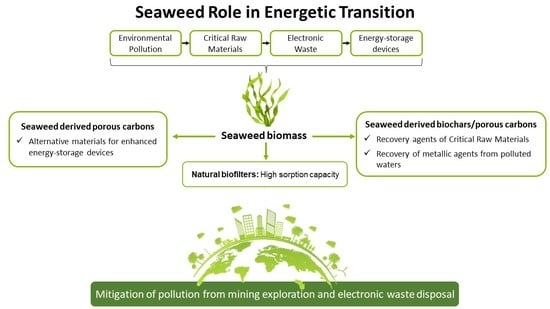
Seaweed’s Role in Energetic Transition—From Environmental Pollution Challenges to Enhanced Electrochemical Devices
Abstract
:Simple Summary
Abstract
1. Introduction
2. Critical Raw Materials Demand
Seaweed-Based Strategies for the Recovery of Critical Raw Materials
3. Electronic Devices’ End of Life—What Next?
Seaweed-Based Strategies for Heavy Metal Bioremediation and Recycling
4. Energy-Storage Devices in the 21st Century
Seaweed—A New Source of Carbons for Electrochemical Applications
5. Final Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NOAA. Global Climate Report—Annual. 2021. Available online: https://www.ncdc.noaa.gov/sotc/global/202113 (accessed on 10 January 2022).
- European Commission. The Paris Agreement. 2015. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 10 January 2022).
- European Commission. Delivering the European Green Deal; European Commission: Brussels, Belgium, 2021; Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal/delivering-european-green-deal_en#key-steps (accessed on 15 January 2022).
- IRENA. Global Energy Transformation: A Roadmap to 2050, 2019 ed.; International Renewable Energy Agency: Masdar City, Abu Dhabi, 2019. [Google Scholar]
- Monnet: A.L.C.Screen—Solutions for Critital Raw Materials—A European Expert Network. Report on Major Trends Affecting Future Demand for Critical Raw Materials. 2018. Available online: https://cordis.europa.eu/project/id/730227 (accessed on 12 January 2022).
- European Commission. Raw Materials for the Transition to Carbon Neutrality: The Demand Will Increase. Available online: https://ec.europa.eu/jrc/en/science-update/increased-demand-raw-materials-energy-transition (accessed on 12 January 2022).
- Pommeret, A.; Ricci, F.; Schubert, K. Critical raw materials for the energy transition. Eur. Econ. Rev. 2022, 141, 103991. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability; COM 474 Final; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- European Commission. Critical Raw Materials for Strategic Technologies and Sectors in the EU–a Foresight Study; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- European Commission. Report: Critical Raw Materials and the Circular Economy. 2018. Available online: https://ec.europa.eu/info/publications/report-critical-raw-materials-and-circular-economy_en (accessed on 12 January 2022).
- Wall, F. Rare Earth Elements. In Encyclopedia of Geology, 2nd ed.; Alderton, D., Elias, S.A., Eds.; Academic Press: Oxford, UK, 2021; pp. 680–693. [Google Scholar]
- Mancheri, N.A. Chinese Monopoly in Rare Earth Elements: Supply–Demand and Industrial Applications. China Rep. 2012, 48, 449–468. [Google Scholar] [CrossRef]
- Keith-Roach, M.; Grundfelt, B.; Höglund, L.O.; Kousa, A.; Pohjolainen, E.; Magistrati, P.; Aggelatou, V.; Olivieri, N.; Ferrari, A. Chapter 18—Environmental Legislation and Best Practice in the Emerging European Rare Earth Element Industry. In Rare Earths Industry; De Lima, I.B., Leal Filho, W., Eds.; Elsevier: Boston, MA, USA, 2016; pp. 279–291. [Google Scholar]
- United Nations. The 17 Sustainable Development Goals. 2015. Available online: https://sdgs.un.org/goals (accessed on 10 January 2022).
- European Commission. 2050 Long-Term Strategy. Available online: https://ec.europa.eu/clima/eu-action/climate-strategies-targets/2050-long-term-strategy_en (accessed on 10 January 2022).
- Keshtkar, A.R.; Moosavian, M.A.; Sohbatzadeh, H.; Mofras, M. La(III) and Ce(III) biosorption on sulfur functionalized marine brown algae Cystoseira indica by xanthation method: Response surface methodology, isotherm and kinetic study. Groundw. Sustain. Dev. 2019, 8, 144–155. [Google Scholar] [CrossRef]
- Costa, M.; Henriques, B.; Pinto, J.; Fabre, E.; Dias, M.; Soares, J.; Carvalho, L.; Vale, C.; Pinheiro-Torres, J.; Pereira, E. Influence of toxic elements on the simultaneous uptake of rare earth elements from contaminated waters by estuarine macroalgae. Chemosphere 2020, 252, 126562. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.; Henriques, B.; Soares, J.; Costa, M.; Dias, M.; Fabre, E.; Lopes, C.B.; Vale, C.; Pinheiro-Torres, J.; Pereira, E. A green method based on living macroalgae for the removal of rare-earth elements from contaminated waters. J. Environ. Manag. 2020, 263, 110376. [Google Scholar] [CrossRef]
- Jacinto, J.; Henriques, B.; Duarte, A.C.; Vale, C.; Pereira, E. Removal and recovery of Critical Rare Elements from contaminated waters by living Gracilaria gracilis. J. Hazard. Mater. 2018, 344, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Henriques, B.; Morais, T.; Cardoso, C.E.D.; Freitas, R.; Viana, T.; Ferreira, N.; Fabre, E.; Pinheiro-Torres, J.; Pereira, E. Can the recycling of europium from contaminated waters be achieved through living macroalgae? Study on accumulation and toxicological impacts under realistic concentrations. Sci. Total Environ. 2021, 786, 147176. [Google Scholar] [CrossRef] [PubMed]
- Fabre, E.; Henriques, B.; Viana, T.; Pinto, J.; Costa, M.; Ferreira, N.; Tavares, D.; Vale, C.; Pinheiro-Torres, J.; Pereira, E. Optimization of Nd(III) removal from water by Ulva sp. and Gracilaria sp. through Response Surface Methodology. J. Environ. Chem. Eng. 2021, 9, 105946. [Google Scholar] [CrossRef]
- Vafajoo, L.; Cheraghi, R.; Dabbagh, R.; McKay, G. Removal of cobalt (II) ions from aqueous solutions utilizing the pre-treated 2-Hypnea Valentiae algae: Equilibrium, thermodynamic, and dynamic studies. Chem. Eng. J. 2018, 331, 39–47. [Google Scholar] [CrossRef]
