Potentially Toxic Metals in the High-Biomass Non-Hyperaccumulating Plant Amaranthus viridis: Human Health Risks and Phytoremediation Potentials
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Sample Preparation
2.3. Digestion of Plant Samples
2.4. Metal Analysis
2.5. Quality Control and Quality Assurance
2.6. Human Health Risk Assessments
2.7. Calculation of Translocation Factor and Bioconcentration Factor
2.8. Statistical Analysis
3. Results
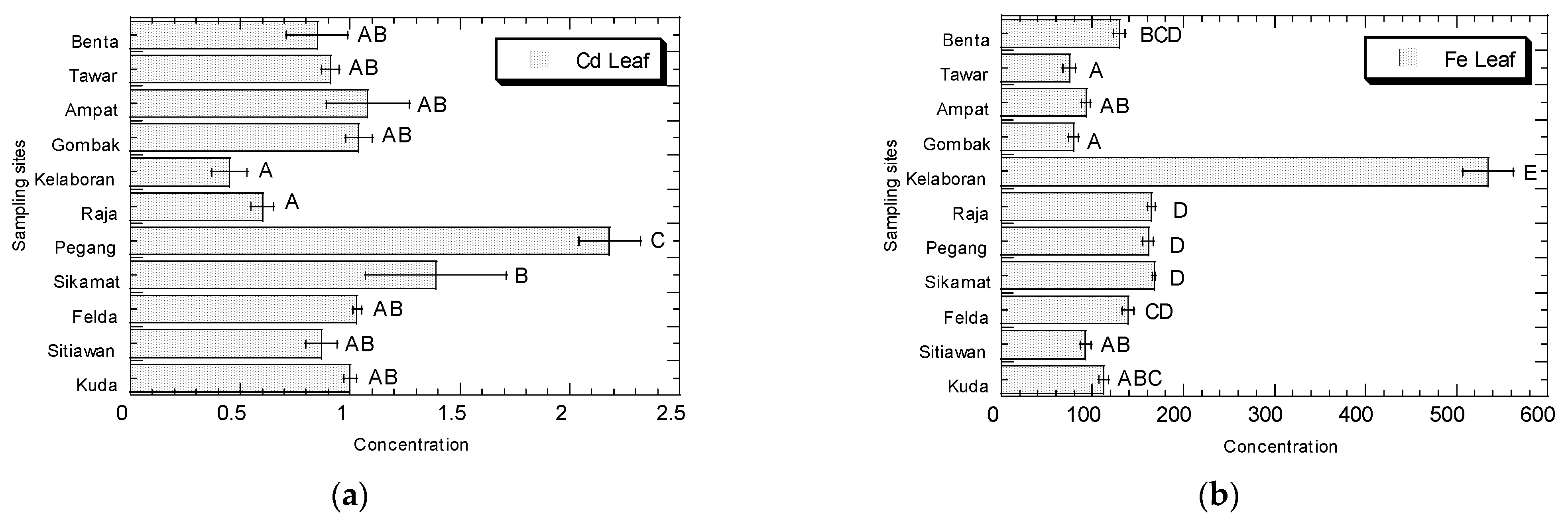
3.1. Potentially Toxic Metals Concentrations in Amaranthus
3.2. Metal Levels in the Habitat Topsoils of Amaranthus
3.3. Correlations of Metals between Amaranthus and the Geochemical Fractions of the Habitat Topsoils
3.4. Human Health Risk of Metals in Amaranthus
3.5. The Translocation Factor and Bioconcentration Factor of Amaranthus
4. Discussion
4.1. Potentially Toxic Metals Concentrations in Amaranthus
4.2. Metal Levels in the Habitat Topsoils of Amaranthus
4.3. Correlations of Metals between Amaranthus and the Geochemical Fractions of the Habitat Topsoils
4.4. Human Health Risk of Metals in Amaranthus
4.5. Amaranthus as Phytoextractor of Ni and Zn
4.6. Amaranthus as a Phytostabiliser of Cd, and Fe
4.7. Comparisons of Bioconcentration Factors of Potentially Toxic Metals in Amaranthus
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adekiya, A.O.; Aboyeji, C.; Dunsin, O.; Asamu, F.F.; Owolabi, A.O. Poultry Manure Addition Affects Production, Plant Nutritional Status and Heavy Metals Accumulation in Green Amaranth (Amaranthus Hybridus). Int. J. Agric. Biol. 2019, 22, 993–1000. [Google Scholar] [CrossRef]
- Oguntade, A.O.; Adetunji, M.T.; Arowolo, T.A.; Salako, F.K.; Azeez, J.O. Use of dye industry effluent for irrigation in Amaranthus cruentus L. production: Effect on growth, root morphology, heavy metal accumulation, and the safety concerns. Arch. Agron. Soil Sci. 2014, 61, 865–876. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA; ISBN 978-0-8493-1575-6.
- Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Cadmium toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 21–34. [Google Scholar] [CrossRef]
- Yap, C.K. Soil Pollution: Sources, Management Strategies and Health Effects; Nova Science Publishers, Incorporated: New York, NY, USA, 2019; ISBN 978-1-5361-3942-6. [Google Scholar]
- Zarcinas, B.A.; Ishak, C.F.; McLaughlin, M.J.; Cozens, G. Heavy metals in soils and crops in Southeast Asia. Environ. Geochem. Health 2004, 26, 343–357. [Google Scholar] [CrossRef]
- Ahmadpour, P.; Nawi, A.M.; Abdu, A.; Abdul-Hamid, H.; Singh, D.K.; Hassan, A.; Majid, N.M.; Jusop, S. Uptake of Heavy Metals by Jatropha curcas L. Planted in Soils Containing Sewage Sludge. Am. J. Appl. Sci. 2010, 7, 1291–1299. [Google Scholar] [CrossRef][Green Version]
- Oguntade, O.A.; Adetunji, M.T.; Salako, F.K.; Arowolo, T.A.; Azeez, J.O. Growth, Dry Matter and Heavy Metal Uptake of Potted Amaranthus Cruentus, L. as Influenced by Dye-Laden Wastewater. Trop. Agric. 2018, 95, 132–145. [Google Scholar]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of Heavy Metals: A Promising Tool for Clean-Up of Polluted Environment? Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef]
- Baker, A.; Brooks, R. Terrestrial Higher Plants Which Hyperaccumulate Metallic Elements, A Review of Their Distribution, Ecol and Phytochem. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Reeves, R.D. Hyperaccumulation of Trace Elements by Plants. In Proceedings of the Phytoremediation of Metal-Contaminated Soils; Morel, J.-L., Echevarria, G., Goncharova, N., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 25–52. [Google Scholar]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting. Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Nielsen, N.E. Studies on the Effect of Heavy Metals (Cd, Pb, Cu, Mn, Zn and Fe) upon the Growth, Productivity and Quality of Lavender (Lavandula angustifolia Mill.) Production. J. Essent. Oil Res. 1996, 8, 259–274. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Nielsen, N.E. Effect of heavy metals on peppermint and cornmint. Plant Soil 1996, 178, 59–66. [Google Scholar] [CrossRef]
- Xu, W.; Lu, G.; Dang, Z.; Liao, C.; Chen, Q.; Yi, X. Uptake and Distribution of Cd in Sweet Maize Grown on Contaminated Soils: A Field-Scale Study. Bioinorg. Chem. Appl. 2013, 2013, 959764. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Mazura, N.A.; Khairulanwar, A. Relationship between Metals in Vegetables with Soils in Farmlands of Kuching, Sarawak. Malays. J. Soil Sci. 2007, 11, 57–69. [Google Scholar]
- Molina; González-Redondo, P.; Montero; Ferrer; Moreno Rojas, R.; Sanchez, A. Effect of Collection Season and Plant Organ on the Metal Content of Amaranthus Dubius Mart. Ex Thell. Interciencia 2011, 36, 386–391. [Google Scholar]
- Azi, F.; Odo, M.O.; Okorie, P.A.; Njoku, H.A.; Nwobasi, V.N.; David, E.; Onu, T.C. Heavy metal and microbial safety assessment of raw and cooked pumpkin and Amaranthus viridis leaves grown in Abakaliki, Nigeria. Food Sci. Nutr. 2018, 6, 1537–1544. [Google Scholar] [CrossRef]
- Lukatkin, A.S.; Bashmakov, D.I.; Al Harbawee, W.E.Q.; Da Silva, J.A.T. Assessment of physiological and biochemical responses of Amaranthus retroflexus seedlings to the accumulation of heavy metals with regards to phytoremediation potential. Int. J. Phytoremediat. 2020, 23, 219–230. [Google Scholar] [CrossRef]
- Oluwatosin, G.A.; Adeoyolanu, O.D.; Ojo, A.O.; Are, K.S.; Dauda, T.O.; Aduramigba-Modupe, V.O. Heavy Metal Uptake and Accumulation by Edible Leafy Vegetable (Amaranthus Hybridus, L.) Grown on Urban Valley Bottom Soils in Southwestern Nigeria. Soil Sediment Contam. Int. J. 2009, 19, 1–20. [Google Scholar] [CrossRef]
- Sahu, M.; Kacholi, D.S. Heavy Metal Levels in Amaranthus Species from Chang’ombe-Mchicha Area in Temeke District, Dar es Salaam, Tanzania. Asian J. Chem. 2016, 28, 1123–1126. [Google Scholar] [CrossRef]
- Majid, N.; Muhamad, N.; Islam, M.M.; Riasmi, Y.; Abdu, A. Assessment of Heavy Metal Uptake and Translocation by Pluchea Indica, L. from Sawdust Sludge Contaminated Soil. J. Food Agric. Environ. 2012, 10, 849–855. [Google Scholar]
- Pachura, P.; Ociepa-Kubicka, A.; Skowron-Grabowska, B. Assessment of the availability of heavy metals to plants based on the translocation index and the bioaccumulation factor. Desalination Water Treat. 2015, 57, 1469–1477. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total. Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Yap, C.; Ismail, A.; Tan, S.; Omar, H. Correlations between speciation of Cd, Cu, Pb and Zn in sediment and their concentrations in total soft tissue of green-lipped mussel Perna viridis from the west coast of Peninsular Malaysia. Environ. Int. 2002, 28, 117–126. [Google Scholar] [CrossRef]
- Badri, M.; Aston, S. Heavy metal occurrence and geochemical fractionation: The relationships of catchment soils to associated estuarine sediments. Environ. Pollut. Ser. B Chem. Phys. 1985, 10, 61–75. [Google Scholar] [CrossRef]
- Badri, M.; Aston, S. Observations on heavy metal geochemical associations in polluted and non-polluted estuarine sediments. Environ. Pollut. Ser. B Chem. Phys. 1983, 6, 181–193. [Google Scholar] [CrossRef]
- Wong, K.W.; Yap, C.K.; Nulit, R.; Hamzah, M.S.; Chen, S.K.; Cheng, W.H.; Karami, A.; Al-Shami, S. Effects of anthropogenic activities on the heavy metal levels in the clams and sediments in a tropical river. Environ. Sci. Pollut. Res. 2016, 24, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Yaacob, A.; Yap, C.K.; Nulit, R.; Omar, H.; Al-Shami, S.A.; Bakhtiari, A.R. Assessment of health risks of the toxic Cd and Pb between leafy and fruit vegetables collected from selected farming areas of Peninsular Malaysia. Integr. Food Nutr. Metab. 2018, 5, 1–9. [Google Scholar] [CrossRef]
- Yaacob, A.; Yap, C.K.; Nulit, R.; Omar, H.; Al-Shami, S.A.; Bakhtiari, A.R. A Comparative Study of Health Risks of Fe and Ni in the Vegetables Collected from Selected Farming Areas of Peninsular Malaysia. J. Aquat. Pollut. Toxicol. 2018, 2, 21. [Google Scholar]
- Yaacob, A.; Yap, C.K.; Omar, H.; Aris, A.Z.; Latif, M.T. Health Risks of Essential Cu and Zn via Consumption of Vegetables and Relationships with the Habitat Topsoils from Three Farming Areas of Peninsular Malaysia. In Soil Pollution: Sources, Management Strategies and Health Effects; Yap, C.K., Ed.; Nova Science Publishers: New York, NY, USA, 2019; pp. 229–260. ISBN 978-1-5361-3941-9. [Google Scholar]
- US EPA. Risk-Based Concentration Table; U.S. Environment Protection Agency: Washington, DC, USA, 2000.
