Magnetic Fluctuations Entrain the Circadian Rhythm of Locomotor Activity in Zebrafish: Can Cryptochrome Be Involved?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Maintenance
2.2. Timed Backlight
2.3. Magnetic Fluctuations
- Natural diurnal geomagnetic variation. This is represented by magnetic fluctuations of about 30 nT within a 24 h period. This variation was recorded in the X-, Y-, and Z-directions throughout the experiment using an NV0302A magnetometer (ENT, St Petersburg, Russia);
- Experimental magnetic fluctuations simulating increased diurnal geomagnetic variation within 26.8 h, 33.76 h, and 36 h periods. We used a sample record of diurnal geomagnetic variation in the X-, Y-, and Z-directions that were made close to the laboratory to generate these magnetic oscillations. The sample record intensity was enhanced to about 100–150 nT for each X-, Y-, and Z-direction. This exposure allowed for more pronounced periodic changes in the magnetic background without exceeding the level of natural geomagnetic storms. The period of the magnetic variation for different experimental groups was also increased to 26.8 h, 33.76 h, or 36 h by a signal prolongation.
- (1)
- A three-component fluxgate magnetometer NV0302A (ENT, St Petersburg, Russia) providing analogous signals proportional to the strength of the geomagnetic field and its variations;
- (2)
- An LTR11 analog-to-digital and an LTR34-4 digital-to-analog signal converter (L-card, Moscow, Russia);
- (3)
- A coil system consisting of three pairs of mutually orthogonal Helmholtz coils (0.5 m in diameter, 700 turns of 0.2 mm copper wire in each coil) made by the Schmidt Institute of Physics of the Earth (www.ifz.ru) (accessed on 10 April 2022).
2.4. Experimental Conditions and Procedure
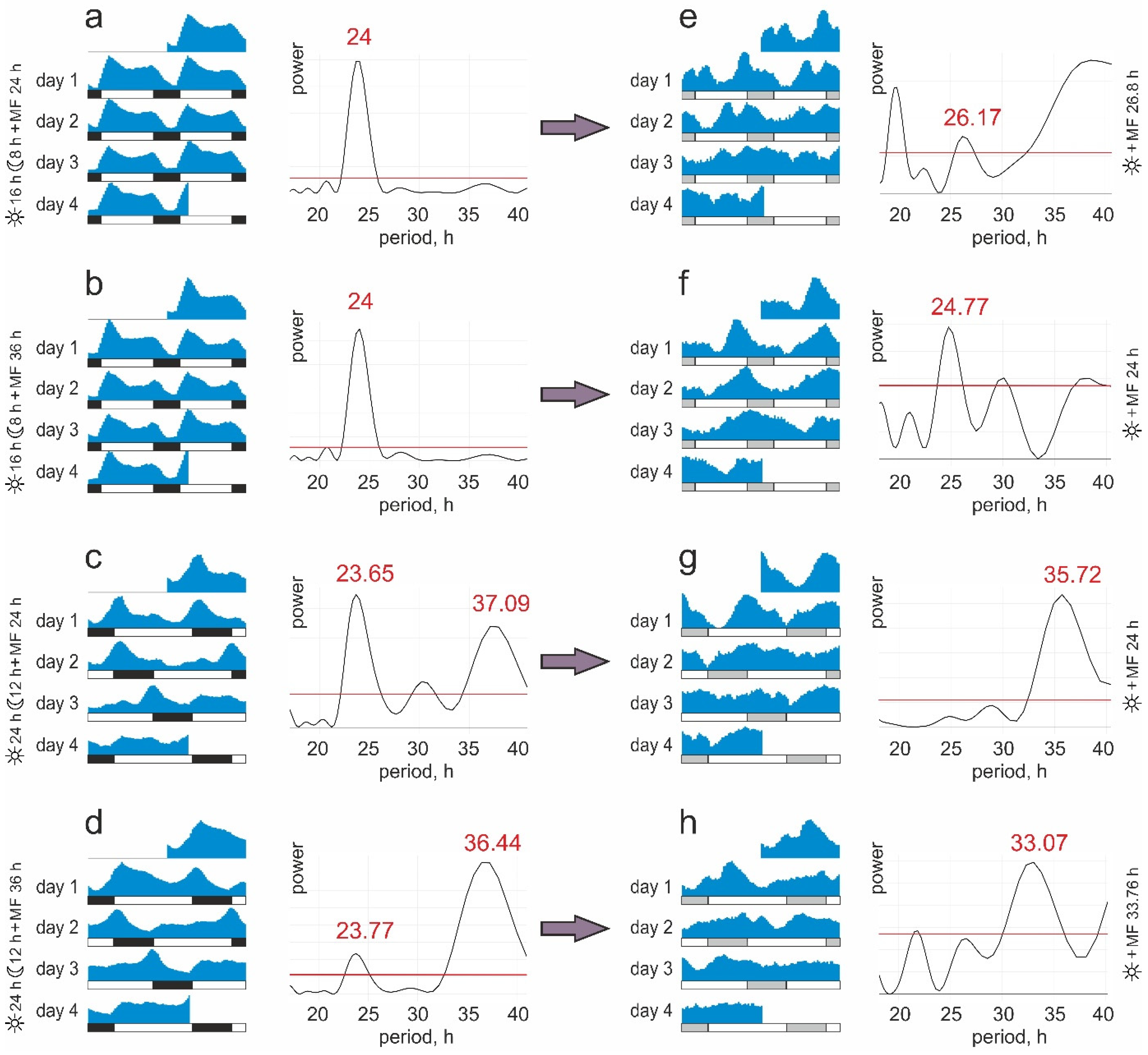
- Group #1. 16:8 light/dark cycle and natural diurnal geomagnetic variation (24 h period). The periods of light and magnetic stimuli coincide and correspond to the natural ones;
- Group #2. 16:8 light/dark cycle and artificially prolonged magnetic variation (36 h period). The light and magnetic stimuli periods do not coincide; the main zeitgeber period corresponds to the natural one;
- Group #3. 24:12 light/dark cycle and natural diurnal geomagnetic variation (24 h period). The light and magnetic stimuli periods do not coincide; the main zeitgeber period does not correspond to the natural one;
- Group #4. 24:12 light/dark cycle and artificially prolonged magnetic variation (36 h period). The periods of light and magnetic stimuli coincide and do not correspond to the natural ones.
2.5. Data Processing
