Simple Summary
Leotiomycetes is one of the most speciose classes of the phylum Ascomycota (Fungi). Its species are mainly apothecioid, paraphysate, and possess active ascospore discharge. Thelebolales are a distinctive order of the Leotiomycetes class whose members have mostly closed ascomata, evanescent asci, and thus passively dispersed ascospores. Within the order, a great diversity of peridia have evolved as adaptations to different dispersal strategies. The genus Thelebolus is an exceptional case of ascomatal evolution within the order. Its species are the most diverse in functional traits, encompassing species with closed ascomata and evanescent asci, and species with open ascomata, active ascospore discharge, and paraphyses. Open ascomata were previously suggested as the ancestral state in the genus, these ascomata depend on mammals and birds as dispersal agents. In our work, we used morphological and phylogenetic methods, as well as the reconstruction of ancestral traits for ascomatal type, asci dehiscence, the presence or absence of paraphyses, and ascospore features to explore evolution within Thelebolales. We demonstrate the apothecial ancestry in Thelebolales and propose a new hypothesis about the evolution of the open ascomata in Thelebolus involving a process of re-evolution where the active dispersal of ascospores appears independently twice within the order. A new family, Holwayaceae, is proposed within Thelebolales, comprising three genera: Holwaya, Patinella, and Ramgea.
Abstract
Closed cleistothecia-like ascomata have repeatedly evolved in non-related perithecioid and apothecioid lineages of lichenized and non-lichenized Ascomycota. The evolution of a closed, darkly pigmented ascoma that protects asci and ascospores is conceived as either an adaptation to harsh environmental conditions or a specialized dispersal strategy. Species with closed ascomata have mostly lost sterile hymenial elements (paraphyses) and the capacity to actively discharge ascospores. The class Leotiomycetes, one of the most speciose classes of Ascomycota, is mainly apothecioid, paraphysate, and possesses active ascospore discharge. Lineages with closed ascomata, and their morphological variants, have evolved independently in several families, such as Erysiphaceae, Myxotrichaceae, Rutstroemiaceae, etc. Thelebolales is a distinctive order in the Leotiomycetes class. It has two widespread families (Thelebolaceae, Pseudeurotiaceae) with mostly closed ascomata, evanescent asci, and thus passively dispersed ascospores. Within the order, closed ascomata dominate and a great diversity of peridia have evolved as adaptations to different dispersal strategies. The type genus, Thelebolus, is an exceptional case of ascomatal evolution within the order. Its species are the most diverse in functional traits, encompassing species with closed ascomata and evanescent asci, and species with open ascomata, active ascospore discharge, and paraphyses. Open ascomata were previously suggested as the ancestral state in the genus, these ascomata depend on mammals and birds as dispersal agents. In this scheme, species with closed ascomata, a lack of paraphyses, and passive ascospore discharge exhibit derived traits that evolved in adaptation to cold ecosystems. Here, we used morphological and phylogenetic methods, as well as the reconstruction of ancestral traits for ascomatal type, asci dehiscence, the presence or absence of paraphyses, and ascospore features to explore evolution within Thelebolales. We demonstrate the apothecial ancestry in Thelebolales and propose a new hypothesis about the evolution of the open ascomata in Thelebolus, involving a process of re-evolution where the active dispersal of ascospores appears independently twice within the order. We propose a new family, Holwayaceae, within Thelebolales, that retains the phenotypic features exhibited by species of Thelebolus, i.e., pigmented capitate paraphyses and active asci discharge with an opening limitation ring.
1. Introduction
Fungi are among the most important organisms in the Earth’s ecosystems, but at the same time, they are one of the most poorly understood and studied groups. Little is known about their diversity; only 148,000 of the estimated 12 million species have been discovered [1,2]. Fungal forms can be simple and microscopic, such as the unicellular yeasts, or complex and multicellular, with intricate and extensive networks of hyphae [3,4,5,6,7]. Ascomycota is the largest and most diverse phylum of Fungi and they play key ecological roles as symbionts and saprobes in all terrestrial and aquatic ecosystems. The synapomorphy for the phylum is the specialized cell, a meiosporangium, called the ascus. Asci can be globose to sac-like to cylindrical, within which meiospores (ascospores) are produced. Unicellular species of the phylum have solitary asci (e.g., yeasts). Multicellular species either produce naked asci directly from hyphae, as in some Saccharomycotina and Taphrinomycotina (basal lineages of Ascomycota), or in multicellular structures, ascomata, that vary in shape, the mode of the presentation of the asci, and the development of associated structures (excipula and paraphyses) [6,8,9,10]. We can differentiate three main types of multicellular ascomata: open (apothecia), closed (chasmothecia, cleistothecia, gymnothecia, stereothecia), and partially closed (perithecia). Ascomatal types were used in the past to establish higher-level classification in Ascomycota [11]. Today, we know that closed ascoma have evolved independently in several apothecial lineages. Therefore, this ascomatal type is a homoplastic feature, driven in part by adaptation to environmental factors, and cannot be used at higher levels in phylogenetically-based classifications [9,11,12,13,14].
Morphological and functional variation in asci, ascospores, the presence of paraphyses, and variation in ascomatal construction are frequently correlated with ascoma type and dispersal strategies [13,15,16,17,18]. Asci have a variety of structural features including wall layering, the thickness of the apex, amyloidity, and dehiscence mechanisms (pore, slit, operculum, etc.). Asci with active ascospore discharge are often linked to open ascoma, where asci are arranged in a palisade manner forming a hymenium. At discharge, their rigid walls and turgor allow the asci to act like water cannons. Interascal space is occupied by paraphyses, suggested as important in contributing to the ejection of ascospores by contributing lateral pressure. For effective dispersal through the air, ascospores need to reach the zone of turbulent air and avoid the layer of still air that surrounds the ascoma [19,20,21], thus allowing them to disperse greater distances. Fritz et al. [22] correlated spore shape and size with ascus pore size. For successful discharge, the pressure within the ascus is proportional to the elasticity of the apical pore. This avoids the disorganized rupture of the ascus during discharge and maximizes launch distance without wasting energy [22]. On the other hand, species with evanescent asci do not actively discharge spores and usually have closed ascoma; the ascal walls are fragile and disintegrate, and the entire ascomata may become the dispersal unit, or spores are released passively by microbial breakdown or the rupture of the ascomatal wall [16,23,24,25,26]. Species with evanescent asci depend on the passive transport of the ascoma or spores, such as being blown off by wind, splashed off by rain or water currents, shaken off, or adhering to or being consumed by animals [16,18,19,27].
Ascospores are structures that function in fungal survival and/or dispersal. They vary in size, shape, wall ornamentation, color, contents, cell number, and the presence or absence of sheaths or appendages [19,27]. The morphological variation of ascospores may be informative about the ecology and dispersal abilities of a fungus [28]. Spore size and shape influence how far spores can be carried before deposition and have been correlated with their trophic status, such as saprobes versus mycorrhizae [18,19,29,30]. For example, large spores tend to be deposited closer (by impaction or sedimentation) to the place of origin, are less sensitive to desiccation and UV radiation because of the surface/volume relationship (i.e., narrow-long spores), and tend to be deposited above ground (i.e., allantoid spores) [16,18,19,29,31,32,33]. On the other hand, small air or water-borne spores have been correlated with long-distance dispersal. Depending on atmospheric factors, these spores can travel even between continents [29,30,34]. Wall thickness, hydrophobicity, and ornamentation are features that vary among species and are correlated with resistance to drying, animal dispersal (internal and external), reducing settling velocities, and trophic strategy [18,28,35,36]. Ascospore color also varies among species. Dark-colored spores vary from light brown to nearly black depending on the melanin content of the walls. Species with dark spores are dominant in certain ecosystems such as hot deserts [37,38]. Wall pigmentation has been correlated with a lower incidence of UV damage in experiments [28,37,38,39]. In general, melanized cells (hyphae, spores, and other propagules) are afforded protection against diverse environmental stresses, such as extremely low temperatures [40,41], hypersalinity [42], heavy metal toxicity [43], resistance to microbial lysis [44,45], and host defenses [46,47].
Leotiomycetes is one of the most diverse classes in the subphylum Pezizomycotina. Leotiomycetes species are widespread in terrestrial and aquatic ecosystems; many are plant pathogens or saprobes, but members of the class are also reported as endophytes, in mycorrhizas, and even with lichenized lifestyles [14,25,48,49,50,51,52,53]. Apothecioid ascomata and asci with active discharge by a pore have been suggested as ancestral features for Pezizomycotina as well for Leotiomycetes; therefore, closed ascoma and evanescent asci represent a repeatedly evolved, homoplastic morphology [13,16,26,54,55,56]. Cleistothecial-like ascomata appear in apothecial-dominated families of Leotiomycetes such as in Rutstroemiaceae (Bicornispora) and Helotiaceae (Amylocarpus). There are species with apothecia in cleistothecial-dominant orders, such as Thelebolales, whereas some families are comprised only of cleistothecial species, e.g., Amorphothecaceae, Erysiphaceae, Myxotrichaceae, Pleuroascaceae, and Pseudeurotiaceae [14,25,57].
Thelebolales is an exceptional case considering ascomatal evolution within Leotiomycetes. Currently, the phylogenetic placement of Thelebolales is clearly inside Leotiomycetes [14]. The order Thelebolales constitutes 11 genera and ca. 30 spp. [25,58,59]. Within Thelebolaceae, Thelebolus is the most speciose genus and the most diverse regarding functional traits. It encompasses species with closed ascomata that have asci without active discharge. Additionally, there are other genera with ascomata that expose asci late in their development, these resemble apothecia and have active ascospore discharge and paraphyses. Hoog et al. [60] provided the first molecular insights and hypothesized about the evolution of these Thelebolus species after species were found to be abundant in Antarctic lakes. Hoog et al. [60] postulated that open ascomata and active ascospore discharge in Thelebolus species (e.g., T. stercoreus and T. microsporus) are ancestral states in the genus, and suggested that lineages with closed ascoma and evanescent asci evolved as new lineages in Antarctica. Examples of the latter are T. ellipsoideus and T. globosus. These were suggested to have evolved in response to the harsh climate and loss of bird or mammal dispersal [60]. Their hypothesis correlates open ascoma with active discharge and agrees with the current knowledge about the ancestral traits of Ascomycota, but they did not consider re-evolution as a possible event inside Thelebolales [13,16,26,54,55,56]. The evolutions of traits should be studied on a larger scale to include several genera and involve both related taxa (ingroups) and outgroups. Given the expanded sampling and new sequences available since their publication, we believe Hoog et al.’s [60] hypothesis about the ancestral traits of Thelebolus should be revisited.
Taxon sampling in molecular phylogenetic studies is limited in Leotiomycetes [14,61,62]. This has hindered the comprehensive study of the systematics, ecology, and evolution in the class. Recent increases in the number and diversity of available DNA sequences for taxa of Leotiomycetes have improved the understanding of relationships among lineages in the class and, for the first time, have provided support for deep nodes. In relationship to the present study, Johnston et al. [14] found the genus Holwaya, previously placed in Tympanidaceae, to be sister to Thelebolales. Holwaya has a unique morphology: large, black, stipitate apothecia growing on coarse woody debris, and long, multiseptated ascospores that produce conidia in a more or less spiral pattern along the main spore axis. This combination of characters makes the genus remarkable and distinguishes it from its closest relatives. Holwaya comprises only two species, H. mucida, well-known worldwide from both its teleomorph and anamorph, and H. byssogena, only known from its anamorph. The asexual fungus most similar to these conidial states is Neocrinula (Neocrinulaceae), as noted by Crous et al. [63]. The second and third authors found a close phylogenetic relationship between Holwaya mucida, Patinella hyalophaea, and Ramgea ozimecii [64]. Ramgea is a coprophilous genus previously included in Thelebolaceae because of its morphological resemblance to Thelebolus [60,65].
Our aim is to investigate in depth the relationships of these open (apothecial) and closed (cleistothecial) ascomata lineages of Thelebolales using phenotypic characters, ecology, and molecular data to provide a reconstruction of ancestral traits. In their study, Fungi are among the most demanding large groups of organisms, and as aforementioned one of the most important groups of organisms for the key roles they play in the biosphere. We elaborate a model aimed at improving our understanding of evolutionary mechanisms in fungi. To accomplish this, we have used every available dataset presumably bearing evolutionary informative signals to provide an “integrative systematics” view of this group and to offer a more stable and functional taxonomy. In so doing, the evolutionary events that have led to high variability in morphology and functional adaptive traits in Thelebolales are explored.
2. Materials and Methods
2.1. Specimens Studied and Bibliographic Review
Fresh material collected by the authors and dried specimens were used for morphological and molecular studies. The new collections are deposited in the Farlow Herbarium (FH). Dried fungal specimens were obtained from the following herbaria/fungaria: C, CNF, CUP, FAMU and PRM (s. Index Herbariorum, http://sweetgum.nybg.org/science/ih (accessed on 1 February 2022)). A bibliographic review of genera and species in Thelebolales was conducted to gather information about the morphology, ecology, and distribution of the taxa. Records were found through HOLLIS (Harvard University’s online library catalog), Web of Knowledge, GBIF (Global Biodiversity Information Facility), and Google Scholar. In assembling the ecological data, we consulted a wide variety of sources, such as taxonomic articles, ecological reviews, checklists, papers concerned with molecular diversity and environmental DNA sampling, and the aforementioned trusted websites. Regarding terminology, we use “teleomorph” for ascigerous morph and “anamorph” for conidial morph, even though the terms “sexual” and “asexual morph” are widely used in newer literature, because in a number of anamorphic fungi, genetic recombination may normally occur via heterokaryosis without the formation of an ascigerous phase, while many conidial morphs function as spermatial producers, cf. Kirschner [66].
