Influence of Apnea Hypopnea Index and the Degree of Airflow Limitation on Endothelial Function in Patients Undergoing Diagnostic Coronary Angiography
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef]
- Bradley, T.D.; Floras, J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009, 373, 82–93. [Google Scholar] [CrossRef]
- Lee, C.-H.; Sethi, R.; Shao-Liang, C.; Thun-How, O.; Hein, T.; Jim, M.-H.; Loo, G.; Koo, C.-Y.; Gao, X.-F.; Chandra, S.; et al. Obstructive Sleep Apnea and Cardiovascular Events After Percutaneous Coronary Intervention. Circulation 2016, 133, 2008–2017. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, S.; Barbé, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef]
- Somuncu, M.U.; Bulut, U.; Karakurt, H.; Utkusavas, A.; Akbay, E.; Kilinc, F.K. The Relationship Between Obstructive Sleep Apnea and Coronary Plaque: A Coronary Computed Tomographic Angiography Study. Acta Cardiol. Sin. 2019, 35, 325–334. [Google Scholar] [CrossRef]
- Tanaka, A.; Tomiyama, H.; Maruhashi, T.; Matsuzawa, Y.; Miyoshi, T.; Kabutoya, T.; Kario, K.; Sugiyama, S.; Munakata, M.; Ito, H.; et al. Physiological Diagnostic Criteria for Vascular Failure. Hypertension 2018, 72, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Wanger, J.; Clausen, J.L.; Coates, A.; Pedersen, O.F.; Brusasco, V.; Burgos, F.; Casaburi, R.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Celermajer, D.S. Reliable Endothelial Function Testing: At our fingertips? Circulation 2008, 117, 2428–2430. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Shpilsky, D.; Erqou, S.; Patel, S.; Kip, K.E.; Ajala, O.; Aiyer, A.; Strollo, P.J.; Reis, S.E.; Olafiranye, O. Association of obstructive sleep apnea with microvascular endothelial dysfunction and subclinical coronary artery disease in a community-based population. Vasc. Med. 2018, 23, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, M.J.; Schaefer, C.A.; Krycki, J.; Schueler, R.; Pizarro, C.; Nickenig, G.; Steinmetz, M.; Skowasch, D.; Tuleta, I. Impairment of vascular strain in patients with obstructive sleep apnea. PLoS ONE 2018, 13, e0193397. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Eraso, C.; Muñoz-Hernández, R.; Cruz, M.I.A.; Luna, R.M.; Bernal, C.C.; López-Campos, J.L.; Stiefel, P.; Armengol, S. Relationship between the endothelial dysfunction and the expression of the β1-subunit of BK channels in a non-hypertensive sleep apnea group. PLoS ONE 2019, 14, e0217138. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Qian, Y.; Guan, J.; Yi, H.; Yin, S. Patients with Obstructive Sleep Apnea Display Decreased Flow-Mediated Dilatation: Evidence from a Meta-Analysis. Med. Sci. Monit. 2017, 23, 1069–1082. [Google Scholar] [CrossRef]
- Wang, J.; Yu, W.; Gao, M.; Zhang, F.; Gu, C.; Yu, Y.; Wei, Y. Impact of Obstructive Sleep Apnea Syndrome on Endothelial Function, Arterial Stiffening, and Serum Inflammatory Markers: An Updated Meta-analysis and Metaregression of 18 Studies. J. Am. Heart Assoc. 2015, 4, e002454. [Google Scholar] [CrossRef]
- Kyotani, Y.; Takasawa, S.; Yoshizumi, M. Proliferative Pathways of Vascular Smooth Muscle Cells in Response to Intermittent Hypoxia. Int. J. Mol. Sci. 2019, 20, 2706. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, Y.; Yamashita, A.; Iwakiri, T.; Sugita, C.; Okuyama, N.; Kitamura, K.; Asada, Y. Vascular wall hypoxia promotes arterial thrombus formation via augmentation of vascular thrombogenicity. Thromb. Haemost. 2015, 114, 158–172. [Google Scholar] [CrossRef]
- Kaczmarek, E.; Bakker, J.P.; Clarke, D.N.; Csizmadia, E.; Kocher, O.; Veves, A.; Tecilazich, F.; O’Donnell, C.; Ferran, C.; Malhotra, A. Molecular Biomarkers of Vascular Dysfunction in Obstructive Sleep Apnea. PLoS ONE 2013, 8, e70559. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Antolín, J.; Carmona-Bernal, C.; Rivero-Valdenebro, V.; Villar, J.; Capote, F.; López-Barneo, J. Maxi-K+ Channel 1 Expression in Sleep Apnea Patients and Its Modulation by CPAP Treatment. Am. J. Hypertens. 2009, 22, 197–202. [Google Scholar] [CrossRef]