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finger, A.M.; Dibner, C.; Kramer, A. Coupled network of the circadian clocks: A driving force of rhythmic physiology. FEBS Lett. 2020, 594, 2734–2769. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Dibner, C.; Schibler, U.; Albrecht, U. The Mammalian Circadian Timing System: Organization and Coordination of Central and Peripheral Clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Jagannath, A.; Hankins, M.W.; Foster, R.G.; Peirson, S.N. Photic Regulation of Clock Systems. Methods Enzymol. 2015, 552, 125–143. [Google Scholar] [CrossRef]
- Lahiri, K.; Vallone, D.; Gondi, S.B.; Santoriello, C.; Dickmeis, T.; Foulkes, N.S. Temperature Regulates Transcription in the Zebrafish Circadian Clock. PLoS Biol. 2005, 3, e351. [Google Scholar] [CrossRef]
- López-Olmeda, J.F.; Madrid, J.A.; Sanchez-Vázquez, F.J. Light and Temperature Cycles as Zeitgebers of Zebrafish (Danio rerio) Circadian Activity Rhythms. Chronobiol. Int. 2006, 23, 537–550. [Google Scholar] [CrossRef]
- Lopez-Olmeda, J.F.; Sánchez-Vázquez, F.J. Zebrafish temperature selection and synchronization of locomotor activity circadian rhythm to ahemeral cycles of light and temperature. Chronobiol. Int. 2009, 26, 200–218. [Google Scholar] [CrossRef]
- López-Olmeda, J.F.; Tartaglione, E.V.; de la Iglesia, H.O.; Sánchez-Vázquez, F.J. Feeding entrainment of food-anticipatory activity and per1 expression in the brain and liver of zebrafish under different lighting and feeding conditions. Chronobiol. Int. 2010, 27, 1380–1400. [Google Scholar] [CrossRef]
- Cavallari, N.; Frigato, E.; Vallone, D.; Fröhlich, N.; López-Olmeda, J.F.; Foà, A.; Berti, R.; Sánchez-Vázquez, F.J.; Bertolucci, C.; Foulkes, N.S. A Blind Circadian Clock in Cavefish Reveals that Opsins Mediate Peripheral Clock Photoreception. PLoS Biol. 2011, 9, e1001142. [Google Scholar] [CrossRef]
- Morbiato, E.; Frigato, E.; Dinarello, A.; Maradonna, F.; Facchinello, N.; Argenton, F.; Carnevali, O.; Dalla Valle, L.; Bertolucci, C. Feeding Entrainment of the Zebrafish Circadian Clock Is Regulated by the Glucocorticoid Receptor. Cells 2019, 8, 1342. [Google Scholar] [CrossRef]
- Krylov, V.V.; Izvekov, E.I.; Pavlova, V.V.; Pankova, N.A.; Osipova, E.A. Circadian rhythms in zebrafish (Danio rerio) behaviour and the sources of their variability. Biol. Rev. 2021, 96, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Audira, G.; Sampurna, B.P.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Han, L.; Hsiao, C.-D. Establishing simple image-based methods and a cost-effective instrument for toxicity assessment on circadian rhythm dysregulation in fish. Biol. Open 2019, 8, bio041871. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Hu, Y.; Li, B.; Chen, M.; Ren, Z. Potential effects of internal physio-ecological changes on the online biomonitoring of water quality: The behavior responses with circadian rhythms of zebrafish (Danio rerio) to different chemicals. Chemosphere 2020, 239, 124752. [Google Scholar] [CrossRef] [PubMed]
