Prescription Patterns, Recurrence, and Toxicity Rates of Adjuvant Treatment for Stage III/IV Melanoma—A Real World Single-Center Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
3.1. Patient and Tumour Characteristics
3.2. Decision on Adjuvant Treatment
3.3. Toxicity and Recurrence Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gershenwald, J.E.; Scolyer, R.A. Melanoma Staging: American Joint Committee on Cancer (AJCC) 8th Edition and Beyond. Ann. Surg. Oncol. 2018, 25, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Del Vecchio, M.; Mandala, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 643–654. [Google Scholar] [CrossRef]
- Dummer, R.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandala, M.; Kirkwood, J.M.; Chiarion Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Five-Year Analysis of Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. N. Engl. J. Med. 2020, 383, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Testori, A.A.E.; Chiellino, S.; van Akkooi, A.C.J. Adjuvant Therapy for Melanoma: Past, Current, and Future Developments. Cancers 2020, 12, 1994. [Google Scholar] [CrossRef] [PubMed]
- Toor, K.; Middleton, M.R.; Chan, K.; Amadi, A.; Moshyk, A.; Kotapati, S. Comparative efficacy and safety of adjuvant nivolumab versus other treatments in adults with resected melanoma: A systematic literature review and network meta-analysis. BMC Cancer 2021, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Ghisoni, E.; Wicky, A.; Bouchaab, H.; Imbimbo, M.; Delyon, J.; Gautron Moura, B.; Gerard, C.L.; Latifyan, S.; Ozdemir, B.C.; Caikovski, M.; et al. Late-onset and long-lasting immune-related adverse events from immune checkpoint-inhibitors: An overlooked aspect in immunotherapy. Eur. J. Cancer 2021, 149, 153–164. [Google Scholar] [CrossRef]