- Ramasamy, D.L.; Porada, S.; Sillanpää, M. Marine algae: A promising resource for the selective recovery of scandium and rare earth elements from aqueous systems. Chem. Eng. J. 2019, 371, 759–768. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, J.; Wu, J. Adsorptive removal of polycyclic aromatic hydrocarbons by detritus of green tide algae deposited in coastal sediment. Sci. Total Environ. 2019, 670, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Neveux, N.; Bolton, J.J.; Bruhn, A.; Roberts, D.A.; Ras, M. The bioremediation potential of seaweeds: Recycling nitrogen, phosphorus, and other waste products. Blue Biotechnol. Prod. Use Mar. Mol. 2018, 1, 217–241. [Google Scholar]
- Rosa, J.; Leston, S.; Crespo, D.; Freitas, A.; Pouca, A.S.V.; Barbosa, J.; Lemos, M.F.L.; Pardal, M.; Ramos, F. Uptake of enrofloxacin from seawater to the macroalgae Ulva and its use in IMTA systems. Aquaculture 2020, 516, 734609. [Google Scholar] [CrossRef]
- Sreekumar, N.; Udayan, A.; Srinivasan, S. 11—Algal Bioremediation of Heavy Metals. In Removal of Toxic Pollutants through Microbiological and Tertiary Treatment; Shah, M.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 279–307. [Google Scholar]
- Arumugam, N.D.O.; Chelliapan, S.; Kamyab, H.; Thirugnana, S.T.; Othman, N.; Nasri, N.S. Treatment of Wastewater Using Seaweed: A Review. Int. J. Environ. Res. Public Health 2018, 15, 2851. [Google Scholar] [CrossRef] [Green Version]
- Pinto, J.; Costa, M.; Henriques, B.; Soares, J.; Dias, M.; Viana, T.; Ferreira, N.; Vale, C.; Pinheiro-Torres, J.; Pereira, E. Competition among rare earth elements on sorption onto six seaweeds. J. Rare Earths 2021, 39, 734–741. [Google Scholar] [CrossRef]
- Abdellatif, M.M.; Soliman, S.M.A.; El-Sayed, N.H.; Abdellatif, F.H.H. Iota-carrageenan based magnetic aerogels as an efficient adsorbent for heavy metals from aqueous solutions. J. Porous Mater. 2020, 27, 277–284. [Google Scholar] [CrossRef]
- Filote, C.; Santos, S.C.R.; Popa, V.I.; Botelho, C.M.S.; Volf, I. Biorefinery of marine macroalgae into high-tech bioproducts: A review. Environ. Chem. Lett. 2021, 19, 969–1000. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Gadkari, S.; Martinez-Hernandez, E.; Ng, K.S.; Shemfe, M.; Torres-Garcia, E.; Lynch, J. Novel macroalgae (seaweed) biorefinery systems for integrated chemical, protein, salt, nutrient and mineral extractions and environmental protection by green synthesis and life cycle sustainability assessments. Green Chem. 2019, 21, 2635–2655. [Google Scholar] [CrossRef]
- Statista. Projected Electronic Waste Generation Worldwide from 2019 to 2030 (In Million Metric Tons). Available online: https://www.statista.com/statistics/1067081/generation-electronic-waste-globally-forecast/ (accessed on 10 January 2022).
- Forti, V.; Baldé, C.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020: Quantities, Flows, and the Circular Economy Potential; United Nations University: Geneva, Switzerland, 2020. [Google Scholar]
- Bloodworth, A. Resources: Track flows to manage technology-metal supply. Nature 2014, 505, 19–20. [Google Scholar] [CrossRef] [Green Version]
- Kiddee, P.; Naidu, R.; Wong, M.H. Electronic waste management approaches: An overview. Waste Manag. 2013, 33, 1237–1250. [Google Scholar] [CrossRef]
- Cucchiella, F.; D’Adamo, I.; Koh, S.C.; Rosa, P. Recycling of WEEEs: An economic assessment of present and future e-waste streams. Renew. Sustain. Energy Rev. 2015, 51, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Ferronato, N.; Torretta, V. Waste Mismanagement in Developing Countries: A Review of Global Issues. Int. J. Environ. Res. Public Health 2019, 16, 1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankit; Saha, L.; Kumar, V.; Tiwari, J.; Sweta; Rawat, S.; Singh, J.; Bauddh, K. Electronic waste and their leachates impact on human health and environment: Global ecological threat and management. Environ. Technol. Innov. 2021, 24, 102049. [Google Scholar] [CrossRef]
- Nithya, R.; Sivasankari, C.; Thirunavukkarasu, A. Electronic waste generation, regulation and metal recovery: A review. Environ. Chem. Lett. 2021, 19, 1347–1368. [Google Scholar] [CrossRef]
- Işıldar, A.; van Hullebusch, E.D.; Lenz, M.; Du Laing, G.; Marra, A.; Cesaro, A.; Panda, S.; Akcil, A.; Kucuker, M.A.; Kuchta, K. Biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment (WEEE)—A review. J. Hazard. Mater. 2019, 362, 467–481. [Google Scholar] [CrossRef]
- Pozdniakova, T.A.; Mazur, L.P.; Boaventura, R.A.R.; Vilar, V.J.P. Brown macro-algae as natural cation exchangers for the treatment of zinc containing wastewaters generated in the galvanizing process. J. Clean. Prod. 2016, 119, 38–49. [Google Scholar] [CrossRef]
- Mazur, L.P.; Pozdniakova, T.A.; Mayer, D.A.; de Souza, S.M.A.G.U.; Boaventura, R.A.R.; Vilar, V.J.P. Cation exchange prediction model for copper binding onto raw brown marine macro-algae Ascophyllum nodosum: Batch and fixed-bed studies. Chem. Eng. J. 2017, 316, 255–276. [Google Scholar] [CrossRef]
- Sarada, B.; Prasad, M.K.; Kumar, K.K.; Murthy, C.R. Cadmium removal by macro algae Caulerpa fastigiata: Characterization, kinetic, isotherm and thermodynamic studies. J. Environ. Chem. Eng. 2014, 2, 1533–1542. [Google Scholar] [CrossRef]
- Pandya, K.Y.; Patel, R.V.; Jasrai, R.T.; Hatt, N.B. Optimization of Cr and Cu biosorption by green marine algae Caulerpa racemosa Var. Cylindracea & Ulva lactuca. Int. J. Adv. Res. 2017, 5, 923–939. [Google Scholar]