- Brown, F.A. Response to Pervasive Geophysical Factors and the Biological Clock Problem. Cold Spring Harb. Symp. Quant. Biol. 1960, 25, 57–71. [Google Scholar] [CrossRef]
- Bliss, V.L.; Heppner, F.H. Circadian activity rhythm influenced by near zero magnetic field. Nature 1976, 261, 411–412. [Google Scholar] [CrossRef]
- Brown, F.A.; Scow, K.M. Magnetic induction of a circadian cycle in hamsters. J. Interdisiplinary Cycle Res. 1978, 9, 137–145. [Google Scholar] [CrossRef]
- Welker, H.A.; Semm, P.; Willig, R.P.; Commentz, J.C.; Wiltschko, W.; Vollrath, L. Effects of an artificial magnetic field on serotonin N-acetyltransferase activity and melatonin content of the rat pineal gland. Exp. Brain Res. 1983, 50, 426–432. [Google Scholar] [CrossRef]
- Bartos, P.; Netusil, R.; Slaby, P.; Dolezel, D.; Ritz, T.; Vacha, M. Weak radiofrequency fields affect the insect circadian clock. J. R. Soc. Interface 2019, 16, 20190285. [Google Scholar] [CrossRef]
- Yoshii, T.; Ahmad, M.; Helfrich-Foerster, C. Cryptochrome mediates light-dependent magnetosensitivity of drosophila’s circadian clock. PLoS Biol. 2009, 7, e1000086. [Google Scholar] [CrossRef]
- Sallam, A.E.-D.; Hassan, S.A.; Hassaneen, E.; Ali, E.M. Environmental stress of mobile phone EM radiation on locomotor activity and melatonin circadian rhythms of rats. Biol. Rhythm. Res. 2016, 47, 597–607. [Google Scholar] [CrossRef]
- Manzella, N.; Bracci, M.; Ciarapica, V.; Staffolani, S.; Strafella, E.; Rapisarda, V.; Valentino, M.; Amati, M.; Copertaro, A.; Santarelli, L. Circadian gene expression and extremely low-frequency magnetic fields: An in vitro study. Bioelectromagnetics 2015, 36, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Agliassa, C.; Maffei, M.E. Reduction of geomagnetic field (GMF) to near null magnetic field (NNMF) affects some Arabidopsis thaliana clock genes amplitude in a light independent manner. J. Plant Physiol. 2019, 232, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Oliva, R.; Jansen, B.; Benscheidt, F.; Sandbichler, A.M.; Egg, M. Nuclear magnetic resonance affects the circadian clock and hypoxia-inducible factor isoforms in zebrafish. Biol. Rhythm Res. 2019, 50, 739–757. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Maute, A. Sq and EEJ—A Review on the Daily Variation of the Geomagnetic Field Caused by Ionospheric Dynamo Currents. Space Sci. Rev. 2017, 206, 299–405. [Google Scholar] [CrossRef]
- Krylov, V.V.; Kantserova, N.P.; Lysenko, L.A.; Osipova, E.A. A simulated geomagnetic storm unsynchronizes with diurnal geomagnetic variation affecting calpain activity in roach and great pond snail. Int. J. Biometeorol. 2019, 63, 241–246. [Google Scholar] [CrossRef]
- Krylov, V.V. Influence of Geomagnetic Disturbances at Different Times of Day on Locomotor Activity in Zebrafish (Danio rerio). Clocks Sleep 2021, 3, 624–632. [Google Scholar] [CrossRef]
