Deep-Dwelling Populations of Mediterranean Corallium rubrum and Eunicella cavolini: Distribution, Demography, and Co-Occurrence
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
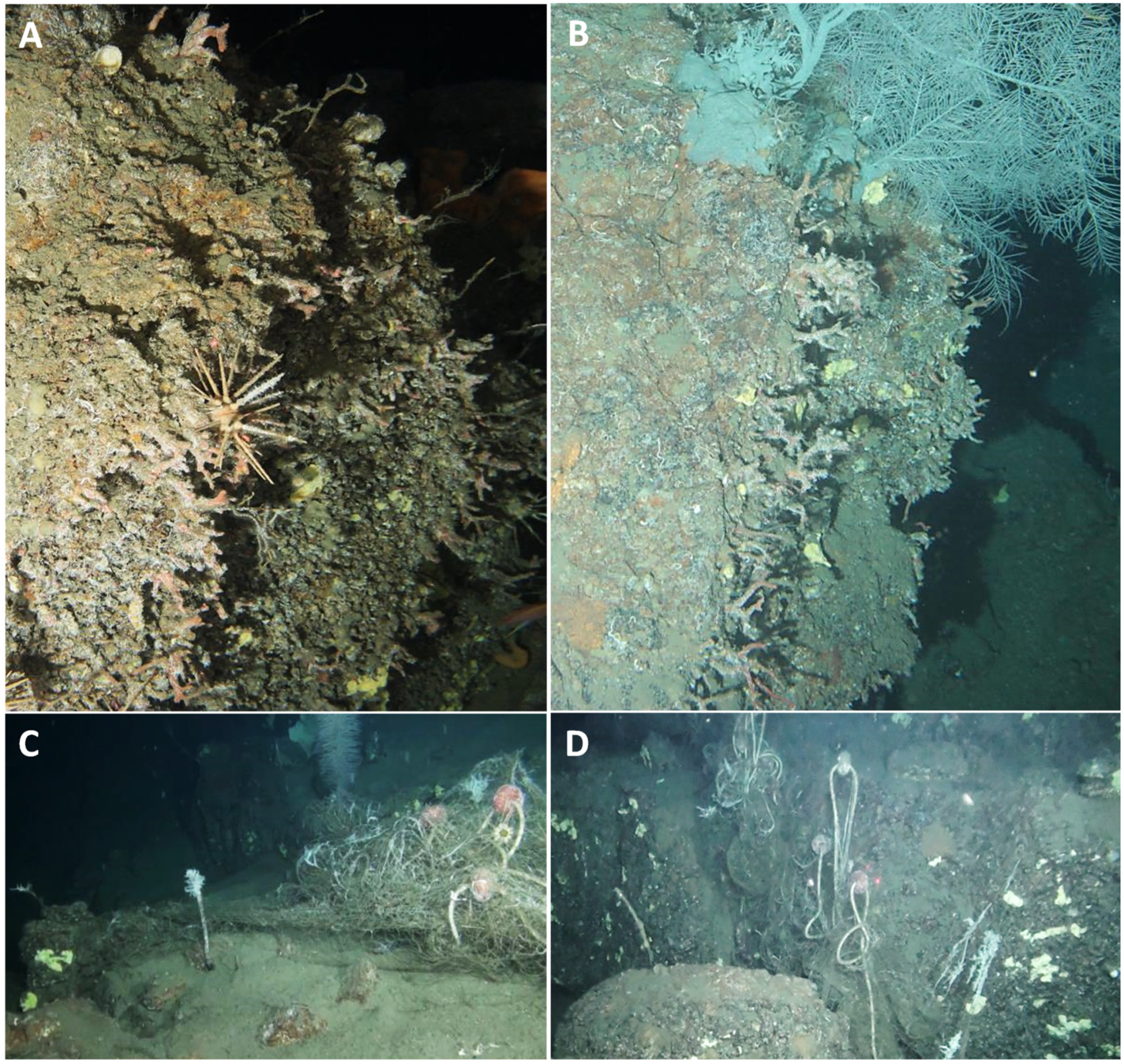
2.2. ROV Surveys—Data Acquisition
2.3. Image Analysis
2.4. Data Analysis
3. Results
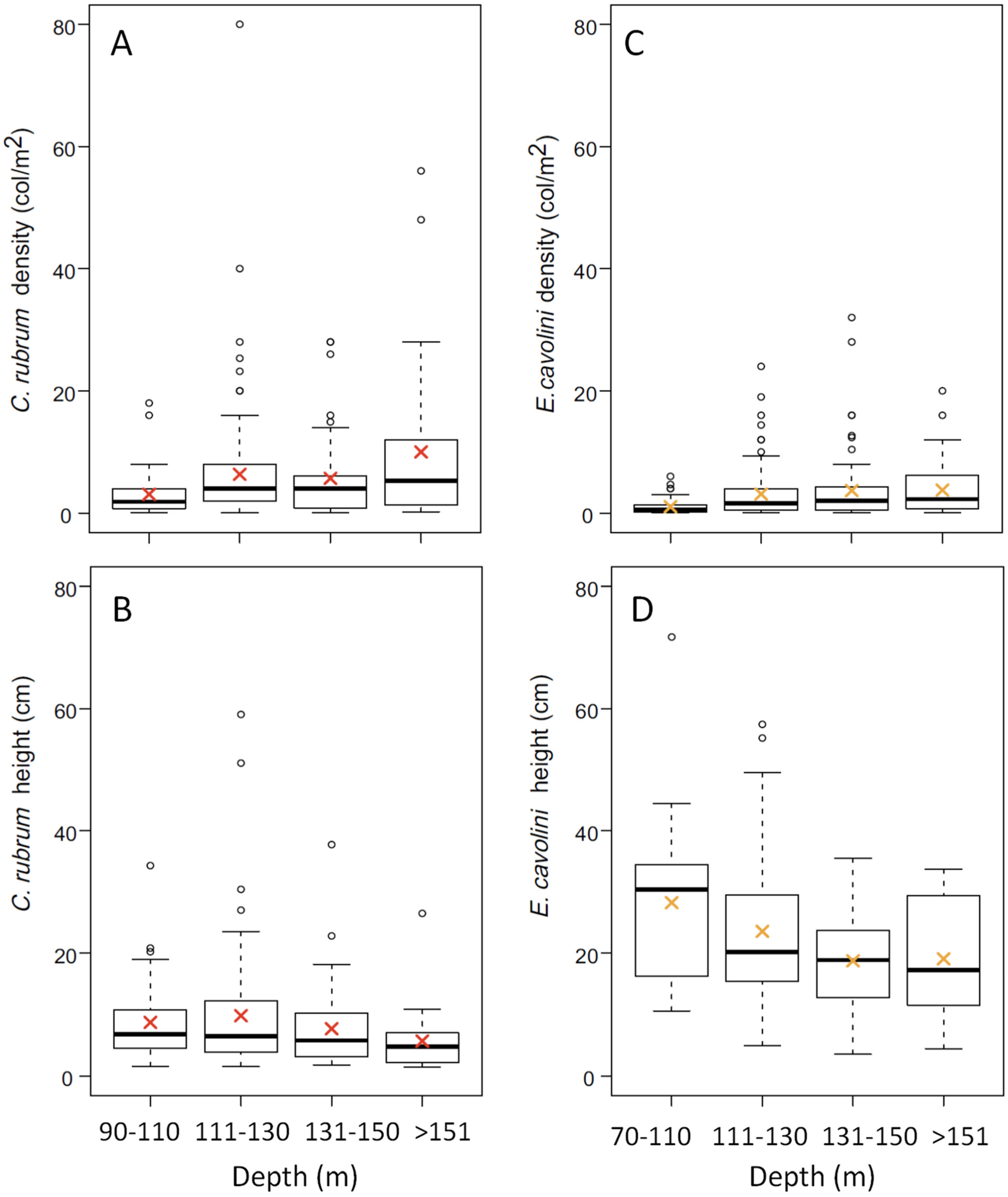
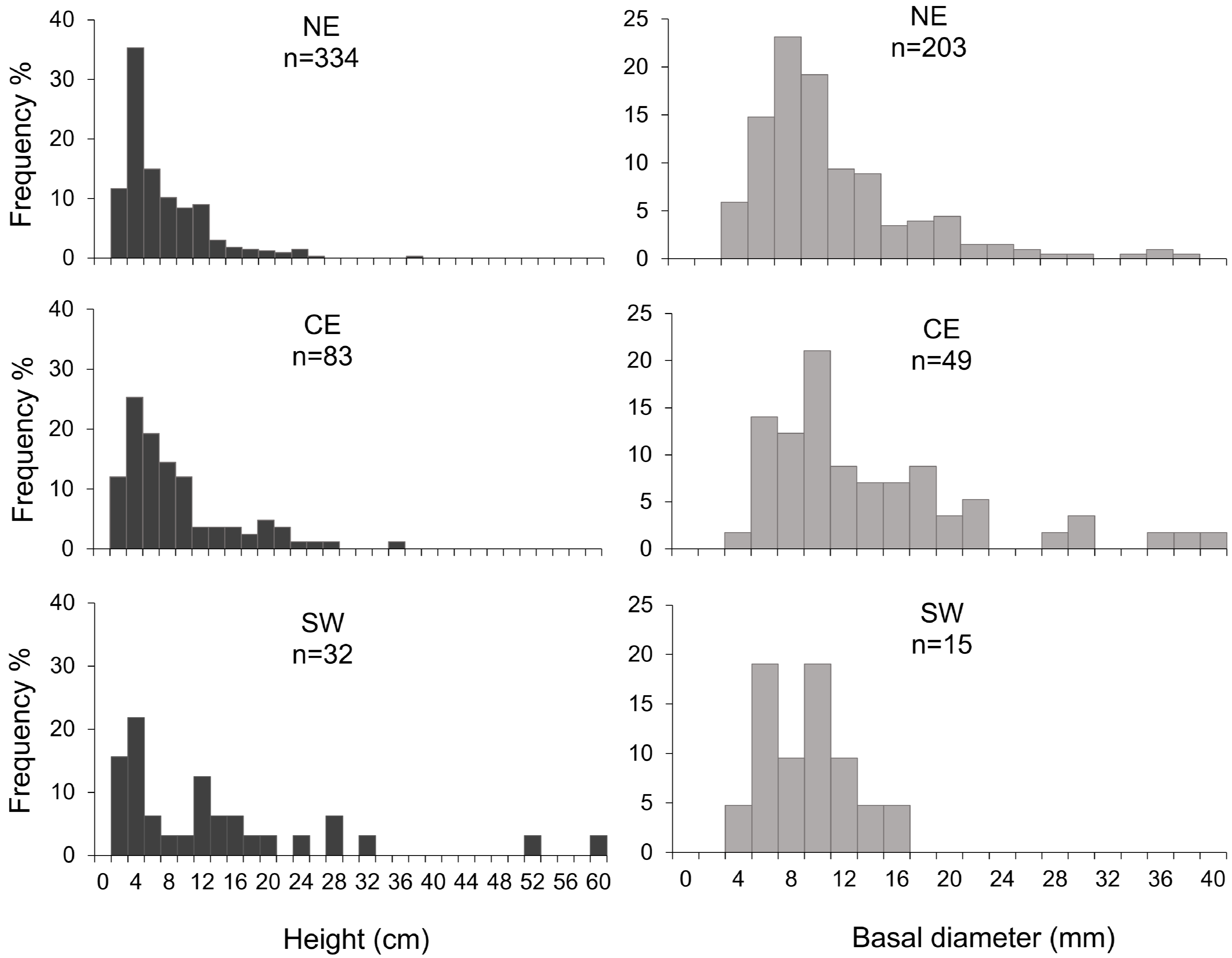
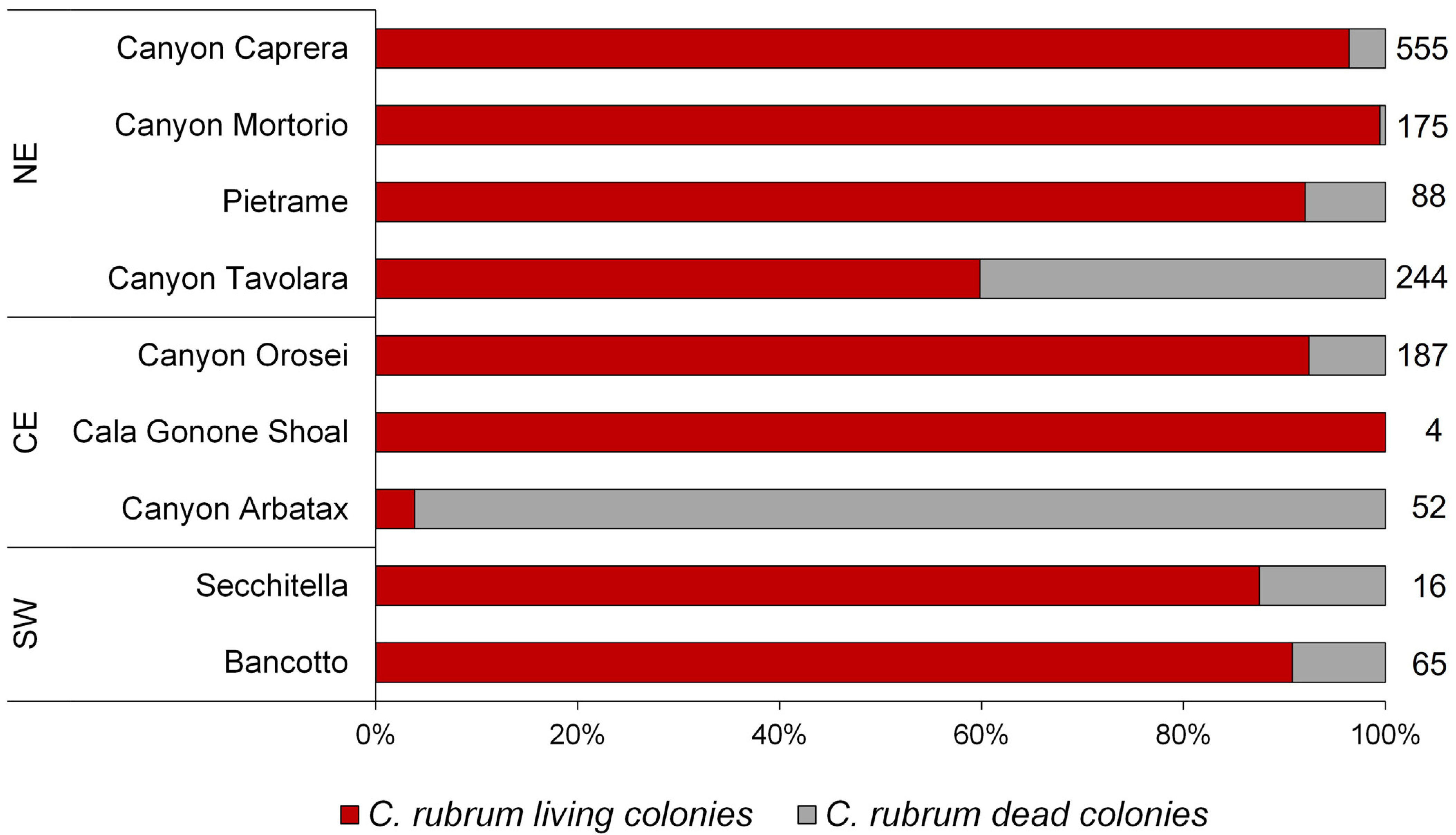
3.1. Corallium Rubrum: Occupancy, Density, and Population Size Structure
3.2. Eunicella cavolini: Occupancy, Density, and Population Size Structure
3.3. Co-Occurrence of C. rubrum with E. cavolini
4. Discussion
4.1. Distribution and Population Structure of Deep-Dwelling C. rubrum
4.2. Demography of Deep-Dwelling E. cavolini
4.3. Patterns of Co-Occurrence between Red Coral and Yellow Gorgonian in the Deep Mediterranean Sea
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. In Ecosystem Management; Springer: New York, NY, USA, 1994; pp. 130–147. [Google Scholar]
- Verdura, J.; Linares, C.; Ballesteros, E.; Coma, R.; Uriz, M.J.; Bensoussan, N.; Cebrian, E. Biodiversity Loss in a Mediterranean Ecosystem due to an Extreme Warming Event Unveils the Role of an Engineering Gorgonian Species. Sci. Rep. 2019, 9, 5911. [Google Scholar] [CrossRef] [PubMed]
- Gili, J.-M.; Coma, R. Benthic Suspension Feeders: Their Paramount Role in Littoral Marine Food Webs. Trends Ecol. Evol. 1998, 13, 316–321. [Google Scholar] [CrossRef]
- De Clippele, L.H.; Buhl-Mortensen, P.; Buhl-Mortensen, L. Fauna Associated with Cold Water Gorgonians and Sea Pens. Cont. Shelf Res. 2015, 105, 67–78. [Google Scholar] [CrossRef]
- Gili, J.M.; Sardá, R.; Madurell, T.; Rossi, S. Zoobenthos 12. The Mediterranean Sea: Its History and Present Challenges; Springer Science & Business Media: Berlin, Germany, 2013; p. 213. [Google Scholar]
- Hill, T.M.; Myrvold, C.R.; Spero, H.J.; Guilderson, T.P. Evidence for Benthic–Pelagic Food Web Coupling and Carbon Export from California Margin Bamboo Coral Archives. Biogeosciences 2014, 11, 3845–3854. [Google Scholar] [CrossRef]
- Sebens, K.P.; Koehl, M.A.R. Predation on Zooplankton by the Benthic Anthozoans Alcyonium siderium (Alcyonacea) and Metridium senile (Actiniaria) in the New England Subtidal. Mar. Biol. 1984, 81, 255–271. [Google Scholar] [CrossRef]
- Giannini, F.; Gili, J.; Santangelo, G. Relationships between the Spatial Distribution of Red Coral Corallium rubrum and Coexisting Suspension Feeders at Medas Islands Marine Protected Area (Spain). Ital. J. Zool. 2003, 70, 233–239. [Google Scholar] [CrossRef]
- Boavida, J.; Paulo, D.; Aurelle, D.; Arnaud-Haond, S.; Marschal, C.; Reed, J.; Gonçalves, J.M.S.; Serrão, E.A. Correction: A Well-Kept Treasure at Depth: Precious Red Coral Rediscovered in Atlantic Deep Coral Gardens (SW Portugal) after 300 Years. PLoS ONE 2016, 11, e0150654. [Google Scholar] [CrossRef]
- Knittweis, L.; Aguilar, R.; Alvarez, H.; Borg, J.A.; Evans, J.; Garcia, S.; Schembri, P.J. New depth record of the precious red coral Corallium rubrum for the Mediterranean. Rapp. Comm. Int. Mer. Méditerra. 2016, 41, 463. [Google Scholar]
