First Modern Data on the Lophophore Nervous System in Adult Novocrania anomala and a Current Assessment of Brachiopod Phylogeny
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Fixation and Microscopy
2.3. Terminology
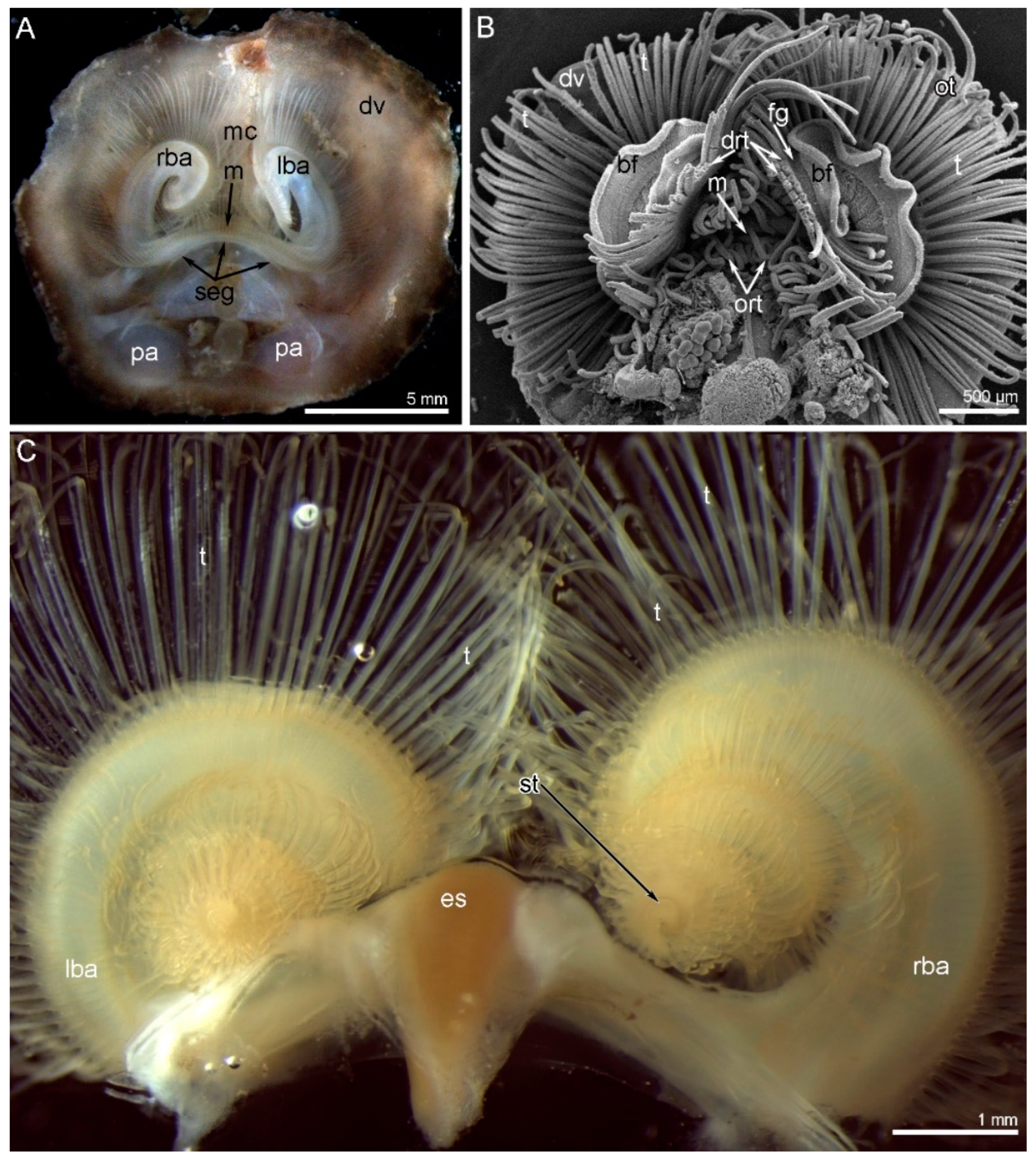
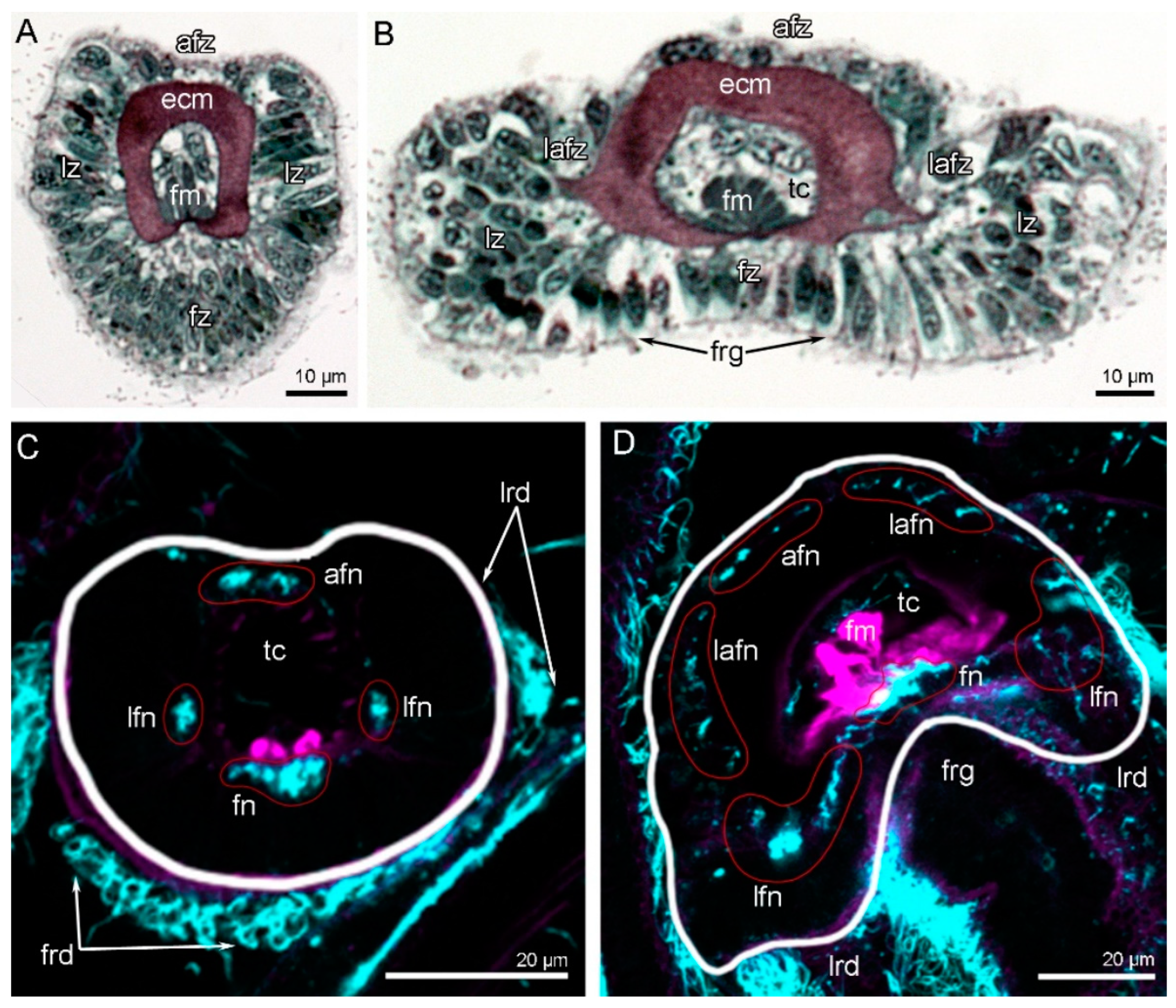
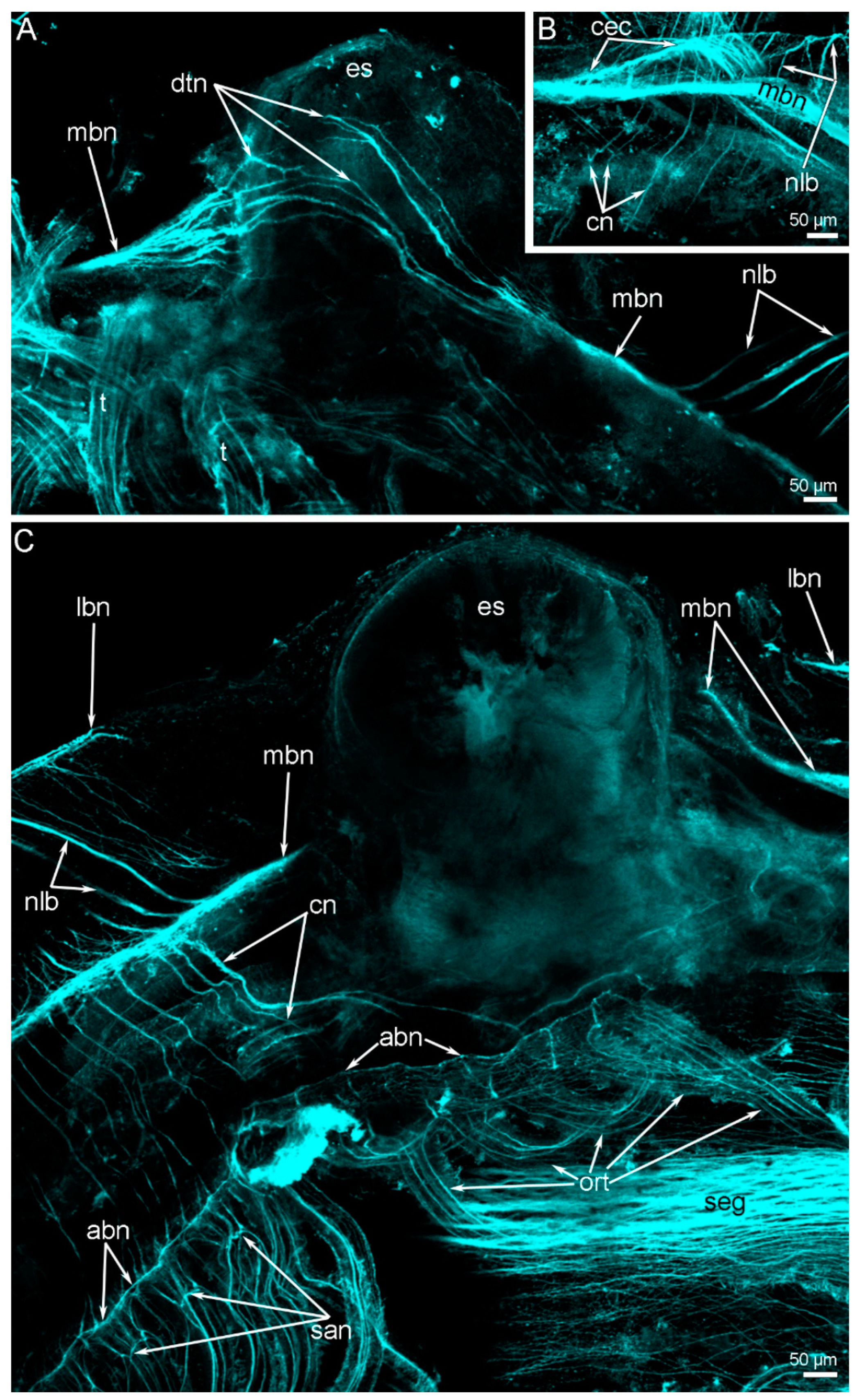
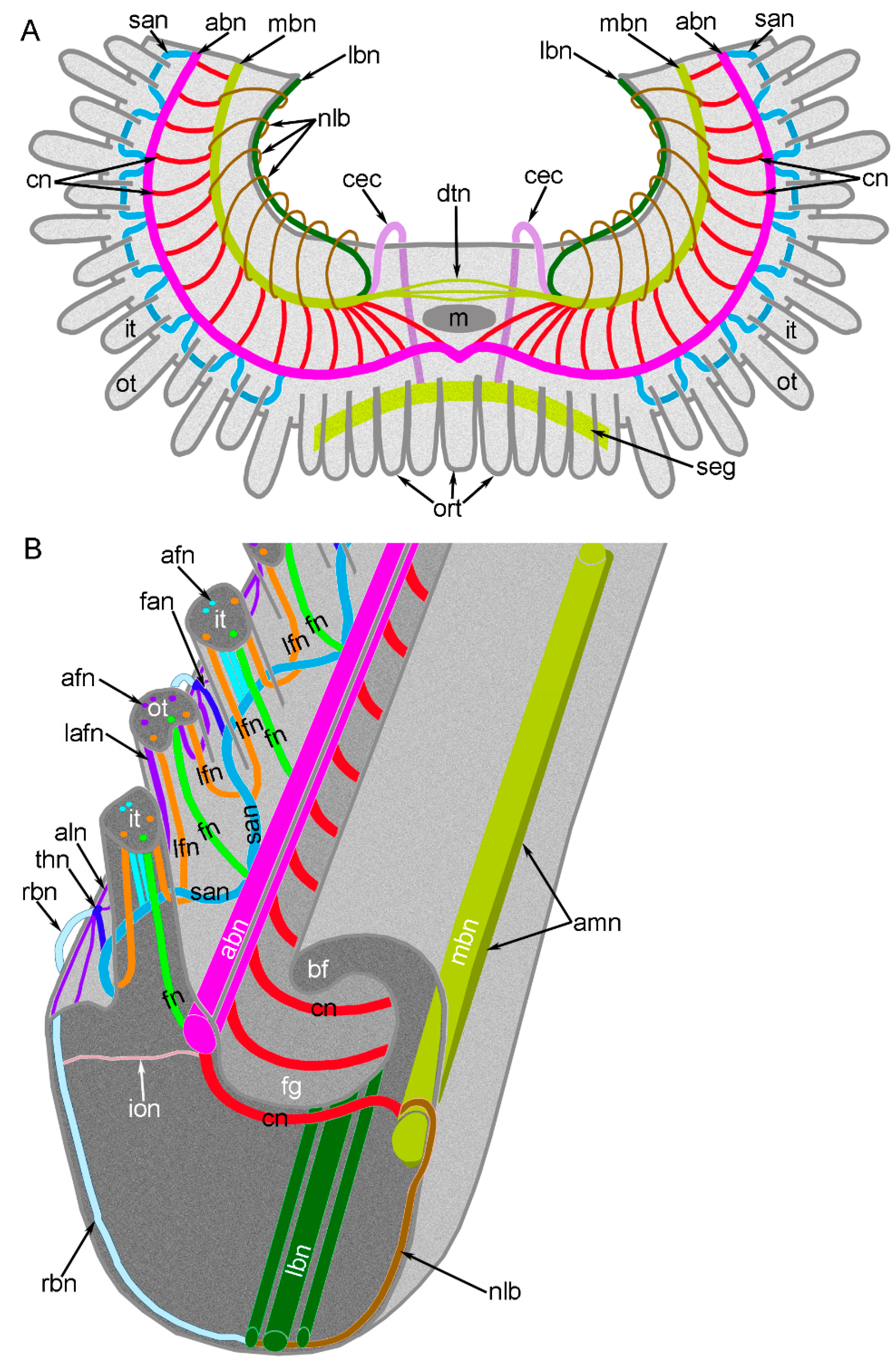
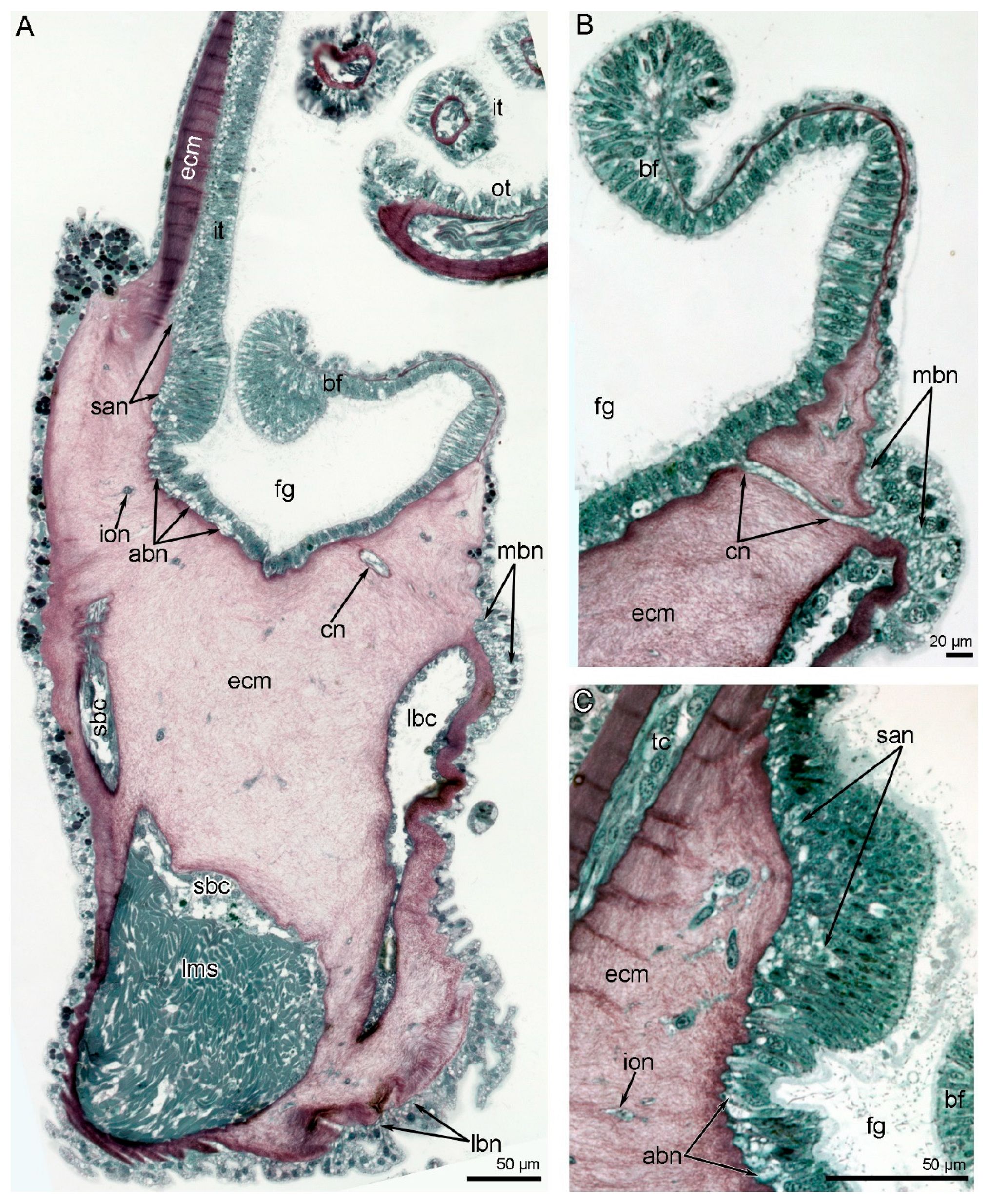
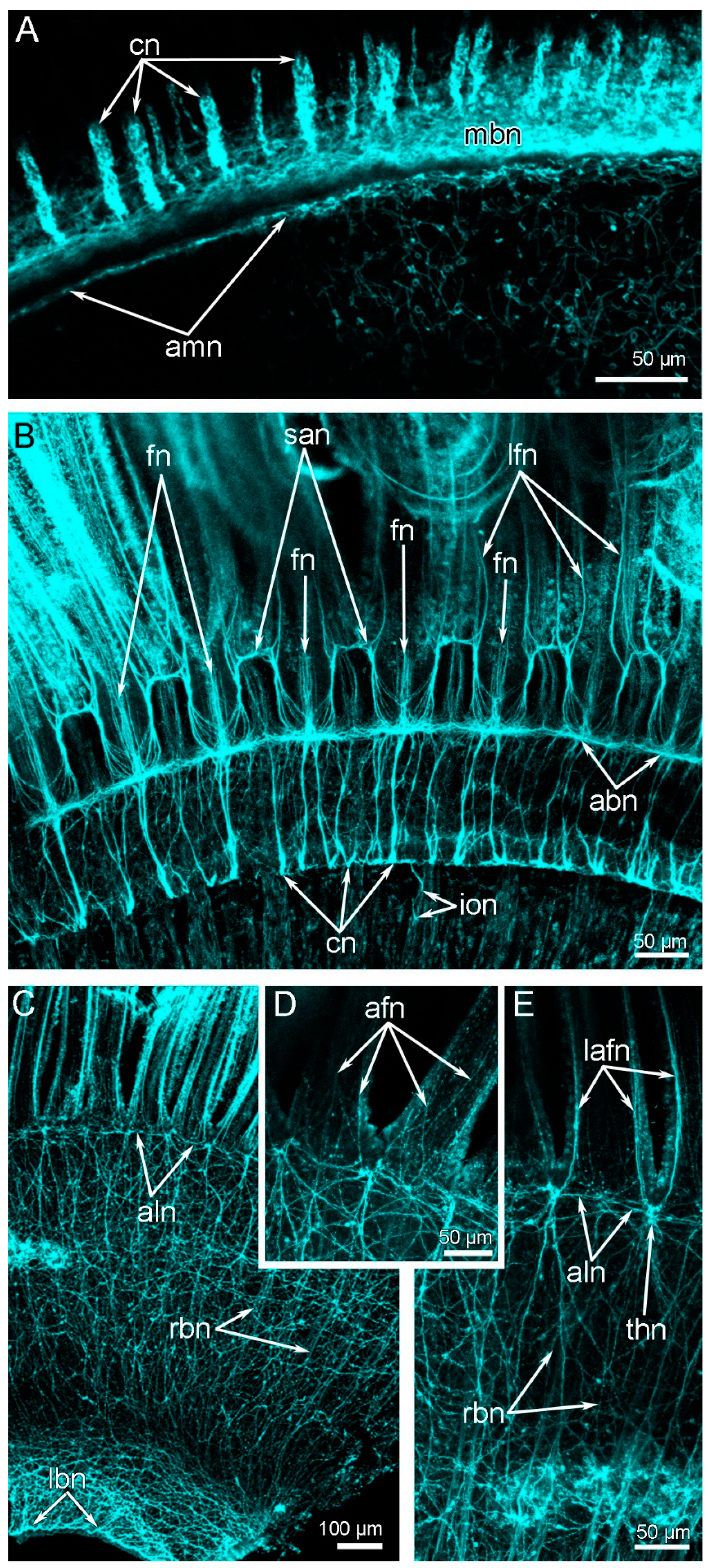
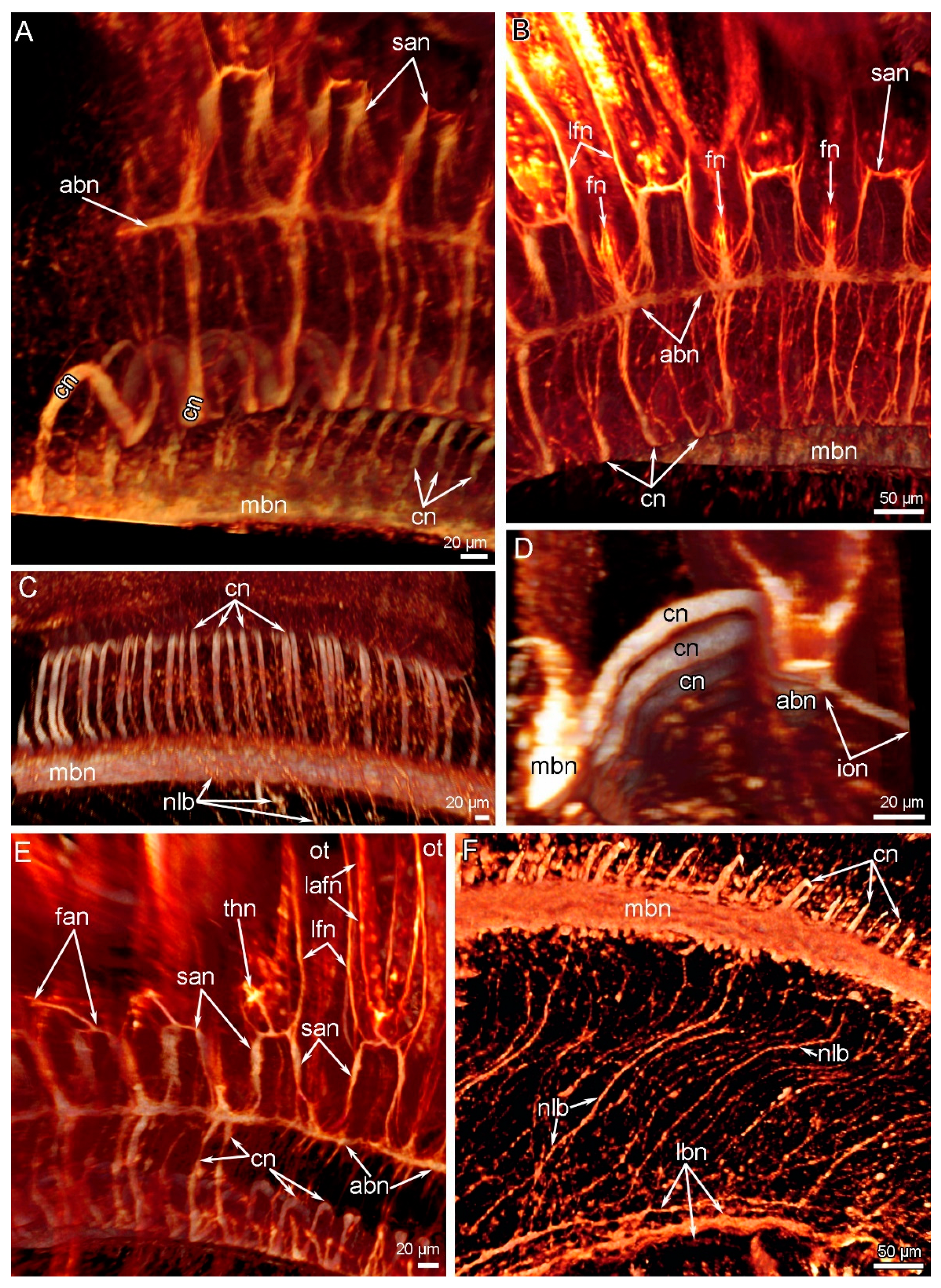
3. Results
3.1. Morphology of the Lophophore and Tentacles
3.2. Main Nerve Elements of the Lophophore
3.3. Innervation of the Brachial (Lophophore) Arms
3.4. Innervation of Tentacles
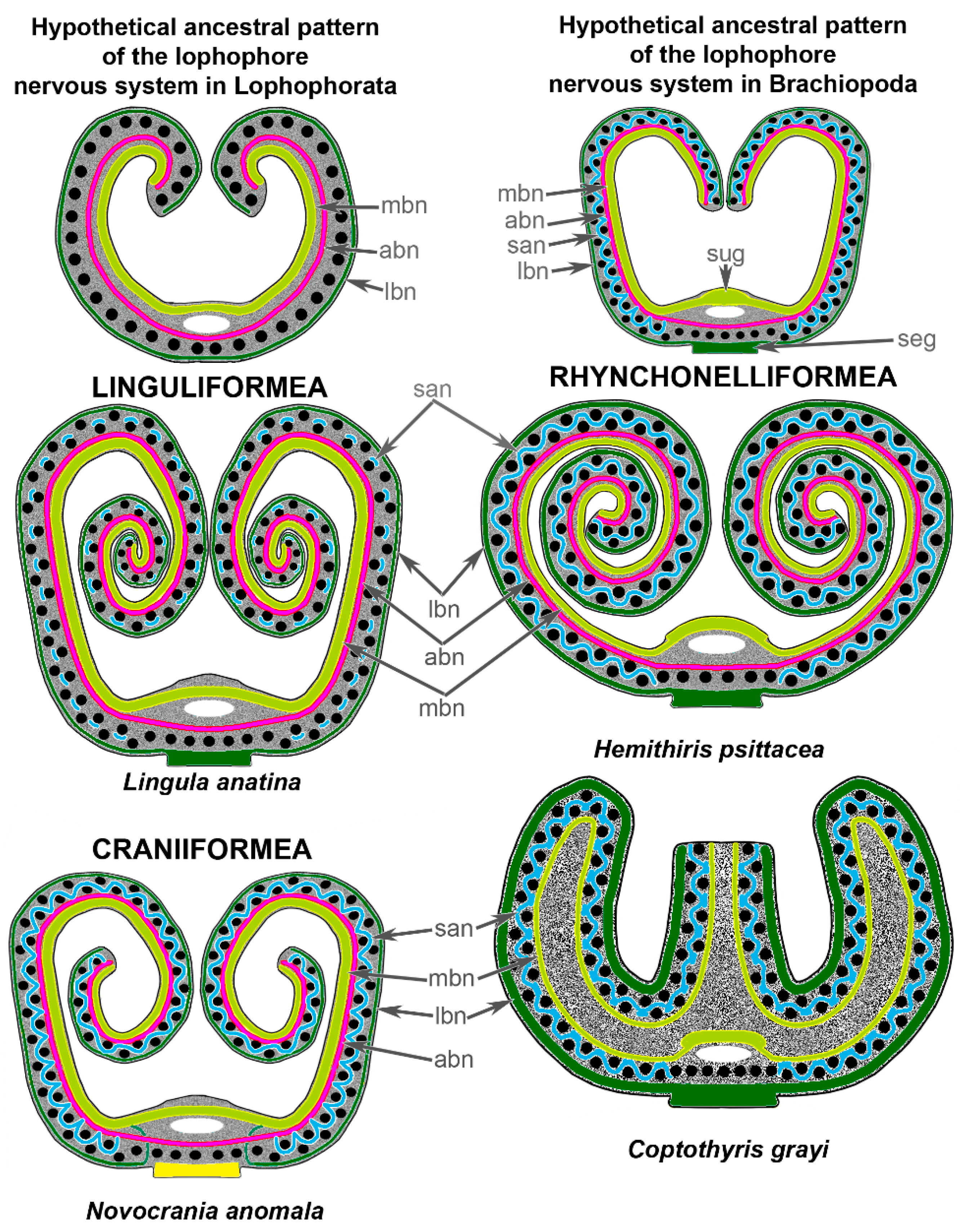
4. Discussion
4.1. Organization of the Central Nervous System in Brachiopods
4.2. Innervation of the Tentacles in Brachiopods
4.3. Evolution of the Lophophore
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, A.; Carlson, S.J.; Branton, C.H.C.; Holmer, L.E.; Popov, L.E. A supra-ordinal classification of the Brachiopoda. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 1996, 35, 1171–1193. [Google Scholar]
- Harper, D.A.T.; Popov, L.; Holmer, L.E. Brachiopods: Origin and early history. Palaeontology 2017, 60, 609–631. [Google Scholar] [CrossRef]
- Temereva, E.N.; Kuzmina, T.V. Spermatogenesis in the deep-sea brachiopod pelagodiscus atlanticus and the phylogenetic significance of spermatozoon structure. J. Morphol. 2018, 279, 1579–1589. [Google Scholar] [CrossRef]
- Cohen, B.L. Rerooting the rDNA gene tree reveals phoronidsto be ‘brachiopods without shells’; dangers ofwide taxon samples in metazoan phylogenetics (Phoronida; Brachiopoda). Zool. J. Linn. Soc. 2013, 167, 82–92. [Google Scholar] [CrossRef]