- Yen, S.T.; Tan, A.K.G.; Feisul, M.I. Consumption of Fruits and Vegetables in Malaysia. Asia Pac. J. Public Health 2012, 27, NP2635–NP2650. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.H.; Lee, S.T.; Ng, S.A.; Khouw, I.; Poh, B.K. Fruit and Vegetable Intake Patterns and Their Associations with Sociodemographic Characteristics, Anthropometric Status and Nutrient Intake Profiles among Malaysian Children Aged 1–6 Years. Nutrients 2017, 9, 723. [Google Scholar] [CrossRef]
- Yap, C.K.; Wong, K.W.; Al-Shami, S.A.; Nulit, R.; Cheng, W.H.; Aris, A.Z.; Sharifinia, M.; Bakhtiari, A.R.; Okamura, H.; Saleem, M.; et al. Human Health Risk Assessments of Trace Metals on the Clam Corbicula javanica in a Tropical River in Peninsular Malaysia. Int. J. Environ. Res. Public Health 2020, 18, 195. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Integrated Risk Information System (IRIS); U.S. Environment Protection Agency: Washington, DC, USA, 2008.
- US EPA. Guidance Manual for Assessing Human Health Risks from Chemically Contaminated, Fish and Shellfish; U.S. Environment Protection Agency: Washington, DC, USA, 1989; ISBN EPA-503/8-89-002.
- Zar, J.H. Biostatistical Analysis, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Hair, J.F. Multivariate Data Analysis: A Global Analysis, 7th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Garson, G.D. Partial Least Squares: Regression and Structural Equation Models; Statistical Associates Publishers: Asheboro, NC, USA, 2016; ISBN 13: 978-1-62638-039-4. [Google Scholar]
- Subha, M.; Srinivas, N. Phytoremediation Potential of Weedy Plants in Heavy Metal Contaminated Benthic Lake Sludge. Int. J. Appl. Eng. Res. 2017, 12, 4534–4538. [Google Scholar]
- AQSIQ Safety Qualification for Agricultural Product-Safety Requirements for Non-Environmental Pollution Vegetable (GB18406.1-2001); General Administration of Quality Supervision, Inspection and Quarantine of China: Beijing, China, 2001.
- Yang, L.; Huang, B.; Hu, W.; Chen, Y.; Mao, M.; Yao, L. The impact of greenhouse vegetable farming duration and soil types on phytoavailability of heavy metals and their health risk in eastern China. Chemosphere 2013, 103, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, Y.; Fu, H.; Cui, Z.; Shi, L.; Wang, L.; Liu, Z. Health risk of heavy metals in food crops grown on reclaimed tidal flat soil in the Pearl River Estuary, China. J. Hazard. Mater. 2012, 227–228, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Huang, B.; Tian, K.; Holm, P.; Zhang, Y. Heavy metals in intensive greenhouse vegetable production systems along Yellow Sea of China: Levels, transfer and health risk. Chemosphere 2016, 167, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, H.; Xue, Z.; Zhang, Q.; Cheng, F. Accumulation characteristics and potential risk of heavy metals in soil-vegetable system under greenhouse cultivation condition in Northern China. Ecol. Eng. 2017, 102, 367–373. [Google Scholar] [CrossRef]
- Xu, L.; Lu, A.; Wang, J.; Ma, Z.; Pan, L.; Feng, X.; Luan, Y. Accumulation status, sources and phytoavailability of metals in greenhouse vegetable production systems in Beijing, China. Ecotoxicol. Environ. Saf. 2015, 122, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.F.; Wang, F.H.; Wang, X.; He, W.; Wen, D.; Wang, Q.F.; Liu, X.X. Soil threshold values of total and available cadmium for vegetable growing based on field data in Guangdong province, South China. J. Sci. Food Agric. 2012, 93, 1967–1973. [Google Scholar] [CrossRef]
- FAO/WHO Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods, 21st Edition; Joint FAO/WHO Expert Committee on Food Additives, 2013. Available online: https://www.fao.org/3/i3243e/i3243e.pdf (accessed on 2 November 2021).