2.2. Morphological Studies
Morphological studies, together with cytological, cytochemical, and histochemical observations, were done following Baral [67], Quijada [68], and Kušan [69]. Macrophotographs of the ascomata on the substrate were taken of fresh samples in situ or after the rehydration of dried specimens. For microphotographs, we employed a Motic B1 compound light microscope with a USB Moticam 2500 camera, a Zeiss Axioskop 40 (Jena, Germany) with a Nikon D750 camera (Minato City, Tokyo) mounted on the microscope’s trinocular tube, and an Olympus BX 51 equipped with an Olympus DP 72 camera at magnifications up to 1000×. Sections for anatomical examination were made by hand using a double-edge safety razor blade or using a freezing microtome. The methods employed for the microtome are detailed by Karakehian et al. [70]. Fresh, living specimens were observed in tap water, Lugol’s solution ≈ 1% I2, 3% KI, aqueous (IKI), or 1% Congo Red (CR). Prior to microscopic analysis, sections made from dried collections were rehydrated in 3% potassium hydroxide (KOH), then treated with other reagents such as Congo Red (CR), Cotton Blue (CB), or Melzer’s reagent (MLZ). For ascomata, we characterized the macroscopic regions by differentiating the disc, margin, and receptacle. In sections, we studied the excipular structure of both the ectal and medullary excipula. In describing the ectal excipulum, the features of the base, lower and upper flank, and margin were characterized. Details of texture, cell shape, and pigmentation are provided. The hymenial elements (asci, ascospores, and paraphyses) were also described in detail and measured. The following abbreviations are also used in the text: * = living state; † = dead state; ↑ = spontaneously opened asci in taxa with forcible spore discharge; DIC = Differential Interference Contrast; OLR = opening limitation ring; TEM = Transmission Electron Microscopy. Color coding refers to Anonymous [71].
2.3. Phylogenetic Studies
Half of one dried apothecium from each Holwaya specimen was used for the molecular studies. DNA isolation, PCR reactions, and PCR amplification profiles followed Karakehian et al. [70]. Two datasets were used for two different analyses. The first dataset included sequences from the 15-gene dataset of Johnston et al. [14], together with Lichinodiaceae sequences from Prieto et al. [53], Micraspidaceae [72], the rDNA sequences available for Neocrinulaceae [63,73], and newly generated ITS, LSU, TEF, and RPB2 sequences from Holwaya mucida specimens: LQH-102 (ITS: OM736084, LSU: OM736099, TEF: OM797039), C-F-91657 (ITS: OM736084, LSU: OM736100, TEF: OM797038), and CNF 2/8749 (ITS: OM282975, LSU: OM282978, RPB2: OM830434). Neocrinulaceae was added because of the morphological similarity between the anamorph of Holwaya and Neocrinula [63]. This dataset was used to explore the position of Holwaya, Patinella, and Ramgea with Thelebolaceae and Pseudeurotiaceae inside Leotiomycetes. The sequences available for each gene were aligned using the MAFFT v7.017 [74] plugin in Geneious 6.1.8 (https://www.geneious.com). The ends were manually trimmed, and introns were removed manually; all remaining data were then concatenated. Maximum likelihood (ML) analyses were run with IQ-TREE v.1.6.6 [75,76] using models selected by ModelFinder [77]; ultrafast bootstrap (BS) analysis with 1000 replicates estimated branch support in the ML tree [78]. Xylaria hypoxylon (Xylariaceae, Xylariales; AFTOL-ID 51, isolate OSC 100004, JGI genome Xylhyp) and Neurospora crassa (Sordariaceae, Sordariales; isolate OR74A, JGI genome Neucr2) were used as outgroups. Alignment, models for each partitioned gene, and sources of the sequence data for the taxa treated are provided as Supplementary Materials through the Manaaki Whenua–Landcare Research Datastore (https://doi.org/10.7931/X93K-H703). Illustrations were prepared in Adobe Illustrator CC (Adobe Systems, San Jose, CA). The second dataset included all taxa in Table 1 but with only one species representing Holwaya, Patinella, and Ramgea. Neocrinula was not included and some genera of Leotiales and related taxa were used as the outgroup (Aotearoamyces, Claussenomyces, Microglossum, Myriodiscus, Thuemenidium, Tympanis). This second dataset was used to perform the character state reconstruction using the morphological features explained in the following chapter.

Table 1.
A list of species, collection numbers, GenBank accession numbers used in this study, and phenotypic features with character states given for each species representative. N/A: not available. Asc = ascoma type; Dis = ascospore discharge; Par = paraphyses; SpMor = ascospore morphology; SpCol = ascospore color; SpOrn = ascospore ornamentation.
2.4. Morphological Character Evolution within Thelebolales
Six discrete phenotypic features (with character states in parentheses) were used to study the evolution inside the order Thelebolales: ascoma type (0 = open ascoma “apothecia”, 1 = closed ascoma “cleistothecia”, 2 = naked asci), ascospore discharge (0 = active discharge, 1 = passive discharge), paraphyses (0 = presence, 1 = absence), ascospore morphology (0 = cylindrical-fusiform-acicular, 1 = ellipsoid-ovoid-fusoid, 2 = globose-subglobose), ascospore color (0 = hyaline-yellowish, 1 = brown-dark), and ascospore ornamentation (0 = smooth, 1 = ornamented). The reconstruction of ancestral characters was performed using Mesquite v.3.6 [79]. The data matrix consisted of single species for each genus representing families in the order. We used 29 ingroup taxa and seven outgroup taxa that represent the closest related taxa according to our multigene phylogeny (Figure 1). ITS and LSU sequences were aligned with MAFFT v7.017 and trimmed with Gblocks v.091b [80]. The final alignment consisted of 1287 bp and is provided as Supplementary Materials through the Manaaki Whenua–Landcare Research Datastore (https://doi.org/10.7931/X93K-H703). We used the “Trace-characters-over-trees” command to calculate ancestral states at each node to plot the morphological traits onto 8000 input trees obtained from the MCMC analyses using MrBayes [81] through Geneious 6.1.7. (https://www.geneious.com) with the settings: 6,000,000 generations with sampling every 1500 generations, and a burn-in phase discarding the first 1000 sampled trees. The reconstruction of characters was performed with maximum parsimony and maximum likelihood using the Mk1 model.
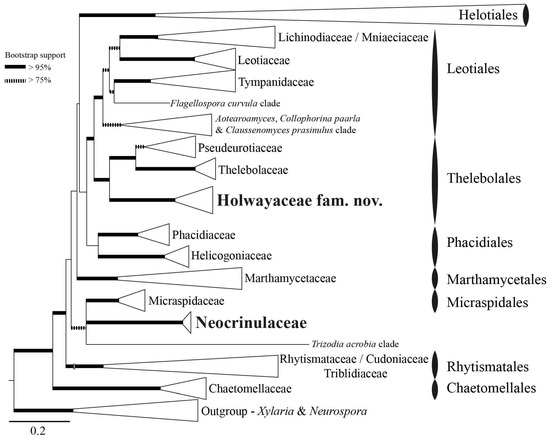
Figure 1.
ML tree from concatenated DNA sequences. In bold we show the position of Holwayaceae fam. nov. and Neocrinulaceae inside Leotiomycetes. The collapsed clades represent the strongly supported family-level and order-level clades accepted by Johnston et al. [14]. Thick branches have bootstrap support (Bps) values > 95% and the dashed branches bootstrap support values 75–95%.
3. Results
3.1. Phylogenetic Results
The overall topology of the ML phylogeny (Figure 1) is consistent with that presented by Johnston et al. [14], Quijada et al. [72], and Batista et al. [82]. The large Helotiales clade is collapsed because Thelebolales is outside of this order, positioned amongst the basal lineages within Leotiomycetes sister to Leotiales (Figure 1). Species of Holwaya, Patinella, and Ramgea form a strongly supported clade, formally named here as Holwayaceae fam. nov. As in the analysis of Johnston et al. [14], this clade has a strongly supported sister relationship to Pseudeurotiaceae and Thelebolaceae and is consequently accepted as a third family within the order Thelebolales. Neocrinulaceae is not related to Holwayaceae and appears isolated without a clear affiliation to any other family in our analysis (Figure 1).
3.2. Reconstruction of Ancestral States
One macro- and five micro-morphological characters were investigated in 16 clades (Figure 2, Figure 3, Figure 4 and Figure 5): A = common ancestor for outgroup and ingroup (Leotiales, Thelebolales); B1 = common ancestor for Thelebolales; B2 = common ancestor for the basal lineage Bettsia, Pseudeurotiaceae s.s. and s.l., and Thelebolaceae; C = common ancestor for Holwayaceae (Holwaya, Patinella, Ramgea); D = common ancestor for Pseudeurotiaceae s.l. (Leuconeurospora, Gymnostellatospora, Pseudogymnoascus); E = Leuconeurospora clade; F = Gymnostellatospora clade; G = common ancestor of Gymnostellatospora and Pseudogymnoascus; H = Pseudogymnoascus clade; I = common ancestor for Pseudeurotiaceae s.l. and s.s. and Thelebolaceae; J = Pseudeurotium clade s.s. (type genus in this clade); K = common ancestor for Pseudeurotiaceae s.s. and Thelebolaceae; L = Antarctomyces clade; M = common ancestor for Thelebolaceae; N = common ancestor for Cleistothelebolus and Thelebolus; and O = Thelebolus clade.
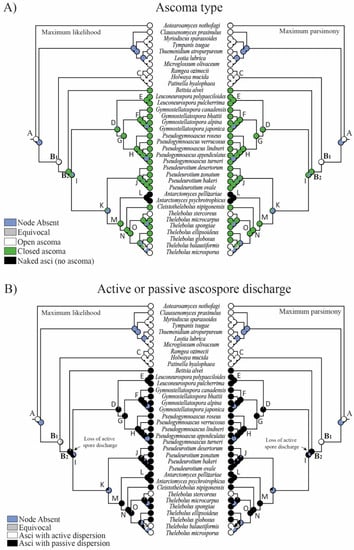
Figure 2.
Evolution of (A) ascoma type and (B) active or passive ascospore discharge using parsimony and likelihood methods across 8000 BMCMC trees obtained from MrBayes. Pie charts at each node illustrate the likelihood (left) and parsimony (right) reconstruction and the proportion of the average received by each character state as the ancestral character of a given clade. Node absent indicates the proportion of nodes with posterior probabilities < 0.95 across trees. Equivocal indicates the same probability for the different features (not resolved).
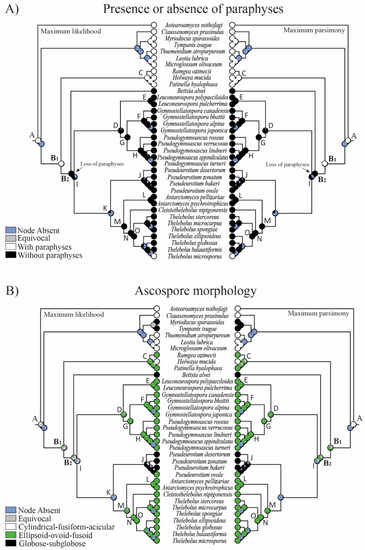
Figure 3.
Evolution of (A) paraphyses and (B) ascospore morphology using parsimony and likelihood methods across 8000 BMCMC trees obtained from MrBayes. Pie charts at each node illustrate the likelihood (left) and parsimony (right) reconstruction and the proportion of the average received by each character state as the ancestral character of a given clade. Node absent indicates the proportion of nodes with posterior probabilities < 0.95 across trees. Equivocal indicates the same probability for the different features (not resolved).
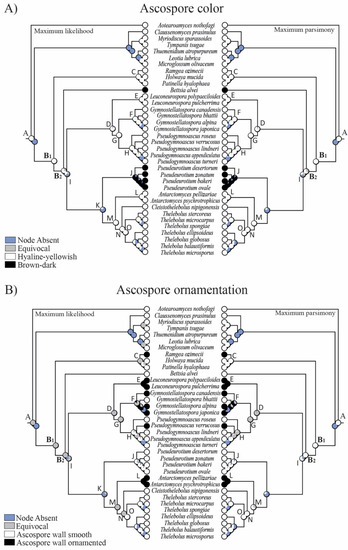
Figure 4.
Evolution of (A) ascospore color and (B) ornamentation using parsimony and likelihood methods across 8000 BMCMC trees obtained from MrBayes. Pie charts at each node illustrate the likelihood (left) and parsimony (right) reconstruction and the proportion of the average received by each character state as the ancestral character of a given clade. Node absent indicates the proportion of nodes with posterior probabilities < 0.95 across trees. Equivocal indicates the same probability for the different features (not resolved).
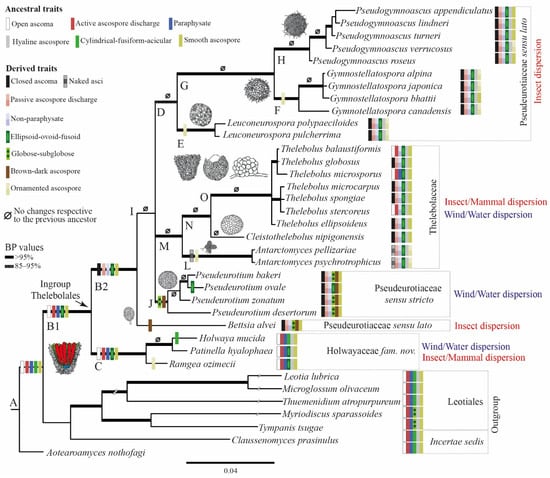
Figure 5.
Hypothetical evolution of traits represented over the consensus Bayesian tree for Thelebolales based on the results of the reconstruction of ancestral states. Thick branches have bootstrap support (Bps) values > 95%. Ascoma morphology, as well as dispersal hypothesis, are represented for each clade.
