732. [Google Scholar] [CrossRef]
- Viana, D.S.; Santamaría, L.; Michot, T.C.; Figuerola, J. Allometric scaling of long-distance seed dispersal by migratory birds. Am. Nat. 2013, 181, 649–662. [Google Scholar] [CrossRef]
- Clench, M.H.; Mathias, J.R. The avian cecum: A review. Wilson Bull. 1995, 107, 93–121. [Google Scholar]
- Reynolds, C.; Cumming, G.S. Defining functional groups using dietary data: Quantitative comparison suggests functional classification for seed-dispersing waterfowl. Basic Appl. Ecol. 2016, 17, 333–343. [Google Scholar] [CrossRef]
- García-Álvarez, A.; van Leeuwen, C.H.A.; Luque, C.J.; Hussner, A.; Vélez-Martín, A.; Pérez-Vázquez, A.; Green, A.J.; Castellanos, E.M. Internal transport of alien and native plants by geese and ducks: An experimental study. Freshw. Biol. 2015, 60, 1316–1329. [Google Scholar] [CrossRef]
- Green, A.J.; Lovas-Kiss, Á.; Stroud, R.A.; Tierney, N.; Fox, A.D. Plant dispersal by Canada geese in arctic Greenland. Polar Res. 2018, 37, 1508268. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; Snijder, M.H.B.; Kranenbarg, A.; Keijsers, E.R.P.; de Jong, E.; Stigsson, L.L. Characterisation and application of NovaFiber lignin. Ind. Crop. Prod. 2004, 20, 191–203. [Google Scholar] [CrossRef]
- Kleyheeg, E.; Nolet, B.A.; Otero-Ojea, S.; Soons, M.B. A mechanistic assessment of the relationship between gut morphology and endozoochorous seed dispersal by waterfowl. Ecol. Evol. 2018, 8, 10857–10867. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.A.; Webb, E.B.; Pierce, R.A.; Bradley, K.W. Evaluating the potential for weed seed dispersal based on waterfowl consumption and seed viability. Pest Manag. Sci. 2017, 73, 2592–2603. [Google Scholar] [CrossRef] [PubMed]
- Kleyheeg, E.; Fiedler, W.; Safi, K.; Waldenström, J.; Wikelski, M.; van Toor, M.L. A comprehensive model for the quantitative estimation of seed dispersal by migratory mallards. Front. Ecol. Evol. 2019, 7, 40. [Google Scholar] [CrossRef]
- Schupp, E.W.; Jordano, P.; Gómez, J.M. Seed dispersal effectiveness revisited: A conceptual review. New Phytol. 2010, 188, 333–353. [Google Scholar] [CrossRef]
- Godínez-Alvarez, H.; Ríos-Casanova, L.; Peco, B. Are large frugivorous birds better seed dispersers than medium- and small-sized ones? Effect of body mass on seed dispersal effectiveness. Ecol. Evol. 2020, 10, 6136–6143. [Google Scholar] [CrossRef]
| Seed Species | Family | Mass (mg) | Length (mm) | Lignin (mg/g) |
|---|---|---|---|---|
| Polygonum aviculare | Polygonaceae | 0.50 ± 0.00 | 0.91 ± 0.01 | 10.03 ± 0.04 |
| Polygonum hydropiper | Polygonaceae | 1.20 ± 0.01 | 1.66 ± 0.02 | 10.33 ± 0.04 |
