Radiomics Metrics Combined with Clinical Data in the Surgical Management of Early-Stage (cT1–T2 N0) Tongue Squamous Cell Carcinomas: A Preliminary Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. CT Protocol
2.3. Image Processing
2.4. Statistical Analysis
2.4.1. Univariate Analysis
2.4.2. Multivariate Analysis
3. Results
3.1. Univariate Analysis
3.2. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nocini, R.; Capocasale, G.; Marchioni, D.; Zotti, F. A Snapshot of Knowledge about Oral Cancer in Italy: A 505 Person Survey. Int. J. Environ. Res. Public Health 2020, 17, 4889. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.C.; Zhang, S.; Ishii, G.; Endoh, Y.; Kodama, K.; Miyamoto, S.; Hayashi, R.; Ebihara, S.; Cho, J.S.; Ochiai, A. Predictive markers for late cervical metastasis in stage I and II invasive squamous cell carcinoma of the oral tongue. Clin. Cancer Res. 2004, 10, 166–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenbrouck, C.; Sancho-Garnier, H.; Chassagne, D.; Saravane, D.; Cachin, Y.; Micheau, C. Elective versus therapeutic radical neck dissection in epidermoid carcinoma of the oral cavity: Results of a randomized clinical trial. Cancer 1980, 46, 386–390. [Google Scholar] [CrossRef]
- Sagheb, K.; Sagheb, K.; Rahimi-Nedjat, R.; Taylor, K.; Al-Nawas, B.; Walter, C. Sentinel lymph node biopsy in T1/T2 squamous cell carcinomas of the tongue: A prospective study. Oncol. Lett. 2016, 11, 600–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piazza, C.; Montalto, N.; Paderno, A.; Taglietti, V.; Nicolai, P. Is it time to incorporate ‘depth of infiltration’ in the T staging of oral tongue and floor of mouth cancer? Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balkwill, F.B.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Kumarasamy, C.; Tiwary, V.; Sunil, K.; Suresh, D.; Shetty, S.; Muthukaliannan, G.K.; Baxi, S.; Jayaraj, R. Prognostic Utility of Platelet-Lymphocyte Ratio, Neutrophil-Lymphocyte Ratio and Monocyte-Lymphocyte Ratio in Head and Neck Cancers: A Detailed PRISMA Compliant Systematic Review and Meta-Analysis. Cancers 2021, 13, 4166. [Google Scholar] [CrossRef] [PubMed]
- Avanzo, M.; Stancanello, J.; El Naqa, I. Beyond imaging: The promise of radiomics. Phys Med. 2017, 38, 122–139. Available online: https://pyradiomics.readthedocs.io/en/latest/features.html (accessed on 15 February 2021). [CrossRef]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Im-age-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Chilling, C.; Stoeckli, S.J.; Haerle, S.K.; Broglie, M.A.; Huber, G.A.; Sorensen, J.A. Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer. Eur. J. Cancer 2015, 51, 2777–2784. [Google Scholar] [CrossRef]