The reconstruction of ancestral characters showed complex macro- and micro-morphological patterns of evolution with the modification of ascomatal development and the gain or loss of different micro-morphological traits (Figure 2, Figure 3 and Figure 4). There are correlations between ascomatal development and active/passive spore discharge and/or presence/absence of paraphyses (Figure 2, Figure 3 and Figure 4). Open ascomata (apothecia) was inferred to be the ancestral state for Thelebolales (Figure 2 and Figure 5, clade B1), an order mostly comprising species with closed ascomata (cleistothecia). The common ancestor for Pseudeurotiaceae and Thelebolaceae was inferred as having a closed ascoma (Figure 2 and Figure 5, clades B2, I). Bettsia alvei produces the simplest closed ascoma, a unicellular cleistothecium (spore cyst), and is sister to all other lineages of Pseudeurotiaceae and Thelebolaceae (Figure 2 and Figure 5, B2). The major part of the descendant lineages of Pseudeurotiaceae and Thelebolaceae produce closed ascomata but with more complex multicellular covering layers than Bettsia (Figure 2 and Figure 5, clades D, E, G, H, J). The common ancestor for Thelebolaceae was inferred as having a closed ascoma (Figure 2 and Figure 5, clade M). Antarctomyces is reduced to naked asci produced directly on the hyphae (Figure 2 and Figure 5, clade L). The ancestor of Cleistothelebolus and Thelebolus was also inferred to produce closed ascomata (Figure 2 and Figure 5, clades N, O). Species with open ascoma are found throughout Thelebolus, e.g., T. stercoreus, T. balaustiformis, and T. microsporus (Figure 2 and Figure 5, clade O). The parsimony reconstruction indicates that the ancestor of Thelebolales had active ascospore discharge (Figure 2 and Figure 5, clade B1). The maximum likelihood reconstructions indicate the same, but 51% of the reconstructions are ambiguous (equivocal). Asci with active discharge appear only in Holwayaceae and some species of Thelebolus (Figure 2 and Figure 5, clades C, O). The ancestor and all the other clades of Thelebolales were inferred with non-active ascospore discharge (Figure 2 and Figure 5, clades B2, D, E, F, G, H, I, J, K, L, M, N, O). The ancestor of Thelebolales was inferred as paraphysate (Figure 3 and Figure 5, clade B1). The ancestor of Holwayaceae was inferred as paraphysate, but Thelebolaceae and Pseudeurotiaceeae were inferred as non-paraphysate (Figure 3 and Figure 5, clade B2). The presence of paraphyses in Thelebolales is linked to species with active ascospore discharge and open ascomata in both Holwayaceae and Thelebolaceae. In the Thelebolus lineage, only T. microsporus has paraphyses (Figure 3 and Figure 5, clades C, O). Maximum likelihood methods for ascospore morphology reconstruction were mostly equivocal for the ancestor of Thelebolales, but the parsimony method shows that 66% of the reconstructions are ellipsoid-ovoid-fusoid (Figure 3, clade B1). The internal nodes for Pseudeurotiaceae s.l. (Figure 3 and Figure 5, clade D) and Thelebolaceae (Figure 3 and Figure 5, clade M) keep the same morphology but the ancestor of Pseudeurotiaceae s.s. is reconstructed with globose-subglobose spores (Figure 3 and Figure 5, clade J). Hyaline-yellowish spore color was inferred as the ancestor of Thelebolales; only Bettsia and the ancestor of Pseudeurotium have brown-dark ascospores. Dark color and a globose-subglobose ascospore shape are known only in Bettsia and Pseudeurotium (Figure 4 and Figure 5, clades J, E). The evolution of ornamented spores appeared several times in independent lineages (Figure 4 and Figure 5): clade C “Ramgea”, clade E “Leuconeurospora”, clade F “Gymnostellatospora”, Clade H “Pseudogymnoascus verrucosus”, and clade L “Antarctomyces”. The common ancestor of Holwayaceae, Pseudeurotiaceae, and Thelebolaceae were inferred as smooth ascospores in the maximum parsimony reconstruction (Figure 4 and Figure 5, clades B, I, K, M, N), those nodes in the maximum likelihood analysis are mostly equivocal.
3.3. Taxonomy
Holwayaceae Quijada, Matočec, and I. Kušan, fam. nov.
Mycobank number: MB 842621, Figure 6.
Type genus: Holwaya Sacc., Syll. fung. (Abellini) 8: 646 (1889)
Etymology: named after the type genus Holwaya.
Other genera included: Patinella Sacc., Ramgea Brumm.
Position in classification: Holwayaceae, Thelebolales, Leotiomycetes, Pezizomycotina, Ascomycota.
Diagnosis: Phylogenetically isolated within Thelebolales. Ascomata open; outer layer of the excipulum with globose to angular cells; capitate paraphyses with intra- or extracellular pigments and asci with forcible spore discharge augmented by an opening limitation ring-type apical apparatus; ascospores hyaline, smooth, or ornamented; either aseptate and ellipsoid-ovoid-fusoid, not producing conidia, or with more than ten septa, acicular-cylindrical-fusiform-acicular, and producing conidia. Ramgea differs from apothecioid members of Thelebolaceae by ornamented ascospores and paraphyses with capitate tips bearing granular, firmly cemented pigment exudates. Patinella and Holwaya differ from Tympanis and Durandiella (Tympanidaceae) and Aotearoamyces and Bulgaria (Phacidiaceae) in excipular composition of mostly globose-angular cells in combination with non-amyloid asci and ascospores.
Description: Ascoma apothecioid, paragymnohymenial or eugymnohymenial, superficial or erumpent, scattered to gregarious, solitary or sharing a common stroma-like base; pulvinate-turbinate to discoid or cup-shaped, pale yellowish to black, hymenium margin and receptacle concolorous, margin indistinct, thin and smooth, disc ± circular when fresh, sometimes slightly irregular by mutual pressure; sessile to short- to rather long-stipitate, stipe tapering downward; surfaces smooth or slightly rugulose due to the protrusion of paraphyses tips in the hymenium or protruding cells in the ectal excipulum. Asci cylindric-clavate, 4–8-spored, (1–)2–4-seriate; apex dome-shaped with a subapical OLR (best visible by the DIC of *ripe unopened and in ↑asci) with its thinner, lateral wall staining in CR but subapical ring and walls at apex unstained, slightly subconical and with moderately to strongly swollen wall in dead state, apex wall also partly laterally thickened; wall IKI and MLZ negative; base narrowed to a short to medium-long stalk and arising from croziers. Ascospores ellipsoid-ovoid-fusoid to cylindrical-fusiform-acicular, hyaline, straight or slightly to moderately curved, smooth to ornamented with coarse ridges or crests that can form a reticulum (KOH stable), eguttulate or multiguttulate (tiny oil drops); aseptate to (10–)16–20 transversal septa when septate and overmature with a small lateral wart-like to rod-shaped protuberance at each cell, on which one or two small conidia are formed. Paraphyses filiform, apically moderately to strongly clavate-capitate, exceeding living mature asci, sparsely branched at lower cells, ±equidistantly and sparingly septate; apical cells hyaline or partly olive-brown, pigmented with or without refractive globules, wall surface smooth to finely warted; cells apically free or agglutinated and embedded in a brownish gelatinous matrix and exudate. Subhymenium composed of compact ascogenous cells. Medullary excipulum very reduced and sometimes undifferentiated from ectal excipulum; if differentiated, then composed of dense textura intricata, slightly gelatinized, of thin-walled cells with a ±vertical orientation to the subhymenium. Ectal excipulum of ±textura globosa–angularis to t. prismatica, cells hyaline to olive-brown; cortical layer with groups of angular to clavate cells slightly protruding, thick-walled, covered by a dark, brownish exudate. Anchoring hyphae at base present or not. Anamorph: Only known for Holwaya. For morphological details see Seifert [83].

Figure 6.
Phenotypic features of Holwayaceae: Holwaya (A1–6,B1–3,C1–6,D1–3,E1–2), Patinella (F1–6,G,H,I,J1–3) [84], and Ramgea (K,L1–2,M1–4,N1–2,O1–3) [64]. Comparative morphological features among genera in the family: A, F, and K. Macromorphology of the apothecia: B, J, and N. Excipulum in transverse section: C, G, and M. Asci and details of ascus apex: D, H, and L. Paraphyses: E, I, and O. Ascospores. A1, A3 from CNF 2/8749; A2, A4–6 from L.Q.H.-102; B1–3, C3–4, D2–E2 from CUP-60122; C1–2, C6, D1 from CUP-A-019509; C5 from CUP-D-02006. A1, A3 phot. N. Matočec, A2, A4–6 phot. J. Warfel, B1–E2 phot. Luis Quijada. Scale bars: A1–6 = 5 mm; F1–6, K = 0.5 mm; B1, N1 = 100 µm; B3, C1–2, D1, E2, J1 = 50 µm; J2 = 20 µm; B2, C3–6, D2–3, E1, G, H, J3, L1–2, M1–4, N2, O1–3 = 10 µm; I = 5 µm.
Specimens examined: Holwaya mucida—CANADA: State and province not recorded, on the bark of Tilia sp., 3 October 1896, J. Macoun (CUP-D-02006).—CROATIA: Primorje - Gorski kotar County, Nadvučnik village, near town Vrbovsko, the vegetation of young Carpinus betulus with Pinus nigra and Picea abies, on cut, semi-decorticated lying trunks of Tilia sp. covered with moss, 6 November 2010, R. Kranjčev, N. Matočec and I. Kušan (CNF 2/8749).—CZECH REPUBLIC: South Bohemian Region, NE of Hluboká nad Vltavou, Libochovka Nature Reserve, a forest of Fagus sylvatica with admixed Picea abies and Tilia sp., on a fallen trunk of Tilia ap. covered with mosses, 16 October 2016, J. Holec (PRM 944637).—DENMARK: Sjælland Region, Ringsted Municipality, Allindemagle Skov, forest with Tilia sp., on Tilia sp., 20 September 2011, M. Vestergaard (det. Thomas Læssøe) (C-F-91657)—SLOVENIA: Gorizia Region, Idrija, 370 m S-SE from the Kres peak (521 m), urban alley of Tilia sp. trees, on a rotten lying trunk of Tilia sp., 23 November 2001, A. Piltaver and N. Matočec (CNF 2/5423).—SWEDEN: Västmanland County, Västerås-Barkarö parish, Flaten island near Ridön island, on a fallen trunk of Tilia cordata, 20 October 1975, S. Ryman (PRM 869874).—USA: New Hampshire State, Carroll County, Chocorua, on Acer sp., September 1910, W.G. Farlow (PRM 685875, as H. gigantea), idem (PRM 685876, as H. gigantea); New York State, Slaterville Springs, Lloyd-Cornell Preserve, on a rotten log of Tilia sp., 25 September 1954, R.P.Korf (PRM 919564); idem, Coy Glen, Ithaca, on unidentified wood, 2 October 1982, T. Capiello and N. Shishkoff (CUP-60122); idem, Tompkins, Enfield, on unidentified wood, 14 October 1905, G. Atkinson (CUP-A-019509); Massachusetts, Concord, Estabrook Woods, on a fallen trunk of Populus sp., 3 Feburary 2019, Luis Quijada (L.Q.H.-102 in FH). Ramgea ozimecii—CROATIA: Lika-Senj County, near Perušić, about 2.6 km E-SE of Gornji Kosinj, pit Čardačina jama, in the dark zone of the karstic pit, on bat dropping, 30 September 2016, R. Ozimec (CNF 2/9997, holotype). Patinella hyalophaea—BOSNIA AND HERZEGOVINA: Sutjeska National Park, riverine canyon bottom, wet dicot woody remnant, 28 June 2015, N. Omerović (FAMU-1390, N.O.280615-03); Sarajevo County, Bijambare Protected Landscape, Brodić stream, periodical karstic watercourse in alti-montane conifer forest, Picea abies wet remnant, 4 August 2014, N. Omerović (no voucher).
4. Discussion
4.1. Diversity and Systematics of Holwayaceae
In recent classifications, Thelebolales has been placed in Leotiomycetes with two families, Pseudeurotiaceae and Thelebolaceae, with 8 and 12 genera, respectively, and three genera incertae sedis [25,59,85,86]. Here, we expand the concept of the order by adding a third family, Holwayaceae, constituting three genera (Holwaya, Patinella, Ramgea) previously placed in Tympanidaceae, Thelebolaceae, or incertae sedis in Helotiales or Leotiomycetes (op cit.). Holwayaceae is the basal lineage of the order (Figure 1 and Figure 5). Holwaya and Ramgea have only two species each, but Patinella has ca. 25 spp. [25,85]. There are no available sequences for H. byssogena (Berk. & Broome) Seifert or R. annulispora Brumm. Our taxonomic studies and bibliographic revision indicate that these two species are properly placed in these genera on morphological grounds. Even though the type species of Patinella is related to H. mucida and R. ozimecii, our unpublished studies of other species of Patinella indicate that the genus is highly polyphyletic, including species that should be placed in various families of Leotiomycetes or Lecanoromycetes (Quijada et al., unpubl. data). For this reason, our concept of Holwayaceae is circumscribed to currently include only one species of Patinella, the type species P. hyalophaea (Figure 6), but all accepted species of Holwaya and Ramgea.