- Cohen, B.L.; Cracraft, J.; Feinstein, J. Monophyly of brachiopods and phoronids: Reconciliation of molecular evidence with Linnaean classification (the subphylum Phoroniformea nov.). Proc. R. Soc. B: Boil. Sci. 2000, 267, 225–231. [Google Scholar] [CrossRef]
- Cohen, B.L.; Weydmann, A. Molecular evidence that phoronids are a subtaxon of brachiopods (Brachiopoda: Phoronata) and that genetic divergence of metazoan phyla began long before the Early Cambrian. Org. Divers. Evol. 2005, 5, 253–273. [Google Scholar] [CrossRef]
- Santagata, S.; Cohen, B.L. Phoronid phylogenetics (Brachiopoda; Phoronata): Evidence from morphological cladistics, small and large subunit rDNA sequences, and mitochondrial cox1. Zool. J. Linn. Soc. 2009, 157, 34–50. [Google Scholar] [CrossRef][Green Version]
- Williams, A. Brachiopoda: Introduction and Integumentary System. In Microscopic Anatomy of Invertebrates; Wiley-Liss, Inc.: New York, NY, USA, 1997; Volume 13, pp. 237–296. [Google Scholar]
- Williams, A.; James, M.A.; Emig, C.C.; Mackay, S.; Rhodes, M.C. Anatomy. In Treatise on Invertebrate Paleontology. Part H. Brachiopoda. Revised. Vol. 1. Introduction; Moore, R.C., Ed.; Geological Society of America Inc.: Boulder, CO, USA, 1997; pp. 7–188. [Google Scholar]
- Nielsen, C. The development of the brachiopod Crania (Neocrania) anomala (O.F. Müller) and its phylogenetic significance. Acta Zool. 1991, 72, 7–28. [Google Scholar] [CrossRef]
- Malakhov, V.V.; Kuzmina, T.V.; Madison, A.A. Classification of planktonic stages of extant brachiopods. Invertebr. Zool. 2021, 18, 95–104. [Google Scholar] [CrossRef]