- Garrabou, J.; Harmelin, J.G. A 20-Year Study on Life-History Traits of a Harvested Long-Lived Temperate Coral in the NW Mediterranean: Insights into Conservation and Management Needs. J. Anim. Ecol. 2002, 71, 966–978. [Google Scholar] [CrossRef]
- Lartaud, F.; Galli, G.; Raza, A.; Priori, C.; Benedetti, M.C.; Cau, A.; Santangelo, G.; Iannelli, M.; Solidoro, C.; Bramanti, L. Growth patterns in long-lived coral species. In Marine Animal Forests; Rossi, S., Bramanti, L., Gori, A., Orejas, C., Eds.; Springer: Cham, Switzerland, 2017; pp. 595–626. [Google Scholar]
- Santangelo, G.; Abbiati, M. Red Coral: Conservation and Management of an Over-Exploited Mediterranean Species. Aquat. Conserv. Mar. Freshw. Ecosyst. 2001, 11, 253–259. [Google Scholar] [CrossRef]
- Rossi, S.; Tsounis, G.; Orejas, C.; Padrón, T.; Gili, J.-M.; Bramanti, L.; Teixidó, N.; Gutt, J. Survey of Deep-Dwelling Red Coral (Corallium rubrum) Populations at Cap de Creus (NW Mediterranean). Mar. Biol. 2008, 154, 533–545. [Google Scholar] [CrossRef]
- Santangelo, G.; Bramanti, L. Quantifying the Decline in Corallium rubrum Populations. Mar. Ecol. Prog. Ser. 2010, 418, 295–297. [Google Scholar] [CrossRef][Green Version]
- Costantini, F.; Rossi, S.; Pintus, E.; Cerrano, C.; Gili, J.-M.; Abbiati, M. Low Connectivity and Declining Genetic Variability along a Depth Gradient in Corallium rubrum Populations. Coral Reefs 2011, 30, 991–1003. [Google Scholar] [CrossRef]
- Angiolillo, M.; Gori, A.; Canese, S.; Bo, M.; Priori, C.; Bavestrello, G.; Salvati, E.; Erra, F.; Greenacre, M.; Santangelo, G. Distribution and Population Structure of Deep-Dwelling Red Coral in the Northwest Mediterranean. Mar. Ecol. 2015, 37, 294–310. [Google Scholar] [CrossRef]
- Costantini, F.; Taviani, M.; Remia, A.; Pintus, E.; Schembri, P.J.; Abbiati, M. Deep-Water Corallium rubrum (L., 1758) from the Mediterranean Sea: Preliminary Genetic Characterisation. Mar. Ecol. 2010, 31, 261–269. [Google Scholar] [CrossRef]
- Priori, C.; Mastascusa, V.; Erra, F.; Angiolillo, M.; Canese, S.; Santangelo, G. Demography of Deep-Dwelling Red Coral Populations: Age and Reproductive Structure of a Highly Valued Marine Species. Estuar. Coast. Shelf Sci. 2013, 118, 43–49. [Google Scholar] [CrossRef]
- Torrents, O.; Tambutté, E.; Caminiti, N.; Garrabou, J. Upper Thermal Thresholds of Shallow vs. Deep Populations of the Precious Mediterranean Red Coral Corallium rubrum (L.): Assessing the Potential Effects of Warming in the NW Mediterranean. J. Exp. Mar. Biol. Ecol. 2008, 357, 7–19. [Google Scholar] [CrossRef]
- Garrabou, J.; Coma, R.; Bensoussan, N.; Bally, M.; ChevaldonnÉ, P.; Cigliano, M.; Diaz, D.; Harmelin, J.G.; Gambi, M.C.; Kersting, D.K.; et al. Mass Mortality in Northwestern Mediterranean Rocky Benthic Communities: Effects of the 2003 Heat Wave. Glob. Change Biol. 2009, 15, 1090–1103. [Google Scholar] [CrossRef]
- Ledoux, J.-B.; Garrabou, J.; Bianchimani, O.; Drap, P.; Féral, J.-P.; Aurelle, D. Fine-Scale Genetic Structure and Inferences on Population Biology in the Threatened Mediterranean Red Coral, Corallium rubrum. Mol. Ecol. 2010, 19, 4204–4216. [Google Scholar] [CrossRef]