- Deniz, F.; Karabulut, A. Biosorption of heavy metal ions by chemically modified biomass of coastal seaweed community: Studies on phycoremediation system modeling and design. Ecol. Eng. 2017, 106, 101–108. [Google Scholar] [CrossRef]
- Fawzy, M.A. Biosorption of copper ions from aqueous solution by Codium vermilara: Optimization, kinetic, isotherm and thermodynamic studies. Adv. Powder Technol. 2020, 31, 3724–3735. [Google Scholar] [CrossRef]
- Ibrahim, W.; Abdel Aziz, Y.; Hamdy, S.; Gad, N. Comparative Study for Biosorption of Heavy Metals from Synthetic Wastewater by Different Types of Marine Algae. J. Bioremediat. Biodegrad. 2018, 9, 425. [Google Scholar] [CrossRef] [Green Version]
- Patel, G.G.; Doshi, H.V.; Thakur, M.C. Biosorption and equilibrium study of copper by marine seaweeds from North West Coast of India. J. Environ. Sci. Toxicol. Food Technol. 2016, 10, 54–64. [Google Scholar]
- Talebian, A.; Keshtkar, A.R.; Moosavian, M.A. Continuous biosorption of U(VI) and Fe(II) using Cystoseira indica biomass packed bed column: Breakthrough curves studies in single, binary and multi-component systems. Korean J. Chem. Eng. 2016, 33, 2205–2214. [Google Scholar] [CrossRef]
- Riazi, M.; Keshtkar, A.R.; Moosavian, M.A. Biosorption of Th(IV) in a fixed-bed column by Ca-pretreated Cystoseira indica. J. Environ. Chem. Eng. 2016, 4, 1890–1898. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Q.; Chen, J.; Yang, J.; Zhang, Y.; Chen, Y.; Li, X.; Du, W.; Liang, A.; Ho, S.-H.; et al. Adsorption behavior of Cr(VI) by magnetically modified Enteromorpha prolifera based biochar and the toxicity analysis. J. Hazard. Mater. 2020, 395, 122658. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Suganya, E.; Lity, A.V.; Selvaraju, N. Equilibrium and kinetics studies of hexavalent chromium biosorption on a novel green macroalgae Enteromorpha sp. Res. Chem. Intermed. 2016, 42, 1275–1294. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y. Removal of Elemental Mercury Using Seaweed Biomass-Based Porous Carbons Prepared from Microwave Activation and H2O2 Modification. Energy Fuels 2021, 35, 2391–2401. [Google Scholar] [CrossRef]
- Rahman, S.M.; Sathasivam, K.V. Heavy metal biosorption potential of a Malaysian Rhodophyte (Eucheuma denticulatum) from aqueous solutions. Int. J. Environ. Sci. Technol. 2016, 13, 1973–1988. [Google Scholar] [CrossRef]
- Filote, C.; Volf, I.; Santos, S.C.R.; Botelho, C.M.S. Bioadsorptive removal of Pb(II) from aqueous solution by the biorefinery waste of Fucus spiralis. Sci. Total Environ. 2019, 648, 1201–1209. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Biosorption of Zn(II) from industrial effluents using sugar beet pulp and F. vesiculosus: From laboratory tests to a pilot approach. Sci. Total Environ. 2017, 598, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Henriques, B.; Lopes, C.B.; Figueira, P.; Rocha, L.S.; Duarte, A.C.; Vale, C.; Pardal, M.A.; Pereira, E. Bioaccumulation of Hg, Cd and Pb by Fucus vesiculosus in single and multi-metal contamination scenarios and its effect on growth rate. Chemosphere 2017, 171, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Figueira, P.; Henriques, B.; Teixeira, A.; Lopes, C.B.; Reis, A.T.; Monteiro, R.J.R.; Duarte, A.C.; Pardal, M.A.; Pereira, E. Comparative study on metal biosorption by two macroalgae in saline waters: Single and ternary systems. Environ. Sci. Pollut. Res. 2016, 23, 11985–11997. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.D.O.; Chelliapan, S.; Thirugnana, S.T.; Jasni, A.B. Optimisation of Heavy Metals Uptake from Leachate Using Red Seaweed Gracilaria changii. J. Environ. Treat. Tech. 2020, 8, 1089–1092. [Google Scholar]
- Abomohra, A.E.-F.; El-Hefnawy, M.E.; Wang, Q.; Huang, J.; Li, L.; Tang, J.; Mohammed, S. Sequential bioethanol and biogas production coupled with heavy metal removal using dry seaweeds: Towards enhanced economic feasibility. J. Clean. Prod. 2021, 316, 128341. [Google Scholar] [CrossRef]
- Cechinel, M.A.P.; Mayer, D.A.; Pozdniakova, T.A.; Mazur, L.P.; Boaventura, R.A.R.; de Souza, A.A.U.; de Souza, S.M.A.G.U.; Vilar, V.J.P. Removal of metal ions from a petrochemical wastewater using brown macro-algae as natural cation-exchangers. Chem. Eng. J. 2016, 286, 1–15. [Google Scholar] [CrossRef]
- Mazur, L.P.; Pozdniakova, T.A.; Mayer, D.A.; Boaventura, R.A.R.; Vilar, V.J.P. Design of a fixed-bed ion-exchange process for the treatment of rinse waters generated in the galvanization process using Laminaria hyperborea as natural cation exchanger. Water Res. 2016, 90, 354–368. [Google Scholar] [CrossRef]
- Hackbarth, F.V.; Maass, D.; de Souza, A.A.U.; Vilar, V.J.P.; de Souza, S.M.A.G.U. Removal of hexavalent chromium from electroplating wastewaters using marine macroalga Pelvetia canaliculata as natural electron donor. Chem. Eng. J. 2016, 290, 477–489. [Google Scholar] [CrossRef]
- Husien, S.; Labena, A.; El-Belely, E.F.; Mahmoud, H.M.; Hamouda, A.S. Adsorption studies of hexavalent chromium [Cr (VI)] on micro-scale biomass of Sargassum dentifolium, Seaweed. J. Environ. Chem. Eng. 2019, 7, 103444. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, S.; Balomajumder, C.; Kumar, S. Efficacy of Sargassum filipendula for the removal of Pb2+, Cd2+ and Ni2+ ions from aqueous solution: A comparative study. Desalin. Water Treat. 2018, 129, 216–226. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, S.; Kumar, S. Biosorption of lead ions from the aqueous solution by Sargassum filipendula: Equilibrium and kinetic studies. J. Environ. Chem. Eng. 2016, 4, 4587–4599. [Google Scholar] [CrossRef]