- Hore, P.J.; Mouritsen, H. The Radical-Pair Mechanism of Magnetoreception. Annu. Rev. Biophys. 2016, 45, 299–344. [Google Scholar] [CrossRef]
- Solov’Yov, I.A.; Chandler, D.E.; Schulten, K. Magnetic Field Effects in Arabidopsis thaliana Cryptochrome-1. Biophys. J. 2007, 92, 2711–2726. [Google Scholar] [CrossRef]
- Close, J.P. The Compass within the Clock—Part 2: Does Cryptochrome Radical-Pair Based Signalling contribute to the Temperature-Robustness of Circadian Systems? Hypothesis 2014, 12, e3. [Google Scholar] [CrossRef][Green Version]
- Kidd, P.B.; Young, M.W.; Siggia, E.D. Temperature compensation and temperature sensation in the circadian clock. Proc. Natl. Acad. Sci. USA 2015, 112, E6284–E6292. [Google Scholar] [CrossRef]
- Qin, S.; Yin, H.; Yang, C.; Dou, Y.; Liu, Z.; Zhang, P.; Yu, H.; Huang, Y.; Feng, J.; Hao, J.; et al. A magnetic protein biocompass. Nat. Mater. 2016, 15, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Peng, X.; Chen, J.; Wu, X.; Wang, Y.; Hong, Y. Identification of zebrafish magnetoreceptor and cryptochrome homologs. Sci. China Life Sci. 2016, 59, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Mandilaras, K.; Missirlis, F. Genes for iron metabolism influence circadian rhythms in Drosophila melanogaster. Metallomics 2012, 4, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Lima-Cabello, E.; Diaz-Casado, M.E.; Guerrero, J.A.; Otalora, B.B.; Escames, G.; Lopez, L.C.; Reiter, R.J.; Acuna-Castroviejo, D. A review of the melatonin functions in zebrafish physiology. J. Pineal Res. 2014, 57, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, D.; Foulkes, N.S.; Strähle, U.; Sassone-Corsi, P. Zebrafish Clock rhythmic expression reveals independent peripheral circadian oscillators. Nat. Neurosci. 1998, 1, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Cermakian, N.; Whitmore, D.; Foulkes, N.S.; Sassone-Corsi, P. Asynchronous oscillations of two zebrafish CLOCK partners reveal differential clock control and function. Proc. Natl. Acad. Sci. USA 2000, 97, 4339–4344. [Google Scholar] [CrossRef]
- Khan, Z.A.; Yumnamcha, T.; Rajiv, C.; Devi, H.S.; Mondal, G.; Devi, S.D.; Bharali, R.; Chattoraj, A. Melatonin biosynthesizing enzyme genes and clock genes in ovary and whole brain of zebrafish (Danio rerio): Differential expression and a possible interplay. Gen. Comp. Endocrinol. 2016, 233, 16–31. [Google Scholar] [CrossRef]
- Abdollahpour, H.; Falahatkar, B.; Lawrence, C. The effect of photoperiod on growth and spawning performance of zebrafish, Danio rerio. Aquac. Rep. 2020, 17, 100295. [Google Scholar] [CrossRef]
- Krylov, V.V.; Zotov, O.D.; Klain, B.I.; Ushakova, N.V.; Kantserova, N.P.; Znobisheva, A.V.; Izyumov, Y.G.; Kuz’Mina, V.V.; Morozov, A.A.; Lysenko, L.A.; et al. An experimental study of the biological effects of geomagnetic disturbances: The impact of a typical geomagnetic storm and its constituents on plants and animals. J. Atmos. Sol-Terr. Phys. 2014, 110–111, 28–36. [Google Scholar] [CrossRef]