- Van Assen, M.; Muscogiuri, G.; Caruso, D.; Lee, S.J.; Laghi, A.; De Cecco, C.N. Artificial intelligence in cardiac radiology. Radiol. Med. 2020, 125, 1186–1199. [Google Scholar] [CrossRef] [PubMed]
- Neri, E.; Coppola, F.; Miele, V.; Bibbolino, C.; Grassi, R. Artificial intelligence: Who is responsible for the diagnosis? Radiol. Med. 2020, 125, 517–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belfiore, M.P.; Urraro, F.; Grassi, R.; Giacobbe, G.; Patelli, G.; Cappabianca, S.; Reginelli, A. Artificial intelligence to codify lung CT in Covid-19 patients. Radiol. Med. 2020, 125, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Cristofaro, M.; Busi Rizzi, E.; Piselli, P.; Pianura, E.; Petrone, A.; Fusco, N.; Di Stefano, F.; Schinina, V. Image quality and radiation dose reduction in chest CT in pulmonary infection. Radiol. Med. 2020, 125, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Gurgitano, M.; Angileri, S.A.; Rodà, G.M.; Liguori, A.; Pandolfi, M.; Ierardi, A.M.; Wood, B.J.; Carrafiello, G. Interventional Radiology ex-machina: Impact of Artificial Intelligence on practice. Radiol. Med. 2021, 126, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Cappabianca, S.; Fusco, R.; de Lisio, A.; Paura, C.; Clemente, A.; Gagliardi, G.; Lombardi, G.; Giacobbe, G.; Russo, G.M.; Belfiore, M.P.; et al. Correction to: Clinical and laboratory data, radiological structured report findings and quantitative evaluation of lung involvement on baseline chest CT in COVID-19 patients to predict prognosis. Radiol. Med. 2021, 126, 643, Erratum in Radiol. Med. 2021, 126, 29–39. [Google Scholar] [CrossRef]
- Coppola, F.; Faggioni, L.; Regge, D.; Giovagnoni, A.; Golfieri, R.; Bibbolino, C.; Miele, V.; Neri, E.; Grassi, R. Artificial intelligence: Radiologists’ expectations and opinions gleaned from a nationwide online survey. Radiol. Med. 2021, 126, 63–71. [Google Scholar] [CrossRef]
- Chianca, V.; Albano, D.; Messina, C.; Vincenzo, G.; Rizzo, S.; Del Grande, F.; Sconfienza, L.M. An update in musculoskeletal tumors: From quantitative imaging to radiomics. Radiol. Med. 2021, 126, 1095–1105. [Google Scholar] [CrossRef]
- Scapicchio, C.; Gabelloni, M.; Barucci, A.; Cioni, D.; Saba, L.; Neri, E. A deep look into radiomics. Radiol. Med. 2021, 126, 1296–1311. [Google Scholar] [CrossRef]
- Benedetti, G.; Mori, M.; Panzeri, M.M.; Barbera, M.; Palumbo, D.; Sini, C.; Muffatti, F.; Andreasi, V.; Steidler, S.; Doglioni, C.; et al. CT-derived radiomics features to discriminate histologic characteristics of pancreatic neuroendocrine tumors. Radiol. Med. 2021, 126, 745–760. [Google Scholar] [CrossRef]