- Chary, N.S.; Kamala, C.; Raj, D.S.S. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol. Environ. Saf. 2008, 69, 513–524. [Google Scholar] [CrossRef]
- Yang, X.; Feng, Y.; He, Z.; Stoffella, P.J. Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. J. Trace Elements Med. Biol. 2005, 18, 339–353. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Y.; Huang, B.; Niedermann, S. Health Risk Assessment of Heavy Metals in Soils and Vegetables from a Typical Greenhouse Vegetable Production System in China. Hum. Ecol. Risk Assess. Int. J. 2014, 20, 1264–1280. [Google Scholar] [CrossRef]
- Li, F.-L.; Shi, W.; Jin, Z.-F.; Wu, H.-M.; Sheng, G.D. Excessive uptake of heavy metals by greenhouse vegetables. J. Geochem. Explor. 2017, 173, 76–84. [Google Scholar] [CrossRef]
- Khan, Z.I.; Ahmad, K.; Yasmeen, S.; Akram, N.A.; Ashraf, M.; Mehmood, N. Potential health risk assessment of potato (Solanum tuberosum L.) grown on metal contaminated soils in the central zone of Punjab, Pakistan. Chemosphere 2017, 166, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Ahmed, K.; Mamun, H.A.; Masunaga, S. Trace metals in soil and vegetables and associated health risk assessment. Environ. Monit. Assess. 2014, 186, 8727–8739. [Google Scholar] [CrossRef] [PubMed]
- Eliku, T.; Leta, S. Heavy metals bioconcentration from soil to vegetables and appraisal of health risk in Koka and Wonji farms, Ethiopia. Environ. Sci. Pollut. Res. 2017, 24, 11807–11815. [Google Scholar] [CrossRef]
- Islam, S.; Ahmed, K.; Mamun, H.A. Metal speciation in soil and health risk due to vegetables consumption in Bangladesh. Environ. Monit. Assess. 2015, 187, 1–15. [Google Scholar] [CrossRef]
- Xue, Z.-J.; Liu, S.-Q.; Liu, Y.-L.; Yan, Y.-L. Health risk assessment of heavy metals for edible parts of vegetables grown in sewage-irrigated soils in suburbs of Baoding City, China. Environ. Monit. Assess. 2011, 184, 3503–3513. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Wang, S.; Li, R.; Wang, J.J.; Zhang, Z. Soil heavy metal contamination and health risks associated with artisanal gold mining in Tongguan, Shaanxi, China. Ecotoxicol. Environ. Saf. 2017, 141, 17–24. [Google Scholar] [CrossRef]
- Liu, X.; Song, Q.; Tang, Y.; Li, W.; Xu, J.; Wu, J.; Wang, F.; Brookes, P.C. Human health risk assessment of heavy metals in soil–vegetable system: A multi-medium analysis. Sci. Total. Environ. 2013, 463–464, 530–540. [Google Scholar] [CrossRef]
- Liu, H.; Probst, A.; Liao, B. Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Sci. Total. Environ. 2005, 339, 153–166. [Google Scholar] [CrossRef]
- Roy, M.; McDonald, L.M. Metal Uptake in Plants and Health Risk Assessments in Metal-Contaminated Smelter Soils. Land Degrad. Dev. 2013, 26, 785–792. [Google Scholar] [CrossRef]
- Qureshi, A.S.; Hussain, M.I.; Ismail, S.; Khan, Q.M. Evaluating heavy metal accumulation and potential health risks in vegetables irrigated with treated wastewater. Chemosphere 2016, 163, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, B.; Dong, L.; Hu, W.; Akhtar, M.S.; Qu, M. Accumulation, sources and health risks of trace metals in elevated geochemical background soils used for greenhouse vegetable production in southwestern China. Ecotoxicol. Environ. Saf. 2017, 137, 233–239. [Google Scholar] [CrossRef]
- Islam, S.; Ahmed, K.; Mamun, H.A. Apportionment of heavy metals in soil and vegetables and associated health risks assessment. Stoch. Environ. Res. Risk Assess. 2015, 30, 365–377. [Google Scholar] [CrossRef]