- Carugati, L.; Cau, A.; Follesa, M.C.; Melis, R.; Moccia, D.; Porcu, C.; Salvadori, S.; Cannas, R. Onboard Scientific Observers Provide a Realistic Picture of Harvesting and Management Priorities for the Precious Red Coral (Corallium rubrum L.). Front. Mar. Sci. 2020, 7, 482. [Google Scholar] [CrossRef]
- Cannas, R.; Sacco, F.; Cau, A.; Coluccia, E.; Follesa, M.C.; Cau, A. New Insights into Connectivity Patterns of Mesophotic Red Coral (Corallium rubrum) Populations. Hydrobiologia 2015, 759, 63–73. [Google Scholar] [CrossRef]
- Cannas, R.; Sacco, F.; Cau, A.; Cuccu, D.; Follesa, M.C.; Cau, A. Genetic Monitoring of Deep-Water Exploited Banks of the Precious Sardinia Coral Corallium rubrum (L., 1758): Useful Data for a Sustainable Management. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 26, 236–250. [Google Scholar] [CrossRef]
- Porcu, C.; Follesa, M.C.; Cannas, R.; Cau, A.; Soldovilla, G.; Pesci, P.; Cau, A. Reproductive Patterns in Deep versus Shallow Populations of the Precious Mediterranean Gorgonian Corallium rubrum (Linnaeus, 1758) (Sardinia, Central-Western Mediterranean). Mediterr. Mar. Sci. 2017, 18, 64. [Google Scholar] [CrossRef]
- Moccia, D.; Cau, A.; Bramanti, L.; Carugati, L.; Canese, S.; Follesa, M.C.; Cannas, R. Spatial Distribution and Habitat Characterization of Marine Animal Forest Assemblages along Nine Submarine Canyons of Eastern Sardinia (Central Mediterranean Sea). Deep. Sea Res. Part I Oceanogr. Res. Pap. 2021, 167, 103422. [Google Scholar] [CrossRef]
- Sini, M.; Kipson, S.; Linares, C.; Koutsoubas, D.; Garrabou, J. The Yellow Gorgonian Eunicella cavolini: Demography and Disturbance Levels across the Mediterranean Sea. PLoS ONE 2015, 10, e0126253. [Google Scholar] [CrossRef]
- Bo, M.; Canese, S.; Spaggiari, C.; Pusceddu, A.; Bertolino, M.; Angiolillo, M.; Giusti, M.; Loreto, M.F.; Salvati, E.; Greco, S.; et al. Deep Coral Oases in the South Tyrrhenian Sea. PLoS ONE 2012, 7, e49870. [Google Scholar] [CrossRef]
- Watremez, P. Canyon heads in the French Mediterranean Overview of results from the MEDSEACAN and CORSEACAN campaigns (2008–2010). In Mediterranean Submarine Canyons: Ecology and Governance; Würtz, M., Ed.; IUCN: Gland, Switzerland; Malaga, Spain, 2012; pp. 105–112. [Google Scholar]
- Cupido, R.; Cocito, S.; Barsanti, M.; Sgorbini, S.; Peirano, A.; Santangelo, G. Unexpected Long-Term Population Dynamics in a Canopy-Forming Gorgonian Coral Following Mass Mortality. Mar. Ecol. Prog. Ser. 2009, 394, 195–200. [Google Scholar] [CrossRef]
- Topçu, E.N.; Öztürk, B. Composition and Abundance of Octocorals in the Sea of Marmara, Where the Mediterranean Meets the Black Sea. Sci. Mar. 2015, 79, 125–135. [Google Scholar] [CrossRef]
- Carugati, L.; Bramanti, L.; Giordano, B.; Pittura, L.; Cannas, R.; Follesa, M.C.; Pusceddu, A.; Cau, A. Colonization of Plastic Debris by the Long-Lived Precious Red Coral Corallium rubrum: New Insights on the “Plastic Benefits” Paradox. Mar. Pollut. Bull. 2021, 165, 112104. [Google Scholar] [CrossRef]