- Madison, A.A.; Kuzmina, T.V.; Temereva, E.N. Analysis of the juvenile shell of Lingula anatina (Brachiopoda: Linguliformea) provides insight into the evolution of life cycles of fossil brachiopods. Paleobiology 2021, 47, 134–148. [Google Scholar] [CrossRef]
- Butler, A.D.; Eitel, M.; Wörheide, G.; Carlson, S.J.; Sperling, E.A. Phylogenomic analysis of Brachiopoda: Revealing the evolutionary history of biomineralization with an integrated palaeontological and molecular approach. In Proceedings of the 8th International Brachiopod Congress, Milan, Italy, 11–14 September 2018; p. 37. [Google Scholar]
- Kuzmina, T.V.; Ratnovskaya, A.A.; Madison, A.A. Lophophore Evolution from the Cambrian to the Present. Paléontol. J. 2021, 55, 37–68. [Google Scholar] [CrossRef]
- Temereva, E.N.; Tsitrin, E.B. Modern Data on the Innervation of the Lophophore in Lingula anatina (Brachiopoda) Support the Monophyly of the Lophophorates. PLoS ONE 2015, 10, e0123040. [Google Scholar] [CrossRef] [PubMed]
- Temereva, E.N.; Kosevich, I.A. The nervous system of the lophophore in the ctenostome Amathia gracilis provides insight into the morphology of ancestral ectoprocts and the monophyly of the lophophorates. BMC Evol. Biol. 2016, 16, 181. [Google Scholar] [CrossRef] [PubMed]
- Temereva, E.N. Innervation of the lophophore suggests that the phoronid Phoronis ovalis is a link between phoronids and bryozoans. Sci. Rep. 2017, 7, 14440. [Google Scholar] [CrossRef] [PubMed]
- Temereva, E.N.; Kosevich, I.A. The nervous system in the cyclostome bryozoan Crisia eburnea as revealed by transmission electron and confocal laser scanning microscopy. Front. Zool. 2018, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Temereva, E.N. First data on the organization of the nervous system in juveniles of Novocrania anomala (Brachiopoda, Craniiformea). Sci. Rep. 2020, 10, 9295. [Google Scholar] [CrossRef] [PubMed]