- Arangalage, D.; Degrauwe, N.; Michielin, O.; Monney, P.; Ozdemir, B.C. Pathophysiology, diagnosis and management of cardiac toxicity induced by immune checkpoint inhibitors and BRAF and MEK inhibitors. Cancer Treat. Rev. 2021, 100, 102282. [Google Scholar] [CrossRef]
- Lodde, G.; Forschner, A.; Hassel, J.; Wulfken, L.M.; Meier, F.; Mohr, P.; Kahler, K.; Schilling, B.; Loquai, C.; Berking, C.; et al. Factors Influencing the Adjuvant Therapy Decision: Results of a Real-World Multicenter Data Analysis of 904 Melanoma Patients. Cancers 2021, 13, 2319. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lee, S.J.; Chmielowski, B.; Ribas, A.; Tarhini, A.A.; Truong, T.-G.; Davar, D.; O’Rourke, M.A.; Curti, B.D.; Brell, J.M.; et al. DREAMseq (Doublet, Randomized Evaluation in Advanced Melanoma Sequencing): A phase III trial—ECOG-ACRIN EA6134. J. Clin. Oncol. 2021, 39, 356154. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Mandala, M.; Ferrucci, P.F.; Rutkowski, P.; Guidoboni, M.; Fernandez, A.M.A.; Ferraresi, V.; Maiello, E.; Guida, M.; Del Vecchio, M.; et al. SECOMBIT: The best sequential approach with combo immunotherapy (ipilimumab/nivolumab) and combo target therapy (encorafenib/binimetinib) in patients with BRAF mutated metastatic melanoma: A phase II randomized study. Ann. Oncol. 2021, 32, S1316–S1317. [Google Scholar] [CrossRef]
- Cooper, Z.A.; Reuben, A.; Spencer, C.N.; Prieto, P.A.; Austin-Breneman, J.L.; Jiang, H.; Haymaker, C.; Gopalakrishnan, V.; Tetzlaff, M.T.; Frederick, D.T.; et al. Distinct clinical patterns and immune infiltrates are observed at time of progression on targeted therapy versus immune checkpoint blockade for melanoma. Oncoimmunology 2016, 5, e1136044. [Google Scholar] [CrossRef] [PubMed]