- Nardone, V.; Reginelli, A.; Grassi, R.; Boldrini, L.; Vacca, G.; D’Ippolito, E.; Annunziata, S.; Farchione, A.; Belfiore, M.P.; Desideri, I.; et al. Delta radiomics: A systematic review. Radiol. Med. 2021, 126, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, T.; Homma, A.; Hatakeyama, H.; Kano, S.; Mizumachi, T.; Kakizaki, T.; Suzuki, T.; Fukuda, S. The role of prophylactic neck dissection and tumor thickness evaluation for patients with cN0 tongue squamous cell carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 3987–3992. [Google Scholar]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greenen, F.L.; Trotti, A. American Joint Committee on Cancer (AJCC). Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017; pp. 1–1854. [Google Scholar]
- Pentenero, M.; Gandolfo, S.; Carrozzo, M. Importance of tumor thickness and depth of invasion in nodal involvement and prognosis of oral squamous cell carcinoma: A review of the literature. Head Neck 2005, 27, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Byers, R.M.; El-Naggar, A.K.; Lee, Y.Y. Can we detect or predict the presence of occult nodal metastases in patients with squamous carcinoma of the oral tongue? Head Neck 1998, 20, 138–144. [Google Scholar] [CrossRef]
- Fukano, H.; Matsuura, H.; Hasegawa, Y.; Nakamura, S. Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head Neck 1997, 19, 205–210. [Google Scholar] [CrossRef]
- Kuan, E.C.; Mallen-St Clair, J.; Badran, K.W.; St John, M.A. How does depth of invasion influence the decision to do a neck dissection in clinically N0 oral cavity cancer? Laryngoscope 2016, 126, 547–548. [Google Scholar] [CrossRef] [Green Version]
- D’Cruz, A.K.; Vaish, R.; Kapre, N. Elective versus therapeutic neck dissection in node-negative oral cancer. N. Engl. J. Med. 2015, 373, 521–529. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, 122–124. [Google Scholar] [CrossRef] [Green Version]
- El-Hag, A.; Clark, R.A. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J. Immunol. 1987, 139, 2406–2413. [Google Scholar]
- Li, J.; Jiang, R.; Liu, W.S.; Liu, Q.; Xu, M.; Feng, Q.S.; Chen, L.Z.; Bei, J.X.; Chen, M.Y.; Zeng, Y.X. A large cohort study reveals the association of elevated peripheral blood lymphocyte-to-monocyte ratio with favorable prognosis in nasopharyngeal carcinoma. PLoS ONE 2013, 8, e83069. [Google Scholar] [CrossRef] [Green Version]