The anamorphs of Holwaya and Neocrinula have similar morphology but the phylogenetic results in Crous et al. [63] did not suggest a close relationship, which was confirmed in other published phylogenies [14,86] as well as in our results (Figure 1). As Johnston et al. [14] stated, it is possible to consider a broader concept of Leotiales to include both apothecial lineages (Holwayaceae, Lichinodiaceae, Mniaeciaceae, Tympanidaceae) and those mostly reduced to cleistothecial ascomata (Pseudeurotiaceae, Thelebolaceae) (Figure 1). We prefer to keep two separate orders, given the similarities found among Holwayaceae, Thelebolaceae, and Pseudeurotiaceae (Figure 6), and to avoid changes in the unstable classification of the class given the lack of taxon sampling [14,85]. Finally, our results for Pseudeurotiaceae suggest polyphyly of the family, although we are only using one specimen per taxa and only teleomorphs (Figure 2 and Figure 5). Other studies that used several specimens with anamorphs and teleomorphs found monophyly of the family, but it did not include Bettsia [86]. For that reason, we here prefer to keep Pseudeurotiaceae and use sensu lato (s.l.) and sensu stricto (s.s.) for the clades.
4.2. Ecology and Distribution in Holwayaceae
Holwayaceae is so far comprised of saprobes on wood or dung [64,87,88,89,90]. Holwaya mucida is well known in the northern hemisphere (Palearctic, Nearctic), with most reports of it on fallen trunks of Tilia, and on other hardwood genera such as Acer, Castanea, Fagus, Fraxinus, Magnolia, Quercus, and Ulmus. The species is found in boreal forests or taiga and temperate broadleaf and mixed forests [87,88,91]. This species prefers old-growth forests, high atmospheric humidity, and anamorphs are more frequently found than teleomorphs [89]. The other species known only from the anamorph, H. byssogena, is found in tropical and subtropical dry or moist broadleaf forests (Neotropic realm) on unidentified wood, Bactris, and Psidium [83,91]. Both species of Holwaya are considered saprobes on wood [87,88,89], but little is known in detail about the ecology.
There are very few reports of Patinella hyalophaea. The discovery of P. hyalophaea in New Brunswick, Canada possibly represented its second collection since the original description of it from Italy in 1875 [84]. These authors observed similar climate and vegetation conditions of their collection site (coastal eastern Canada) and the type locality in montane northeastern Italy, including Fagus as the dominant forest tree species. Baral and Carter [84] also suggested an association with semi-aquatic habitats subjected to occasional flooding, which is in line given the recent report of P. hyalophaea from a torrent watercourse in a bog complex of an altimontane karst polje in the Dinaric Alps [92]. Patinella hyalophaea was isolated from historic wood in Deception Island, Antarctica [93], and most recently from buried wooden artifacts at five sites in Western Greenland and lacustrine sediment cores from a lake in King George Island, Antarctica [94,95]. Ramgea annulispora was described from pheasant (Phasianus colchicus) dung in the Netherlands [90], and R. ozimecii was described from bat droppings in Croatia [64]. A significant point in the evolution of Holwayaceae occurred between the basal Ramgea lineage and Patinella-Holwaya clade (Figure 5), when nutrition strategy might have switched from fimicolous saprotrophy to lignicolous saprotrophy. The evolution and nutritional switch in Holwayaceae are accompanied by several phenotypic changes (Figure 6) discussed below.
4.3. Evolution in Holwayaceae
Our analyses indicate that the ancestral state of the group is apothecial-paraphysate with forcible spore discharge, as seen in the monophyletic family Holwayaceae (Figure 2, Figure 3, Figure 4 and Figure 5 clade C). The closed, non-paraphysate ascoma of Thelebolaceae and Pseudeurotiaceae, with the passive release of spores, is a derived condition (Figure 2, Figure 3, Figure 4 and Figure 5, clade B2). Holwaya, Patinella, and Ramgea share several traits: (1) open ascoma, (2) outer layer of the excipulum with globose/angular cells, (3) capitate paraphyses with intra- or extracellular pigments, and (4) asci with forcible spore discharge augmented by an OLR-type apical apparatus (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7). Here, we show that nutritional switches and habitat preferences in Holwayaceae are accompanied by several phenotypic modifications.
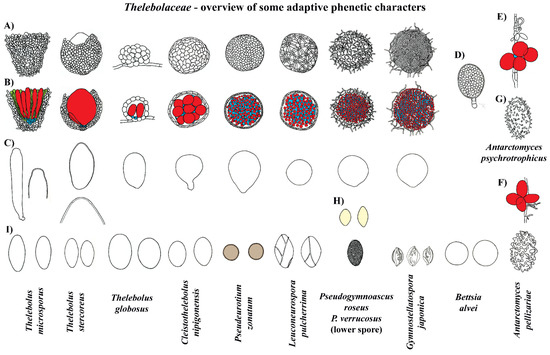
Figure 7.
Thelebolaceae-an overview of some adaptive phenotypic characters. (A) Ascomata-side view. (B) Ascomata in section. (C) Asci (in Thelebolus microsporus and T. stercoreus, also enlarged view of ascus tips). (D) Cyst-like ascoma with ascospores inside. (E,F) Naked asci. (G,H,I) Ascospores. Color code: Red = asci; blue = ascogenous cells; green = hamathecium. Compiled drawings are not presented to scale in order to fit the single plate. Del. N. Matočec.
Fruitbody size is variable in the Holwayaceae. Changes in fruiting body size have been correlated with lifestyles in fungi [96]. Large fruiting bodies typically live longer, are more resistant to desiccation due to the lower surface-to-volume ratio, and increase the number of asci and ascospores per fruiting body, thus enabling a much higher production of propagules [97,98]. In Holwayaceae, larger ascomata occur in the woody saprobes (Holwaya, Patinella) compared to the dung saprobes (Ramgea spp.). Dung saprobes, such as Ramgea, must develop more rapidly to maturity due to the transient nature of their substrate and the spatial competition for limited resources [99]. The larger apothecia—up to 15 mm in diameter [87]—of Holwaya mucida are a striking deviation from the ascomata of Ramgea (0.04–0.36 mm diameter) [64,90]. Patinella hyalophaea produces apothecia that are intermediate in size (up to 1.2 mm in diameter) [84]. The production of relatively large, energy-demanding ascomata in Holwaya and Patinella is probably permitted by their ability to colonize wood, a substrate that is nutritionally, temporally, and spatially less restrictive compared to the ephemeral animal dung where the constrained colonies produced by Ramgea can develop only small fruiting bodies [99,100]. Remarkable differences in the ascomatal morphology of closely related taxa with contrasting trophic strategies can be seen in other Leotiomycetes taxa [61,101]. For example, Bulgaria inquinans (Phacidiaceae, Phacidiales) is a lignicolous saprotroph that produces exceptionally large and morphologically divergent ascomata compared to the more reduced ascomata of the endophytic and parasitic species typical of this family [102]. Another example is found in Leotiales, the sister order to Thelebolales (Figure 1), where small fruiting bodies are dominant in pathogens such as Tympanidaceae and Mniaeciaceae, but large fruiting bodies evolved in ectomycorrhizal lineages such as Leotia [14].
The ancestor of Holwayaceae was reconstructed with active discharge asci (OLR) and hyaline ellipsoid-ovoid-fusoid ascospores (Figure 2, Figure 3, Figure 4 and Figure 5). Unlike the majority of Leotiomycetes, species of Holwayaceae do not possess a complex and stable structured plug-like apical pore, like in Helotiales [25,101], but rather a subapical wall reinforcement, like a ring (OLR) [65]. Its function is to limit tearing below the ring level, allowing opening above an irregular tear (Figure 8). Patinella and Ramgea retained the ancestral traits of the family. Their ascospores have the same shape (Figure 2 and Figure 5) and their apical opening is similar to, although wider than, Holwaya (Figure 8).
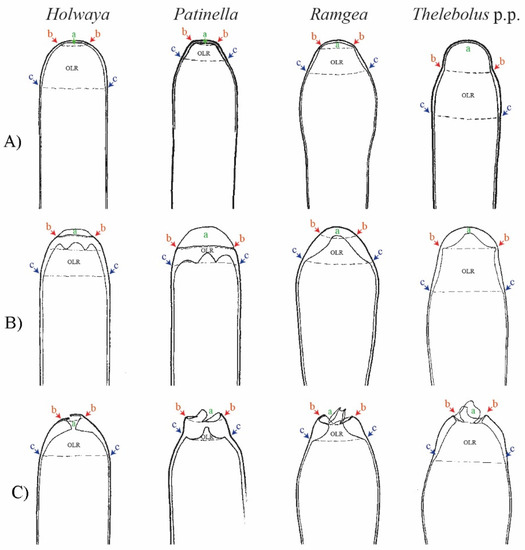
Figure 8.
Asci apices with OLR-type dehiscence in Thelebolales species with forcible discharge asci: (A) mature ascus (ascal wall compressed by ascoplasm turgor); (B) mature ascus (ascal wall relaxed due to loss of turgor); (C) ↑ mature ascus displaying irregular (asymmetric) tear above OLR after ascospore discharge; a—opening apical zone above OLR; b—upper OLR line; c—lower OLR line. Compiled drawings are not presented to scale in order to fit the single plate. Del. N. Matočec.
Ramgea is the only genus in the family with ornamented ascospores (Figure 6), a feature that is present in many animal-associated fungi [15,18,103], and discussed later here under the evolution of Thelebolales. Holwaya is the most divergent lineage inside the family and has developed several apomorphies in adaptation to its ecology. The OLR is positioned closer to the apex, producing a narrower opening. This correlates with the ascospores shape (narrow-long) (Figure 6) that has evolved to be less sensitive to desiccation (high surface/volume relationship), travel further in calm air, deposit above the ground by impaction [28], and resist wash-out by precipitation [18]. Ramgea and Patinella have ellipsoid, smaller ascospores that presumably can travel further than Holwaya [19,29,30]. However, Holwaya has a unique apomorphy: each cell of the ascospore produces up to several secondary spores (Figure 6), very small conidia (Figure 6, E1, E2. 3 × 0.5 µm). These might be specialized to penetrate deeper into the host substrate (e.g., bark furrows), to enable a further increase in propagule productivity per fruiting body and secure better long-distance dispersal [28,29,30,34], or to serve as spermatia. The conidia, if produced on ascospores that have landed on an unsuitable substrate, may facilitate subsequent dispersal to possibly more appropriate hosts.
Changes in pigmentation are also observed in Holwayaceae (Figure 6). Dark pigments might have evolved to protect against UV radiation, temperature fluctuation, desiccation, digestion by hydrolytic enzymes, or to absorb heat and warm ascomata quickly during a brief winter thaw [26,104,105]. The ascomata of the basal lineage Ramgea are hyaline or light-colored (Figure 6) [64,90], as are the fimicolous species in Thelebolus [60]. Holwaya and Patinella are completely black (Figure 6).
The most diagnostic synapomorphy of the Holwayaceae is found in the paraphyses, where protective KOH-inert pigmentation is present (Figure 6). More subtly defined apomorphies in the pigment location are confined to each genus either inside or outside the cell walls. In Ramgea, a light-yellowish paraphysal pigment is distributed within the wall and inside the cells, but a dark pigment is found only on the wall in Patinella and only outside the cell walls in Holwaya (Figure 6). In addition, paraphyses in Holwayaceae are abruptly enlarged at their tips. Brummelen [90] and Baral et al. [26] proposed that this shape could help to preserve the hydrated intercellular system of the hymenium and protect immature asci during their development. The latter authors also suggested that rough exudate surfaces over the paraphyses might assist condensation from atmospheric humidity (op. cit.). The large ascomata of Holwaya can survive under snow and reproduce during the autumn-winter thaw in the northern hemisphere [87] (Quijada pers. obs.). Its black tissues and paraphysal apomorphies effectively resist the desiccation of the hymenial layer, making the organism more capable of colonizing and reproducing in drier habitats [89] such as the surface of fallen trunks exposed to sunlight. Patinella hyalophaea dwells in more humid or even flooded habitats and does not exhibit, or consequently require, such an efficient paraphysal protective layer. On the other hand, the ascomata of Ramgea, with lighter pigmentation, are ephemeral and therefore can survive light exposure if at all only for a short time. Their ascomata develop in shaded or permanently dark ecosystems such as karstic pits and caves (R. ozimecii) [64], where UV radiation could be less problematic during the development of the ascospores.
4.4. Evolution in Thelebolales Lineages
Based on morphology and ecology, it is initially surprising that Holwaya and Patinella (Holwayaceae) are related to Thelebolaceae-Pseudeurotiaceae lineages; however, the phylogenetic evidence in our multigene analyses is clear (Figure 1) and confirms previous results [14]. There are several interesting examples of homoplasy among Holwayaceae and genera in Pseudeurotiaceae and Thelebolaceae. Holwaya and Patinella species have asci with OLR and paraphyses with enlarged apices, something that evolved independently in some Thelebolus species with active ascospore discharge (Figure 2, Figure 3, Figure 5 and Figure 8). However, the most striking similarity is between T. microsporus (Thelebolaceae) and the oldest lineage in Holwayaceae, the genus Ramgea. Both share the following traits [60,64,90,106]: (1) paragymnohymenial ontogeny and closed ascoma in early development that opens late upon ascospore maturation (Figure 2, Figure 6 and Figure 7); (2) thin, lateral, simply structured excipular flanks composed of ± isodiametric, slightly yellowish-grey cell walls (Figure 5, Figure 6 and Figure 7); (3) abruptly enlarged apices of the paraphyses with similar pigmentation inside the cells (Figure 5 and Figure 6); (4) the presence of a wall reinforcement in the asci that limits the area of the apical opening (OLR) (Figure 6 and Figure 8); (5) thick-walled, hyaline, aseptate ascospores (Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7); (6) the presence of gluing substances or TEM microtubules among the ascospores to facilitate the active release of all spores as a single unit [107]; and (7) ecology (dung saprobes). Due to these similarities, in the past, these genera were placed together within the same family (Thelebolaceae) [65]. However, this convergent evolution is likely a response to similar lifestyles [108]: habitat (cold environments), substrate (dung), ecology (saprotrophs), and a similar mechanism for dispersal (asci with OLR, Figure 8). The ascospores of these dung inhabitors need to actively escape their habitat, “sinking islands”, an ephemeral source of nutrients, and reach a distance to avoid the zone of repugnance and be ingested/transported by animals [16,60,99,109]. Due to OLR asci and the presence of adhering ascospores [16,64,106,107], ascospores are ejected as a single projectile, which helps increase shooting distance, a common phenomenon in coprophilous fungi [16,19,21,26,32]. In this case, the thick wall of the ascospores protects against hazards of gut passage (pH, digestive enzymes, etc.) [60,110,111].