- Gide, T.N.; Quek, C.; Menzies, A.M.; Tasker, A.T.; Shang, P.; Holst, J.; Madore, J.; Lim, S.Y.; Velickovic, R.; Wongchenko, M.; et al. Distinct Immune Cell Populations Define Response to Anti-PD-1 Monotherapy and Anti-PD-1/Anti-CTLA-4 Combined Therapy. Cancer Cell 2019, 35, 238–255.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newell, F.; Pires da Silva, I.; Johansson, P.A.; Menzies, A.M.; Wilmott, J.S.; Addala, V.; Carlino, M.S.; Rizos, H.; Nones, K.; Edwards, J.J.; et al. Multiomic profiling of checkpoint inhibitor-treated melanoma: Identifying predictors of response and resistance, and markers of biological discordance. Cancer Cell 2021, 40, 88–102e7. [Google Scholar] [CrossRef] [PubMed]
- Gide, T.N.; Silva, I.P.; Quek, C.; Ahmed, T.; Menzies, A.M.; Carlino, M.S.; Saw, R.P.M.; Thompson, J.F.; Batten, M.; Long, G.V.; et al. Close proximity of immune and tumor cells underlies response to anti-PD-1 based therapies in metastatic melanoma patients. Oncoimmunology 2020, 9, 1659093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Yao, Z.; Yang, H.; Liang, N.; Zhang, X.; Zhang, F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020, 18, 87. [Google Scholar] [CrossRef]
- Xing, P.; Zhang, F.; Wang, G.; Xu, Y.; Li, C.; Wang, S.; Guo, Y.; Cai, S.; Wang, Y.; Li, J. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: A systematic review and meta-analysis. J. Immunother. Cancer 2019, 7, 341. [Google Scholar] [CrossRef] [PubMed]
- Ben-Betzalel, G.; Baruch, E.N.; Boursi, B.; Steinberg-Silman, Y.; Asher, N.; Shapira-Frommer, R.; Schachter, J.; Markel, G. Possible immune adverse events as predictors of durable response to BRAF inhibitors in patients with BRAF V600-mutant metastatic melanoma. Eur. J. Cancer 2018, 101, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.N.; McQuade, J.L.; Gopalakrishnan, V.; McCulloch, J.A.; Vetizou, M.; Cogdill, A.P.; Khan, M.A.W.; Zhang, X.; White, M.G.; Peterson, C.B.; et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 2021, 374, 1632–1640. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillere, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2017, 359, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Wang, Q.; Tang, X.; Xu, R.; Zhang, L.; Chen, X.; Xue, Q.; Wang, Z.; Shi, R.; Wang, F.; et al. Correlation between patients’ age and cancer immunotherapy efficacy. Oncoimmunology 2019, 8, e1568810. [Google Scholar] [CrossRef]
- Jain, V.; Hwang, W.T.; Venigalla, S.; Nead, K.T.; Lukens, J.N.; Mitchell, T.C.; Shabason, J.E. Association of Age with Efficacy of Immunotherapy in Metastatic Melanoma. Oncologist 2020, 25, e381–e385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Irelli, A.; Sirufo, M.M.; D’Ugo, C.; Ginaldi, L.; De Martinis, M. Sex and Gender Influences on Cancer Immunotherapy Response. Biomedicines 2020, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Kugel, C.H., 3rd; Douglass, S.M.; Webster, M.R.; Kaur, A.; Liu, Q.; Yin, X.; Weiss, S.A.; Darvishian, F.; Al-Rohil, R.N.; Ndoye, A.; et al. Age Correlates with Response to Anti-PD1, Reflecting Age-Related Differences in Intratumoral Effector and Regulatory T-Cell Populations. Clin. Cancer Res. 2018, 24, 5347–5356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozdemir, B.C.; Dotto, G.P. Sex Hormones and Anticancer Immunity. Clin. Cancer Res. 2019, 25, 4603–4610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, A.; Webster, M.R.; Marchbank, K.; Behera, R.; Ndoye, A.; Kugel, C.H., 3rd; Dang, V.M.; Appleton, J.; O’Connell, M.P.; Cheng, P.; et al. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature 2016, 532, 250–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascierto, P.A.; Lewis, K.D.; Di Giacomo, A.M.; Demidov, L.; Mandala, M.; Bondarenko, I.; Herbert, C.; Mackiewicz, A.; Rutkowski, P.; Guminski, A.; et al. Prognostic impact of baseline tumour immune infiltrate on disease-free survival in patients with completely resected, BRAF(v600) mutation-positive melanoma receiving adjuvant vemurafenib. Ann. Oncol. 