- Bayram, N.A.; Ciftci, B.; Keles, T.; Durmaz, T.; Turhan, S.; Bozkurt, E.; Peker, Y. Endothelial Function in Normotensive Men with Obstructive Sleep Apnea Before and 6 Months After CPAP Treatment. Sleep 2009, 32, 1257–1263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pamidi, S.; Wroblewski, K.; Stepien, M.; Sharif-Sidi, K.; Kilkus, J.; Whitmore, H.; Tasali, E. Eight Hours of Nightly Continuous Positive Airway Pressure Treatment of Obstructive Sleep Apnea Improves Glucose Metabolism in Patients with Prediabetes. A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2015, 192, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Bratton, D.J.; Stradling, J.R.; Barbé, F.; Kohler, M. Effect of CPAP on blood pressure in patients with minimally symptomatic obstructive sleep apnoea: A meta-analysis using individual patient data from four randomised controlled trials. Thorax 2014, 69, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.A.; García-Río, F.; Alonso-Fernández, A.; Martínez, I.; Villamor, J. Pulmonary hypertension in obstructive sleep apnoea: Effects of continuous positive airway pressure: A randomized, controlled cross-over study. Eur. Heart J. 2006, 27, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, W.T.; bin Nasir, U.; Alqalyoobi, S.; O’Neal, W.; Mawri, S.; Sabbagh, S.; Soliman, E.Z.; Al-Mallah, M. Meta-Analysis of Continuous Positive Airway Pressure as a Therapy of Atrial Fibrillation in Obstructive Sleep Apnea. Am. J. Cardiol. 2015, 116, 1767–1773. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Barbé, F.; Durán-Cantolla, J.; Sanchez-De-La-Torre, M.; Martinez-Alonso, M.; Carmona, C.; Barceló, A.; Chiner, E.; Masa, J.F.; González, M.; Marín, J.M.; et al. Effect of Continuous Positive Airway Pressure on the Incidence of Hypertension and Cardiovascular Events in Nonsleepy Patients with Obstructive Sleep Apnea: A randomized controlled