The ancestor of Thelebolaceae/Pseudeurotiaceae was reconstructed to have had a closed ascoma, without paraphyses, and with evanescent asci that contain ellipsoid-fusoid ascospores (Figure 2, Figure 3, Figure 5 and Figure 7). The Thelebolaceae clade has achieved adaptive radiation in all traits analyzed compared with all other lineages, where a smaller number of changes through their evolution were found. Only the shape and color of ascospores were constant in Thelebolaceae. Species in the family evolved different types of ascomata (open, closed, or naked), asci (active or passive discharge), the presence or absence of paraphyses, and changes in ascospore number and ornamentation (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 7). Cleistothelebolus retains all the ancestral traits of Thelebolales, as does Pseudeurotium (Figure 2, Figure 3, Figure 4 and Figure 5, clade N and J). Species of both genera have closed ascomata that are considered cleistothecia (Figure 7), with a membranaceous peridium composed of polygonal cells, and the ascogenous system spread irregularly throughout the ascomatal interior [112]. In other closed ascomatal lineages, such as Bettsia, Gymnostellatospora, Leuconeurospora, and Pseudogymnoascus (Pseudeurotiaceae s.l.), the peridium evolved differently, although they also have a similar ascogenous arrangement [17]. Conversely, species with closed ascoma in Thelebolus have an ascogenous system regularly arranged in a hymenial layer [60]. The loss of active ascospore discharge in Cleistothelebolus and Pseudeurotium likely afforded more effective protection during ascosporogenesis, opening only after the ascospores are completely mature and capable of withstanding environmental stresses [112,113]. Cleistothelebolus is sister to Thelebolus, and both genera have similar ecologies (mostly isolated from dung), and its morphology is similar to those species of Thelebolus with closed ascoma (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7). However, Cleistothelebolus has a narrow optimum temperature for fruiting of around 25 °C and evolved in adaptation to warmer environments compared to the more psychrotolerant/psychrophilic Thelebolus [60,112]. Although they are not closely related, Cleistothelebolus and Pseudeurotium share all traits except ascospore color (hyaline vs. dark) and the composition of the cover layer of the ascoma (i.e., arrangement of cells), which is one layer in Cleistothelebolus and several in Pseudeurotium (Figure 4, Figure 5 and Figure 7). They also dwell in different habitats. Cleistothelebolus has been only recorded a few times from carnivore dung (op. cit.), but Pseudeurotium is a cosmopolitan genus that has been reported as an endophyte in algae, isolated from grass, wood, freshwater, marine sponges, soil (including permafrost), diesel fuel, dung, and sea-bottom sediments [113,114,115,116,117,118]. Despite these many reports, the ecology of Pseudeurotium, as well as its dispersal mechanism, remains unclear. Pseudeurotium species have evolved in adaptation to harsh habitats (heat, cold, osmotic pressures, etc.), where whole ascomata and ascospores are likely dispersed by wind/water. Hoog et al. [60] suggested water dispersal for species of Thelebolus with similar traits. Species of Pseudeurotium have two interesting apomorphies: small globose ascospores that are dark-walled (Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7). These spore traits are found in species that evolved long-distance dispersal [29,30,37,38,40,41,44,45,47].
Thelebolus species are psychrophilic and have been isolated from dung, mud, soil, fresh or saltwater, the digestive tracts of birds, marine sponges, and living plant tissue such as endophytes. Ascospore production is enhanced by temperatures below 15 °C [60,106]. Our analyses inferred the ancestor of Thelebolus as having a closed ascoma, without paraphyses, and with passive ascospore dispersal (Figure 2, Figure 3, Figure 4 and Figure 5, clade O). Most species of Thelebolus have these traits [60,106,119], although there are some species with open ascoma that actively discharge ascospores. Hoog et al. [60] postulated that species with open ascomata and active ascospore discharge have an ancestral position in the genus due to their broad distribution and correlation with animal dispersal (birds, mammals). They considered that species with closed ascomata (T. ellipsoideus, T. globosus) evolved in response to cold environments and the loss of bird vectors. Our results and evolutionary hypothesis regarding Thelebolus disagree with Hoog et al. [60]. We found that the closed ascoma is an ancestral trait in Thelebolaceae (Figure 2 and Figure 5 clade M) and in Thelebolus (Figure 2 and Figure 5, clade O). Therefore, the reappearance of apothecial ascomatal configuration with forcible spore discharge in some Thelebolus species (Figure 2, Figure 5 and Figure 7) is an apomorphy. When complex phenotypic traits are lost in evolution, evolutionary reversals are unlikely (i.e., Dollo’s law of irreversibility); for example, the loss of the flagellum was one of the major changes in fungal evolution, a trait that has not been gained again after its loss [56]. However, examples of evolutionary reversals are known, e.g., in Animalia: [120,121,122,123]. The loss of active ascospore discharge is correlated with closed ascoma development and has evolved multiple times in several lineages of Leotiomycetes (Amorphothecaceae, Helotiaceae, Myxotrichaceae, Pseudeurotiaceae, Rutstroemiaceae, Thelebolaceae), as well as in other lineages of lichenized and non-lichenized Ascomycota [11,14,25,54]. Regaining a trait represents a rare reversal phenomenon that could be designated as a “re-evolution” [121,122,123,124]. We have been unable to find any example in Leotiomycetes that represents a re-evolution. Therefore, it is likely that some Thelebolus species could provide the first known example of re-evolution in this class (Figure 2, Figure 3, Figure 4 and Figure 5, Figure 7 and Figure 8).
Our analyses showed another homoplastic trait, ornamented ascospores, which evolved multiple times in different lineages: Holwayaceae (Ramgea), Pseudeurotiaceae (Gymnostellatospora, Leuconeurospora, Pseudogymnoascus), and Thelebolaceae (Antarctomyces) (Figure 4, Figure 5, Figure 6 and Figure 7). Ornamented ascospores are not common in Leotiomycetes [25]. Ornaments of spores are generally hydrophobic and tend to facilitate attachment to substrates [125,126]. Ornamented ascospores are present in many diverse, animal-associated fungi with enclosed or partially enclosed ascomata, i.e.,: Onygenales (Arthrodermataceae, Gymnoascaceae, Myxotrichaceae, Onygenaceae), Pezizales (Tuberaceae), Cephalothecales (Cephalothecaceae), and Eurotiales (Elaphomycetaceae) [15,18,103]. We can observe a similar evolution pattern in some lineages of Pseudeurotiaceae (Figure 4 and Figure 5 clade D). Vanderwolf et al. [127] found that arthropods contribute to the dispersal of Pseudogymnoascus and Leuconeurospora; species of both genera have been also isolated from the fur and skin of hibernating bats [128]. Leuconeurospora has closed ascomata with a peridial cover modified into groups of radiating cells with well-defined lines of dehiscence (Figure 7). This special peridial covering is called cephalothecoid and has been correlated with arthropod dispersal [103]. Pseudogymnoascus and Gymnostellatospora have cage- or mesh-like closed ascomata (Figure 7) that are very similar to the reticuloperidial gymnothecia in Myxotrichaceae. These highly reduced ascomata have also evolved to be dispersed by insects [15]. An example of extreme simplification in Thelebolales adapted to zoochory is the beehive-dwelling Bettsia. This genus, and others that share the same phenotypic traits have a predilection for high-sugar substrates (osmophilic) where water activity is low and solute concentration is high [17,129]. Wynns [17] showed the convergent evolution between Ascosphaera (Eurotiomycetes) vs. Bettsia (Thelebolales, Leotiomycetes) and Eremascus (Eurotiomycetes) vs. Skoua (Myxotrichiaceae, Leotiomycetes). These taxa evolved independently to produce highly simplified closed ascomata. In Ascosphaera and Bettsia, the closed ascoma is a cyst-like cell envelope with evanescent asci instead of a more complex multicellular peridium (Figure 7). A further reduction is found in a species of Eremascus and Skoua, in which ascomata are absent and asci are produced directly on hyphae, such as in Antarctomyces (Figure 2, Figure 5 and Figure 7). Wynns [17] suggested that cyst-like and naked asci are reduced forms of cage- or mesh-like closed ascomata (i.e., reticuloperidial ascomata). Our results showed Bettsia as a basal lineage of Pseudeurotiaceae/Thelebolaceae and Antarctomyces as a basal lineage of Thelebolaceae. Therefore, we have not found the evolution pattern for Bettsia and Antarctomyces (Figure 2 and Figure 5 clade B2, L) suggested by Wynns [17] for Ascosphaera and Eremascus. We believe that more sequence data and taxon sampling from those genera currently without sequences in Thelebolales are needed to determine the real affinities of these two genera inside the order.
4.5. Known Anamorphs and Conidia Dispersal
Anamorphs in Holwayaceae are known only for Holwaya. The anamorph of H. mucida is conspicuous and readily identifiable in the field, where it occurs scattered gregariously and sometimes covering large areas (up to several meters long) of host logs [89] (Matočec and Kušan pers. obs.). Its determinate synnemata are 2 mm in diameter and up to 11 mm tall, with shiny black stipes and grey fertile heads comprised of branched, hyaline conidiophores with phialides producing aseptate, ellipsoidal, hyaline, smooth conidia, in a slimy mass. The conidia frequently germinate by budding to form microconidia [87,89,130]. The dispersal mode of Holwaya conidia is unknown, but its slimy conidia are likely dispersed by insects, rain splash, and surface water films [131,132]. Furthermore, the ability of Holwaya species to inhabit logs with reduced or fluctuating water availability may be enhanced by this conidial phase, which enables it to produce propagules in less favorable ecological conditions when apothecial development is suppressed [133,134]. The synnemata of Holwaya are distinct from all other known anamorphs in Thelebolales. Thelebolaceae anamorphs are morphologically reduced, with Thelebolus ellipsoideus and T. globosus forming yeast-like morphs involving integrated, intercalary conidiogenous cells producing aseptate, subglobose or ellipsoid, hyaline, thin-walled, smooth conidia [60]. Similarly, Inopinatum lactosum colonies are dimorphic and yeast-like, producing blastoconidia [59]. Cleistothelebolus nipigonensis also forms a yeast-like anamorph, with integrated, intercalary, or peg-like conidiogenouscells producing aseptate, ellipsoidal to short cylindrical, hyaline, thin-walled, smooth conidia in basipetal order from protruding scars; the conidia adhere to the conidiogenous cells in slimy globules and often turn into budding cells that produce daughter cells [112,135]. Antarctomyces psychotrophicus forms a sporothrix-like morph consisting of enteroblastic, integrated conidiogenous cells producing aseptate, subglobose to irregularly cylindrical, hyaline, thick-walled, smooth conidia that aggregate in slimy masses [136]. Antarctomyces pellizariae forms an anamorph similar to A. psychotrophicus and both species also produce abundant irregular, 1–2-celled chlamydospores singly or in long chains [137]. The formation of yeast-like colonies is apparently rare in Leotiomycetes [138], but examples exist in Thelebolaceae, as listed above. The production of yeast-like, budding conidia in slimy masses may be an adaptation for dispersal to new sources of dung via coprophilous arthropods. For example, some strains of Cleistothelebolus nipigonensis produce wine- or cheese-like odors that might function as an insect attractant [135,139]. In Thelebolus spp. isolated from birdless lakes covered by ice for all or most of the year, Hoog et al. [60] suggested that the loss of bird vectors led to an increasing predominance of conidial production, and a reduction in asci or the absence of sexual reproduction. The authors also suggested that T. globosus thrived in submerged conditions for a major part of its life cycle, evinced by its loss of active ascospore discharge and abundantly produced simple, slimy conidia that are water dispersed.
Geomyces (Pseudeurotiaceae) anamorphs typically consist of aseptate, globose, barrel-shaped, pyriform, or clavate conidia that are hyaline (sometimes yellow), thin-walled, smooth, or echinulate. The conidia are borne apically (aleurioconidia) and often intergraded with intercalary conidia produced from short, distinct, branching (sometimes verticillately) conidiophores. Geomyces-like anamorphs are also found in Pseudogymnoascus and Solomyces [86,140]. Pseudeurotium anamorphs are sporothrix-like, with conidiophores consisting of a single terminal or intercalary conidiogenous cells with short side branches bearing aseptate, globose, obovoid to ellipsoidal, hyaline conidia (sometimes turning dark brown after prolonged storage) borne singly or in clusters from short denticles at the apices [141]. Reduced malbranchea-like alternate arthroconidia are reported for Gymnostellatospora [142]. Leuconeurospora species may form both arthroconidia and dichotomously branched conidiophores with annellides that produce chains of aseptate, obovate to pyriform, basally truncate, thin- or thick-walled, hyaline to dark green, smooth conidia [128,143,144]. Bettsia alvei and B. fastidia (Pseudeurotiaceae) anamorphs were previously described in Chrysosporium and consist of solitary aleurioconidia borne on short pedicels and intercalary chlamydospores or arthroconidia [129]. Animals (arthropods, birds, mammals including humans) and air currents appear to be important dispersal agents of Pseudeurotiaceae conidia [128,145,146,147]. The majority of reticuloperidial fungi (including Pseudeurotiaceae) produce dry, cylindrical arthroconidia, which may enhance their electrostatic attraction to arthropods compared to spherical conidia with lower surface area [15].