- Lion, G.; Olowoyo, J. Population health risk due to dietary intake of toxic heavy metals from Spinacia oleracea harvested from soils collected in and around Tshwane, South Africa. S. Afr. J. Bot. 2013, 88, 178–182. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, M.; Liu, Y.; Zhu, Y. Health risk assessment of heavy metals in soils and vegetables from wastewater irrigated area, Beijing-Tianjin city cluster, China. J. Environ. Sci. 2012, 24, 690–698. [Google Scholar] [CrossRef]
- Favas, P.J.C.; Morais, I.; Campos, J.; Pratas, J. Nickel phytoextraction by a native population of Alyssum serpyllifolium subsp. lusitanicum on ultramafic soils (Portugal): Prospects for phytomining. Comun. Geol. 2020, 107, 115–117. [Google Scholar]
- Shang, K.; Hu, Y.H.; Vincent, G.; Labrecque, M. Biomass and phytoextraction potential of three ornamental shrub species tested over three years on a large-scale experimental site in Shanghai, China. Int. J. Phytoremediat. 2020, 22, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Santoyo-Martínez, M.; Mussali-Galante, P.; Hernández-Plata, I.; Valencia-Cuevas, L.; Flores-Morales, A.; Ortiz-Hernández, M.L.; Flores-Trujillo, K.; Ramos-Quintana, F.; Tovar-Sánchez, E. Heavy metal bioaccumulation and morphological changes in Vachellia campechiana (Fabaceae) reveal its potential for phytoextraction of Cr, Cu, and Pb in mine tailings. Environ. Sci. Pollut. Res. 2020, 27, 11260–11276. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Naqvi, N.; Gul, I.; Yaqoob, K.; Bilal, M.; Kallerhoff, J. Lead phytoextraction by Pelargonium hortorum: Comparative assessment of EDTA and DIPA for Pb mobility and toxicity. Sci. Total. Environ. 2020, 748, 141496. [Google Scholar] [CrossRef] [PubMed]
- Petrović, J.V.; Alagić, S.; Milić, S.M.; Tošić, S.B.; Bugarin, M.M. Chemometric characterization of heavy metals in soils and shoots of the two pioneer species sampled near the polluted water bodies in the close vicinity of the copper mining and metallurgical complex in Bor (Serbia): Phytoextraction and biomonitoring contexts. Chemosphere 2020, 262, 127808. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Barceló, J.; Poschenrieder, C. Phytoremediation: Principles and Perspectives. Contrib. Sci. 2003, 2, 333–344. [Google Scholar]
- Patra, D.K.; Pradhan, C.; Patra, H.K. An in situ study of growth of Lemongrass Cymbopogon flexuosus (Nees ex Steud.) W. Watson on varying concentration of Chromium (Cr6+) on soil and its bioaccumulation: Perspectives on phytoremediation potential and phytostabilisation of chromium toxicity. Chemosphere 2018, 193, 793–799. [Google Scholar] [CrossRef]
- Drozdova, I.; Alekseeva-Popova, N.; Dorofeyev, V.; Bech, J.; Belyaeva, A.; Roca, N. A comparative study of the accumulation of trace elements in Brassicaceae plant species with phytoremediation potential. Appl. Geochem. 2019, 108, 104377. [Google Scholar] [CrossRef]
- Alaboudi, K.A.; Ahmed, B.; Brodie, G. Phytoremediation of Pb and Cd contaminated soils by using sunflower (Helianthus annuus) plant. Ann. Agric. Sci. 2018, 63, 123–127. [Google Scholar] [CrossRef]