- Nascimento, W.J., Jr.; Silva, M.G.C.; Vieira, M.G.A. Competitive fixed-bed biosorption of Ag(I) and Cu(II) ions on Sargassum filipendula seaweed waste. J. Water Process Eng. 2020, 36, 101294. [Google Scholar] [CrossRef]
- Tabaraki, R.; Heidarizadi, E. Simultaneous biosorption of Arsenic (III) and Arsenic (V): Application of multiple response optimizations. Ecotoxicol. Environ. Saf. 2018, 166, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, V.; Govindaradjane, S.; Rajamohan, N.; Rajasimman, M. Biosorption potential of brown algae, Sargassum polycystum, for the removal of toxic metals, cadmium and zinc. Environ. Sci. Pollut. Res. 2021, 1–14. [Google Scholar] [CrossRef]
- Mahmood, Z.; Zahra, S.; Iqbal, M.; Raza, M.A.; Nasir, S. Comparative study of natural and modified biomass of Sargassum sp. for removal of Cd2+ and Zn2+ from wastewater. Appl. Water Sci. 2017, 7, 3469–3481. [Google Scholar] [CrossRef] [Green Version]
- Benaisa, S.; Arhoun, B.; El Mail, R.; Rodriguez-Maroto, J. Potential of brown algae biomass as new biosorbent of Iron: Kinetic, equilibrium and thermodynamic study. Proteins 2018, 3, 18. [Google Scholar]
- Mohammadi, M.; Izadbakhsh, E.; Ehsandoost, E. Biosorption of Cadmium as Toxic Metal from Aqueous Solutions by Marine Green Algae Ulva compressa (Linnaeus). Res. J. Environ. Toxicol. 2017, 11, 28–34. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Hamouda, R.A.; Mousa, I.E.; Abdel-Hamid, M.S.; Rabei, N.H. Statistical optimization for cadmium removal using Ulva fasciata biomass: Characterization, immobilization and application for almost-complete cadmium removal from aqueous solutions. Sci. Rep. 2018, 8, 12456. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, W.M.; Hassan, A.F.; Azab, Y.A. Biosorption of toxic heavy metals from aqueous solution by Ulva lactuca activated carbon. Egypt. J. Basic Appl. Sci. 2016, 3, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Henriques, B.; Teixeira, A.; Figueira, P.; Reis, A.T.; Almeida, J.; Vale, C.; Pereira, E. Simultaneous removal of trace elements from contaminated waters by living Ulva lactuca. Sci. Total Environ. 2019, 652, 880–888. [Google Scholar] [CrossRef]
- Bastos, E.; Schneider, M.; de Quadros, D.P.C.; Welz, B.; Batista, M.B.; Horta, P.A.; Rörig, L.R.; Barufi, J.B. Phytoremediation potential of Ulva ohnoi (Chlorophyta): Influence of temperature and salinity on the uptake efficiency and toxicity of cadmium. Ecotoxicol. Environ. Saf. 2019, 174, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Bădescu, I.S.; Bulgariu, D.; Bulgariu, L. Alternative utilization of algal biomass (Ulva sp.) loaded with Zn(II) ions for improving of soil quality. J. Appl. Phycol. 2017, 29, 1069–1079. [Google Scholar] [CrossRef]
- Romera, E.; González, F.; Ballester, A.; Blázquez, M.L.; Muñoz, J.A. Comparative study of biosorption of heavy metals using different types of algae. Bioresour. Technol. 2007, 98, 3344–3353. [Google Scholar] [CrossRef] [PubMed]
- Saldarriaga-Hernandez, S.; Hernandez-Vargas, G.; Iqbal, H.M.N.; Barceló, D.; Parra-Saldívar, R. Bioremediation potential of Sargassum sp. biomass to tackle pollution in coastal ecosystems: Circular economy approach. Sci. Total Environ. 2020, 715, 136978. [Google Scholar] [CrossRef] [PubMed]
- Pinteus, S.; Lemos, M.; Alves, C.; Neugebauer, A.; Silva, J.; Thomas, O.; Botana, L.; Gaspar, H.; Pedrosa, R. Marine invasive macroalgae: Turning a real threat into a major opportunity—the biotechnological potential of Sargassum muticum and Asparagopsis armata. Algal Res. 2018, 34, 217–234. [Google Scholar] [CrossRef]
- Macoi. Macoi—Portuguese Seaweeds Website. 2008. Available online: http://macoi.ci.uc.pt/ (accessed on 15 January 2022).
- Singh, A.; Sharma, R.; Pant, D.; Malaviya, P. Engineered algal biochar for contaminant remediation and electrochemical applications. Sci. Total Environ. 2021, 774, 145676. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Katiyar, R.; Patel, A.K.; Nguyen, T.-B.; Singhania, R.R.; Chen, C.-W.; Dong, C.-D. Adsorption of copper (II) in aqueous solution using biochars derived from Ascophyllum nodosum seaweed. Bioresour. Technol. 2021, 328, 124829. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Xin, J.; Sun, P.; Wu, D. Adsorption behavior and mechanism of Cr(VI) by modified biochar derived from Enteromorpha prolifera. Ecotoxicol. Environ. Saf. 2018, 164, 440–447. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Tian, J.; Liu, J.; Chen, G. Comparison of adsorption properties for cadmium removal from aqueous solution by Enteromorpha prolifera biochar modified with different chemical reagents. Environ. Res. 2020, 186, 109502. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Z.; Song, S.; Han, J.; Chen, H.; Wang, X.; Sun, R.; Cheng, J. Adsorption of copper(II) and lead(II) from seawater using hydrothermal biochar derived from Enteromorpha. Mar. Pollut. Bull. 2019, 149, 110586. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.L.; Paul, N.A.; de Nys, R.; Roberts, D.A. Simultaneous biosorption of selenium, arsenic and molybdenum with modified algal-based biochars. J. Environ. Manag. 2016, 165, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Son, E.-B.; Poo, K.-M.; Chang, J.-S.; Chae, K.-J. Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Sci. Total Environ. 2018, 615, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.-S. Adsorption characteristics of phenol and heavy metals on biochar from Hizikia fusiformis. Environ. Earth Sci. 2017, 76, 782. [Google Scholar] [CrossRef]