- Temereva, E.N. Myoanatomy of the phoronid Phoronis ovalis: Functional and phylogenetic implications. Zoology 2019, 133, 27–39. [Google Scholar] [CrossRef]
- Temereva, E.N. Morphology evidences the lophophorates monophyly: Brief review of studies on the lophophore innervation. Invertebr. Zool. 2017, 14, 85–91. [Google Scholar] [CrossRef]
- Laumer, C.E.; Fernández, R.; Lemer, S.; Combosch, D.; Kocot, K.M.; Riesgo, A.; Andrade, S.C.S.; Sterrer, W.; Sørensen, M.V.; Giribet, G. Revisiting metazoan phylogeny with genomic sampling of all phyla. Proc. Biol. Sci. 2019, 286, 20190831. [Google Scholar] [CrossRef]
- Marlétaz, F.; Peijnenburg, K.T.C.A.; Goto, T.; Satoh, N.; Rokhsar, D.S. A new spiralian phylogeny places the enigmatic arrow worms among gnathiferans. Curr. Biol. 2019, 29, 312–318.e3. [Google Scholar] [CrossRef]
- Zverkov, O.A.; Mikhailov, K.V.; Isaev, S.V.; Rusin, L.Y.; Popova, O.V.; Logacheva, M.D.; Penin, A.A.; Moroz, L.L.; Panchin, Y.V.; Lyubetsky, V.; et al. Dicyemida and Orthonectida: Two Stories of Body Plan Simplification. Front. Genet. 2019, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Temereva, E.N. Myoanatomy of the lophophore in adult phoronids and the evolution of the phoronid lophophore. Biol. Bull. 2019, 237, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Isaeva, M.A.; Kosevich, I.A.; Temereva, E.N. Peculiarities of tentacle innervation of Flustrellidra hispida and evolution of lophophore in bryozoa. Dokl. Biol. Sci. 2021, 496, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Temereva, E.N.; Kuzmina, T.V. The nervous system of the most complex lophophore provides new insights into the evolution of brachiopoda. Sci. Rep. 2021, 11, 16192. [Google Scholar] [CrossRef]