2020, 31, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Versluis, J.M. The prognostic value of the interferon-gamma (IFNγ) signature in patients with macroscopic stage III melanoma treated with and without adjuvant systemic therapy. J. Clin. Oncol. 2021, 39, 9579. [Google Scholar] [CrossRef]
- Vuletic, A.; Jovanic, I.; Jurisic, V.; Milovanovic, Z.; Nikolic, S.; Spurnic, I.; Konjevic, G. Decreased Interferon gamma Production in CD3+ and CD3- CD56+ Lymphocyte Subsets in Metastatic Regional Lymph Nodes of Melanoma Patients. Pathol. Oncol. Res. 2015, 21, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.N.; Shoushtari, A.N.; Chauhan, D.; Palmieri, D.J.; Lee, B.; Rohaan, M.W.; Mangana, J.; Atkinson, V.; Zaman, F.; Young, A.; et al. Management of early melanoma recurrence despite adjuvant anti-PD-1 antibody therapy. Ann. Oncol. 2020, 31, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Bhave, P.; Pallan, L.; Long, G.V.; Menzies, A.M.; Atkinson, V.; Cohen, J.V.; Sullivan, R.J.; Chiarion-Sileni, V.; Nyakas, M.; Kahler, K.; et al. Melanoma recurrence patterns and management after adjuvant targeted therapy: A multicentre analysis. Br. J. Cancer 2021, 124, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, E.; Newsom-Davis, T.; Morganstein, D.L. Immunotherapy-induced endocrinopathies: Assessment, management and monitoring. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819896182. [Google Scholar] [CrossRef] [PubMed]
- Scovell, J.M.; Benz, K.; Samarska, I.; Kohn, T.P.; Hooper, J.E.; Matoso, A.; Herati, A.S. Association of Impaired Spermatogenesis with the Use of Immune Checkpoint Inhibitors in Patients with Metastatic Melanoma. JAMA Oncol. 2020, 6, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, B.C. Immune checkpoint inhibitor-related hypogonadism and infertility: A neglected issue in immuno-oncology. J. Immunother. Cancer 2021, 9, e002220. [Google Scholar] [CrossRef] [PubMed]