- Salzano, G.; Dell’Aversana Orabona, G.; Abbate, V.; Vaira, L.A.; Committeri, U.; Bonavolontà, P.; Piombino, P.; Maglitto, F.; Russo, C.; Russo, D.; et al. The prognostic role of the pre-treatment neutrophil to lymphocyte ratio (NLR) and tumor depth of invasion (DOI) in early-stage squamous cell carcinomas of the oral tongue. Oral Maxillofac. Surg. 2021, 26, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Abbate, V.; Dell’Aversana Orabona, G.; Salzano, G.; Bonavolontà, P.; Maglitto, F.; Romano, A.; Tarabbia, F.; Turri-Zanoni, M.; Attanasi, F.; Di Lauro, A.E.; et al. Pre-treatment Neutrophil to Lymphocyte Ratio as a predictor for occult cervical metastasis in early stage (T1-T2 cN0) squamous cell carcinoma of the oral tongue. Surg. Oncol. 2018, 27, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Chang, K.P.; Ho, T.Y.; Chou, W.C.; Hung, S.P.; Fan, K.H.; Chiang, Y.Y.; Chou, Y.C.; Tsang, N.M. Comparative prognostic value of different preoperative complete blood count cell ratios in patients with oral cavity cancer treated with surgery and postoperative radiotherapy. Cancer Med. 2021, 10, 1975–1988. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, N.; Wang, S.; Zou, W.; He, Y.; Ma, J.A.; Liu, P.; Liu, X.; Hu, C.; Hou, T. Systemic Inflammation Response Index Is a Predictor of Poor Survival in Locally Advanced Nasopharyngeal Carcinoma: A Propensity Score Matching Study. Front. Oncol. 2020, 10, 575417. [Google Scholar] [CrossRef]
- Valero, C.; Pardo, L.; Sansa, A.; Garcia Lorenzo, J.; López, M.; Quer, M.; León, X. Prognostic capacity of Systemic Inflammation Response Index (SIRI) in patients with head and neck squamous cell carcinoma. Head Neck 2020, 42, 336–343. [Google Scholar] [CrossRef]
- Romeo, V.; Cuocolo, R.; Ricciardi, C.; Ugga, L.; Cocozza, S.; Verde, F.; Stanzione, A.; Napolitano, V.; Russo, D.; Improta, G.; et al. Prediction of Tumor Grade and Nodal Status in Oropharyngeal and Oral Cavity Squamous-cell Carcinoma Using a Radiomic Approach. Anticancer Res. 2020, 40, 271–280. [Google Scholar] [CrossRef]
- Shan, J.; Jiang, R.; Chen, X.; Zhong, Y.; Zhang, W.; Xie, L.; Cheng, J.; Jiang, H. Machine Learning Predicts Lymph Node Metastasis in Early-Stage Oral Tongue Squamous Cell Carcinoma. J. Oral Maxillofac. Surg. 2020, 78, 2208–2218. [Google Scholar] [CrossRef]
- Tomita, H.; Yamashiro, T.; Heianna, J.; Nakasone, T.; Kimura, Y.; Mimura, H.; Murayama, S. Nodal-based radiomics analysis for identifying cervical lymph node metastasis at levels I and II in patients with oral squamous cell carcinoma using contrast-enhanced computed tomography. Eur. Radiol. 2021, 31, 7440–7449. [Google Scholar] [CrossRef]

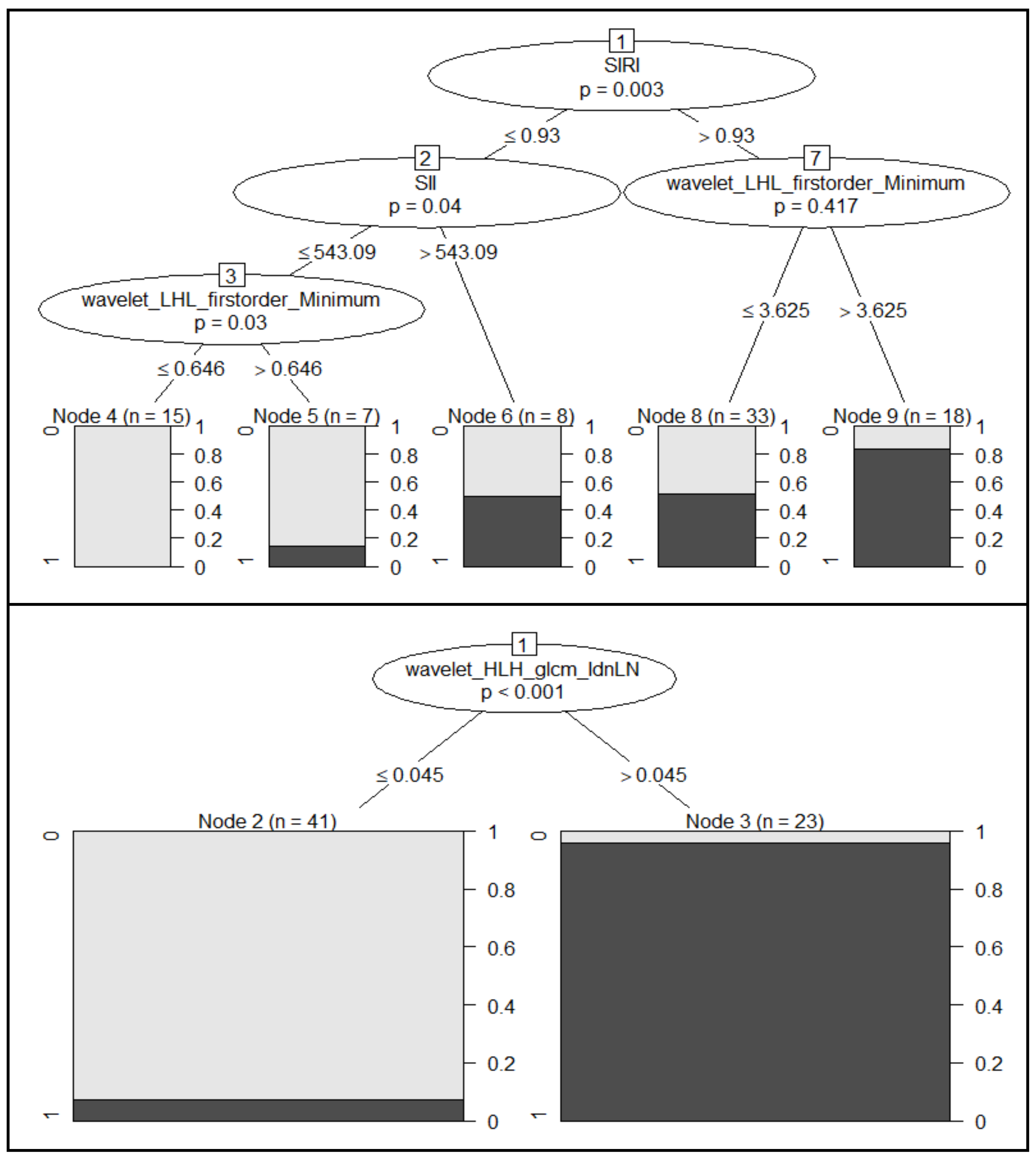
| Tumor Grading | |||||||