- Temereva, E.N.; Kuzmina, T.V. The first data on the innervation of the lophophore in the rhynchonelliform brachiopod Hemithiris psittacea: What is the ground pattern of the lophophore in lophophorates? BMC Evol. Biol. 2017, 17, 172. [Google Scholar] [CrossRef]
- Blochmann, F. Die Anatomie von Crania anomala (Müller). In Untersuchungen über den Bau der Brachiopoden I; Blochmann, F., Ed.; Gustav Fischer: Jena, Germany, 1892; pp. 1–65. [Google Scholar]
- Hancock, A. On the organization of the Brachiopoda. Proc. R. Soc. Lond. 1857, 148, 791–869. [Google Scholar]
- Richter, S.; Loesel, R.; Purschke, G.; Schmidt-Rhaesa, A.; Scholtz, G.; Stach, T.; Vogt, L.; Wanninger, A.; Brenneis, G.; Döring, C.; et al. Invertebrate neurophylogeny: Suggested terms and definitions for a neuroanatomical glossary. Front. Zool. 2010, 7, 29. [Google Scholar] [CrossRef]
- Kuzmina, T.V.; Temereva, E.N. First description of ultrastructure of brachiopod ganglia and its phylogenetic significance. J. Zool. Syst. Evol. Res. 2020, 59, 376–386. [Google Scholar] [CrossRef]
- Blochmann, F. Die Anatomie von Discinisca lamellose (Broderip) und Lingula anatina (Bruguiére). In Untersuchungen uber den Bau der Brachiopoden II; Blochmann, F., Ed.; Gustav Fischer: Jena, Germany, 1900; pp. 1–124. [Google Scholar]
- Van Bemmelen, J.F.V. Untersuchungen uber den anatomichen und histologichen Bau der Brachiopoda Testicardina. Jena Z Naturwiss 1883, 16, 88–161. [Google Scholar]