Although the complex synnemata of Holwaya distinguish Holwayaceae from all other Thelebolales taxa, culture studies are needed to better understand the morphological diversity of the anamorphs of Holwayaceae and Thelebolales. The frequent isolation of Thelebolales species from environmental samples is suggestive of psychrotolerance, halotolerance, xerotolerance, dimorphism, and temperature-controlled sporulation [148]. Culture studies should include different salt, osmolyte, and temperature treatments to attempt to induce sporulation and provide physiological data that may in turn infer ecological traits.
5. Conclusions
Apothecial ancestry and evolution patterns in Thelebolales are revealed by the inclusion of the new family Holwayaceae. Holwayaceae contains three genera (Holwaya, Patinella, Ramgea) with wide morphological and ecological diversity, for example, the apothecial and conidial morphologies of the wood saprobe Holwaya diverge from other Thelebolales taxa. In Thelebolales, evanescent asci are surrounded by an extraordinary variety of peridial walls from different types of closed ascomata (cleistothecial, gymnothecial). Species with closed ascomata protect ascospores, which are passively discharged as an adaptation to similar ecologies (soil, dung saprobes), harsh macroclimate conditions (cold, hot), and microenvironments (osmotic pressures). The water and wind dispersal of ascospores is suggested in some lineages, but clear examples of phenotypic adaptation to animal dispersal are shown in Pseudeurotiaceae and Thelebolaceae. In response to the absence of animal (bird) vectors, Thelebolus spp. are believed to produce cleistothecial ascomata with evanescent asci and to rely increasingly on asexual reproduction. Yeast-like conidial morphs are relatively rare in Leotiomycetes, but several examples can be found in Thelebolaceae and Pseudeurotiaceae, which might be an adaption to dispersal by invertebrates and/or a stress response to cold and marine habitats. Many Thelebolales species occupy extreme habitats and exhibit corresponding adaptations. Highly reduced ascomata, from cyst-like (in adaptation to low water-activity substrates in Bettsia) to naked asci in cold environments (Antarctomyces) evolved independently and are not derived from cage- or mesh-like closed ascomata in Thelebolales. Holwayaceae, the basal family of the order, retained all phenotypic traits of the ancestor of Thelebolales, namely apothecioid, paraphysate ascomata with actively discharging asci. Phenotypic variation in ascoma size, cell pigmentation, ascus, and ascospore shape and biometry are correlated with nutritional strategies in this family. Furthermore, the dung saprobes Ramgea (Holwayaceae) and Thelebolus (Thelebolaceae) evolved independently but share multiple traits, including the homoplasic mechanism of ascus opening (OLR) and actively discharging asci, which, in Thelebolus, represents the first report of the phenomenon of re-evolution in Leotiomycetes.
Supplementary Materials
The following supporting information can be downloaded at: https://doi.org/10.7931/X93K-H703.
Author Contributions
The individual contributions are as follows: L.Q., N.M., and I.K. conceptualized the study, performed microscopical examinations of fungal specimens, wrote, edited, and reviewed the manuscript. P.R.J. and L.Q. conducted the phylogenetic studies. J.B.T. wrote, reviewed, and edited the manuscript. L.Q. and N.M. prepared figures. D.H.P. and P.R.J. provided supervision and reviewed the manuscript. A.M. reviewed the manuscript and provided funding. All authors have read and agreed to the published version of the manuscript.
Funding
The first author thanks the support of the Farlow Fellowship, Royal T. Moore Awards, the Department of Organismic and Evolutionary Biology at Harvard University, Harvard University Herbaria, and the Fundacion Ramón Areces. This work is part of the project “DNA barcoding for plant-pathogens diagnostic and monitoring. Forest diseases and turbo-taxonomy in Tympanidaceae as a case of study”, fellowship program: Becas Fundación Ramón Areces para Estudios Postdoctorales-XXIX Convocatoria para la Ampliación de Estudios en el Extranjero en Ciencia de la Vida y de la Materia. Peter Johnston was supported through the Manaaki Whenua–Landcare Research Biota Portfolio with funding from the Science and Innovation Group of the New Zealand Ministry of Business, Innovation and Employment. The second, third, and sixth authors were supported by the Croatian Science Foundation under the project ForFungiDNA (IP-2018-01-1736).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in Manaaki Whenua–Landcare Research Datastore at https://doi.org/10.7931/X93K-H703. Publicly available datasets were also analyzed in this study. This data can be found here: www.ncbi.nlm.nih.gov/genbank/ (accessed on 1 July 2021).
Acknowledgments
Curators and staff of the C, CUP, FAMU and PRM are appreciated for material loan. The collection staff of FH, Michaela Schmull, Genevieve E. Tocci and Hannah Merchant, are also thanked for their aid in searching the collections and processing loans from other institutions. We thank Radovan Kranjčev and Danijel Balaško for sharing the locality of the recent Croatian collection of Holwaya mucida and Margita Jadan (Ruđer Bošković Institute) for sequencing it. Nihad Omerović (MycoBH) is greatly appreciated for insight into his data on Patinella hyalophaea. We thank Hans-Otto Baral and Adrian Carter for sharing their photographs of P. hyalophaea and Joseph Warfel for providing his macrophotographs of H. mucida. We are grateful to Ondřej Koukol (Charles University, Prague) for help with the equipment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, B.; Hussain, M.; Zhang, W.; Stadler, M.; Liu, X.; Xiang, M. Current insights into fungal species diversity and perspective on naming the environmental DNA sequences of fungi. Mycology 2019, 10, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Fry, C.; Smith, R.J.; Simmonds, M.S.J.; Kersey, P.J.; Pritchard, H.W.; Abbo, M.S.; Acedo, C.; Adams, J.; Ainsworth, A.M.; et al. State of the World’s Plants and Fungi 2020; Royal Botanic Gardens, Kew: London, UK, 2020; pp. 1–96. [Google Scholar] [CrossRef]
- Hawksworth, D.L. The magnitude of fungal diversity: The 1.5 million species estimate revisited. Mycol. Res. 2001, 105, 1422–1432. [Google Scholar] [CrossRef]
- Hibbett, D.S.; Ohman, A.; Glotzer, D.; Nuhn, M.; Kirk, P.; Nilsson, R.H. Progress in molecular and morphological taxon discovery in Fungi and options for formal classification of environmental sequences. Fungal Biol. Rev. 2011, 25, 38–47. [Google Scholar] [CrossRef]
- Monastersky, R. Biodiversity: Life—A status report. Nature 2014, 516, 158–161. [Google Scholar] [CrossRef]
- Cannon, P.; Aguirre-Hudson, B.; Aime, M.C.; Ainsworth, A.M.; Bidartondo, M.I.; Gaya, E.; Hawksworth, D.; Kirk, P.; Leitch, I.J.; Lücking, R. Definition and diversity. In State of the World’s Fungi. Report; Willis, K.J., Ed.; Royal Botanic Gardens, Kew: London, UK, 2018; pp. 4–11. [Google Scholar]
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. In The Fungal Kingdom; Heitman, J., Howlett, B.J., Crous, P.W., Stukenbrock, E.H., James, T.Y., Eds.; American Society for Microbiology: Washington, DC, USA, 2018; pp. 1–1136. [Google Scholar]
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Dictionary of the Fungi, 10th ed.; CABI: Wallingford, UK, 2008. [Google Scholar]
- Liu, Y.J.; Hall, B.D. Body plan evolution of ascomycetes, as inferred from an RNA polymerase II phylogeny. Proc. Natl. Acad. Sci. USA 2004, 101, 4507–4512. [Google Scholar] [CrossRef]
- Taylor, T.N.; Krings, M.; Taylor, E.L. Fossil Fungi; Academic Press: London, UK, 2015; pp. 1–384. [Google Scholar]
- Schmitt, I.; Prado, R.; Grube, M.; Lumbsch, H.T. Repeated evolution of closed fruiting bodies is linked to ascoma development in the largest group of lichenized fungi (Lecanoromycetes, Ascomycota). Mol. Phylogenet. Evol. 2009, 52, 34–44. [Google Scholar] [CrossRef]
- Berbee, M.L.; Taylor, J.W. Two ascomycete classes based on fruiting-body characters and ribosomal DNA sequence. Mol. Biol. Evol. 1992, 9, 278. [Google Scholar]
- Schoch, C.L.; Sung, G.H.; López-Giráldez, F.; Townsend, J.P.; Miadlikowska, J.; Hofstetter, V.; Robbertse, B.; Matheny, P.B.; Kauff, F.; Wang, Z.; et al. The Ascomycota tree of life: A phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Syst. Biol. 2009, 58, 224–239. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.R.; Quijada, L.; Smith, C.A.; Baral, H.O.; Hosoya, T.; Baschien, C.; Pärtel, K.; Zhuang, W.Y.; Haelewaters, D.; Park, D.; et al. A multigene phylogeny toward a new phylogenetic classification of Leotiomycetes. IMA Fungus 2019, 10, 1–22. [Google Scholar] [CrossRef]
- Greif, M.D.; Currah, R.S. A functional interpretation of the role of the reticuloperidium in whole-ascoma dispersal by arthropods. Mycol. Res. 2003, 107, 77–81. [Google Scholar] [CrossRef]
- Trail, F. Fungal cannons: Explosive spore discharge in the Ascomycota. FEMS Microbiol. Lett. 2007, 276, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Wynns, A.A. Convergent evolution of highly reduced fruiting bodies in Pezizomycotina suggests key adaptations to the bee habitat. BMC Evol. Biol. 2015, 15, 145. [Google Scholar] [CrossRef] [PubMed]
- Calhim, S.; Halme, P.; Petersen, J.H.; Læssøe, T.; Bässler, C.; Heilmann-Clausen, J. Fungal spore diversity reflects substrate-specific deposition challenges. Sci. Rep. 2018, 8, 5356. [Google Scholar] [CrossRef] [PubMed]
- Ingold, C.T. Fungal Spores: Their Liberation and Dispersal; Oxford University Press: New York, NY, USA, 1971; pp. 1–302. [Google Scholar]
- Roper, M.; Pepper, R.E.; Brenner, M.P.; Pringle, A. Explosively launched spores of ascomycete fungi have drag-minimizing shapes. Proc. Natl. Acad. Sci. USA 2008, 105, 20583–20588. [Google Scholar] [CrossRef]
- Pringle, A.; Brenner, M.P.; Fritz, J.A.; Roper, M.; Seminara, A. Reaching the wind: Boundary layer escape as a constraint on ascomycete spore dispersal. In The Fungal Community: Its Organization and Role in the Ecosystem, 4th ed.; Dighton, J., White, J.F., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2017; pp. 309–320. [Google Scholar]
- Fritz, J.A.; Seminara, A.; Roper, M.; Pringle, A.; Brenner, M.P. A natural O-ring optimizes the dispersal of fungal spores. J. R. Soc. Interface 2013, 10, 20130187. [Google Scholar] [CrossRef] [PubMed]
- van Brummelen, J. A world-monograph of the genera Ascobolus and Saccobolus (Ascomycetes, Pezizales). Persoonia 1967, 1, 1–260. [Google Scholar]
- Plishka, M.J.R.; Tsuneda, A.; Currah, R.S. Evidence of apothecial ancestry in the cleistothecial ascomata of Pleuroascus nicholsonii. Mycol. Res. 2008, 112, 1319–1326. [Google Scholar] [CrossRef]
- Jaklitsch, W.; Baral, H.-O.; Lücking, R.; Lumbsch, H.T. Ascomycota. In Syllabus of Plant Families-Adolf Engler’s Syllabus der Pflanzenfamilien. Part 1/2 Ascomycota, 13th ed.; Frey, W., Ed.; Borntraeger Science Publishers: Stuttgart, Germany, 2016; pp. 1–322. [Google Scholar]
- Baral, H.O.; Weber, E.; Marson, G. Monograph of Orbiliomycetes (Ascomycota) based on vital taxonomy; National Museum of Natural History: Luxembourg, 2020; Volume Part I and II, pp. 1–1752. [Google Scholar]
- Wyatt, T.T.; Wösten, H.A.B.; Dijksterhuis, J. Fungal spores for dispersion in space and time. Adv. Appl. Microbiol. 2013, 85, 43–91. [Google Scholar]
- Magyar, D.; Vass, M.; Li, D.W. Dispersal strategies of microfungi. In Biology of Microfungi; Li, D.W., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 315–371. [Google Scholar]