| Polygonum orientale | Polygonaceae | 7.01 ± 0.07 | 2.98 ± 0.03 | 9.55 ± 0.09 |
| Rumex dentatus | Polygonaceae | 0.51 ± 0.00 | 0.94 ± 0.04 | 8.73 ± 0.17 |
| Euphrasia pectinata | Orobanchaceae | 3.36 ± 0.07 | 1.88 ± 0.03 | 6.73 ± 0.06 |
| Vallisneria natans | Hydrocharitaceae | 0.14 ± 0.00 | 1.86 ± 0.02 | 9.51 ± 0.13 |
| Carex cinerasce | Cyperaceae | 1.14 ± 0.00 | 1.19 ± 0.01 | 10.11 ± 0.14 |
| Ranunculus polii | Ranunculaceae | 1.21 ± 0.01 | 2.18 ± 0.02 | 6.87 ± 0.08 |
| Seed Species | Retention Time | Retrievability% | Germination% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GWT | BT | GWT | BT | GWT | BT | Control | |||||||
| Mean | Med | Max | Gmax | Mean | Med | Max | Gmax | ||||||
| P. avi | 4.71 ± 0.33 | 4 | 24 | 24 | 5.51 ± 0.95 | 4 | 24 | 24 | 24 ± 1 | 30 ± 6 | 25 ± 2 | 25 ± 0 | 16 ± 7 |
| P. hyd | 6.99 ± 0.36 | 4 | 30 | 30 | 7.94 ± 0.25 | 6 | 30 | 20 | 10 ± 1 | 24 ± 4 | 41 ± 2 | 28 ± 2 | 26 ± 7 |
| P. ori | 8.17 ± 0.25 | 8 | 16 | 16 | 8.33 ± 0.83 | 8 | 24 | 20 | 9 ± 1 | 19 ± 1 | 46 ± 2 | 31 ± 3 | 26 ± 6 |
| R. den | 4.43 ± 0.30 | 4 | 10 | 8 | 4.84 ± 0.19 | 4 | 10 | 8 | 8 ± 1 | 16 ± 1 | 40 ± 6 | 37 ± 2 | 22 ± 9 |
| E. pec | 4.00 ± 0.69 | 4 | 10 | 10 | 6.32 ± 1.15 | 5 | 16 | 16 | 4 ± 1 | 8 ± 2 | 50 ± 6 | 56 ± 9 | 48 ± 9 |
| V. nat | 5.70 ± 0.15 | 5 | 16 | 16 | 4.71 ± 0.27 | 4 | 20 | 8 | 7 ± 2 | 12 ± 2 | 55 ± 10 | 32 ± 4 | 12 ± 5 |
| C. cin | 5.36 ± 0.32 | 4 | 20 | 20 | 6.69 ± 0.31 | 5 | 24 | 20 | 10 ± 2 | 20 ± 6 | 39 ± 6 | 37 ± 7 | 22 ± 5 |
| R. pol | 5.00 ± 0.19 | 4 | 10 | 10 | 6.10 ± 0.24 | 4 | 16 | 16 | 3 ± 0.3 | 14 ± 4 | 39 ± 6 | 52 ± 4 | 36 ± 12 |
| LMM1a | Tave | CIs | LMM1b | Tmax | CIs | ||||||
| Variables | β ± SE | 2.5% | 97.5% | p-value | R2 | Variables | β ± SE | 2.5% | 97.5% | p-value | R2 |
| (Intercept) | 1.79 ± 0.06 | 1.67 | 1.91 | <0.001 | (Intercept) | 2.66 ± 0.09 | 2.49 | 2.83 | <0.001 | ||
| BS(GWT) | −0.13 ± 0.07 | −0.27 | 0.02 | 0.088 | mR2 | BS(GWT) | −0.24 ± 0.10 | −0.44 | −0.04 | 0.02 | mR2 |
| SL | 0.15 ± 0.04 | 0.08 | 0.23 | <0.001 | 0.514 | SLI | 0.27 ± 0.09 | 0.09 | 0.46 | 0.004 | 0.469 |
| SLI | 0.11 ± 0.04 | 0.02 | 0.20 | 0.015 | BS(GWT)*SLI | 0.18 ± 0.10 | −0.02 | 0.38 | 0.075 | ||
| BS(GWT)*SLI | 0.09 ± 0.05 | −0.01 | 0.19 | 0.062 | cR2 | SL | 0.10 ± 0.07 | −0.04 | 0.23 | 0.152 | cR2 |
| BS(GWT)*SL | 0.06 ± 0.05 | −0.04 | 0.15 | 0.257 | 0.632 | 0.554 | |||||
| GLMM2 | Retrieval | CIs | GLMM3 | Germination | CIs | ||||||
| Variables | β ± SE | 2.5% | 97.5% | p-value | R2 | Variables | β ± SE | 2.5% | 97.5% | p-value | R2 |
| (Intercept) | −1.82 ± 0.10 | −2.02 | −1.62 | <0.001 | (Intercept) | −1.01 ± 0.09 | −1.19 | −0.84 | <0.001 | ||