- Bavestrello, G.; Bo, M.; Canese, S.; Sandulli, R.; Cattaneo-Vietti, R. The Red Coral Populations of the Gulfs of Naples and Salerno: Human Impact and Deep Mass Mortalities. Ital. J. Zool. 2014, 81, 552–563. [Google Scholar] [CrossRef]
- Sulli, A. Structural Framework and Crustal Characteristics of the Sardinia Channel Alpine Transect in the Central Mediterranean. Tectonophysics 2000, 324, 321–336. [Google Scholar] [CrossRef]
- Mascle, G.H.; Tricart, P.; Torelli, L.; Bouillin, J.-P.; Rolfo, F.; Lapierre, H.; Monié, P.; Depardon, S.; Mascle, J.; Peis, D. Evolution of the Sardinia Channel (Western Mediterranean): New Constraints from a Diving Survey on Cornacya Seamount off SE Sardinia. Mar. Geol. 2001, 179, 179–201. [Google Scholar] [CrossRef]
- Harris, P.T.; Whiteway, T. Global Distribution of Large Submarine Canyons: Geomorphic Differences between Active and Passive Continental Margins. Mar. Geol. 2011, 285, 69–86. [Google Scholar] [CrossRef]
- Millot, C. Levantine Intermediate Water Characteristics: An Astounding General Misunderstanding! Sci. Mar. 2013, 77, 217–232. [Google Scholar] [CrossRef]
- Astraldi, M.; Conversano, F.; Civitarese, G.; Gasparini, G.P.; Ribera d’Alcalà, M.; Vetrano, A. Water Mass Properties and Chemical Signatures in the Central Mediterranean Region. J. Mar. Syst. 2002, 33–34, 155–177. [Google Scholar] [CrossRef]
- Kohler, K.E.; Gill, S.M. Coral Point Count with Excel Extensions (CPCe): A Visual Basic Program for the Determination of Coral and Substrate Coverage Using Random Point Count Methodology. Comput. Geosci. 2006, 32, 1259–1269. [Google Scholar] [CrossRef]
- Follesa, M.C.; Cannas, R.; Cau, A.; Pedoni, C.; Pesci, P.; Cau, A. Deep-Water Red Coral from the Island of Sardinia (North-Western Mediterranean): A Local Example of Sustainable Management. Mar. Freshw. Res. 2013, 64, 706–715. [Google Scholar] [CrossRef]
- Tsounis, G.; Rossi, S.; Aranguren, M.; Gili, J.-M.; Arntz, W. Effects of Spatial Variability and Colony Size on the Reproductive Output and Gonadal Development Cycle of the Mediterranean Red Coral (Corallium rubrum L.). Mar. Biol. 2006, 148, 513–527. [Google Scholar] [CrossRef]
- Brazeau, D.A.; Lasker, H.R. Inter- and Intraspecific Variation in Gorgonian Colony Morphology: Quantifying Branching Patterns in Arborescent Animals. Coral Reefs 1988, 7, 139–143. [Google Scholar] [CrossRef]
- Benedetti, M.C.; Priori, C.; Erra, F.; Santangelo, G. Growth Patterns in Mesophotic Octocorals: Timing the Branching Process in the Highly-Valuable Mediterranean Corallium rubrum. Estuar. Coast. Shelf Sci. 2016, 171, 106–110. [Google Scholar] [CrossRef]
- Angiolillo, M.; Fortibuoni, T. Impacts of Marine Litter on Mediterranean Reef Systems: From Shallow to Deep Waters. Front. Mar. Sci. 2020, 7, 826. [Google Scholar] [CrossRef]
- Canals, M.; Pham, C.K.; Bergmann, M.; Gutow, L.; Hanke, G.; van Sebille, E.; Angiolillo, M.; Buhl-Mortensen, L.; Cau, A.; Ioakeimidis, C.; et al. The Quest for Seafloor Macrolitter: A Critical Review of Background Knowledge, Current Methods and Future Prospects. Environ. Res. Lett. 2020, 16, 023001. [Google Scholar] [CrossRef]