- Zhao, N.; Huang, H.; Lv, X.; Li, J.; Guo, G.; Liu, Y. Removal of Cr(III) from aqueous solutions using waste kelp-derived biochar. Desalin. Water Treat. 2020, 188, 223–231. [Google Scholar] [CrossRef]
- Park, S.H.; Cho, H.J.; Ryu, C.; Park, Y.-K. Removal of copper(II) in aqueous solution using pyrolytic biochars derived from red macroalga Porphyra tenera. J. Ind. Eng. Chem. 2016, 36, 314–319. [Google Scholar] [CrossRef]
- Poo, K.-M.; Son, E.-B.; Chang, J.-S.; Ren, X.; Choi, Y.-J.; Chae, K.-J. Biochars derived from wasted marine macro-algae (Saccharina japonica and Sargassum fusiforme) and their potential for heavy metal removal in aqueous solution. J. Environ. Manag. 2018, 206, 364–372. [Google Scholar] [CrossRef]
- Yacou, C.; Altenor, S.; Carene, B.; Gaspard, S. Chemical structure investigation of tropical Turbinaria turbinata seaweeds and its derived carbon sorbents applied for the removal of hexavalent chromium in water. Algal Res. 2018, 34, 25–36. [Google Scholar] [CrossRef]
- Beom-Sik, K. Removal of Cu2+ by biochars derived from green macroalgae. Environ. Sci. Pollut. Res. Int. 2016, 23, 985–994. [Google Scholar] [CrossRef]
- Shah, K.; Brahmbhatt, N.; Thaker, P. Study of Green Seaweed Biochar for Lead Adsorption from Aqueous Solution. Orient. J. Chem. 2021, 37, 1242–1247. [Google Scholar] [CrossRef]
- Senthilkumar, R.; Prasad, D.M.R.; Govindarajan, L.; Saravanakumar, K.; Prasad, B.S.N. Synthesis of green marine algal-based biochar for remediation of arsenic(V) from contaminated waters in batch and column mode of operation. Int. J. Phytoremed. 2020, 22, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.A.; Paul, N.A.; Dworjanyn, S.A.; Bird, M.I.; de Nys, R. Biochar from commercially cultivated seaweed for soil amelioration. Sci. Rep. 2015, 5, 9665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, M.I.; Wurster, C.M.; de Paula Silva, P.H.; Bass, A.M.; de Nys, R. Algal biochar—production and properties. Bioresour. Technol. 2011, 102, 1886–1891. [Google Scholar] [CrossRef]
- Kidgell, J.T.; De Nys, R.; Paul, N.A.; Roberts, D.A. The Sequential Application of Macroalgal Biosorbents for the Bioremediation of a Complex Industrial Effluent. PLoS ONE 2014, 9, e101309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Zhong, L.; Duan, J.; Feng, Y.; Yang, B.; Yang, L. Bioremediation of Wastewater by Iron Oxide-Biochar Nanocomposites Loaded with Photosynthetic Bacteria. Front. Microbiol. 2017, 8, 823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, M.Z.; Aziz, U. Supercapattery: Merging of battery-supercapacitor electrodes for hybrid energy storage devices. J. Energy Storage 2022, 46, 103823. [Google Scholar] [CrossRef]
- Zhou, H.; Li, H.; Li, L.; Liu, T.; Chen, G.; Zhu, Y.; Zhou, L.; Huang, H. Structural composite energy storage devices—A review. Mater. Today Energy 2022, 24, 100924. [Google Scholar] [CrossRef]
- Inagaki, M. Chapter 2.1—Advanced Carbon Materials. In Handbook of Advanced Ceramics, 2nd ed.; Somiya, S., Ed.; Academic Press: Oxford, UK, 2013; pp. 25–60. [Google Scholar]
- Libin, W.; Hu, X. Recent Advances in Porous Carbon Materials for Electrochemical Energy Storage. Chem. Asian J. 2018, 13, 1518–1529. [Google Scholar] [CrossRef]
- Long, W.; Fang, B.; Ignaszak, A.; Wu, Z.; Wang, Y.-J.; Wilkinson, D. Biomass-derived nanostructured carbons and their composites as anode materials for lithium ion batteries. Chem. Soc. Rev. 2017, 46, 7176–7190. [Google Scholar] [CrossRef]
- Perez-Salcedo, K.Y.; Ruan, S.; Su, J.; Shi, X.; Kannan, A.M.; Escobar, B. Seaweed-derived KOH activated biocarbon for electrocatalytic oxygen reduction and supercapacitor applications. J. Porous Mater. 2020, 27, 959–969. [Google Scholar] [CrossRef]
- Pourhosseini, S.E.M.; Norouzi, O.; Naderi, H.R. Study of micro/macro ordered porous carbon with olive-shaped structure derived from Cladophora glomerata macroalgae as efficient working electrodes of supercapacitors. Biomass Bioenergy 2017, 107, 287–298. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, K.; Wang, H.; Yuan, C.; Cao, B.; Qian, L.; Wang, S.; Zafar, F.F.; Ding, K.; Wang, Q. Preparation and property of N-doped porous carbon material by one-step pyrolysis of protein-rich algal biomass. J. Anal. Appl. Pyrolysis 2021, 157, 105221. [Google Scholar] [CrossRef]
- Du, W.; Wang, X.; Ju, X.; Xu, K.; Gao, M.; Zhang, X. Carbonized Enteromorpha prolifera with porous architecture and its polyaniline composites as high-performance electrode materials for supercapacitors. J. Electroanal. Chem. 2017, 802, 15–21. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Zhu, S.; Sun, D.; Jin, Y. Enteromorpha prolifera-derived carbon as a high-performance cathode material for lithium–sulfur batteries. J. Appl. Electrochem. 2017, 47, 631–639. [Google Scholar] [CrossRef]
- Wu, X.; Tian, Z.; Hu, L.; Huang, S.; Cai, J. Macroalgae-derived nitrogen-doped hierarchical porous carbons with high performance for H2 storage and supercapacitors. RSC Adv. 2017, 7, 32795–32805. [Google Scholar] [CrossRef] [Green Version]
- Ren, M.; Jia, Z.; Tian, Z.; Lopez, D.; Cai, J.; Titirici, M.-M.; Jorge, A.B. High Performance N-Doped Carbon Electrodes Obtained via Hydrothermal Carbonization of Macroalgae for Supercapacitor Applications. ChemElectroChem 2018, 5, 2686–2693. [Google Scholar] [CrossRef]
- Xie, T.; Wang, J.; Liu, X.; Shang, Y.; Ma, C.; Su, L.; Gong, L. Hierarchical porous activated carbon derived from Enteromorpha prolifera for superior electrochemical capacitive behavior. Ionics 2020, 26, 403–413. [Google Scholar] [CrossRef]