- Bernal, M.P.; Gómez, X.; Chang, R.; Arco-Lázaro, E.; Clemente, R. Strategies for the use of plant biomass obtained in the phytostabilisation of trace-element-contaminated soils. Biomass-Bioenergy 2019, 126, 220–230. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, Z.; Li, X.; Xu, Y.; Sun, X.; Liang, Q. Formation of iron plaque in the roots of Spartina alterniflora and its effect on the immobilization of wastewater-borne pollutants. Ecotoxicol. Environ. Saf. 2018, 168, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Mataruga, Z.; Jarić, S.; Kostić, O.; Marković, M.; Jakovljevic, K.; Mitrović, M.; Pavlović, P. The potential of elm trees (Ulmus glabra Huds.) for the phytostabilisation of potentially toxic elements in the riparian zone of the Sava River. Environ. Sci. Pollut. Res. 2019, 27, 4309–4324. [Google Scholar] [CrossRef]
- Santos, E.; Abreu, M.M.; Peres, S.; Magalhães, C.; Leitão, S.; Pereira, A.S.; Cerejeira, M.J. Potential of Tamarix africana and other halophyte species for phytostabilisation of contaminated salt marsh soils. J. Soils Sediments 2015, 17, 1459–1473. [Google Scholar] [CrossRef]
- Varun, M.; Jaggi, D.; D’Souza, R.; Paul, M.S.; Kumar, B. Abutilon indicum L.: A prospective weed for phytoremediation. Environ. Monit. Assess. 2015, 187, 527. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Pandey, S. Status of Phytoremediation in World Scenario. Int. J. Environ. Bioremediat. Biodegrad. 2014, 2, 178–191. [Google Scholar] [CrossRef]
- Erakhrumen, A.A. Phytoremediation: An Environmentally Sound Technology for Pollution Prevention, Control and Remediation in Developing Countries. Educ. Res. Rev. 2007, 2, 151–156. [Google Scholar]
- Jeddi, K.; Chaieb, M. Evaluation of the potential of Erodium glaucophyllum L. for phytoremediation of metal-polluted arid soils. Environ. Sci. Pollut. Res. 2018, 25, 36636–36644. [Google Scholar] [CrossRef] [PubMed]


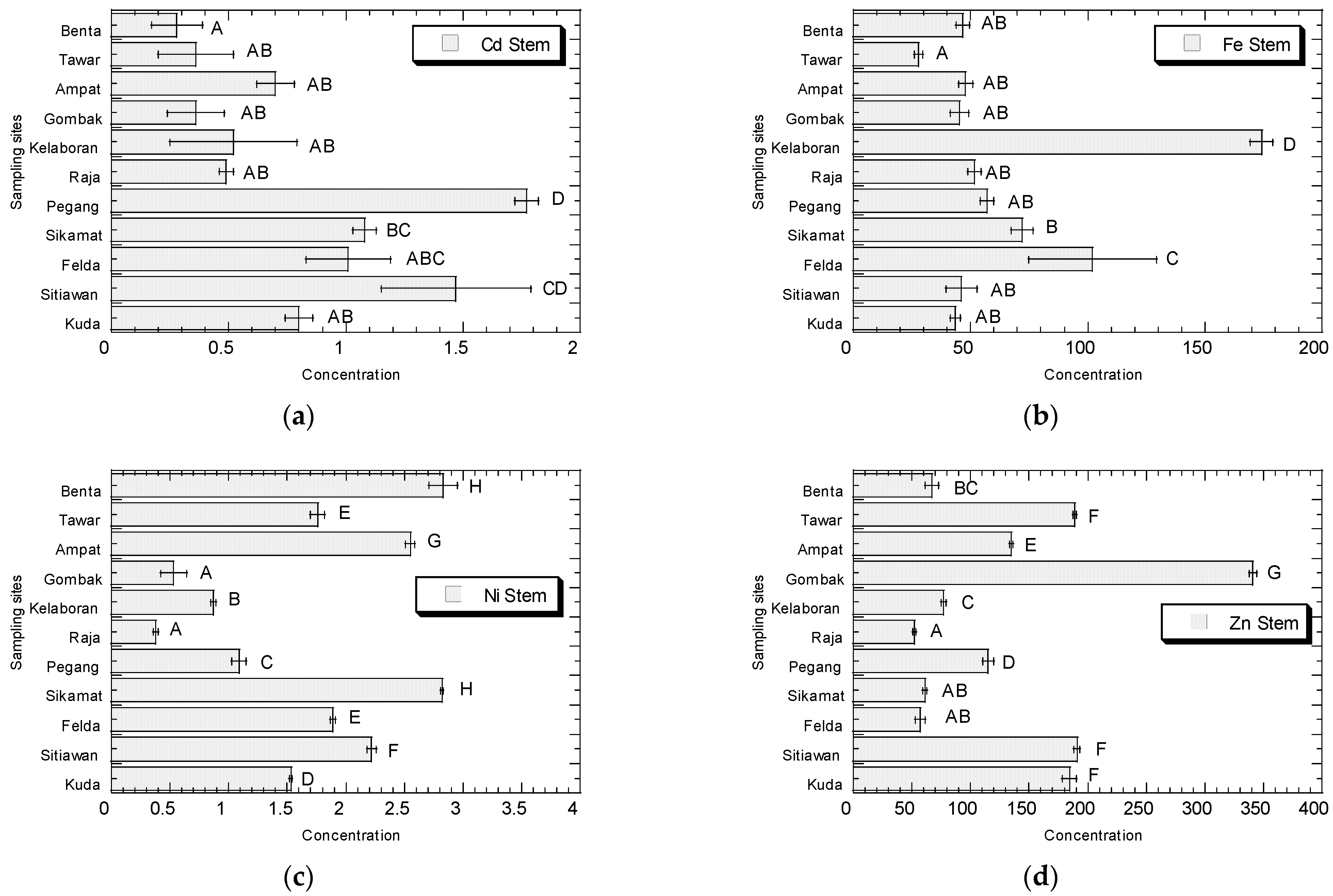

| No | Sampling Sites | Sampling Date | GPS | Received Wastewater | Site Descriptions |
|---|---|---|---|---|---|
| 1 | Ara Kuda (Penang) | 12 Sep 2017 | N 05°27′09″ | Yes | Agriculture area and palm oil plantation |
| E 100°31′12″ | |||||
| 2 | Kg Sitiawan (Perak) | 14 Sep 2017 | N 04°15′18″ | Yes | Near coastal region and residential area |
| E 100°42′27″ | |||||
| 3 | Felda Taib Andak | 4 Nov 2017 | N 01°43′55″ | No | Palm oil plantation |
| (Johor) | E 103°38′23″ | ||||
| 4 | Sikamat (N.Sembilan) | 15 Oct 2017 | N 02°46′24″ | No | Residential area and road side |
| E 101°59′28″ | |||||
| 5 | Kuala Pegang | 16 Sep 2017 | N 05°39′13″ | Yes | Agriculture area |
| (Kedah) | E 100°54′35″ | ||||
| 6 | Kg Raja (Terengganu) | 17 Jan 2018 | N 05°48′19″ | No | Residential area and road side |
| E 102°35′09″ | |||||
| 7 | Kelaboran (Kelantan) | 18 Jan 2018 | N 06°11′14″ | Yes | Residential area and road side |
| E 102°10′25″ | |||||
| 8 | Batu 12 Gombak (Selangor) | 9 Oct 2017 | N 03°15′46″ | Yes | Residential area and road side |
| E 101°43′22″ | |||||
| 9 | Simpang Ampat | 16 Oct 2017 | N 02°26′44″ | No | Residential area and highway |
| (Melaka) | E 102°11′12″ | ||||
| 10 | Kampung Tawar | 21 Dec 2017 | N 05°26′39″ | Yes | Agriculture area |
| (Perak) | E 101°07′13″ | ||||
| 11 | Benta (Pahang) | 14 Nov 2017 | N 03°47′15″ | Yes | Rubber plantation |
| E 101°51′31″ |
| EFLE | AR | OO | RES | SUM | |
|---|---|---|---|---|---|
| Cd | |||||
| Min | 0.18 | 0.24 | 0.26 | 1.12 | 2.11 |
| Max | 0.45 | 0.71 | 1.33 | 4.00 | 5.17 |
| Mean | 0.28 | 0.46 | 0.64 | 2.37 | 3.75 |
| SE | 0.03 | 0.05 | 0.09 | 0.27 | 0.30 |
| Skewness | 0.67 | 0.16 | 0.79 | 0.48 | −0.04 |
| Kurtosis | −0.49 | −1.10 | 0.22 | −0.71 | −1.16 |
| Fe | |||||
| Min | 0.40 | 26.5 | 105 | 8084 | 8430 |
| Max | 1.39 | 216 | 244 | 58,216 | 58,375 |