- Sokal, R.R.; James Rohlf, F. Biometry; W.H. Freeman & Co: New York, NY, USA, 1995. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, R.K. Permanova+ for Primer: Guide to Software and Statisticl Methods; Primer-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Anderson, M.J. A New Method for Non-Parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Anderson, M.J.; Robinson, J. Generalized Discriminant Analysis Based on Distances. Aust. N. Z. J. Stat. 2003, 45, 301–318. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User manual. Tutor. PRIMER-E Plymouth 2015, 1–20. [Google Scholar]
- McArdle, B.H.; Anderson, M.J. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology 2001, 82, 290–297. [Google Scholar] [CrossRef]
- Miyanishi, K.; Hoy, A.R.; Cavers, P.B. A Generalized Law of Self-Thinning in Plant Populations (Self-Thinning in Plant Populations). J. Theor. Biol. 1979, 78, 439–442. [Google Scholar] [CrossRef]
- Cau, A.; Bramanti, L.; Cannas, R.; Follesa, M.C.; Angiolillo, M.; Canese, S.; Bo, M.; Cuccu, D.; Guizien, K. Habitat Constraints and Self-Thinning Shape Mediterranean Red Coral Deep Population Structure: Implications for Conservation Practice. Sci. Rep. 2016, 6, 23322. [Google Scholar] [CrossRef]
- Santangelo, G.; Carletti, E.; Maggi, E.; Bramanti, L. Reproduction and Population Sexual Structure of the Overexploited Mediterranean Red Coral Corallium rubrum. Mar. Ecol. Prog. Ser. 2003, 248, 99–108. [Google Scholar] [CrossRef]
- Torrents, O.; Garrabou, J.; Marschal, C.; Harmelin, J.G. Age and Size at First Reproduction in the Commercially Exploited Red Coral Corallium rubrum (L.) in the Marseilles Area (France, NW Mediterranean). Biol. Conserv. 2005, 121, 391–397. [Google Scholar] [CrossRef]
- Linares, C.; Bianchimani, O.; Torrents, O.; Marschal, C.; Drap, P.; Garrabou, J. Marine Protected Areas and the Conservation of Long-Lived Marine Invertebrates: The Mediterranean Red Coral. Mar. Ecol. Prog. Ser. 2010, 402, 69–79. [Google Scholar] [CrossRef]
- Linares, C.; Coma, R.; Garrabou, J.; Díaz, D.; Zabala, M. Size Distribution, Density and Disturbance in Two Mediterranean Gorgonians:Paramuricea ClavataandEunicella Singularis. J. Appl. Ecol. 2008, 45, 688–699. [Google Scholar] [CrossRef]
- Bramanti, L.; Vielmini, I.; Rossi, S.; Tsounis, G.; Iannelli, M.; Cattaneo-Vietti, R.; Priori, C.; Santangelo, G. Demographic Parameters of Two Populations of Red Coral (Corallium rubrum L. 1758) in the North Western Mediterranean. Mar. Biol. 2014, 161, 1015–1026. [Google Scholar] [CrossRef]
- Cau, A.; Alvito, A.; Moccia, D.; Canese, S.; Pusceddu, A.; Rita, C.; Angiolillo, M.; Follesa, M.C. Submarine Canyons along the Upper Sardinian Slope (Central Western Mediterranean) as Repositories for Derelict Fishing Gears. Mar. Pollut. Bull. 2017, 123, 357–364. [Google Scholar] [CrossRef]
- Bo, M.; Bava, S.; Canese, S.; Angiolillo, M.; Cattaneo-Vietti, R.; Bavestrello, G. Fishing Impact on Deep Mediterranean Rocky Habitats as Revealed by ROV Investigation. Biol. Conserv. 2014, 171, 167–176. [Google Scholar] [CrossRef]