trial. JAMA 2012, 307, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Peker, Y.; Glantz, H.; Eulenburg, C.; Wegscheider, K.; Herlitz, J.; Thunström, E. Effect of Positive Airway Pressure on Cardiovascular Outcomes in Coronary Artery Disease Patients with Nonsleepy Obstructive Sleep Apnea. The RICCADSA Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2016, 194, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Duran, C.A.; Rahman, H.; Lekkala, M.; Saleem, M.A.; Kaluski, E. A meta-analysis of continuous positive airway pressure therapy in prevention of cardiovascular events in patients with obstructive sleep apnoea. Eur. Heart J. 2017, 39, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.J.; Herrington, D.M.; Redline, S.; Benjamin, E.J.; Robbins, J.A. Sleep Apnea and Markers of Vascular Endothelial Function in a Large Community Sample of Older Adults. Am. J. Respir. Crit. Care Med. 2004, 169, 354–360. [Google Scholar] [CrossRef]
- Seif, F.; Patel, S.; Walia, H.; Rueschman, M.; Bhatt, D.L.; Gottlieb, D.J.; Lewis, E.F.; Patil, S.P.; Punjabi, N.M.; Babineau, D.C.; et al. Association between obstructive sleep apnea severity and endothelial dysfunction in an increased background of cardiovascular burden. J. Sleep Res. 2013, 22, 443–451. [Google Scholar] [CrossRef]
- Bironneau, V.; Tamisier, R.; Trzepizur, W.; Andriantsitohaina, R.; Berger, M.; Goupil, F.; Joyeux-Faure, M.; Jullian-Desayes, I.; Launois, S.; Le Vaillant, M.; et al. Sleep apnoea and endothelial dysfunction: An individual patient data meta-analysis. Sleep Med. Rev. 2020, 52, 101309. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of Sleep Apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef]
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet 2013, 383, 736–747. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Gurubhagavatula, I.; Teff, K.; Rader, D.J.; Wadden, T.A.; Townsend, R.; Foster, G.D.; Maislin, G.; Saif, H.; Broderick, P.; et al. CPAP, Weight Loss, or Both for Obstructive Sleep Apnea. N. Engl. J. Med. 2014, 370, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Lyons, O.; Bradley, T.D. Heart Failure and Sleep Apnea. Can. J. Cardiol. 2015, 31, 898–908. [Google Scholar] [CrossRef]