- Norros, V.; Rannik, Ü.; Hussein, T.; Petäjä, T.; Vesala, T.; Ovaskainen, O. Do small spores disperse further than large spores? Ecology 2014, 95, 1612–1621. [Google Scholar] [CrossRef]
- Golan, J.J.; Pringle, A. Long-distance dispersal of fungi. Microbiol. Spectr. 2017, 5, 1–24. [Google Scholar] [CrossRef]
- Buller, A.H.R. Researches on Fungi. An Account of the Production, Liberation and Dispersion of the Spores of Hymenomycetes Treated Botanically and Physically. Also Some Observations upon the Discharge and Dispersion of the Spores of Ascomycetes and Pilobolus; Longmans, Green and Co.: London, UK; New York, NY, USA, 1909. [Google Scholar]
- Landvik, S.; Schumacher, T.K.; Eriksson, O.E.; Moss, S.T. Morphology and ultrastructure of Neolecta species. Mycol. Res. 2003, 107, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Norros, V.; Karhu, E.; Nordén, J.; Vähätalo, A.V.; Ovaskainen, O. Spore sensitivity to sunlight and freezing can restrict dispersal in wood-decay fungi. Ecol. Evol. 2015, 5, 3312–3326. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, C.A.; Griffin, D.W. Aerobiology and the global transport of desert dust. Trends Ecol. Evol. 2006, 21, 638–644. [Google Scholar] [CrossRef]
- Pringle, A.; Vellinga, E.; Peay, K. The shape of fungal ecology: Does spore morphology give clues to a species’ niche? Fungal Ecol. 2015, 17, 213–216. [Google Scholar] [CrossRef]
- Zanatta, F.; Patiño, J.; Lebeau, F.; Massinon, M.; Hylander, K.; Hann, M.; Ballings, P.; Degreef, J.; Vanderpoorten, A. Measuring spore settling velocity for an improved assessment of dispersal rates in mosses. Ann. Bot. 2016, 118, 197–206. [Google Scholar] [CrossRef]
- Durrell, L.W. The composition and structure of walls of dark fungus spores. Mycopathol. Mycol. Appl. 1964, 23, 339–345. [Google Scholar] [CrossRef]
- Deveautour, C.; Chieppa, J.; Nielsen, U.N.; Boer, M.M.; Mitchell, C.; Horn, S.; Power, S.A.; Guillen, A.; Bennet, A.; Powell, J.R. Biogeography of arbuscular mycorrhizal fungal spore traits along an aridity gradient, and responses to experimental rainfall manipulation. Fungal Ecol. 2020, 46, 100899. [Google Scholar] [CrossRef]
- Kawamura, C.; Tsujimoto, T.; Tsuge, T. Targeted disruption of a melanin biosynthesis gene affects conidial development and UV tolerance in the Japanese pear pathotype of Alternaria alternata. Mol. Plant-Microbe Interact. 1999, 12, 59–63. [Google Scholar] [CrossRef]
- Robinson, C.H. Cold adaptation in Arctic and Antarctic fungi. New Phytol. 2001, 151, 341–353. [Google Scholar] [CrossRef]
- Gessler, N.N.; Egorova, A.S.; Belozerskaya, T.A. Melanin pigments of fungi under extreme environmental conditions. Appl. Biochem. Microbiol. 2014, 50, 105–113. [Google Scholar] [CrossRef]
- Kejžar, A.; Gobec, S.; Plemenitaš, A.; Lenassi, M. Melanin is crucial for growth of the black yeast Hortaea werneckii in its natural hypersaline environment. Fungal Biol. 2013, 117, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, R.V.; Tobin, J.M. Fungal melanins and their interactions with metals. Enzyme Microb. Technol. 1996, 19, 311–317. [Google Scholar] [CrossRef]
- Bloomfield, B.J.; Alexander, M. Melanins and resistance of fungi to lysis. J. Bacteriol. 1967, 93, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.J.; Alexander, M. Inhibition of the lysis of fungi by melanins. J. Bacteriol. 1967, 94, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.M.; Butler, M.J.; Day, A.W. The dark side of the mycelium: Melanins of phytopathogenic fungi. Annu. Rev. Phytopathol. 1999, 37, 447–471. [Google Scholar] [CrossRef]
- Nosanchuk, J.D.; Casadevall, A. The contribution of melanin to microbial pathogenesis. Cell. Microbiol. 2003, 5, 203–223. [Google Scholar] [CrossRef]
- Berbee, M.L. The phylogeny of plant and animal pathogens in the Ascomycota. Physiol. Mol. Plant Pathol. 2001, 59, 165–187. [Google Scholar] [CrossRef]
- Mueller, G.M.; Foster, M.S.; Bills, G.F. Biodiversity of Fungi: Inventory and Monitoring Methods; Elsevier Academic Press: Burlington, MA, USA, 2004; pp. 1–777. [Google Scholar]
- O’Brien, H.E.; Parrent, J.L.; Jackson, J.A.; Moncalvo, J.M.; Vilgalys, R. Fungal community analysis by large-scale sequencing of environmental samples. Appl. Environ. Microbiol. 2005, 71, 5544–5550. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Sung, G.-H.; Johnson, D.; Hesse, C.; O’Rourke, B.; Serdani, M.; Spotts, R.; Lutzoni, F.; Hofstetter, V.; Miadlikowska, J.; et al. A five-gene phylogeny of Pezizomycotina. Mycologia 2006, 98, 1018–1028. [Google Scholar] [CrossRef]
- Sieber, T.N. Endophytic fungi in forest trees: Are they mutualists? Fungal Biol. Rev. 2007, 21, 75–89. [Google Scholar] [CrossRef]
- Prieto, M.; Schultz, M.; Olariaga, I.; Wedin, M. Lichinodium is a new lichenized lineage in the Leotiomycetes. Fungal Divers. 2019, 94, 23–39. [Google Scholar] [CrossRef]
- Suh, S.O.; Blackwell, M. Molecular phylogeny of the cleistothecial fungi placed in Cephalothecaceae and Pseudeurotiaceae. Mycologia 1999, 91, 836–848. [Google Scholar] [CrossRef]
- Gernandt, D.S.; Platt, J.L.; Stone, J.K.; Spatafora, J.W.; Holst-Jensen, A.; Hamelin, R.C.; Kohn, L. Phylogenetics of Helotiales and Rhytismatales based on partial small subunit nuclear ribosomal DNA sequences. Mycologia 2001, 93, 915–933. [Google Scholar] [CrossRef]
- Stajich, J.E.; Berbee, M.L.; Blackwell, M.; Hibbett, D.S.; James, T.Y.; Spatafora, J.W.; Taylor, J.W. The Fungi. Curr. Biol. 2009, 19, R840–R845. [Google Scholar] [CrossRef] [PubMed]
- Untereiner, W.; Yue, Q.; Chen, L.; Li, Y.; Bills, G.; Štěpánek, V.; Réblová, M. Phialophora section Catenulatae disassembled: New genera, species, and combinations and a new family encompassing taxa with cleistothecial ascomata and phialidic asexual states. Mycologia 2019, 111, 998–1027. [Google Scholar] [CrossRef]
- Rodríguez-Andrade, E.; Stchigel, A.M.; Terrab, A.; Guarro, J.; Cano-Lira, J.F. Diversity of xerotolerant and xerophilic fungi in honey. IMA Fungus 2019, 10, 20. [Google Scholar] [CrossRef]
- Haelewaters, D.; Peterson, R.A.; Nevalainen, H.; Aime, M.C. Inopinatum lactosum gen. & comb. nov., the first yeast-like fungus in Leotiomycetes. Int. J. Syst. Evol. Microbiol. 2021, 71, 1–7. [Google Scholar]
- Hoog, G.S.; Göttlich, E.; Platas, G.; Genilloud, O.; Leotta, G.; Brummelen, J. van Evolution, taxonomy and ecology of the genus Thelebolus in Antarctica. Stud. Mycol. 2005, 51, 33–76. [Google Scholar]
- Wang, Z.; Binder, M.; Schoch, C.L.; Johnston, P.R.; Spatafora, J.W.; Hibbett, D.S. Evolution of helotialean fungi (Leotiomycetes, Pezizomycotina): A nuclear rDNA phylogeny. Mol. Phylogenet. Evol. 2006, 41, 295–312. [Google Scholar] [CrossRef]
- LoBuglio, K.F.; Pfister, D.H. Placement of Medeolaria farlowii in the Leotiomycetes, and comments on sampling within the class. Mycol. Prog. 2010, 9, 361–368. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.E.S.J.; Crane, C.; Barrett, S.; Cano-Lira, J.F.; Le Roux, J.J.; Thangavel, R.; Guarro, J.; et al. Fungal Planet description sheets: 469–557. Persoonia 2016, 37, 218–403. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; Norphanphoun, C.; Abreu, V.P.; Bazzicalupo, A.; Chethana, K.W.T.; Clericuzio, M.; Dayarathne, M.C.; Dissanayake, A.J.; Ekanayaka, A.H.; He, M.Q.; et al. Fungal diversity notes 603–708: Taxonomic and phylogenetic notes on genera and species. Fungal Divers. 2017, 87, 1–235. [Google Scholar] [CrossRef]
- Brummelen, J. van Reconsideration of relationships within the Thelebolaceae based on ascus ultrastructure. Persoonia 1998, 16, 119–125. [Google Scholar]
- Kirschner, R. Sex does not sell: The argument for using the terms “anamorph” and “teleomorph” for fungi. Mycol. Prog. 2018, 18, 305–312. [Google Scholar] [CrossRef]
- Baral, H.O. Vital versus herbarium taxonomy: Morphological differences between living and dead cells of ascomycetes, and their taxonomic implications. Mycotaxon 1992, 44, 333–390. [Google Scholar]
- Quijada, L. Estudio de los órdenes Helotiales s.l. y Orbiliales (Ascomycota, Fungi) en la Isla de Tenerife. Ph.D. Thesis, Departamento de Botánica, Ecología y Fisiología Vegetal, Universidad de La Laguna, La Laguna, Spain, 18 September 2015. [Google Scholar]
- Kušan, I. Contribution of “Vital taxonomy” Methods to the Ascomycota Taxonomy. Ph.D. Thesis, Josip Juraj Strossmayer University of Osijek, Osijek, Croatia, 25 September 2015. [Google Scholar]
- Karakehian, J.M.; Quijada, L.; Friebes, G.; Tanney, J.B.; Pfister, D.H. Placement of Triblidiaceae in Rhytismatales and comments on unique ascospore morphologies in Leotiomycetes (Fungi, Ascomycota). MycoKeys 2019, 54, 99–133. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. ISCC-NBS Color-Name Charts Illustrated with Centroid Colors. Inter-Society Color Council; National Bureau of Standards: Washington, DC, USA, 1976. [Google Scholar]
- Quijada, L.; Tanney, J.B.; Popov, E.; Johnston, P.R.; Pfister, D.H. Cones, needles and wood: Micraspis (Micraspidaceae, Micraspidales fam. et ord. nov.) speciation segregates by host plant tissues. Fungal Syst. Evol. 2020, 5, 99–111. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Carnegie, A.J.; St.J. Hardy, G.E.; Smith, D.; Summerell, B.A.; Cano-Lira, J.F.; Guarro, J.; Houbraken, J.; et al. Fungal Planet description sheets: 625–715. Persoonia 2017, 39, 270–467. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Chernomor, O.; von Haeseler, A.; Minh, B.Q. Terrace aware data structure for phylogenomic inference from supermatrices. Syst. Biol. 2016, 65, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Version 3.70. 2021. Available online: http://www.mesquiteproject.org (accessed on 12 August 2021).
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Batista, T.M.; Hilario, H.O.; De Brito, G.A.M.; Moreira, R.G.; Furtado, C.; De Menezes, G.C.A.; Rosa, C.A.; Rosa, L.H.; Franco, G.R. Whole-genome sequencing of the endemic Antarctic fungus Antarctomyces pellizariae reveals an ice-binding protein, a scarce set of secondary metabolites gene clusters and provides insights on Thelebolales phylogeny. Genomics 2020, 112, 2915–2921. [Google Scholar] [CrossRef]
- Seifert, K.A. A monograph of Stilbella and some allied Hyphomycetes. Stud. Mycol. 1985, 27, 1–225. [Google Scholar]
- Baral, H.O.; Carter, A. Patinella hyalophaea Sacc.–Rediscovered in New Brunswick, Canada. Ascomycete 2013, 5, 91–96. [Google Scholar]
- Wijayawardene, N.N.; Hyde, K.D.; Al-Ani, L.K.T.; Tedersoo, L.; Haelewaters, D.; Rajeshkumar, K.C.; Zhao, R.L.; Aptroot, A.; Leontyev, D.V.; Saxena, R.K.; et al. Outline of Fungi and fungi-like taxa. Mycosphere 2020, 11, 1060–1456. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, C.; Chen, W.; Mou, Q.; Lu, X.; Han, Y.; Huang, J.; Liang, Z. The enigmatic Thelebolaceae (Thelebolales, Leotiomycetes): One new genus Solomyces and five new species. Front. Microbiol. 2020, 11, 572596. [Google Scholar] [CrossRef]
- Korf, R.P.; Abawi, G.S. On Holwaya, Crinula, Claussenomyces, and Corynella. Can. J. Bot. 1971, 49, 1879–1883. [Google Scholar] [CrossRef]
- Krieglsteiner, G.J.; Häffner, J. Über Holwaya mucida (S. Schulzer von Müggenburg 1860) R.P. Korf et G.S. Abawi 1971, subspec. mucida Korf et Abawi 1971 und ihr Vorkommen in Europa. Z. Mykol. 1985, 51, 131–138. [Google Scholar]
- Aronsson, G. Lindskål, Holwaya mucida, i Sverige. Sven. Bot. Tidskr. 1991, 85, 9–18. [Google Scholar]
- Brummelen, J. van Ramgea, a new genus of Pezizales from the Netherlands. Persoonia 1992, 14, 577–582. [Google Scholar]
- GBIF–Global Biodiversity Information Facility. Available online: https://www.gbif.org (accessed on 25 December 2021).