- Hurd, M.W.; DeBruyne, J.; Straume, M.; Cahill, G.M. Circadian Rhythms of Locomotor Activity in Zebrafish. Physiol. Behav. 1998, 65, 465–472. [Google Scholar] [CrossRef]
- Pérez-Escudero, A.; Vicente-Page, J.; Hinz, R.C.; Arganda, S.; de Polavieja, G.G. idTracker: Tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 2014, 11, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Noldus, L.P.; Spink, A.J.; Tegelenbosch, R.A. EthoVision: A versatile video tracking system for automation of behavioral experiments. Behav. Res. Methods Instrum. Comput. 2001, 33, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Abhilash, L.; Sheeba, V. RhythmicAlly: Your R and Shiny–Based Open-Source Ally for the Analysis of Biological Rhythms. J. Biol. Rhythm. 2019, 34, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Tackenberg, M.C.; Hughey, J.J. The risks of using the chi-square periodogram to estimate the period of biological rhythms. PLOS Comput. Biol. 2021, 17, e1008567. [Google Scholar] [CrossRef]
- del Pozo, A.; Sánchez-Férez, J.A.; Sánchez-Vázquez, F.J. Circadian rhythms of self-feeding and locomotor activity in zebrafish (Danio rerio). Chronobiol. Int. 2011, 28, 39–47. [Google Scholar] [CrossRef]
- Maeda, K.; Robinson, A.J.; Henbest, K.B.; Hogben, H.J.; Biskup, T.; Ahmad, M.; Schleicher, E.; Weber, S.; Timmel, C.R.; Hore, P.J. Magnetically sensitive light-induced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor. Proc. Natl. Acad. Sci. USA 2012, 109, 4774–4779. [Google Scholar] [CrossRef]
- Zoltowski, B.D.; Chelliah, Y.; Wickramaratne, A.; Jarocha, L.; Karki, N.; Xu, W.; Mouritsen, H.; Hore, P.J.; Hibbs, R.E.; Green, C.B.; et al. Chemical and structural analysis of a photoactive vertebrate cryptochrome from pigeon. Proc. Natl. Acad. Sci. USA 2019, 116, 19449–19457. [Google Scholar] [CrossRef]
- Wan, G.; Hayden, A.N.; Iiams, S.E.; Merlin, C. Cryptochrome 1 mediates light-dependent inclination magnetosensing in monarch butterflies. Nat. Commun. 2021, 12, 771. [Google Scholar] [CrossRef]
- Liu, C.; Hu, J.; Qu, C.; Wang, L.; Huang, G.; Niu, P.; Zhong, Z.; Hong, F.; Wang, G.; Postlethwait, J.H.; et al. Molecular evolution and functional divergence of zebrafish (Danio rerio) cryptochrome genes. Sci. Rep. 2015, 5, 8113. [Google Scholar] [CrossRef]
- Steindal, I.A.F.; Whitmore, D. Circadian clocks in fish—What have we learned so far? Biology 2019, 8, 17. [Google Scholar] [CrossRef]