- Yu, W.; Wang, H.; Liu, S.; Mao, N.; Liu, X.; Shi, J.; Liu, W.; Chen, S.; Wang, X. N, O-codoped hierarchical porous carbons derived from algae for high-capacity supercapacitors and battery anodes. J. Mater. Chem. A 2016, 4, 5973–5983. [Google Scholar] [CrossRef]
- Sun, N.; Li, Z.; Zhang, X.; Qin, W.; Zhao, C.; Zhang, H.; Ng, D.H.L.; Kang, S.; Zhao, H.; Wang, G. Hierarchical Porous Carbon Materials Derived from Kelp for Superior Capacitive Applications. ACS Sustain. Chem. Eng. 2019, 7, 8735–8743. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Wang, Q.; Zheng, J.; Meng, C. Kelp-derived three-dimensional hierarchical porous N, O-doped carbon for flexible solid-state symmetrical supercapacitors with excellent performance. Appl. Surf. Sci. 2018, 447, 876–885. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, X.; Wang, Q.; Xu, X.; Zhou, X.; Bao, J. Kelp-derived hard carbons as advanced anode materials for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 5761–5769. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, L.; Fang, C.; Li, T.; Chen, J.; Yang, M.; Zhang, Q. Synthesis of porous carbon materials derived from Laminaria japonica via simple carbonization and activation for supercapacitors. J. Mater. Res. Technol. 2020, 9, 3261–3271. [Google Scholar] [CrossRef]
- Wang, J.; Qin, F.; Guo, Z.; Shen, W. Oxygen- and Nitrogen-Enriched Honeycomb-Like Porous Carbon from Laminaria japonica with Excellent Supercapacitor Performance in Aqueous Solution. ACS Sustain. Chem. Eng. 2019, 7, 11550–11563. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Hariram, M.; Vivekanandhan, S.; Navia, R. Sustainable biocarbon materials derived from Lessonia trabeculata macroalgae biomass residue for supercapacitor applications. Energy Storage 2021, 3, e222. [Google Scholar] [CrossRef]
- Wang, C.; Liu, T. Nori-based N, O, S, Cl co-doped carbon materials by chemical activation of ZnCl2 for supercapacitor. J. Alloys Compd. 2017, 696, 42–50. [Google Scholar] [CrossRef]
- Ouyang, H.; Ma, Y.; Gong, Q.; Li, C.; Huang, J.; Xu, Z.; Wei, B. Tailoring porous structure and graphitic degree of seaweed-derived carbons for high-rate performance lithium-ion batteries. J. Alloys Compd. 2020, 823, 153862. [Google Scholar] [CrossRef]
- Li, J.; Han, K.; Li, S. Porous carbons from Sargassum muticum prepared by H3PO4 and KOH activation for supercapacitors. J. Mater. Sci. Mater. Electron. 2018, 29, 8480–8491. [Google Scholar] [CrossRef]
- Jia, X.; Guo, F.; Zhan, Y.; Zhou, H.; Jiang, X.; Qian, L. Synthesis of porous carbon materials with mesoporous channels from Sargassum as electrode materials for supercapacitors. J. Electroanal. Chem. 2020, 873, 114353. [Google Scholar] [CrossRef]
- Guo, F.; Zhan, Y.; Jia, X.; Zhou, H.; Liang, S.; Qian, L. Fabrication of nitrogen-doped hierarchical porous carbons from Sargassum as advanced electrode materials for supercapacitors. New J. Chem. 2021, 45, 15514–15524. [Google Scholar] [CrossRef]
- Escobar, B.; Pérez-Salcedo, K.Y.; Alonso-Lemus, I.L.; Pacheco, D.; Barbosa, R. N-doped porous carbon from Sargassum spp. as metal-free electrocatalysts for oxygen reduction reaction in alkaline media. Int. J. Hydrogen Energy 2017, 42, 30274–30283. [Google Scholar] [CrossRef]
- Divya, P.; Prithiba, A.; Rajalakshmi, R. Biomass derived functional carbon from Sargassum Wightii seaweed for supercapacitors. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Tamil Nadu, India, 12–13 April 2019; Volume 561, p. 012078. [Google Scholar] [CrossRef] [Green Version]
- Divya, P.; Rajalakshmi, R. Facile synthesis of micro/mesoporous functional carbon from Turbinaria conoides seaweed for high performance of supercapacitors. Mater. Today Proc. 2020, 27, 44–53. [Google Scholar] [CrossRef]
- Chaudhary, J.P.; Gupta, R.; Mahto, A.; Vadodariya, N.; Dharmalingm, K.; Kotrappanavar, N.S.; Meena, R. Self-Doped Interwoven Carbon Network Derived from Ulva fasciata for All-Solid Supercapacitor Devices: Solvent-Free Approach to a Scalable Synthetic Route. ACS Sustain. Chem. Eng. 2019, 7, 174–186. [Google Scholar] [CrossRef]
- Gang, B.; Zhang, F.; Li, X.; Zhai, B.; Wang, X.; Song, Y. A Ulva lactuca-derived porous carbon for high-performance electrode materials in supercapacitor: Synergistic effect of porous structure and graphitization degree. J. Energy Storage 2021, 33, 102132. [Google Scholar] [CrossRef]
- Hencz, L.; Gu, X.; Zhou, X.; Martens, W.; Zhang, S. Highly porous nitrogen-doped seaweed carbon for high-performance lithium–sulfur batteries. J. Mater. Sci. 2017, 52, 12336–12347. [Google Scholar] [CrossRef]
- Senthil, C.; Park, J.W.; Shaji, N.; Sim, G.S.; Lee, C.W. Biomass seaweed-derived nitrogen self-doped porous carbon anodes for sodium-ion batteries: Insights into the structure and electrochemical activity. J. Energy Chem. 2022, 64, 286–295. [Google Scholar] [CrossRef]
- Vergés, A.; Campbell, A.H. Kelp forests. Curr. Biol. 2020, 30, R919–R920. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, G.; Rallo, H.; Casals, L.C.; Merino, A.; Amante, B. Comparison of the state of Lithium-Sulphur and lithium-ion batteries applied to electromobility. J. Environ. Manag. 2018, 226, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gissi, E.; Manea, E.; Mazaris, A.D.; Fraschetti, S.; Almpanidou, V.; Bevilacqua, S.; Coll, M.; Guarnieri, G.; Lloret-Lloret, E.; Pascual, M.; et al. A review of the combined effects of climate change and other local human stressors on the marine environment. Sci. Total Environ. 2021, 755, 142564. [Google Scholar] [CrossRef]
- McNutt, M. Climate Change Impacts. Science 2013, 341, 435. [Google Scholar] [CrossRef] [Green Version]