| DOI | NLR | PLR | LMR | SII | SIRI | SIZE | |
| AUC | 0.72 | 0.65 | 0.66 | 0.34 | 0.73 | 0.70 | 0.74 |
| Sensitivity | 0.68 | 0.89 | 0.51 | 0.97 | 0.68 | 0.86 | 1.00 |
| Specificity | 0.73 | 0.43 | 0.86 | 0.09 | 0.73 | 0.57 | 0.39 |
| PPV | 0.68 | 0.57 | 0.76 | 0.47 | 0.68 | 0.63 | 0.58 |
| NPV | 0.73 | 0.83 | 0.68 | 0.80 | 0.73 | 0.83 | 1.00 |
| Accuracy | 0.70 | 0.64 | 0.70 | 0.49 | 0.70 | 0.70 | 0.67 |
| Cut-off | 5.43 | 2.11 | 153.33 | 2.67 | 563.26 | 0.93 | 19.00 |
| Metastatic Lymph Nodes | |||||||
| DOI | NLR | PLR | LMR | SII | SIRI | SIZE | |
| AUC | 0.82 | 0.73 | 0.76 | 0.22 | 0.72 | 0.74 | 0.70 |
| Sensitivity | 0.89 | 0.74 | 0.80 | 0.06 | 0.77 | 0.74 | 0.86 |
| Specificity | 0.72 | 0.83 | 0.85 | 0.96 | 0.78 | 0.80 | 0.57 |
| PPV | 0.70 | 0.76 | 0.80 | 0.50 | 0.73 | 0.74 | 0.60 |
| NPV | 0.89 | 0.81 | 0.85 | 0.57 | 0.82 | 0.80 | 0.84 |
| Accuracy | 0.79 | 0.79 | 0.83 | 0.57 | 0.78 | 0.78 | 0.69 |
| Cut-off | 4.76 | 2.92 | 142.02 | 7.47 | 563.26 | 1.42 | 23.00 |
| Perineural Infiltration | |||||||
| DOI | NLR | PLR | LMR | SII | SIRI | SIZE | |
| AUC | 0.77 | 0.57 | 0.65 | 0.34 | 0.67 | 0.65 | 0.62 |
| Sensitivity | 0.74 | 0.69 | 0.59 | 0.03 | 0.69 | 0.64 | 0.36 |
| Specificity | 0.74 | 0.55 | 0.74 | 1.00 | 0.74 | 0.74 | 0.90 |
| PPV | 0.73 | 0.59 | 0.68 | 1.00 | 0.71 | 0.69 | 0.78 |
| NPV | 0.76 | 0.66 | 0.66 | 0.53 | 0.72 | 0.69 | 0.60 |
| Accuracy | 0.74 | 0.62 | 0.67 | 0.53 | 0.72 | 0.69 | 0.64 |
| Cut-off | 5.11 | 2.41 | 145.55 | 7.52 | 563.23 | 1.37 | 32.00 |
| Vascular Infiltration | |||||||
| DOI | NLR | PLR | LMR | SII | SIRI | SIZE | |
| AUC | 0.69 | 0.64 | 0.53 | 0.40 | 0.64 | 0.65 | 0.58 |
| Sensitivity | 0.93 | 0.50 | 0.54 | 0.96 | 0.64 | 0.68 | 0.93 |
| Specificity | 0.49 | 0.85 | 0.62 | 0.08 | 0.72 | 0.70 | 0.25 |
| PPV | 0.49 | 0.64 | 0.43 | 0.36 | 0.55 | 0.54 | 0.39 |
| NPV | 0.93 | 0.76 | 0.72 | 0.80 | 0.79 | 0.80 | 0.87 |
| Accuracy | 0.64 | 0.73 | 0.59 | 0.38 | 0.69 | 0.69 | 0.48 |
| Cut-off | 3.64 | 3.26 | 142.02 | 2.67 | 578.01 | 1.42 | 18.00 |
| Performance Results | Tumor Gradin—Tumor Area | Metastatic Lymph Nodes—Lymph Node Area | Perineural Infiltration—Tumor Area | Vascular Infiltration—Tumor Area |
|---|---|---|---|---|
| Original_Glszm_Highgraylevelzoneemphasis | Wavelet_HHH_Glrlm_Lowgraylevelrunemphasis | Wavelet_HHH_Glcm_Maximumprobability | Wavelet_LLL_Glszm_Highgraylevelzoneemphasis | |
| AUC | 0.66 | 0.93 | 0.65 | 0.62 |
| Sensitivity | 0.76 | 0.94 | 0.69 | 0.29 |
| Specificity | 0.66 | 0.98 | 0.67 | 0.98 |
| PPV | 0.65 | 0.97 | 0.66 | 0.89 |
| NPV | 0.76 | 0.96 | 0.70 | 0.72 |
| Accuracy | 0.70 | 0.96 | 0.68 | 0.74 |