| Mean | 0.73 | 143 | 175 | 25,513 | 25,832 |
| SE | 0.10 | 19.4 | 14.5 | 5178 | 5160 |
| Skewness | 0.96 | −0.92 | 0.14 | 0.80 | 0.79 |
| Kurtosis | −0.33 | −0.29 | −1.40 | −0.78 | −0.79 |
| Ni | |||||
| Min | 0.25 | 0.20 | 2.43 | 2.94 | 8.07 |
| Max | 1.70 | 4.27 | 9.53 | 14.4 | 24.8 |
| Mean | 0.99 | 1.16 | 5.24 | 7.07 | 14.5 |
| SE | 0.14 | 0.36 | 0.70 | 1.08 | 1.81 |
| Skewness | 0.26 | 1.74 | 0.36 | 1.02 | 0.67 |
| Kurtosis | −0.93 | 2.32 | −1.03 | −0.12 | −0.83 |
| Zn | |||||
| Min | 1.38 | 10.7 | 15.8 | 14.8 | 42.7 |
| Max | 5.90 | 57.6 | 93.5 | 117 | 246 |
| Mean | 2.75 | 34.2 | 51.7 | 56.7 | 145 |
| SE | 0.48 | 3.96 | 8.44 | 10.3 | 21.4 |
| Skewness | 1.33 | 0.25 | 0.13 | 0.71 | 0.40 |
| Kurtosis | 0.26 | −0.18 | −1.51 | −0.91 | −1.12 |
| EFLE | AR | OO | RES | SUM | |
|---|---|---|---|---|---|
| Cd | |||||
| Leaf | 0.65 | −0.10 | −0.18 | 0.32 | 0.24 |
| Stem | 0.48 | −0.04 | 0.13 | 0.58 | 0.57 |
| Root | 0.55 | 0.46 | 0.34 | 0.73 | 0.86 |
| Fe | |||||
| Leaf | 0.20 | −0.27 | −0.37 | 0.32 | 0.32 |
| Stem | 0.17 | −0.55 | −0.57 | 0.48 | 0.48 |
| Root | 0.25 | −0.60 | −0.50 | 0.69 | 0.68 |
| Ni | |||||
| Leaf | 0.04 | 0.08 | −0.20 | 0.57 | 0.24 |
| Stem | 0.08 | 0.10 | 0.01 | 0.57 | 0.34 |
| Root | −0.53 | −0.50 | −0.02 | −0.40 | −0.35 |
| Zn | |||||
| Leaf | 0.31 | 0.21 | −0.27 | 0.03 | −0.05 |
| Stem | 0.33 | 0.28 | −0.23 | 0.00 | −0.04 |
| Root | 0.31 | 0.30 | −0.08 | 0.14 | 0.09 |
| Dependent Variables | Independent Variables Selected | R | R2 |
|---|---|---|---|
| Cd-leaf | 0.14 + 2.35 (EFLE) − 0.42 (OO) | 0.74 | 0.55 |
| Cd-stem | −0.12 + 0.45 (RES) + 1.21 (EFLE) | 0.65 | 0.42 |
| Cd-root | −0.16 + 0.81 (SUM) | 0.86 | 0.73 |
| Fe-leaf | 3.73 − 0.72 (OO) | 0.37 | 0.14 |
| Fe-stem | 2.53 − 0.79 (OO) + 0.23 (RES) | 0.65 | 0.42 |
| Fe-root | −0.69 + 0.72 (RES) | 0.69 | 0.47 |
| Ni-leaf | 0.51 + 0.64 (RES) − 0.44 (OO) | 0.71 | 0.51 |
| Ni-stem | −0.03 + 0.49 (RES) | 0.57 | 0.32 |
| Ni-root | 0.52 − 1.07 (EFLE) + 0.81 (OO) − 0.64 (AR) | 0.78 | 0.60 |
| Zn-leaf | No significant variable was selected. | - | - |
| Zn-stem | 1.72 + 0.26 (EFLE) − 0.73 (OO) + 0.92 (AR) | 0.63 | 0.40 |
| Zn-root | No significant variable was selected. | - | - |
| EDI Child | THQ Child | EDI Adult | THQ Adult | EDI Child | THQ Child | EDI Adult | THQ Adult | |
|---|---|---|---|---|---|---|---|---|
| Zn | Zn | Zn | Zn | Fe | Fe | Fe | Fe | |
| Min | 8.53 | 0.028 | 3.85 | 0.013 | 9.78 | 0.014 | 4.41 | 0.006 |
| Max | 68.19 | 0.227 | 30.74 | 0.102 | 69.95 | 0.100 | 31.54 | 0.045 |
| Mean | 31.03 | 0.103 | 13.99 | 0.047 | 20.79 | 0.030 | 9.37 | 0.013 |
| SD | 7.10 | 0.024 | 3.20 | 0.011 | 5.10 | 0.007 | 2.30 | 0.003 |
| Skewness | 0.54 | 0.538 | 0.54 | 0.531 | 2.49 | 2.493 | 2.49 | 2.475 |
| Kurtosis | −1.40 | −1.398 | −1.40 | −1.411 | 4.92 | 4.925 | 4.92 | 4.869 |
| Cd | Cd | Cd | Cd | Ni | Ni | Ni | Ni | |
| Min | 0.06 | 0.059 | 0.03 | 0.027 | 0.26 | 0.013 | 0.12 | 0.006 |
| Max | 0.29 | 0.285 | 0.13 | 0.129 | 0.97 | 0.049 | 0.44 | 0.022 |
| Mean | 0.14 | 0.136 | 0.06 | 0.061 | 0.61 | 0.031 | 0.28 | 0.014 |
| SD | 0.02 | 0.018 | 0.01 | 0.008 | 0.07 | 0.004 | 0.030 | 0.002 |
| Skewness | 1.51 | 1.410 | 1.71 | 1.431 | −0.08 | −0.058 | −0.08 | −0.054 |
| Kurtosis | 2.16 | 1.948 | 2.57 | 1.995 | −1.30 | −1.294 | −1.30 | −1.292 |
| BCFleaf/EFLE | BCFleaf/SUM | BCFstem/EFLE | BCFstem/SUM | BCFroot/EFLE | BCFroot/SUM | |
|---|---|---|---|---|---|---|
| Cd | Cd | Cd | Cd | Cd | Cd | |
| Min | 2.06 | 0.10 | 1.02 | 0.10 | 2.52 | 0.24 |