- Marín, A.; Goya, D. Mining—The dark side of the energy transition. Environ. Innov. Soc. Transit. 2021, 41, 86–88. [Google Scholar] [CrossRef]
| Antimony | Coking Coal | LREEs * | PGMs * | Tungsten |
|---|---|---|---|---|
| Baryte | Fluorspar | Indium | Phosphate rock | Vanadium |
| Beryllium | Gallium | Magnesium | Phosphorus | Bauxite |
| Bismuth | Germanium | Natural Graphite | Scandium | Lithium |
| Borate | Hafnium | Natural Rubber | Silicon metal | Titanium |
| Cobalt | HREEs * | Niobium | Tantalum | Strontium |
| Seaweed | Metal | Maximum Uptake Capacity | Reference |
|---|---|---|---|
| Cystoseira indica (xanthated) | La | 38.26 mg/g | [16] |
| Cystoseira indica (xanthated) | Ce | 41.44 mg/g | [16] |
| Fucus spiralis (live) | Y, La, Ce, Pr, Nd, Eu, Gd, Tb, Dy | ranging from 37% to 61% | [17] |
| Fucus vesiculosus (live) | La, Ce and Eu | >60% | [18] |
| Fucus vesiculosus (live) | Y, La, Ce, Pr, Nd, Eu, Gd, Tb, Dy | 55–74% | [17] |
| Gracilaria gracilis (live) | La, Ce, Pr, Gd, and Nd | >60% | [18] |
| Gracilaria gracilis (live) | Y, Ce, Nd, Eu and La. | 100% | [19] |
| Gracilaria sp. (live) | Eu | >85% | [20] |
| Gracilaria sp. | Y, La, Ce, Pr, Nd, Eu, Gd, Tb, Dy | ranging from 60 to 93% | [17] |
| Gracilaria sp. (live) | Nd | >90% | [21] |
| Hypnea valentiae | Co | 47.44 mg/g | [22] |
| Osmundea pinnatifida (live) | La, Ce | >60% | [18] |
| Osmundea pinnatifida (live) | Y, La, Ce, Pr, Nd, Eu, Gd, Tb, Dy | ranging from 35 to 61% | [17] |
| Posidonia oceanica | Sc | 66.81 mg/g | [23] |
| Ulva intestinalis (live) | La, Ce | >60% | [18] |
| Ulva intestinalis (live) | Y, La, Ce, Pr, Nd, Eu, Gd, Tb, Dy | ranging from 63 to 88% | [17] |
| Ulva lactuca (live) | Y, La, Ce, Pr, Nd, Eu, Gd, Tb, Dy | >60% | [18] |
| Ulva lactuca (live) | Eu | >85% | [20] |
| Ulva lactuca (live) | Y, La, Ce, Pr, Nd, Eu, Gd, Tb, Dy | ranging from 80 to 98% | [17] |
| Ulva sp. (live) | Nd | >90% | [21] |
| Seaweed | Metal | Maximum Uptake Capacity | Reference |
|---|---|---|---|
| Ascophyllum nodosum | Zn(II) | 2.34 mmol/g | [42] |
| Ascophyllum nodosum | Cu(II) | 20.00 mg/L | [43] |
| Caulerpa fastigiata | Cd(II) | 16.48 mg/g (92.01%) | [44] |
| Caulerpa racemosa | Cr | 20% | [45] |
| Caulerpa racemosa | Cu | 43% | [45] |
| Chaetomorpha sp., Polysiphonia sp., Ulva sp. and Cystoseira sp. (combined) | Zn(II) | 115.20 mg/g | [46] |
| Codium vermilara | Cu(II) | >85% | [47] |
| Colpomenia sinuosa | Ni(II) Pb(II) Cd(II) | 89% | [48] |
| Cystoseira indica | Cu(II) | 30.86 mg/g | [49] |
| Cystoseira indica | U(VI) | 250.00 mg/L | [50] |
| Cystoseira indica | Fe(II) | 900.00 mg/L | [50] |
| Cystoseira indica | Th(VI) | 90.00 mg/L | [51] |
| Enteromorpha prolifera | Cr(VI) | 95.25 mg/g | [52] |
| Enteromorpha sp. | Cr(VI) | 5.35 mg/g | [53] |
| Enteromorpha sp. | Hg | 5.357 mg/g | [54] |
| Eucheuma denticulatum | Pb(II) | 81.87 mg/g | [55] |
| Eucheuma denticulatum | Cu(II) | 66.23 mg/g | [55] |
| Eucheuma denticulatum | Fe(II) | 51.02 mg/g | [55] |
| Eucheuma denticulatum | Zn(II) | 43.48 mg/g | [55] |
| Fucus spiralis | Zn(II) | 2.04 mmol/g | [42] |
| Fucus spiralis (waste) | Pb(II) | 132.00 mg/g | [56] |
| Fucus vesiculosus | Zn(II) | 400.00 mg/L | [57] |
| Fucus vesiculosus | Cd | 22–76% | [58] |
| Fucus vesiculosus | Pb | 65% | [58] |
| Fucus vesiculosus | Pb | 86% | [59] |
| Fucus vesiculosus (live seaweed) | Hg | 95% | [58] |
| Gracilaria changii | Fe(II) | 45% | [60] |
| Gracilaria changii | Cr(VI) | 35% | [60] |
| Gracilaria changii | Ni(II) | 30% | [60] |
| Gracilaria spp. | Cu(II) | 42% | [61] |
| Halimeda tuna | Cu(II) | 17.92 mg/g | [49] |
| Lyengaria stellata | Cu(II) | 46.29 mg/g | [49] |
| Jania rubens | Ni(II) Pb(II) Cd(II) | 91% | [48] |
| Laminaria hyperborea | Zn(II) | 2.22 mmol/g | [42] |
| Laminaria hyperborea | Cu(II) | 2.50 mg/L | [62] |
| Laminaria hyperborea | Zn(II) | 4.30 mg/L | [62] |
| Laminaria hyperborea | Ni(II) | 4.20 mg/L | [62] |
| Laminaria hyperborea | Zn(II) | 21.5 mg/L | [63] |
| Lobophora variegata | Cu(II) | 38.02 mg/g | [49] |
| Pelvetia caniculata | Cr(VI) | 2.10 mmol/g | [64] |
| Pelvetia caniculata | Zn(II) | 1373.00 mg/L | [64] |
| Pelvetia caniculata | Fe(II) | 44.70 mg/L | [64] |
| Pelvetia caniculata | Zn(II) | 2.22 mmol/g | [42] |
| Sargassum cinereum | Cu(II) | 34.01 mg/g | [49] |
| Sargassum dentifolium | Cr(VI) | ~100% | [65] |
| Sargassum filipendula | Cd(II) | 103.50 mg/g | [66] |
| Sargassum filipendula | Ni(II) | 34.30 mg/g | [66] |
| Sargassum filipendula | Pb(II) | 96% | [67] |
| Sargassum filipendula | Ag(I) | 0.39 mmol/g | [68] |
| Sargassum filipendula | Cu(II) | 0.64 mmol/g | [68] |
| Sargassum filipendula | Pb(II) | 367.94 mg/g | [66] |
| Sargassum filipendula | Pb(II) | 285.00 mg/g | [67] |
| Sargassum glaucescens | As(III) | 207.30 mg/g | [69] |
| Sargassum glaucescens | As(III) | 116.60 mg/g | [69] |
| Sargassum glaucescens | As(V) | 207.30 mg/g | [69] |
| Sargassum glaucescens | As(V) | 116.00 mg/g | [69] |
| Sargassum polycystum | Cd(II) | 105.26 mg/g | [70] |
| Sargassum polycystum | Zn(II) | 116.20 mg/g | [70] |
| Sargassum sp. | Cd(II) | 2.89 mg/g (95%) | [71] |
| Sargassum sp. | Zn(II) | 1.85 mg/g (90%) | [71] |
| Sargassum sp. | Cu(II) | 95% | [61] |
| Sargassum tenerrimum | Cu(II) | 39.84 mg/g | [49] |
| Sargassum vulgare | Fe(III) | 20.82 mg/g | [72] |
| Ulva compressa | Cd(II) | 95% | [73] |
| Ulva fasciata | Cd(II) | ~100% | [74] |
| Ulva lactuca | Cu(II) | 60.97 mg/g | [49] |
| Ulva lactuca | Cr | 62% | [45] |
| Ulva lactuca | Cu | 70% | [45] |
| Ulva lactuca | Hg | 96–99% | [59] |
| Ulva lactuca | Pb | 86% | [59] |