| BS(GWT) | −0.68 ± 0.10 | −0.87 | −0.49 | <0.001 | mR2 | SLI | −0.22 ± 0.09 | −0.40 | −0.04 | 0.010 | mR2 |
| SLI | 0.35 ± 0.11 | 0.13 | 0.56 | 0.001 | 0.679 | BS(GWT) | 0.14 ±0.16 | −0.19 | 0.47 | 0.410 | 0.216 |
| BS(GWT)* SLI | 0.18 ± 0.11 | −0.04 | 0.40 | 0.110 | SL | 0.06 ± 0.08 | −0.10 | 0.22 | 0.460 | ||
| SL | −0.03 ± 0.11 | −0.26 | 0.18 | 0.730 | cR2 | cR2 | |||||
| BS(GWT)* SL | −0.17 ± 0.09 | −0.35 | 0.01 | 0.070 | 0.802 | 0.216 | |||||
| SL* SLI | −0.24 ± 0.16 | −0.57 | 0.09 | 0.150 | |||||||
| GLMM4a | RT|Retrieval | CIs | GLMM4b | RT|Germination | CIs | ||||||
| Variables | β ± SE | 2.5% | 97.5% | p-value | R2 | Variables | β ± SE | 2.5% | 97.5% | p-value | R2 |
| (Intercept) | −3.53 ± 0.07 | −3.67 | −3.39 | <0.001 | mR2 | (Intercept) | −3.57 ± 0.10 | −3.76 | −3.35 | <0.001 | mR2 |
| BS(GWT) | −0.65 ± 0.11 | −0.87 | −0.43 | <0.001 | 0.913 | BS(GWT) | −0.51 ± 0.15 | −0.80 | −0.22 | <0.001 | 0.870 |
| RT | 0.45 ±0.24 | −0.04 | 0.93 | 0.071 | RT2 | −0.70 ± 0.10 | −0.89 | −0.51 | <0.001 | ||
| RT2 | −0.99 ± 0.28 | −1.56 | −0.42 | 0.001 | cR2 | cR2 | |||||
| BS(GWT)*RT | −0.23 ± 0.16 | −0.56 | 0.09 | 0.161 | 0.958 | 0.870 | |||||
| GLMM5 | TT | CIs | |||||||||
| Variables | β ± SE | 2.5% | 97.5% | p-value | R2 | ||||||
| (Intercept) | −1.68 ± 0.27 | −2.20 | −1.15 | <0.001 | |||||||
| SS (R. den) | 0.36 ± 0.25 | −0.13 | 0.86 | 0.144 | mR2 | ||||||
| SS (P. ori) | 0.39 ± 0.24 | −0.09 | 0.87 | 0.110 | 0.257 | ||||||
| SS (V. nat) | 0.19 ± 0.27 | −0.35 | 0.73 | 0.486 | |||||||
| SS (R. pol) | 0.73 ± 0.25 | 0.24 | 1.22 | 0.003 | cR2 | ||||||
| SS (P. hyd) | 0.30 ± 0.24 | −0.17 | 0.77 | 0.211 | 0.257 | ||||||
| SS (C. cin) | 0.30 ± 0.24 | −0.17 | 0.78 | 0.214 | |||||||
| SS (E. pec) | 0.95 ± 0.25 | 0.45 | 1.45 | <0.001 | |||||||
| TT (ingested) | 0.38 ± 0.14 | 0.09 | 0.67 | 0.011 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, S.; Zhou, L.; Xu, W. Effect of Seed Traits and Waterbird Species on the Dispersal Effectiveness of Wetland Plants. Biology 2022, 11, 629. https://doi.org/10.3390/biology11050629
Nie S, Zhou L, Xu W. Effect of Seed Traits and Waterbird Species on the Dispersal Effectiveness of Wetland Plants. Biology. 2022; 11(5):629. https://doi.org/10.3390/biology11050629
Chicago/Turabian StyleNie, Shenghong, Lizhi Zhou, and Wenbin Xu. 2022. "Effect of Seed Traits and Waterbird Species on the Dispersal Effectiveness of Wetland Plants" Biology 11, no. 5: 629. https://doi.org/10.3390/biology11050629
APA StyleNie, S., Zhou, L., & Xu, W. (2022). Effect of Seed Traits and Waterbird Species on the Dispersal Effectiveness of Wetland Plants. Biology, 11(5), 629. https://doi.org/10.3390/biology11050629