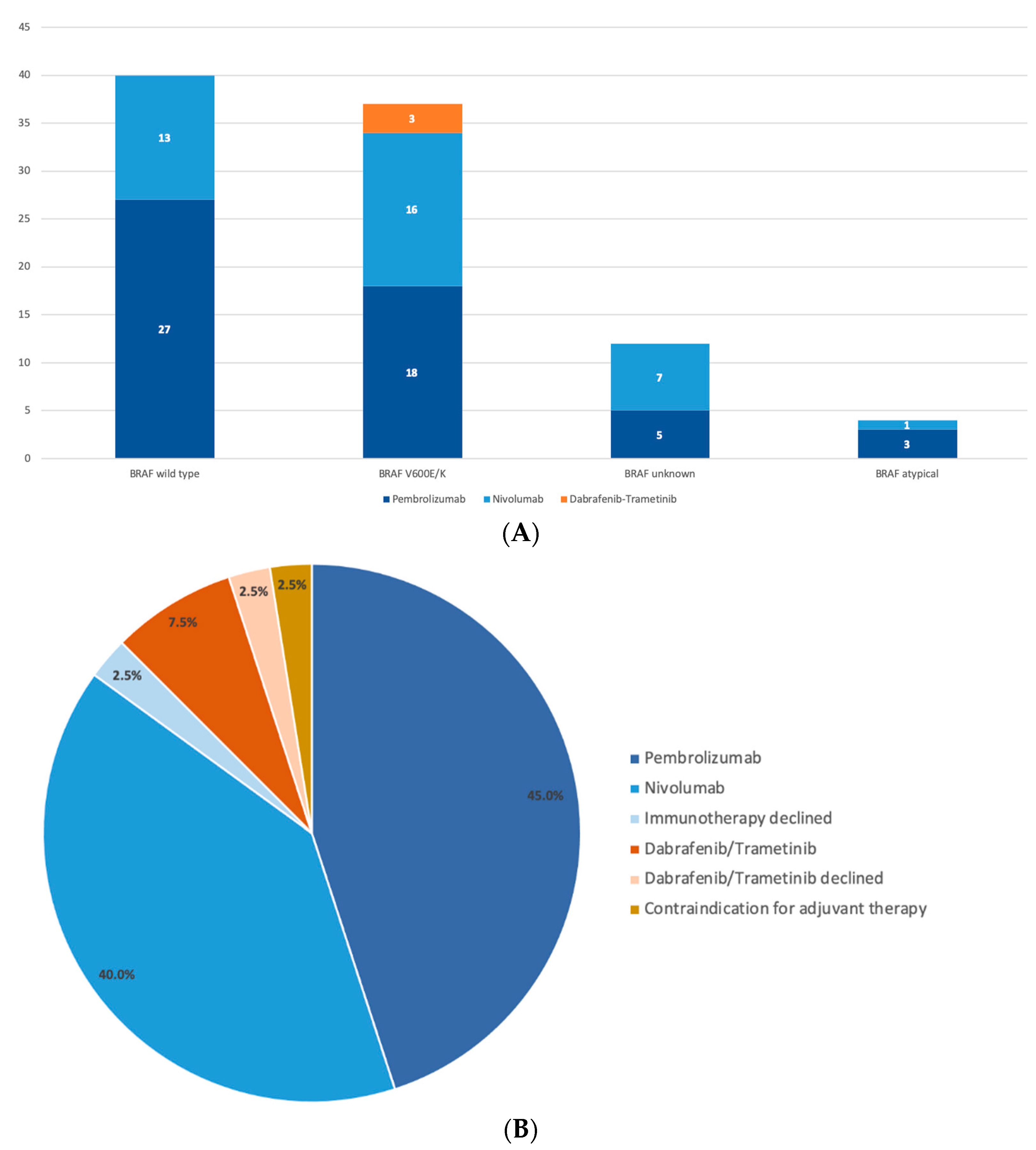
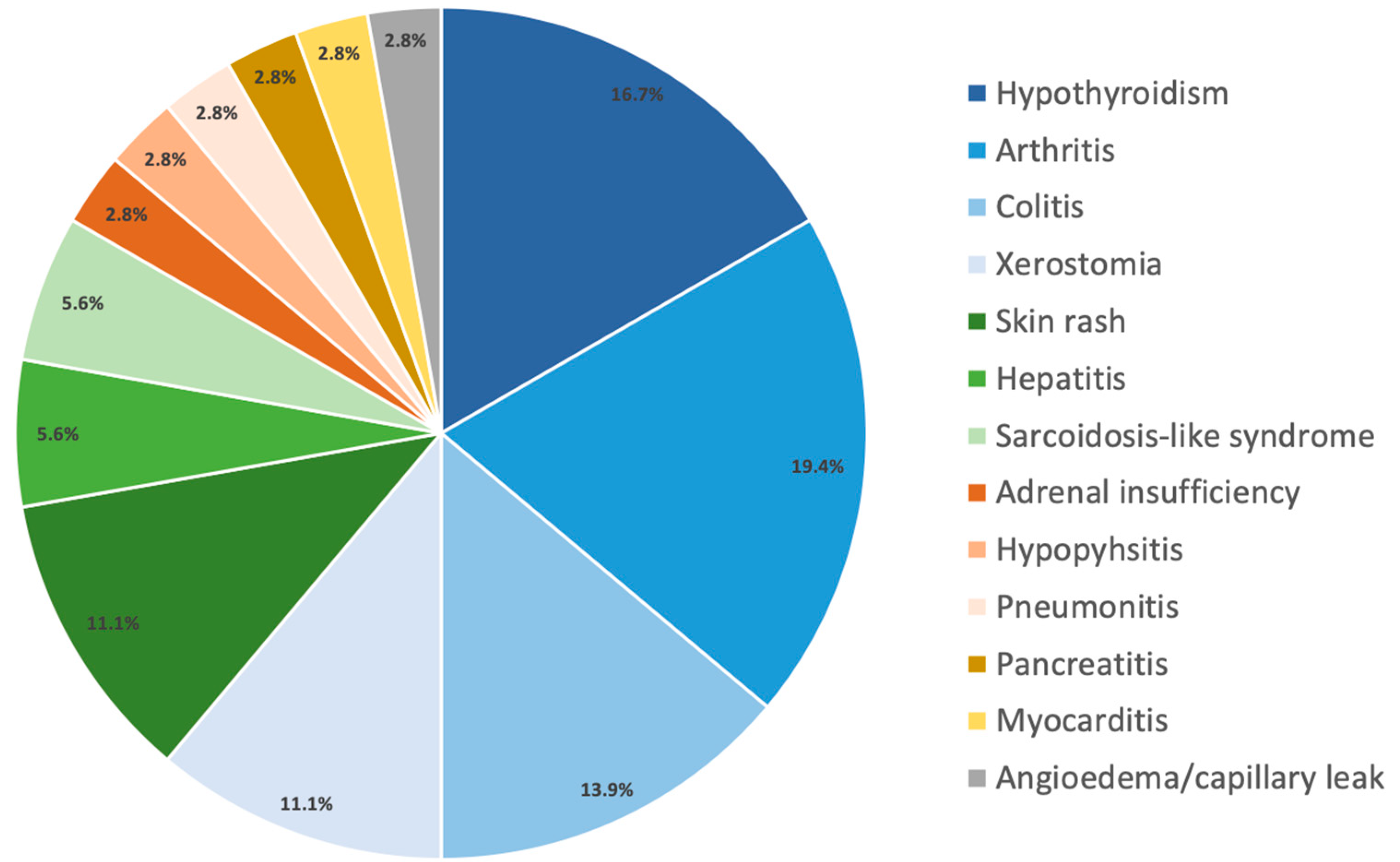
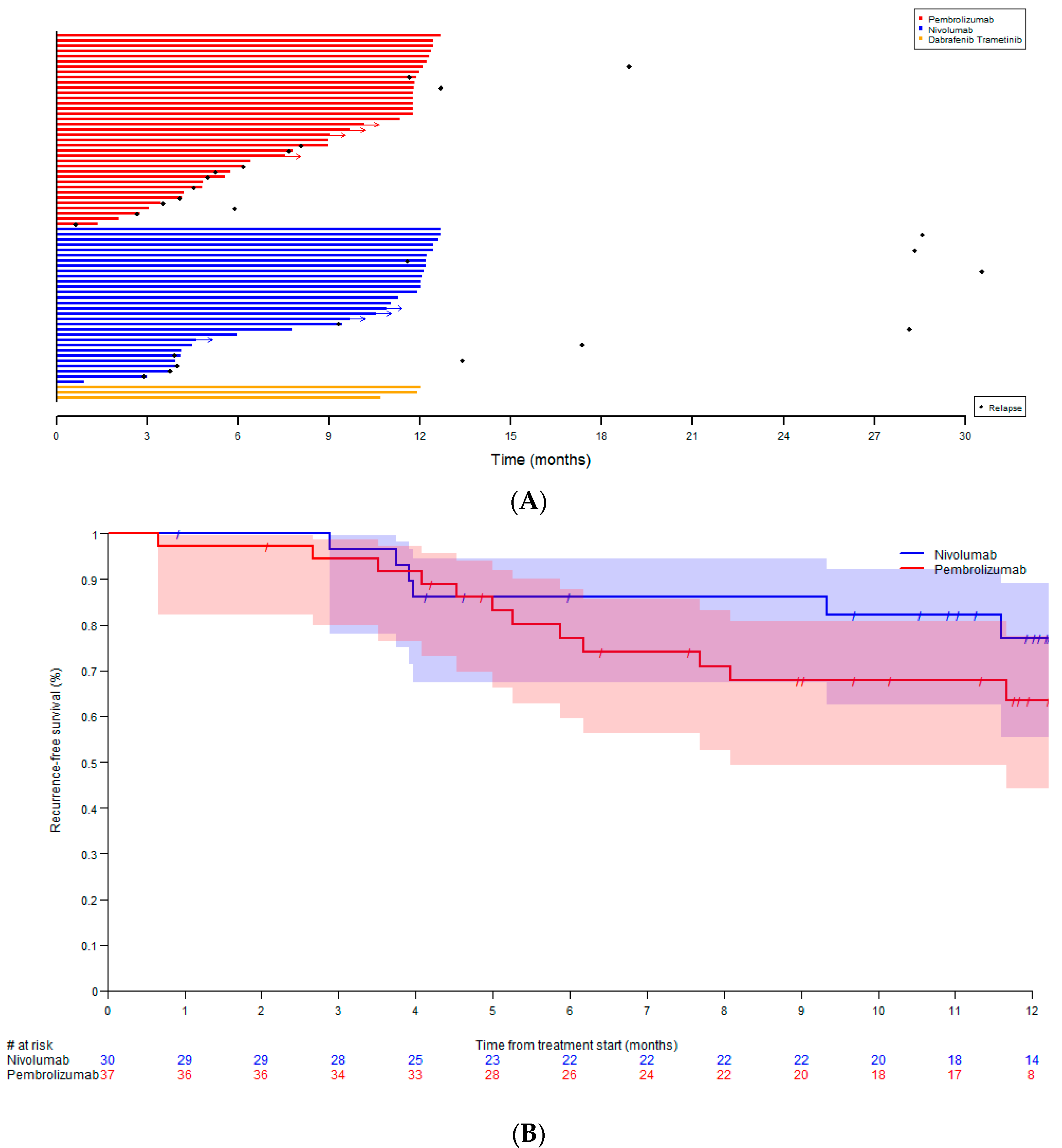
| Patients Qualifying for Adjuvant Treatment, N | 109 |
|---|---|
| Sex | |
| Male | 71 (65.1%) |
| Female | 38 (34.9%) |
| Age (years) | |
| median (range) | 60 (28–82) |
| BRAF status all patients/male/female | |
| BRAF wild type | 47 (43.1%)/30/17 |
| BRAF V600E/K | 40 (36.7%)/25/15 |
| BRAF atypical | 4 (3.7%)/3/1 |
| BRAF unknown | 18 (16.5%)/13/5 |
| Stage | |
| IIIA | 7 (6.4%) |
| IIIB | 44 (40.4%) |
| IIIC | 50 (45.9%) |
| IIID | 3 (2.8%) |
| IV | 5 (4.6%) |
| Melanoma type | |
| Cutaneous | 96 (88.1%) |
| Unknown origin | 13 (11.9%) |
| Breslow thickness (mm) | |
| Median (range) | 2.6 (0.2–12) |
| Tumor ulceration | |
| Yes | 43 (39.4%) |
| No | 66 (60.6%) |
| Adjuvant treatment not recommended | 5 (4.6%) |
| Adjuvant treatment not received | 11 (10.1%) |
| Progression before start | 1 (0.9%) |
| Adjuvant treatment declined | 10 (9.2%) |
| ICI | 9 (8.3%) |
| Dabrafenib/Trametinib | 1 (0.9%) |
| Adjuvant treatment at different hospital | 23 (21.1%) |
| Pembrolizumab | 16 (14.7%) |
| Nivolumab | 7 (6.4%) |
| Dabrafenib/Trametinib | 0 (0.0%) |
| Adjuvant treatment received at our center | 70 (64.2%) |
| Pembrolizumab | 37 (52.9%) |
| Nivolumab | 30 (42.9%) |
| Dabrafenib/Trametinib | 3 (4.3%) |
| Pembrolizumab N = 37 | Nivolumab N = 30 | Dabrafenib/Trametinib N = 3 | |
|---|---|---|---|
| Median number of cycles, range | 15 (2–19) | 20.5 (2–27) | 12 |
| Median duration of treatment (months), range | 11.3 (1.3–12.7) | 11.6 (0.8–12.7) | 12.0 |
| Toxicity all grade | 20 (54.1%) | 16 (53.3%) | 0 (0%) |
| Toxicity grade 3–4 | 7 (18.9%) | 5 (16.7%) | 0 (0%) |