- Mendelson, M.; Lyons, O.; Yadollahi, A.; Inami, T.; Oh, P.; Bradley, T.D. Effects of exercise training on sleep apnoea in patients with coronary artery disease: A randomised trial. Eur. Respir. J. 2016, 48, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.; Klein, R.; Jerosch-Herold, M.; Hoffman, E.A.; Ahmed, F.S.; Jacobs, D.R.; Klein, B.E.K.; Wong, T.Y.; Lima, J.A.C.; Cotch, M.F.; et al. The Association of Systemic Microvascular Changes with Lung Function and Lung Density: A Cross-Sectional Study. PLoS ONE 2012, 7, e50224. [Google Scholar] [CrossRef]
- Ghavampour, S.; Kleefeldt, F.; Bömmel, H.; Volland, J.; Paus, A.; Horst, A.; Pfeiffer, V.; Hübner, S.; Wagner, N.; Rueckschloss, U.; et al. Endothelial barrier function is differentially regulated by CEACAM1-mediated signaling. FASEB J. 2018, 32, 5612–5625. [Google Scholar] [CrossRef]
- Kyomoto, Y.; Kanazawa, H.; Tochino, Y.; Watanabe, T.; Asai, K.; Kawaguchi, T. Possible role of airway microvascular permeability on airway obstruction in patients with chronic obstructive pulmonary disease. Respir. Med. 2019, 146, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Heijink, I.H.; Noordhoek, J.A.; Timens, W.; Van Oosterhout, A.J.M.; Postma, D.S. Abnormalities in Airway Epithelial Junction Formation in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2014, 189, 1439–1442. [Google Scholar] [CrossRef]
| Normal Endothelial Function n = 70 | Endothelial Dysfunction * n = 43 | p Value | |
|---|---|---|---|
| Age [years] | 64.8 ± 11.8 | 64.1 + 12.8 | 0.77 |
| Male | 73% | 81% | 0.21 |
| Previous MI | 53% | 37% | 0.08 |
| Previous PCI | 56% | 42% | 0.11 |
| Previous CABG | 14% | 9.3% | 0.32 |
| Previous stroke | 8.6% | 9.3% | 0.57 |
| Peripheral artery disease | 11% | 16% | 0.32 |