- Ferrigno, F.; Appolloni, L.; Rendina, F.; Donnarumma, L.; Russo, G.F.; Sandulli, R. Red Coral (Corallium rubrum) Populations and Coralligenous Characterization within “Regno Di Nettuno MPA” (Tyrrhenian Sea, Italy). Eur. Zool. J. 2020, 87, 203–213. [Google Scholar] [CrossRef]
- Ferrigno, F.; Appolloni, L.; Russo, G.F.; Sandulli, R. Impact of Fishing Activities on Different Coralligenous Assemblages of Gulf of Naples (Italy). J. Mar. Biol. Assoc. U. K. 2017, 98, 41–50. [Google Scholar] [CrossRef]
- Cannas, R.; Follesa, M.C.; Cau, A.; Cau, A.; Friedman, K. Global Report on the Biology, Fishery and Trade of Precious Corals; FAO: Rome, Italy, 2019; pp. 1–254. [Google Scholar]
- Weinbauer, M.G.; Velimirov, B. Population Dynamics and Overgrowth of the Sea Fan Eunicella cavolini (Coelenterata: Octocorallia). Estuar. Coast. Shelf Sci. 1996, 42, 583–595. [Google Scholar] [CrossRef]
- Bo, M.; Bertolino, M.; Borghini, M.; Castellano, M.; Covazzi Harriague, A.; Di Camillo, C.G.; Gasparini, G.; Misic, C.; Povero, P.; Pusceddu, A.; et al. Characteristics of the Mesophotic Megabenthic Assemblages of the Vercelli Seamount (North Tyrrhenian Sea). PLoS ONE 2011, 6, e16357. [Google Scholar] [CrossRef]
- Velimirov, B. Orientation in the sea fan Eunicella cavolinii related to water movement. Helgoländer Wiss. Meeresunters. 1973, 24, 163–173. [Google Scholar] [CrossRef][Green Version]
- Velimirov, B. Variations in growth forms of Eunicella cavolinh Koch (Octocorallia) related to intensity of water movement. J. Exp. Mar. Biol. Ecol. 1976, 21, 109–117. [Google Scholar] [CrossRef]
- Weinbauer, M.G.; Velimirov, B. Morphological variations in the Mediterranean Sea fan Eunicella cavolini (Coelenterata: Gorgonacea) in relation to exposure, colony size and colony region. Bull. Mar. Sci. 1995, 56, 283–295. [Google Scholar]
- Canals, M.; Puig, P.; de Madron, X.D.; Heussner, S.; Palanques, A.; Fabres, J. Flushing Submarine Canyons. Nature 2006, 444, 354–357. [Google Scholar] [CrossRef]
- Zabala i Limousin, M.; Ballesteros i Sagarra, E. Surface-dependent strategies and energy flux in benthic marine communities or, why corals do not exist in the Mediterranean. Sci. Mar. 1989, 53, 3–17. [Google Scholar]
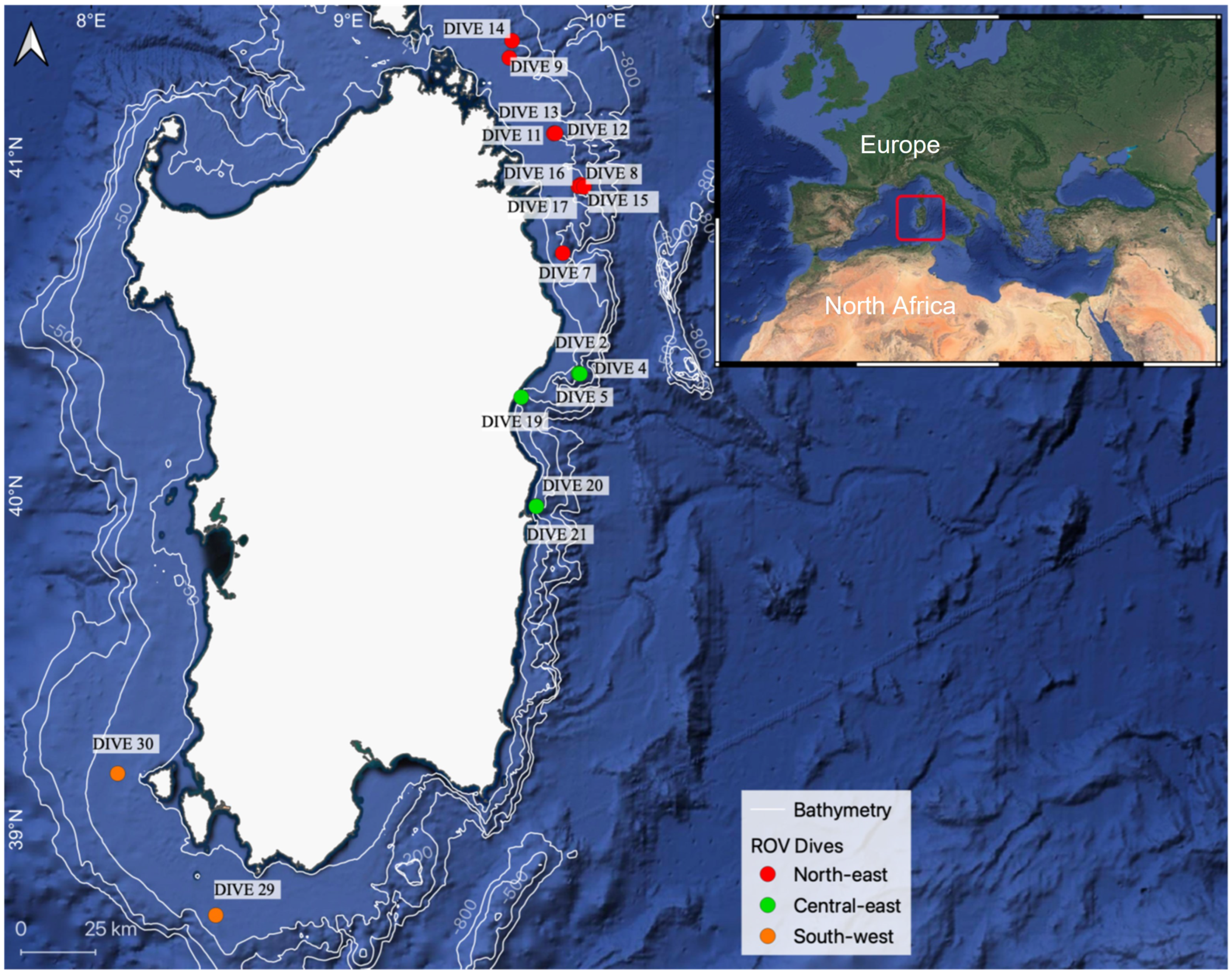


| Geographic Area | Site | Dive ID Number | Depth Range (m) | Coordinates | Surveyed Area (m2) | N Frames | |
|---|---|---|---|---|---|---|---|
| Lat (N) | Long (E) | ||||||
| North-east | Canyon Caprera | 9 | 112–220 | 41°17′427″ | 9°37′481″ | 52 | 10 |
| Canyon Caprera | 10 | 127–190 | 41°18′357″ | 9°38′021″ | 84 | 28 | |
| Canyon Caprera | 14 | 121–165 | 41°20′433″ | 9°38′121″ | 1068 | 79 | |
| Canyon Mortorio | 11 | 51–123 | 41°04′217″ | 9°47′899″ | 107 | 87 | |
| Canyon Mortorio | 12 | 110–152 | 41°04′152″ | 9°47′845″ | 140 | 114 | |
| Canyon Mortorio | 13 | 126–163 | 41°04′200″ | 9°48′224″ | 122 | 48 | |
| Pietrame | 7 | 144–150 | 40°43′080″ | 9°49′933″ | 86 | 35 | |
| Canyon Tavolara | 8 | 106–190 | 40°55′169″ | 9°54′140″ | 456 | 69 | |
| Canyon Tavolara | 15 | 90–103 | 40°54′858″ | 9°53′592″ | 97 | 20 | |