- Reed, C.G.; Cloney, R.A. Brachiopod tentacles: Ultrastructure and functional significance of the connective tissue and myoepithelial cells in Terebratalia. Cell Tissue Res. 1977, 185, 17–42. [Google Scholar] [CrossRef]
- Gilmour, T.H.J. Ciliation and function of the food-collecting and waste-rejecting organs of lophophorates. Can. J. Zool. 1978, 56, 2142–2155. [Google Scholar] [CrossRef]
- Kuzmina, T.V.; Temereva, E.N. Tentacle muscles in brachiopods: Ultrastructure and relation to peculiarities of life style. J. Exp. Zool. Part B Mol. Dev. Evol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hay-Schmidt, A. Ultrastructure and immunocytochemistry of the nervous system of the larvae of Lingula anatina and Glottidia sp. (Brachiopoda). Zoomorphology 1992, 112, 189–205. [Google Scholar] [CrossRef]
- Santagata, S. Evaluating neurophylogenetic patterns in the larval nervous systems of brachiopods and their evolutionary significance to other bilaterian phyla. J. Morphol. 2011, 272, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Altenburger, A.; Wanninger, A. Neuromuscular development in Novocrania anomala: Evidence for the presence of serotonin and a spiralian-like apical organ in lecithotrophic brachiopod larvae. Evol. Dev. 2010, 12, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Altenburger, A.; Martinez, P.; Wanninger, A. Homeobox gene expression in Brachiopoda: The role of Not and Cdx in bodyplan patterning, neurogenesis, and germ layer specification. Gene Expr. Patterns 2011, 11, 427–436. [Google Scholar] [CrossRef]
- Malakhov, V.V.; Bogomolova, E.V.; Kuzmina, T.V.; Temereva, E.N. Evolution of metazoan life cycles and the origin of pelagic larvae. Russ. J. Dev. Biol. 2019, 50, 303–316. [Google Scholar] [CrossRef]