| Ulva lactuca | Cd | <20% | [59] |
| Ulva lactuca | Ni(II) Cd(II) Pb(II) | 85% | [48] |
| Ulva lactuca | Cd(II) | 62.5 mg/g | [75] |
| Ulva lactuca | Pb(II) | 68.9 mg/g | [75] |
| Ulva lactuca | Cr(III) | 60.9 mg/g | [75] |
| Ulva lactuca | Cu(II) | 64.5 mg/g | [75] |
| Ulva lactuca (live seaweed) | Hg | 98% | [76] |
| Ulva lactuca (live seaweed) | Pb | 87% | [76] |
| Ulva lactuca (live seaweed) | Cu | 86% | [76] |
| Ulva lactuca (live seaweed) | Ni | 77% | [76] |
| Ulva lactuca (live seaweed) | Mn | 74% | [76] |
| Ulva lactuca (live seaweed) | Cr | 72% | [76] |
| Ulva lactuca (live seaweed) | Cd | 56% | [76] |
| Ulva lactuca (live seaweed) | As | 48% | [76] |
| Ulva ohnoi | Cd | 81% | [77] |
| Ulva sp. | Zn | 29.63 mg/g | [78] |
| Ulva spp. | Cu(II) | 65% | [61] |
| Seaweed | Metal | Maximum Uptake Capacity | Reference |
|---|---|---|---|
| Ascophyllum nodosum biochar | Cu(II) | 223.00 mg/g (>99% removal) | [85] |
| Enteromorpha prolifera (magnetically modified biochar) | Cr(VI) | 88.17 mg/g | [86] |
| Enteromorpha prolifera (H3PO4 modified biochar) | Cd(II) | 423.00 mg/g | [87] |
| Enteromorpha sp biochar | Cu(II) | 91% | [88] |
| Enteromorpha sp biochar | Pb(II) | 54% | [88] |
| Gracilaria sp. waste (Fe biochar) | As | 62.50 mg/g | [89] |
| Gracilaria sp. waste (Fe biochar) | Mo | 78.50 mg/g | [89] |
| Gracilaria sp. waste (Fe biochar) | Se | 14.90 mg/g | [89] |
| Hizikia sp. (engineered biochar) | Cd(II) | 19.40 mg/g | [90] |
| Hizikia sp. (engineered biochar) | Cu(II) | 47.75 mg/g | [90] |
| Hizikia sp. (engineered biochar) | Zn(II) | 19.13 mg/g | [90] |
| Hizikia fusiformis biochar | Ni(II) | 12.10 mg/g | [91] |
| Hizikia fusiformis biochar | Zn(II) | 22.20 mg/g | [91] |
| Hizikia fusiformis biochar | Cu(II) | 2.24 mg/g | [91] |
| Hizikia fusiformis biochar | Ld(II) | 2.89 mg/g | [91] |
| Hizikia fusiformis biochar | Cd(II) | 22.00 mg/g | [91] |
| Kelp (engineered biochar) | Cd(II) | 23.16 mg/g | [90] |
| Kelp (engineered biochar) | Cu(II) | 55.86 mg/g | [90] |
| Kelp (engineered biochar) | Zn(II) | 22.22 mg/g | [90] |
| Kelp biochar | Cr(III) | 39.16 mg/g (91.13%) | [92] |
| Oedogonium sp. (Fe biochar) | Mo | 67.40 mg/g | [89] |
| Oedogonium sp. (Fe biochar) | As | 80.70 mg/g | [89] |
| Oedogonium sp. (Fe biochar) | Se | 36.80 mg/g | [89] |
| Porphyra tenera biochar | Cu(II) | 75.10 mg/g | [93] |
| Porphyra tenera biochar (steam activated) | Cu(II) | ~78.00 mg/g | [93] |
| Porphyra tenera biochar (KOH-activated) | Cu(II) | 75.10 mg/g | [93] |
| Saccharina japonica biochar | Cu(II | 98.60 mg/g (>98%) | [94] |
| Saccharina japonica biochar | Cd(II) | 60.70 mg/g (>98%) | [94] |
| Saccharina japonica biochar | Zn(II) | 84.30 mg/g (>98%) | [94] |
| Sargassum fusiforme biochar | Cu(II | 94.10 mg/g (>86%) | [94] |
| Sargassum fusiforme biochar | Cd(II) | 37.20 mg/g (>86%) | [94] |
| Sargassum fusiforme biochar | Zn(II) | 43.00 mg/g (>86%) | [94] |
| Sargassum sp. | Hg | 7.41 mg/g | [54] |
| Turbinaria turbinata biochar | Cr(VI) | 12.60 mg/g | [95] |
| Ulva compressa biochar (steam activated) | Cu(II) | 137.00 mg/g | [96] |
| Ulva lactuca KOH activated carbon | Cu (II) | 84.70 mg/g | [75] |
| Ulva lactuca KOH activated carbon | Cr(III) | 81.90 mg/g | [75] |
| Ulva lactuca KOH activated carbon | Cd(II) | 84.60 mg/g | [75] |
| Ulva lactuca KOH activated carbon | Pb(II) | 83.30 mg/g | [75] |
| Ulva lactuca biochar | Pb(II) | 3.49 mg/g | [97] |
| Ulva reticulata biochar | Ar (V) | 8.12 mg/g | [98] |
| Seaweed | Preparation of Seaweed Biochar | Main Achievements | Reference |
|---|---|---|---|
| Ascophyllum nodosum |
|
| [108] |
| Cladophora glomerata |
|
| [109] |
| Enteromorpha clathrate |
|
| [110] |
| Enteromorpha prolifera |
|
| [111] |
| Enteromorpha prolifera |
|
| [112] |
| Enteromorpha prolifera |
|
| [113] |
| Enteromorpha prolifera |
|
| [114] |
| Enteromorpha prolifera |
|
| [115] |
| Enteromorpha sp. |
|
| [116] |
| Kelp |
|
| [117] |
| Kelp |
|
| [118] |
| Kelp |
|
| [119] |
| Laminaria japonica |
|
| [120] |
| Laminaria japonica |
|
| [121] |
| Lessonia trabeculata |
|
| [122] |
| Nori |
|
| [123] |
| Porphyra sp. |
|
| [124] |
| Sargassum muticum |
|
| [125] |
| Sargassum sp. |
|
| [126] |
| Sargassum sp. |
|
| [127] |
| Sargassum spp. |
|
| [128] |
| Sargassum wightii |
|
| [129] |
| Turbinaria cunoides |
|
| [130] |
| Ulva fasciata |
|
| [131] |
| Ulva lactuca |
|
| [132] |
| Brown seaweed |
|
| [133] |
| Seaweed Biomass |
|
| [134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinteus, S.; Susano, P.; Alves, C.; Silva, J.; Martins, A.; Pedrosa, R. Seaweed’s Role in Energetic Transition—From Environmental Pollution Challenges to Enhanced Electrochemical Devices. Biology 2022, 11, 458. https://doi.org/10.3390/biology11030458
Pinteus S, Susano P, Alves C, Silva J, Martins A, Pedrosa R. Seaweed’s Role in Energetic Transition—From Environmental Pollution Challenges to Enhanced Electrochemical Devices. Biology. 2022; 11(3):458. https://doi.org/10.3390/biology11030458
Chicago/Turabian StylePinteus, Susete, Patrícia Susano, Celso Alves, Joana Silva, Alice Martins, and Rui Pedrosa. 2022. "Seaweed’s Role in Energetic Transition—From Environmental Pollution Challenges to Enhanced Electrochemical Devices" Biology 11, no. 3: 458. https://doi.org/10.3390/biology11030458
APA StylePinteus, S., Susano, P., Alves, C., Silva, J., Martins, A., & Pedrosa, R. (2022). Seaweed’s Role in Energetic Transition—From Environmental Pollution Challenges to Enhanced Electrochemical Devices. Biology, 11(3), 458. https://doi.org/10.3390/biology11030458