| Canyon Tavolara | 16 | 107–170 | 40°54′860″ | 9°54′041″ | 225 | 63 | |
| Canyon Tavolara | 17 | 158–290 | 40°54′768″ | 9°54′908″ | 264 | 70 | |
| Central-east | Canyon Orosei | 2 | 76–120 | 40°21′825″ | 9°53′670″ | 661 | 89 |
| Canyon Orosei | 4 | 96–120 | 40°21′767″ | 9°53′657″ | 18 | 5 | |
| Canyon Orosei | 5 | 156–187 | 40°21′692″ | 9°53′950″ | 171 | 57 | |
| Cala Gonone Shoal | 19 | 54–217 | 40°17′544″ | 9°40′252″ | 71 | 32 | |
| Canyon Arbatax | 20 | 108–147 | 39°58′136″ | 9°43′734″ | 73 | 14 | |
| Canyon Arbatax | 21 | 121–181 | 39°58′080″ | 9°43′770″ | 213 | 43 | |
| South-west | Secchitella | 33 | 143–156 | 39°10′122″ | 8°06′133″ | 78 | 25 |
| Bancotto | 29 | 108–140 | 38°44′425″ | 8°29′025″ | 208 | 49 | |
| Area | Site | Depth Range (m) | Colony Density (col/m2) | Height (cm) | Basal Diameter (mm) | Max Order Branches | Orientation of Colonies (%) | Colonies Occurring with E. cavolini (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0° | 45° | 90° | 135° | 180° | ||||||||
| North-East | Canyon Caprera | 116–152 | 2.1 ± 1.9 | 5.3 ± 4.4 | 0.84 ± 0.41 | fifth | 0 | 0 | 30 | 56 | 14 | 85 |
| Canyon Mortorio | 108–146 | 6.9 ± 8.7 | 6.14.9 | 0.95 ± 0.37 | fourth | 6 | 6 | 55 | 31 | 2 | 19 | |
| Pietrame | 145–149 | 3.2 ± 0.4 | 9.4 ± 6.8 | 1.70 ± 0.90 | fourth | 0 | 0 | 21 | 48 | 31 | 93 | |
| Canyon Tavolara | 108–257 | 3.7 ± 3.6 | 6.5 ± 4.6 | 1.04 ± 0.56 | fourth | 0 | 1 | 30 | 62 | 7 | 29 | |
| Central-East | Canyon Orosei | 91–176 | 6.2 ± 10.8 | 8.5 ± 6.9 | 1.34 ± 0.90 | third | 0 | 1 | 13 | 63 | 23 | 37 |
| Cala Gonone Shoal | 132 | 4 * | 3.5 ± 0.8 | 0.75 * | third | 0 | 0 | 0 | 100 | 0 | 100 | |
| Canyon Arbatax | 131–148 | 8.1 ± 7.5 | 10.8 ± 4 | 1.91 ± 0.24 | na | 0 | 4 | 50 | 44 | 2 | 0 | |
| South-west | Secchitella | 148–152 | 1.6 ± 2.1 | 7.9 ± 9.1 | 0.76 ± 0.56 | third | 0 | 11 | 22 | 56 | 11 | 19 |
| Bancotto | 112–123 | 3.5 ± 1.6 | 13.9 ± 14.9 | 0.86 ± 0.28 | fifth | 9 | 6 | 42 | 26 | 17 | 34 | |
| (A) Variable | Source | df | MS | Pseudo-F | p (MC) |
| Density | Ar | 2 | 69.010 | 1.022 | ns |
| Si(Ar) | 6 | 53.380 | 0.443 | ns | |
| Pr(Si(Ar)) | 7 | 138.100 | 1.799 | ns | |
| Residual | 233 | 76.720 | |||
| Total | 248 | ||||
| Height | Ar | 2 | 174.170 | 3.362 | * |
| Si(Ar) | 6 | 56.693 | 0.351 | ns | |
| Pr(Si(Ar)) | 7 | 233.400 | 6.345 | *** | |
| Residual | 433 | 36.785 | |||
| Total | 448 | ||||
| Basal diameter | Ar | 2 | 0.430 | 1.0020 | ns |
| Si(Ar) | 6 | 0.542 | 0.859 | ns | |
| Pr(Si(Ar)) | 7 | 0.786 | 2.162 | * | |
| Residual | 251 | 0.364 | |||
| Total | 266 | ||||
| Maximum branching level | Ar | 2 | 3.027 | 1.627 | ns |
| Si(Ar) | 6 | 5.701 | 1.795 | ns | |
| Pr(Si(Ar)) | 7 | 3.988 | 5.186 | *** | |
| Residual | 1352 | 0.769 | |||
| Total | 1367 | ||||
| Colony orientation | Ar | 2 | 1899.5 | 0.480 | ns |
| Si(Ar) | 6 | 14543 | 1.551 | ns | |
| Pr(Si(Ar)) | 7 | 12243 | 13.546 | *** | |
| Residual | 1352 | 903.82 | |||
| Total | 1367 | ||||
| (B) Variable | Source | df | MS | Pseudo-F | p (MC) |
| Density | Ar | 2 | 3.295 | 0.145 | ns |
| Si(Ar) | 7 | 30.741 | 0.967 | ns | |