- Temereva, E.N. Development and structure of the nervous system in phoronids: Evolutionary significance. Neurosci. Behav. Physiol. 2022, 52, 77–85. [Google Scholar] [CrossRef]
- Voronezhskaya, E.E.; Khabarova, M.Y. Function of the apical sensory organ in the development of invertebrates. Dokl. Biol. Sci. 2003, 390, 231–234. [Google Scholar] [CrossRef]
- Rudwick, M.J.S. Living and Fossil Brachiopods; Hutchinson & Co. Ltd.: London, UK, 1970. [Google Scholar]
- Emig, C.C. Functional disposition of the lophophore in living Brachiopoda. Lethaia 1992, 25, 291–302. [Google Scholar] [CrossRef]
- Kuzmina, T.V.; Temereva, E.N. Organization of the lophophore in the deep-sea brachiopod Pelagodiscus atlanticus and evolution of the lophophore. Org. Divers. Evol. 2019, 19, 31–39. [Google Scholar] [CrossRef]

| Number | Feature | Rhynchonelliformea [27,28] | Craniiformea [17,29], herein | Linguliformea [15,33] |
|---|---|---|---|---|
| 1a | supraenteric ganglionin juvenile | + | + | ― |
| 1b | supraenteric ganglionin adult | + | ― | ― |
| 2 | subenteric ganglion | + | + | + |
| 3 | organization of subenteric ganglion | a portion of the nerve | a portion of the nerve | ganglion |
| 4 | main brachial nerve | + | + | + |
| 5 | accessory brachial nerve | ― | + | + |
| 6 | second accessory nerve | + | + | ― |
| 7a | lower brachial nerve structure: nerve net | + | + | + |
| 7b | lower brachial nerve structure: solid nerve | + | + | ― |
| 8 | origin of the lower brachial nerve | ganglionic | circumenteric | circumenteric |
| Innervation of inner tentacles | ||||
| 9 | frontal nerve | from the cross nerves | from accessory brachial nerve | from accessory brachial nerve |
| 10 | laterofrontal nerves | from second accessory nerve | from second accessory nerve | from intertentacular nerves |