| Hypertension | 80% | 79% | 0.54 |
| Diabetes | 31% | 42% | 0.18 |
| Chronic kidney disease | 5.7% | 9.3% | 0.36 |
| Hypercholesterolemia | 33% | 30% | 0.47 |
| COPD | 4.3% | 7.0% | 0.41 |
| Asthma | 1.4% | 2.3% | 0.62 |
| Atrial fibrillation | 7.1% | 19% | 0.06 |
| Smoking | 24% | 21% | 0.43 |
| Chest pain | 81% | 79% | 0.76 |
| BMI [kg/m2] | 28.5 ± 4.3 | 30.5 ± 6.1 | 0.049 |
| Waist circumference [cm] | 80 ± 43 | 83 ± 48 | 0.75 |
| Systolic blood pressure [mm Hg] | 130 ± 20 | 130 ± 20 | 0.64 |
| Diastolic blood pressure [mm Hg] | 70 ± 10 | 71 ± 10 | 0.71 |
| Heart rate [beats per minute] | 65 ± 14 | 65 ± 10 | 0.59 |
| Pharmacotherapy | |||
| Aspirin | 93% | 84% | 0.11 |
| P2Y12 inhibitor | 40% | 35% | 0.37 |
| β-adrenergic receptor inhibitor | 84% | 84% | 0.57 |
| ACE inhibitor | 74% | 61% | 0.09 |
| ARB | 14% | 19% | 0.36 |
| Calcium blocker | 20% | 14% | 0.29 |
| MRA | 13% | 7% | 0.26 |
| Thiazide diuretics | 13% | 16% | 0.4 |
| Loop diuretics | 11% | 35% | 0.003 |
| Statins | 80% | 79% | 0.54 |
| Fibrates | 2.9% | 4.7% | 0.49 |
| Sulphonylureas | 13% | 4.7% | 0.13 |
| Metformin | 19% | 32% | 0.07 |
| Insulin | 5.7% | 11% | 0.22 |
| LABA | 4.3% | 2.3% | 0.51 |
| LAMA | 1.4% | 0% | 0.62 |
| Inhaled corticosteroids | 1.4% | 0% | 0.62 |
| SABA | |||
| SAMA | 1.4% | 2.3% | 0.62 |
| Vitamin K antagonists | 0% | 4.7% | 0.14 |
| NOAC | 2.9% | 7% | 0.28 |
| α-adrenergic receptor inhibitor | 1.4% | 7% | 0.15 |
| Nitrates | 2.9% | 7% | 0.28 |
| Normal Endothelial Function n = 70 | Endothelial Dysfunction * n = 43 | p Value | |
|---|---|---|---|
| Coronary angiography | |||
| No significant coronary artery disease | 13% | 23% | 0.53 |
| Single-vessel disease | 19% | 19% | |
| Two-vessel disease | 31% | 28% | |
| Three-vessel disease | 37% | 30% | |
| Echocardiography | |||
| Left ventricle diastolic diameter [cm] | 5.03 ± 0.64 | 5.12 ± 0.57 | 0.44 |
| Intraventricular septum [cm] | 1.14 ± 0.22 | 1.21 ± 0.15 | 0.049 |