| Cut-off | −0.19 | 0.39 | 4.97 | 0.03 |
| Performance Results | Clinical Features | Radiomics Features | Combination of Both Clinical and Radiomics Features | ||
|---|---|---|---|---|---|
| Logistic Regression | Logistic Regression | Logistic Regression | CART | CIDT | |
| Accuracy | 0.65 | 0.76 | 0.59 | 0.82 | 0.65 |
| Sensitivity | 0.44 | 0.78 | 0.56 | 0.78 | 0.33 |
| Specificity | 0.87 | 0.75 | 0.62 | 0.87 | 1.00 |
| No. of features | 3 | 2 | 6 | 4 | 4 |
| Performance Results | Clinical Features | Radiomics Features | Combination of Both Clinical and Radiomics Features | ||
|---|---|---|---|---|---|
| Logistic Regression | Logistic Regression | Logistic Regression | CART | CIDT | |
| Accuracy | 0.76 | 0.94 | 0.88 | 1.00 | 1.00 |
| Sensitivity | 0.57 | 1.00 | 0.86 | 1.00 | 1.00 |
| Specificity | 0.90 | 0.90 | 0.90 | 1.00 | 1.00 |
| No. of features | 4 | 15 | 20 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Committeri, U.; Fusco, R.; Di Bernardo, E.; Abbate, V.; Salzano, G.; Maglitto, F.; Dell’Aversana Orabona, G.; Piombino, P.; Bonavolontà, P.; Arena, A.; et al. Radiomics Metrics Combined with Clinical Data in the Surgical Management of Early-Stage (cT1–T2 N0) Tongue Squamous Cell Carcinomas: A Preliminary Study. Biology 2022, 11, 468. https://doi.org/10.3390/biology11030468
Committeri U, Fusco R, Di Bernardo E, Abbate V, Salzano G, Maglitto F, Dell’Aversana Orabona G, Piombino P, Bonavolontà P, Arena A, et al. Radiomics Metrics Combined with Clinical Data in the Surgical Management of Early-Stage (cT1–T2 N0) Tongue Squamous Cell Carcinomas: A Preliminary Study. Biology. 2022; 11(3):468. https://doi.org/10.3390/biology11030468
Chicago/Turabian StyleCommitteri, Umberto, Roberta Fusco, Elio Di Bernardo, Vincenzo Abbate, Giovanni Salzano, Fabio Maglitto, Giovanni Dell’Aversana Orabona, Pasquale Piombino, Paola Bonavolontà, Antonio Arena, and et al. 2022. "Radiomics Metrics Combined with Clinical Data in the Surgical Management of Early-Stage (cT1–T2 N0) Tongue Squamous Cell Carcinomas: A Preliminary Study" Biology 11, no. 3: 468. https://doi.org/10.3390/biology11030468
APA StyleCommitteri, U., Fusco, R., Di Bernardo, E., Abbate, V., Salzano, G., Maglitto, F., Dell’Aversana Orabona, G., Piombino, P., Bonavolontà, P., Arena, A., Perri, F., Maglione, M. G., Setola, S. V., Granata, V., Iaconetta, G., Ionna, F., Petrillo, A., & Califano, L. (2022). Radiomics Metrics Combined with Clinical Data in the Surgical Management of Early-Stage (cT1–T2 N0) Tongue Squamous Cell Carcinomas: A Preliminary Study. Biology, 11(3), 468. https://doi.org/10.3390/biology11030468