| 11 | abfrontal nerve | from second accessory nerve | from second accessory nerve | from intertentacular perikarya |
| 12 | lateroabfrontal nerves | ― | ― | ― |
| 13 | immunoreactive peritoneal neurites | + | ― | ― |
| Innervation of outer tentacles | ||||
| 14 | frontal nerve | from second accessory nerve | from second accessory nerve | from intertentacular nerves |
| 15 | laterofrontal nerves | from second accessory nerve | from second accessory nerve | from intertentacular nerves |
| 16 | abfrontal nerve | from lower brachial nerve | from lower brachial nerve | from lower brachial nerve |
| 17 | lateroabfrontal nerves | from lower brachial nerve | from lower brachial nerve | ― |
| 18 | immunoreactive peritoneal neurites | + | ― | ― |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temereva, E. First Modern Data on the Lophophore Nervous System in Adult Novocrania anomala and a Current Assessment of Brachiopod Phylogeny. Biology 2022, 11, 406. https://doi.org/10.3390/biology11030406
Temereva E. First Modern Data on the Lophophore Nervous System in Adult Novocrania anomala and a Current Assessment of Brachiopod Phylogeny. Biology. 2022; 11(3):406. https://doi.org/10.3390/biology11030406
Chicago/Turabian StyleTemereva, Elena. 2022. "First Modern Data on the Lophophore Nervous System in Adult Novocrania anomala and a Current Assessment of Brachiopod Phylogeny" Biology 11, no. 3: 406. https://doi.org/10.3390/biology11030406
APA StyleTemereva, E. (2022). First Modern Data on the Lophophore Nervous System in Adult Novocrania anomala and a Current Assessment of Brachiopod Phylogeny. Biology, 11(3), 406. https://doi.org/10.3390/biology11030406