| Left atrium [cm] | 4.09 ± 0.49 | 4.31 ± 0.64 | 0.05 |
| Right ventricle [cm] | 3.0 ± 0.37 | 3.0 ± 0.38 | 0.98 |
| Left ventricle ejection fraction [%] | 53.09 ± 9.23 | 52.70 ± 9.23 | 0.83 |
| Posterior wall thickness [cm] | 0.95 ± 0.17 | 1.05 ± 0.14 | 0.004 |
| Ascending aorta diameter [cm] | 3.36 ± 0.36 | 3.45 ± 0.4 | 0.31 |
| E’ medial | 6.89 ± 1.69 | 6.22 ± 1.84 | 0.09 |
| E’ lateral | 8.46 ± 2.58 | 8.05 ± 2.32 | 0.46 |
| E | 73.23 ± 18.2 | 74.94 ± 18.1 | 0.67 |
| A | 80.20 ± 17.8 | 87.46 ± 18.2 | 0.07 |
| E/A | 0.93 ± 0.27 | 0.95 ± 0.50 | 0.87 |
| Deceleration time [ms] | 211 ± 65 | 223.5 ± 66 | 0.5 |
| TRPG [mmHg] | 22.05 ± 5.1 | 23.96 ± 9.8 | 0.36 |
| TAPSE [mm] | 23.40 ± 4.1 | 23.95 ± 4.3 | 0.5 |
| Laboratory tests | |||
| GFR [mL/min/m2] | 60 ± 4.2 | 60 ± 13.2 | 0.32 |
| CRP [mg/L] | 1.5 ± 1.6 | 1.9 ± 3.7 | 0.15 |
| NT pro BNP [pg/mL] | 539.42 ± 1406 | 467.83 ± 791 | 0.76 |
| Normal Endothelial Function n = 70 | Endothelial Dysfunction * n = 43 | p Value | |
|---|---|---|---|
| Epworth sleepiness scale | 5.36 ± 3.9 | 6.42 ± 5 | 0.24 |
| PAT respiratory disturbance index | 14.8 ± 13.7 | 27.32 ± 22.9 | 0.001 |
| PAT apnea and hypopnea index | 10.30 ± 12.8 | 24.6 ± 24.7 | <0.001 |
| Oxygen desaturation index | 5.23 ± 7.0 | 13.73 ± 17.2 | 0.002 |
| CAT questionnaire | 10.03 ± 5.8 | 10.93 ± 7.3 | 0.47 |
| FEV1 (L) | 2.64 ± 0.8 | 2.46 ± 0.8 | 0.26 |
| FEV1% pred. | 89.01 ± 18.4 | 81.17 ± 19.6 | 0.04 |
| FVC (L) | 3.85 ± 1 | 3.65 ± 1 | 0.36 |
| FVC% pred. | 100.02 ± 17 | 93.18 ± 19 | 0.05 |
| FEV1%FVC | 68.56 ± 8 | 67.55 ± 10 | 0.55 |
| PEF (L/S) | 7.3 ± 2.3 | 7.0 ± 2.3 | 0.5 |
| PEF% pred. | 93.36 ± 27 | 89.35 ± 24 | 0.44 |
| TLC (L) | 6.76 ± 1.4 | 6.87 ± 1.3 | 0.7 |
| TLC% pred. | 110.17 ± 13 | 106.84 ± 13 | 0.21 |
| RV (L) | 2.86 ± 0.6 | 3.01 ± 0.8 | 0.3 |
| RV% pred. | 125.02 ± 25 | 126.6 ± 29 | 0.77 |
| RV/TLC | 43.24 ± 9 | 44.06 ± 8 | 0.65 |
| RV/TLC% pred. | 108.21 ± 18 | 111.98 ± 19 | 0.31 |
| FRC (L) | 3.97 ± 1 | 4.02 ± 1 | 0.75 |