- Matočec, N.; Jukić, N.; Omerović, N.; Kušan, I. Dinaric karst poljes and their importance for mycobiota. In Dinaric Karst Poljes-Nature Conservation and Rural Development; Sackl, P., Ferger, S., Sarajlić, N., Kotrošan, D., Topić, G., Eds.; Ornitološko društvo “Naše ptice”: Sarajevo, Bosnia and Herzegovina, 2019; pp. 27–49. [Google Scholar]
- Held, B.W.; Blanchette, R.A. Deception Island, Antarctica, harbors a diverse assemblage of wood decay fungi. Fungal Biol. 2017, 121, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Ogaki, M.B.; Vieira, R.; Muniz, M.C.; Zani, C.L.; Alves, T.M.; Junior, P.A.; Murta, S.M.; Barbosa, E.C.; Oliveira, J.G.; Ceravolo, I.P.; et al. Diversity, ecology, and bioprospecting of culturable fungi in lakes impacted by anthropogenic activities in Maritime Antarctica. Extremophiles 2020, 24, 637–655. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.B.; Matthiesen, H.; Blanchette, R.A.; Alfredsen, G.; Held, B.W.; Westergaard-Nielsen, A.; Hollesen, J. Fungal attack on archaeological wooden artefacts in the Arctic—Implications in a changing climate. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Bässler, C.; Halbwachs, H.; Karasch, P.; Holzer, H.; Gminder, A.; Krieglsteiner, L.; Gonzalez, R.S.; Müller, J.; Brandl, R. Mean reproductive traits of fungal assemblages are correlated with resource availability. Ecol. Evol. 2016, 6, 582–592. [Google Scholar] [CrossRef]
- Fischer, M.W.; Money, N.P. Why mushrooms form gills: Efficiency of the lamellate morphology. Fungal Biol. 2010, 114, 57–63. [Google Scholar] [CrossRef][Green Version]
- Bässler, C.; Heilmann-Clausen, J.; Karasch, P.; Brandl, R.; Halbwachs, H. Ectomycorrhizal fungi have larger fruit bodies than saprotrophic fungi. Fungal Ecol. 2015, 17, 205–212. [Google Scholar] [CrossRef]
- Halbwachs, H.; Bässler, C. No bull: Dung-dwelling mushrooms show reproductive trait syndromes different from their non-coprophilous allies. Mycol. Prog. 2020, 19, 817–824. [Google Scholar] [CrossRef]
- Moore, D.; Gange, A.C.; Gange, E.G.; Boddy, L. Fruit bodies: Their production and development in relation to environment. In Ecology of Saprotrophic Basidiomycetes; Boddy, L., Frankland, J.C., van West, P., Eds.; Mycological Society Symposia Series; Elsevier Ltd.: London, UK, 2008; Volume 28, pp. 79–103. [Google Scholar]
- Wang, Z.; Johnston, P.R.; Yang, Z.L.; Townsend, J.P. Evolution of reproductive morphology in leaf endophytes. PLoS ONE 2009, 4, e4246. [Google Scholar] [CrossRef] [PubMed]
- Tanney, J.B.; Seifert, K.A. Phacidiaceae endophytes of Picea rubens in Eastern Canada. Botany 2018, 96, 555–588. [Google Scholar] [CrossRef]
- Greif, M.D.; Tsuneda, A.; Currah, R.S. The peridial development and dehiscence mechanism of Cryptendoxyla hypophloia, a cleistothecial ascomycete isolated from the bodies of arthropods. Int. J. Plant Sci. 2004, 165, 957–964. [Google Scholar] [CrossRef]
- Sherwood, M. Convergent evolution in discomycetes from bark and wood. Bot. J. Linn. Soc. 1981, 82, 15–34. [Google Scholar] [CrossRef]
- Rehnstrom, A.L.; Free, S.J. The isolation and characterization of melanin-deficient mutants of Monilinia fructicola. Physiol. Mol. Plant Pathol. 1996, 49, 321–330. [Google Scholar] [CrossRef]
- Wicklow, D.T.; Malloch, D. Studies in the genus Thelebolus: Temperature optima for growth and ascocarp development. Mycologia 1971, 63, 118–131. [Google Scholar] [CrossRef]
- Czymmek, K.; Klomparens, K. The ultrastructure of ascosporogenesis in freeze-substituted Thelebolus crustaceus: Enveloping membrane system and ascospore initial development. Canad. J. Bot. 1992, 70, 1669–1683. [Google Scholar] [CrossRef]
- Torruella, G.; De Mendoza, A.; Grau-Bové, X.; Antó, M.; Chaplin, M.A.; Del Campo, J.; Eme, L.; Pérez-Cordón, G.; Whipps, C.M.; Nichols, K.M.; et al. Phylogenomics reveals convergent evolution of lifestyles in close relatives of animals and fungi. Curr. Biol. 2015, 25, 2404–2410. [Google Scholar] [CrossRef]
- Wicklow, D.T. The coprophilous fungal community: An experimental system. In The Fungal Community, Its Organization and Role in the Ecosystem; Carrol, G.C., Wicklow, D.T., Eds.; Marcel Dekker: New York, NY, USA, 1992; pp. 715–728. [Google Scholar]
- Garnica, S.; Weiss, M.; Walther, G.; Oberwinkler, F. Reconstructing the evolution of agarics from nuclear gene sequences and basidiospore ultrastructure. Mycol. Res. 2007, 111, 1019–1029. [Google Scholar] [CrossRef]
- Halbwachs, H.; Brandl, R.; Bässler, C. Spore wall traits of ectomycorrhizal and saprotrophic agarics may mirror their distinct lifestyles. Fungal Ecol. 2015, 17, 197–204. [Google Scholar] [CrossRef]
- Malloch, D.; Cain, R.F. Four new genera of cleistothecial Ascomycetes with hyaline ascospores. Can. J. Bot. 1971, 49, 847–854. [Google Scholar] [CrossRef]
- Doveri, F.; Sarrocco, S.; Vannacci, G. Studies on three rare coprophilous plectomycetes from Italy. Mycotaxon 2013, 124, 279–300. [Google Scholar] [CrossRef]
- Pivkin, M.; Aleshko, S.; Krasokhin, V.; Khudyakova, Y. Fungal assemblages associated with sponges of the southern coast of Sakhalin Island. Russ. J. Mar. Biol. 2006, 32, 207–213. [Google Scholar] [CrossRef]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi, 2nd ed.; taxonomically revised by W Gams; IHW-Verlag: Eching, Germany, 2007; pp. 1–672. [Google Scholar]
- Bubnova, E. Fungal diversity in bottom sediments of the Kara Sea. Bot. Mar. 2010, 53, 595–600. [Google Scholar] [CrossRef]
- Adhikari, M.; Kim, S.; Yadav, D.; Um, Y.; Kim, H.; Lee, H.; Lee, Y. A new record of Pseudeurotium bakeri from crop field soil in Korea. Korean J. Mycol. 2016, 44, 145–149. [Google Scholar]
- Da Silva, T.H.; Saraiva Silva, D.A.; De Oliveira, F.S.; Goncalves Reynaud Schaefer, C.E.; Rosa, C.A.; Rosa, L.H. Diversity, distribution, and ecology of viable fungi in permafrost and active layer of Maritime Antarctica. Extremophiles 2020, 24, 565–576. [Google Scholar] [CrossRef]
- Bovio, E.; Garzoli, L.; Poli, A.; Prigione, V.; Firsova, D.; McCormack, G.; Varese, G. The culturable mycobiota associated with three Atlantic sponges, including two new species: Thelebolus balaustiformis and T. spongiae. Fungal Syst. Evol. 2018, 1, 141–167. [Google Scholar] [CrossRef]
- Collin, R.; Cipriani, R. Dollo’s law and the re-evolution of shell coiling. Proc. Royal Soc. B Biol. Sci. 2003, 270, 2551–2555. [Google Scholar] [CrossRef]
- Wiens, J.J. Re-evolution of lost mandibular teeth in frogs after more than 200 million years, and re-evaluating Dollo’s law. Evolution 2011, 65, 1283–1296. [Google Scholar] [CrossRef]
- Seher, T.; Ng, C.; Signor, S.; Podlaha, O.; Barmina, O.; Kopp, A. Genetic basis of a violation of Dollo’s Law: Re-evolution of rotating sex combs in Drosophila bipectinata. Genetics 2012, 192, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Recknagel, H.; Kamenos, N.; Elmer, K. Common lizards break Dollo’s law of irreversibility: Genome-wide phylogenomics support a single origin of viviparity and re-evolution of oviparity. Mol. Phylogenet. Evol. 2018, 127, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Kleisner K Re-semblance and re-evolution: Paramorphism and semiotic co-option may explain the re-evolution of similar phenotypes. Sign Syst. Stud. 2010, 38, 378–392. [CrossRef]
- Gregory, P.H. Microbiology of the Atmosphere, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1973. [Google Scholar]
- Jennings, D.H.; Lysek, G. Fungal Biology: Understanding the Fungal Lifestyle, 2nd ed.; Bios Scientific Publishers Ltd.: Oxford, UK, 1999; pp. 1–166. [Google Scholar]
- Vanderwolf, K.J.; Malloch, D.; McAlpine, D.F. Ectomycota associated with arthropods from bat hibernacula in Eastern Canada, with particular reference to Pseudogymnoascus destructans. Insects 2016, 7, 16. [Google Scholar] [CrossRef]
- Malloch, D.; Sigler, L.; Hambleton, S.; Vanderwolf, K.; Gibas, C.; McAlpine, D. Fungi associated with hibernating bats in New Brunswick caves: The genus Leuconeurospora. Botany 2016, 94, 1171–1181. [Google Scholar] [CrossRef]
- Pitt, J.I.; Lantz, H.; Pettersson, O.V.; Leong, S.L. Xerochrysium gen. nov. and Bettsia, genera encompassing xerophilic species of Chrysosporium. IMA Fungus 2013, 4, 229–241. [Google Scholar] [CrossRef]
- Seifert, K.; Morgan-Jones, G.; Gams, W.; Kendrick, B. The Genera of Hyphomycetes; CBS Biodiversity Series, no. 9; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2011; pp. 1–997. [Google Scholar]
- Bandoni, R.J.; Koske, R.E. Monolayers and microbial dispersal. Science 1974, 183, 1079–1081. [Google Scholar] [CrossRef]
- Wicklow, D.T. Biogeography and conidial fungi. In Biology of Conidial Fungi; Cole, G.T., Kendrick, B., Eds.; Academic Press: New York, NY, USA, 1981; pp. 417–447. [Google Scholar]
- Arpin, N.; Bouillant, M.L. Light and mycosporines. In The Fungal Spore: Morphogenetic Controls; Turian, G., Hohl, H.R., Eds.; Academic Press: London, UK; New York, NY, USA; Toronto, ON, Canada, 1981; pp. 435–454. [Google Scholar]
- Matočec, N. Cave fungi. Ascomycota, Basidiomycota and Anamorphic (Mitosporic) fungi. An Overview of the Cave and Interstitial Biota of Croatia. Natura Croatica 2002, 11 (Suppl. 1), 21–27. [Google Scholar]
- Hoog, G.S.; Smith, M.T. Hyphozyma, a new genus of yeast-like Hyphomycetes. Antonie van Leeuwenhoek 1981, 47, 339–352. [Google Scholar] [CrossRef]
- Stchigel, A.M.; Cano, J.; Mac Cormack, W.; Guarro, J. Antarctomyces psychrotrophicus gen. et sp. nov., a new ascomycete from Antarctica. Mycol. Res. 2001, 105, 377–382. [Google Scholar] [CrossRef]
- Menezes, G.C.; Godinho, V.M.; Porto, B.A.; Gonçalves, V.N.; Rosa, L.H. Antarctomyces pellizariae sp. nov., a new, endemic, blue, snow resident psychrophilic ascomycete fungus from Antarctica. Extremophiles 2017, 21, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Tanney, J.B.; Quijada, L. Comments on the occurrence of yeast-like morphologies in Leotiomycetes. Int. J. Syst. Evol. Microbiol. 2021, 71, 005141. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.G.; Hagman, A.; Verschut, V.; Chakraborty, A.; Rozpędowska, E.; Lebreton, S.; Bengtsson, M.; Flick, G.; Witzgall, P.; Piškur, J. Chemical signaling and insect attraction is a conserved trait in yeasts. Ecol. Evol. 2018, 8, 2962–2974. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, P.; Vásquez, G.; Gil-Durán, C.; Oliva, V.; Díaz, A.; Henríquez, M.; Alvarez, E.; Laich, F.; Chávez, R.; Vaca, I. Description of the first four species of the genus Pseudogymnoascus from Antarctica. Front. Microbiol. 2021, 12, 713189. [Google Scholar] [CrossRef]
- Sogonov, M.V.; Schroers, H.J.; Gams, W.; Dijksterhuis, J.; Summerbell, R.C. The hyphomycete Teberdinia hygrophila gen. nov., sp. nov. and related anamorphs of Pseudeurotium species. Mycologia 2005, 97, 695–709. [Google Scholar] [CrossRef]
- Sigler, L.; Lumley, T.C.; Currah, R.S. New species and records of saprophytic ascomycetes (Myxotrichaceae) from decaying logs in the boreal forest. Mycoscience 2000, 41, 495–502. [Google Scholar] [CrossRef]
- Smith, G. Polypaecilum gen. nov. Trans. Brit. Mycol. Soc. 1961, 44, 437–440. [Google Scholar] [CrossRef]
- Von Arx, J.A. Notes on Microascaceae with the description of two new species. Persoonia 1978, 10, 23–31. [Google Scholar]
- Rice, A.V.; Currah, R.S. Oidiodendron: A survey of the named species and related anamorphs of Myxotrichum. Stud. Mycol. 2005, 53, 83–120. [Google Scholar] [CrossRef]
- Hayes, M.A. The Geomyces fungi: Ecology and distribution. BioScience 2012, 62, 819–823. [Google Scholar] [CrossRef]
- Vanderwolf, K.J.; McAlpine, D.F.; Malloch, D.; Forbes, G.J. Ectomycota associated with hibernating bats in eastern Canadian caves prior to the emergence of white-nose syndrome. Northeast. Nat. 2013, 20, 115–130. [Google Scholar] [CrossRef]
- Sazanova, K.V.; Senik, S.V.; Kirtsideli, I.Y.; Shavarda, A.L. Metabolomic profiling and lipid composition of Arctic and Antarctic strains of Micromycetes Geomyces pannorum and Thelebolus microsporus grown at different temperatures. Microbiology 2019, 88, 282–291. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).