| Discontinuation | |||
| For toxicity grade 3–4 | 6 (16.2%) | 5 (16.7%) | 0 (0%) |
| Recurrence | 14 (37.8%) | 12 (40.0%) | 0 (0%) |
| During adjuvant treatment | 12 (32.4%) | 6 (20.0%) | |
| After stopping adjuvant treatment | 2 (5.4%) | 6 (20.0%) | |
| Site of first recurrence | |||
| Locoregional | 5 (13.5%) | 4 (13.3%) | |
| Distant | 9 (24.3%) | 8 (26.7%) | |
| Subsequent first treatment at recurrence | N/A | ||
| Ipilimumab-Nivolumab | 10 (71.4%) | 2 (16.7%) | |
| Pembrolizumab | 0 (0%) | 2 (16.7%) | |
| Nivolumab | 0 (0%) | 0 (0%) | |
| Dabrafenib-Trametinib | 3 (21.4%) | 3 (25.0%) | |
| Encorafenib-Binimetinib | 0 (0%) | 1 (8.3%) | |
| TVEC | 0 (0%) | 0 (0%) | |
| Clinical trial | 0 (0%) | 1 (8.3%) | |
| Other (surgery, local treatment) | 1 (7.1%) | 3 (25.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, M.; Hayoz, S.; Özdemir, B.C. Prescription Patterns, Recurrence, and Toxicity Rates of Adjuvant Treatment for Stage III/IV Melanoma—A Real World Single-Center Analysis. Biology 2022, 11, 422. https://doi.org/10.3390/biology11030422
Hoffmann M, Hayoz S, Özdemir BC. Prescription Patterns, Recurrence, and Toxicity Rates of Adjuvant Treatment for Stage III/IV Melanoma—A Real World Single-Center Analysis. Biology. 2022; 11(3):422. https://doi.org/10.3390/biology11030422
Chicago/Turabian StyleHoffmann, Michèle, Stefanie Hayoz, and Berna C. Özdemir. 2022. "Prescription Patterns, Recurrence, and Toxicity Rates of Adjuvant Treatment for Stage III/IV Melanoma—A Real World Single-Center Analysis" Biology 11, no. 3: 422. https://doi.org/10.3390/biology11030422
APA StyleHoffmann, M., Hayoz, S., & Özdemir, B. C. (2022). Prescription Patterns, Recurrence, and Toxicity Rates of Adjuvant Treatment for Stage III/IV Melanoma—A Real World Single-Center Analysis. Biology, 11(3), 422. https://doi.org/10.3390/biology11030422