| FRC% pred. | 121.80 ± 26 | 117.97 ± 25 | 0.47 |
| Β-Coefficient | 95% CI | p Value | |
|---|---|---|---|
| BMI | 0.003 | −0.024–0.024 | 0.98 |
| Previous MI | −0.036 | −0.277–0.193 | 0.72 |
| Atrial fibrillation | 0.021 | −0.313–0.387 | 0.83 |
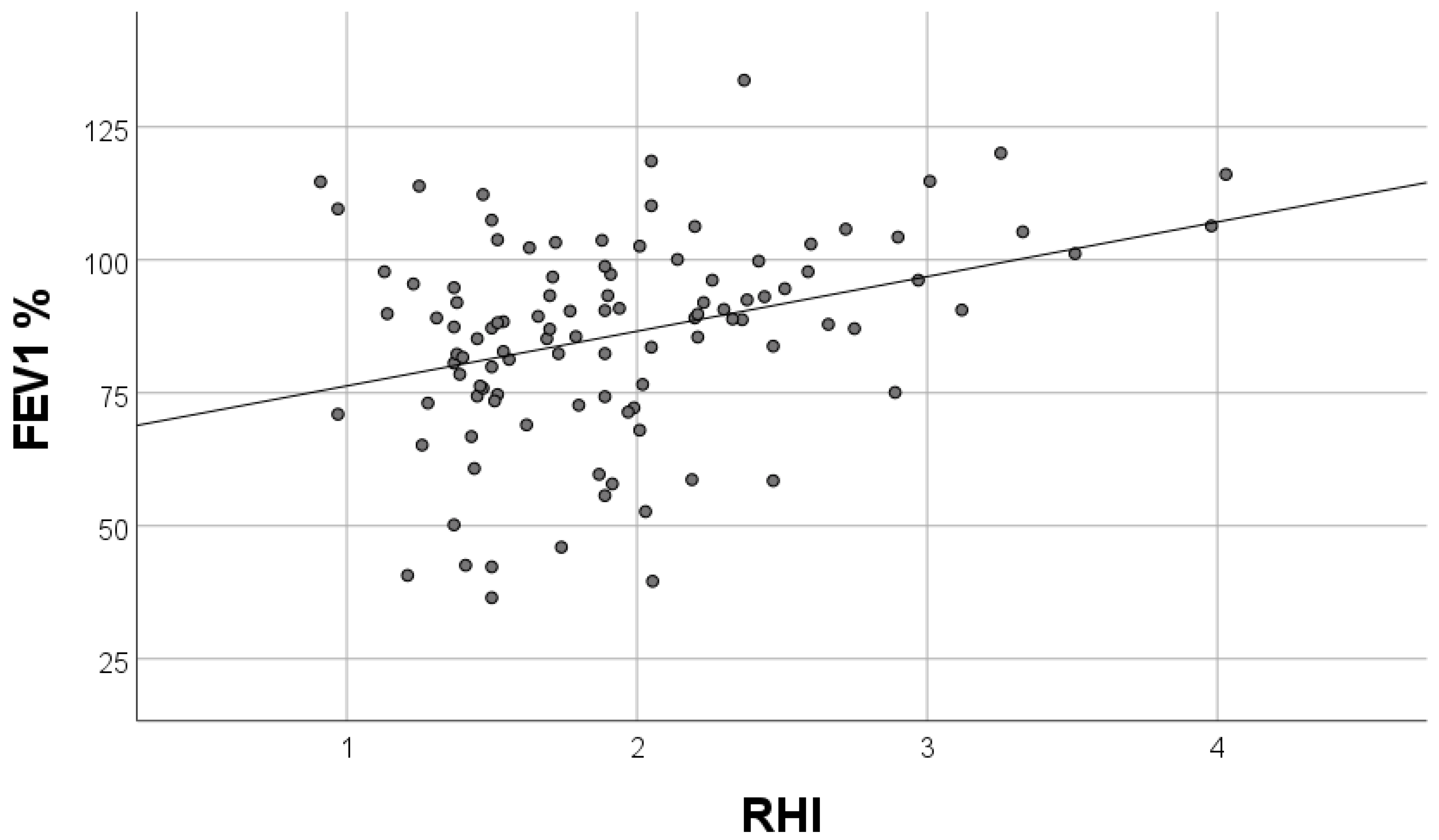
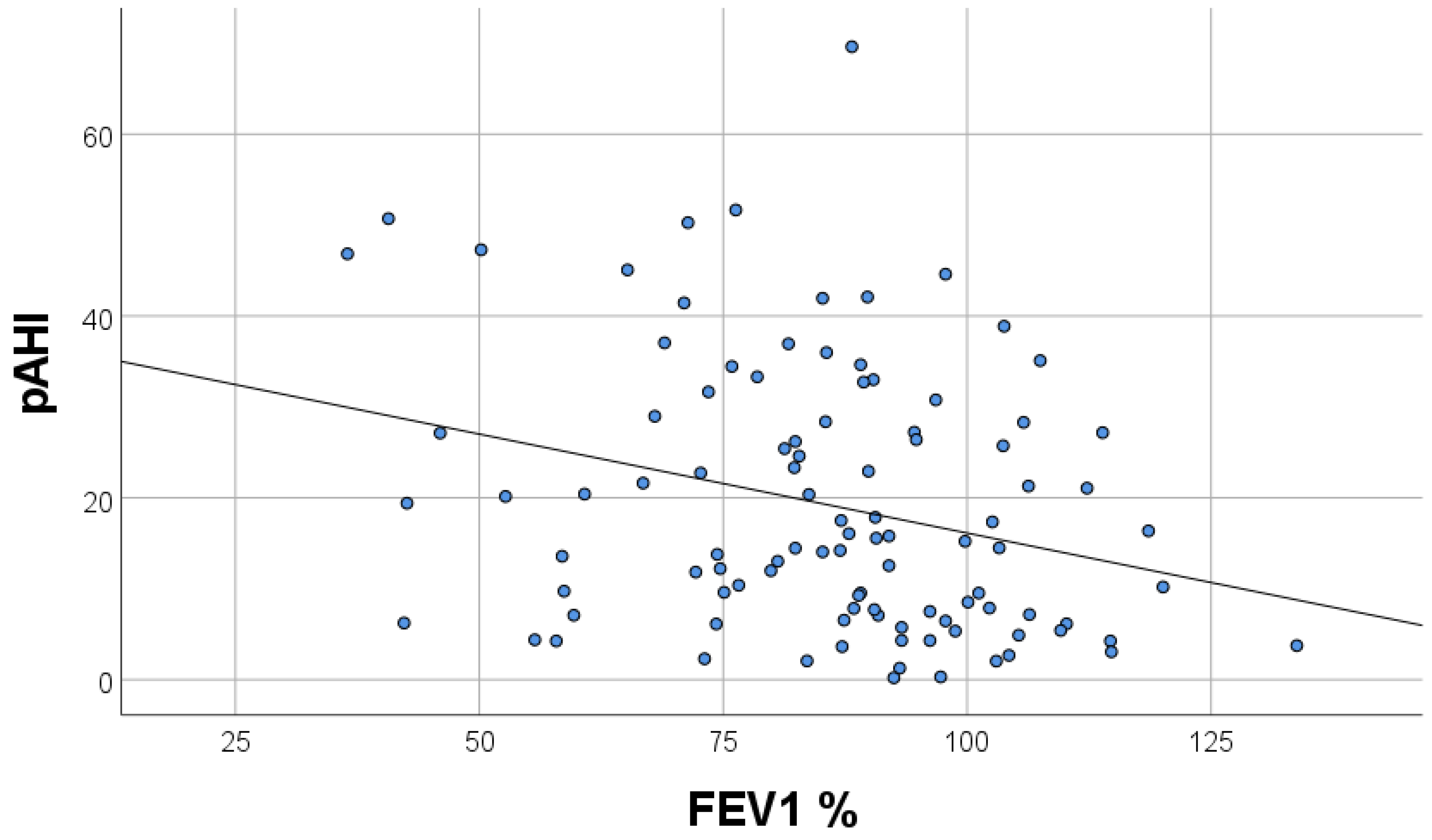
| FEV1% pred. | 0.219 | 0.001–0.013 | 0.03 |
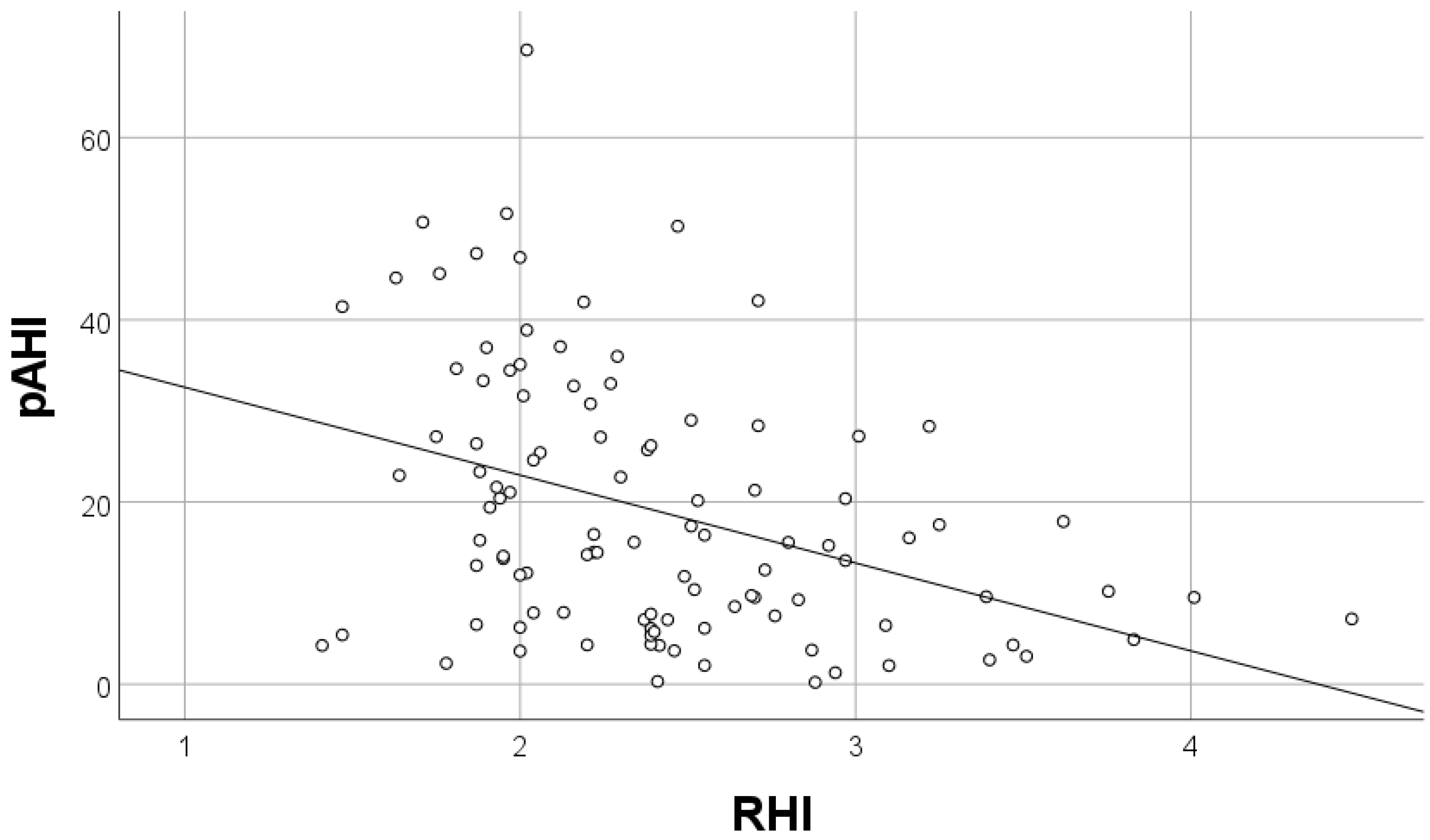
| pAHI | −0.313 | −0.021–0.004 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochijewicz, D.; Rdzanek, A.; Przybyłowski, T.; Rubinsztajn, R.; Budnik, M.; Pędzich, E.; Białek-Gosk, K.; Bielicki, P.; Kapłon-Cieślicka, A. Influence of Apnea Hypopnea Index and the Degree of Airflow Limitation on Endothelial Function in Patients Undergoing Diagnostic Coronary Angiography. Biology 2022, 11, 457. https://doi.org/10.3390/biology11030457
Ochijewicz D, Rdzanek A, Przybyłowski T, Rubinsztajn R, Budnik M, Pędzich E, Białek-Gosk K, Bielicki P, Kapłon-Cieślicka A. Influence of Apnea Hypopnea Index and the Degree of Airflow Limitation on Endothelial Function in Patients Undergoing Diagnostic Coronary Angiography. Biology. 2022; 11(3):457. https://doi.org/10.3390/biology11030457
Chicago/Turabian StyleOchijewicz, Dorota, Adam Rdzanek, Tadeusz Przybyłowski, Renata Rubinsztajn, Monika Budnik, Ewa Pędzich, Katarzyna Białek-Gosk, Piotr Bielicki, and Agnieszka Kapłon-Cieślicka. 2022. "Influence of Apnea Hypopnea Index and the Degree of Airflow Limitation on Endothelial Function in Patients Undergoing Diagnostic Coronary Angiography" Biology 11, no. 3: 457. https://doi.org/10.3390/biology11030457
APA StyleOchijewicz, D., Rdzanek, A., Przybyłowski, T., Rubinsztajn, R., Budnik, M., Pędzich, E., Białek-Gosk, K., Bielicki, P., & Kapłon-Cieślicka, A. (2022). Influence of Apnea Hypopnea Index and the Degree of Airflow Limitation on Endothelial Function in Patients Undergoing Diagnostic Coronary Angiography. Biology, 11(3), 457. https://doi.org/10.3390/biology11030457