| Max | 5.70 | 0.51 | 5.73 | 0.37 | 7.06 | 0.51 |
| Mean | 3.81 | 0.29 | 2.91 | 0.21 | 5.36 | 0.38 |
| SD | 0.39 | 0.04 | 0.44 | 0.03 | 0.46 | 0.02 |
| Skewness | 0.18 | 0.35 | 0.39 | 0.41 | −0.72 | −0.25 |
| Kurtosis | −1.34 | −0.48 | −0.58 | −1.42 | −0.66 | 0.59 |
| Fe | Fe | Fe | Fe | Fe | Fe | |
| Min | 97.09 | 0.00 | 37.2 | 0.00 | 153 | 0.00 |
| Max | 972.74 | 0.03 | 317 | 0.01 | 1277 | 0.03 |
| Mean | 253.70 | 0.01 | 103 | 0.00 | 485 | 0.01 |
| SD | 74.00 | 0.00 | 23.3 | 0.00 | 116 | 0.00 |
| Skewness | 2.56 | 1.79 | 2.08 | 1.64 | 1.18 | 0.93 |
| Kurtosis | 5.14 | 2.55 | 3.56 | 2.02 | −0.02 | 0.00 |
| Ni | Ni | Ni | Ni | Ni | Ni | |
| Min | 2.13 | 0.14 | 0.51 | 0.02 | 0.73 | 0.05 |
| Max | 22.12 | 0.83 | 7.56 | 0.35 | 11.7 | 0.75 |
| Mean | 6.34 | 0.37 | 2.22 | 0.13 | 5.31 | 0.32 |
| SD | 1.74 | 0.07 | 0.61 | 0.03 | 1.09 | 0.06 |
| Skewness | 2.01 | 1.09 | 1.72 | 1.46 | 0.18 | 0.48 |
| Kurtosis | 3.20 | −0.14 | 2.43 | 1.68 | −1.07 | −0.14 |
| Zn | Zn | Zn | Zn | Zn | Zn | |
| Min | 13.8 | 0.33 | 9.10 | 0.22 | 9.72 | 0.23 |
| Max | 206 | 5.09 | 80.8 | 2.00 | 101.07 | 2.18 |
| Mean | 93.5 | 2.02 | 52.7 | 1.12 | 59.10 | 1.21 |
| SD | 19.2 | 0.49 | 7.04 | 0.20 | 7.46 | 0.21 |
| Skewness | 0.65 | 0.64 | −0.35 | −0.18 | −0.23 | −0.15 |
| Kurtosis | −0.90 | −0.80 | −0.79 | −1.52 | −0.16 | −1.43 |
| TFleaf/root | TFstem/root | TFleaf/root | TFstem/root | |
|---|---|---|---|---|
| Cd | Cd | Ni | Ni | |
| Min | 0.30 | 0.28 | 0.44 | 0.08 |
| Max | 1.23 | 0.86 | 5.34 | 2.23 |
| Mean | 0.76 | 0.53 | 1.77 | 0.62 |
| SD | 0.09 | 0.06 | 0.47 | 0.19 |
| Skewness | 0.15 | 0.22 | 1.22 | 1.71 |
| Kurtosis | −0.74 | −1.35 | 0.41 | 2.09 |
| Fe | Fe | Zn | Zn | |
| Min | 0.14 | 0.10 | 0.88 | 0.61 |
| Max | 0.94 | 0.38 | 2.61 | 1.04 |
| Mean | 0.59 | 0.24 | 1.47 | 0.89 |
| SD | 0.07 | 0.03 | 0.15 | 0.04 |
| Skewness | −0.29 | 0.00 | 1.01 | −0.80 |
| Kurtosis | −0.49 | −0.95 | 0.49 | −0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yap, C.K.; Yaacob, A.; Tan, W.S.; Al-Mutairi, K.A.; Cheng, W.H.; Wong, K.W.; Berandah Edward, F.; Ismail, M.S.; You, C.-F.; Chew, W.; et al. Potentially Toxic Metals in the High-Biomass Non-Hyperaccumulating Plant Amaranthus viridis: Human Health Risks and Phytoremediation Potentials. Biology 2022, 11, 389. https://doi.org/10.3390/biology11030389
Yap CK, Yaacob A, Tan WS, Al-Mutairi KA, Cheng WH, Wong KW, Berandah Edward F, Ismail MS, You C-F, Chew W, et al. Potentially Toxic Metals in the High-Biomass Non-Hyperaccumulating Plant Amaranthus viridis: Human Health Risks and Phytoremediation Potentials. Biology. 2022; 11(3):389. https://doi.org/10.3390/biology11030389
Chicago/Turabian StyleYap, Chee Kong, Aziran Yaacob, Wen Siang Tan, Khalid Awadh Al-Mutairi, Wan Hee Cheng, Koe Wei Wong, Franklin Berandah Edward, Mohamad Saupi Ismail, Chen-Feng You, Weiyun Chew, and et al. 2022. "Potentially Toxic Metals in the High-Biomass Non-Hyperaccumulating Plant Amaranthus viridis: Human Health Risks and Phytoremediation Potentials" Biology 11, no. 3: 389. https://doi.org/10.3390/biology11030389
APA StyleYap, C. K., Yaacob, A., Tan, W. S., Al-Mutairi, K. A., Cheng, W. H., Wong, K. W., Berandah Edward, F., Ismail, M. S., You, C.-F., Chew, W., Nulit, R., Ibrahim, M. H., Amin, B., & Sharifinia, M. (2022). Potentially Toxic Metals in the High-Biomass Non-Hyperaccumulating Plant Amaranthus viridis: Human Health Risks and Phytoremediation Potentials. Biology, 11(3), 389. https://doi.org/10.3390/biology11030389