| Pr(Si(Ar)) | 8 | 42.990 | 2.412 | ** | |
| Residual | 274 | 17.822 | |||
| Total | 291 | ||||
| Height | Ar | 2 | 101.430 | 0.812 | ns |
| Si(Ar) | 7 | 130.450 | 1.043 | ns | |
| Pr(Si(Ar)) | 8 | 128.550 | 1.052 | ns | |
| Residual | 246 | 122.250 | |||
| Total | 263 | ||||
| Colony orientation | Ar | 2 | 4606.100 | 0.360 | ns |
| Si(Ar) | 7 | 44923.000 | 7.227 | *** | |
| Pr(Si(Ar)) | 8 | 16681.000 | 9.614 | *** | |
| Residual | 1426 | 1735.100 | |||
| Total | 1443 |
| Area | Site | Depth Range (m) | Colony Density (col/m2) | Height (cm) | Orientation of Colonies (%) | Colonies Occurring with C. rubrum (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0° | 45° | 90° | 135° | 180° | ||||||
| North-East | Canyon Caprera | 122–152 | 4.5 ± 5.6 | 19.1 ± 10.4 | 30 | 18 | 32 | 20 | 0 | 55 |
| Canyon Mortorio | 109–151 | 1.9 ± 1.4 | 24.9 ± 12.3 | 3 | 22 | 49 | 27 | 0 | 18 | |
| Pietrame | 142–149 | 2.1 ± 2.3 | 21.2 ± 7.7 | 0 | 17 | 41 | 41 | 2 | 67 | |
| Canyon Tavolara | 109–199 | 2.5 ± 6 | 18.5 ± 9.5 | 22 | 15 | 54 | 9 | 0 | 40 | |
| Central-East | Canyon Orosei | 76–187 | 2.5 ± 3.6 | 24.1 ± 13.6 | 5 | 7 | 48 | 39 | 1 | 14 |
| Cala Gonone Shoal | 114–175 | 3.6 ± 4.6 | 26.9 ± 12.5 | 0 | 11 | 78 | 11 | 0 | 7 | |
| South-west | Secchitella | 149–152 | 3.5 ± 4.3 | 10.9 ± 4.8 | 0 | 0 | 5 | 74 | 21 | 42 |
| Bancotto | 108–126 | 2.6 ± 2.8 | 24.8 ± 11.4 | 74 | 23 | 3 | 0 | 0 | 28 | |
| Variables | R2 | p-Value | Prop. (%) | Cumul. (%) |
|---|---|---|---|---|
| (A) C. rubrum density | ||||
| Density of E. cavolini | 0.267 | *** | 26.7 | 26.7 |
| Depth | 0.299 | * | 3.2 | 29.9 |
| Height of E. cavolini | 0.299 | ns | 0.04 | 29.9 |
| (B) E. cavolini density | ||||
| Density of C. rubrum | 0.194 | *** | 19.4 | 19.4 |
| Depth | 0.227 | * | 3.3 | 22.7 |
| Height of C. rubrum | 0.231 | ns | 0.4 | 23.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carugati, L.; Moccia, D.; Bramanti, L.; Cannas, R.; Follesa, M.C.; Salvadori, S.; Cau, A. Deep-Dwelling Populations of Mediterranean Corallium rubrum and Eunicella cavolini: Distribution, Demography, and Co-Occurrence. Biology 2022, 11, 333. https://doi.org/10.3390/biology11020333
Carugati L, Moccia D, Bramanti L, Cannas R, Follesa MC, Salvadori S, Cau A. Deep-Dwelling Populations of Mediterranean Corallium rubrum and Eunicella cavolini: Distribution, Demography, and Co-Occurrence. Biology. 2022; 11(2):333. https://doi.org/10.3390/biology11020333
Chicago/Turabian StyleCarugati, Laura, Davide Moccia, Lorenzo Bramanti, Rita Cannas, Maria Cristina Follesa, Susanna Salvadori, and Alessandro Cau. 2022. "Deep-Dwelling Populations of Mediterranean Corallium rubrum and Eunicella cavolini: Distribution, Demography, and Co-Occurrence" Biology 11, no. 2: 333. https://doi.org/10.3390/biology11020333
APA StyleCarugati, L., Moccia, D., Bramanti, L., Cannas, R., Follesa, M. C., Salvadori, S., & Cau, A. (2022). Deep-Dwelling Populations of Mediterranean Corallium rubrum and Eunicella cavolini: Distribution, Demography, and Co-Occurrence. Biology, 11(2), 333. https://doi.org/10.3390/biology11020333