- Hirayama, J.; Alifu, Y.; Hamabe, R.; Yamaguchi, S.; Tomita, J.; Maruyama, Y.; Asaoka, Y.; Nakahama, K.-I.; Tamaru, T.; Takamatsu, K.; et al. The clock components Period2, Cryptochrome1a, and Cryptochrome2a function in establishing light-dependent behavioral rhythms and/or total activity levels in zebrafish. Sci. Rep. 2019, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jarocha, L.E.; Zollitsch, T.; Konowalczyk, M.; Henbest, K.B.; Richert, S.; Golesworthy, M.J.; Schmidt, J.; Déjean, V.; Sowood, D.J.C.; et al. Magnetic sensitivity of cryptochrome 4 from a migratory songbird. Nature 2021, 594, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.N.; Prato, F.S. Biological effects of the hypomagnetic field: An analytical review of experiments and theories. PLoS ONE 2017, 12, e0179340. [Google Scholar] [CrossRef] [PubMed]
- Wiltschko, W. Further analysis of the magnetic compass of migratory birds. In Animal Migration, Navigation and Homing; Springer: Berlin, Germany, 1978. [Google Scholar]

| Experimental Group | Average Swimming Speed (cm/s) | Meandering (°/cm) | Average Angular Velocity (°/s) | Freezing Time (%) | Swimming Time (%) | Rapid Movement Time (%) |
|---|---|---|---|---|---|---|
| #1 16-8 L/D MF period 24 h | 2.33 ± 0.24 0.96 ± 0.10 * | 35.31 ± 1.77 69.77 ± 4.99 * | 78.60 ± 9.77 47.56 ± 4.19 * | 26.41 ± 4.33 71.46 ± 2.45 * | 72.44 ± 4.06 28.29 ± 2.40 * | 1.15 ± 0.43 0.25 ± 0.07 |
| #2 16-8 L/D MF period 36 h | 2.34 ± 0.23 1.05 ± 0.08 * | 39.04 ± 3.74 60.50 ± 5.82 * | 70.80 ± 5.25 47.48 ± 2.49 * | 33.88 ± 5.74 67.69 ± 2.81 * | 64.11 ± 5.66 32.05 ± 2.78 * | 2.01 ± 0.41 0.26 ± 0.04 * |
| #3 24-12 L/D MF period 24 h | 1.93 ± 0.18 1.97 ± 0.14 | 59.27 ± 12.10 45.35 ± 2.79 | 77.22 ± 7.40 86.41 ± 8.24 | 43.44 ± 5.52 40.87 ± 3.25 | 55.16 ± 5.56 58.39 ± 3.19 | 1.38 ± 0.22 0.74 ± 0.19 |
| #4 24-12 L/D MF period 36 h | 2.19 ± 0.19 1.54 ± 0.10 * | 42.92 ± 3.22 52.84 ± 8.09 | 70.15 ± 5.27 63.36 ± 4.53 | 33.04 ± 5.15 52.70 ± 3.01 * | 65.71 ± 4.98 46.55 ± 3.05 * | 1.25 ± 0.23 0.66 ± 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krylov, V.V.; Izvekov, E.I.; Pavlova, V.V.; Pankova, N.A.; Osipova, E.A. Magnetic Fluctuations Entrain the Circadian Rhythm of Locomotor Activity in Zebrafish: Can Cryptochrome Be Involved? Biology 2022, 11, 591. https://doi.org/10.3390/biology11040591
Krylov VV, Izvekov EI, Pavlova VV, Pankova NA, Osipova EA. Magnetic Fluctuations Entrain the Circadian Rhythm of Locomotor Activity in Zebrafish: Can Cryptochrome Be Involved? Biology. 2022; 11(4):591. https://doi.org/10.3390/biology11040591
Chicago/Turabian StyleKrylov, Viacheslav V., Evgeny I. Izvekov, Vera V. Pavlova, Natalia A. Pankova, and Elena A. Osipova. 2022. "Magnetic Fluctuations Entrain the Circadian Rhythm of Locomotor Activity in Zebrafish: Can Cryptochrome Be Involved?" Biology 11, no. 4: 591. https://doi.org/10.3390/biology11040591
APA StyleKrylov, V. V., Izvekov, E. I., Pavlova, V. V., Pankova, N. A., & Osipova, E. A. (2022). Magnetic Fluctuations Entrain the Circadian Rhythm of Locomotor Activity in Zebrafish: Can Cryptochrome Be Involved? Biology, 11(4), 591. https://doi.org/10.3390/biology11040591






