Identification of DPP4/CTNNB1/MET as a Theranostic Signature of Thyroid Cancer and Evaluation of the Therapeutic Potential of Sitagliptin
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Microarray Data Acquisition and Identification of Differentially Expressed Genes (DEGs)
2.2. Differential Expression of the THCA Gene Hub
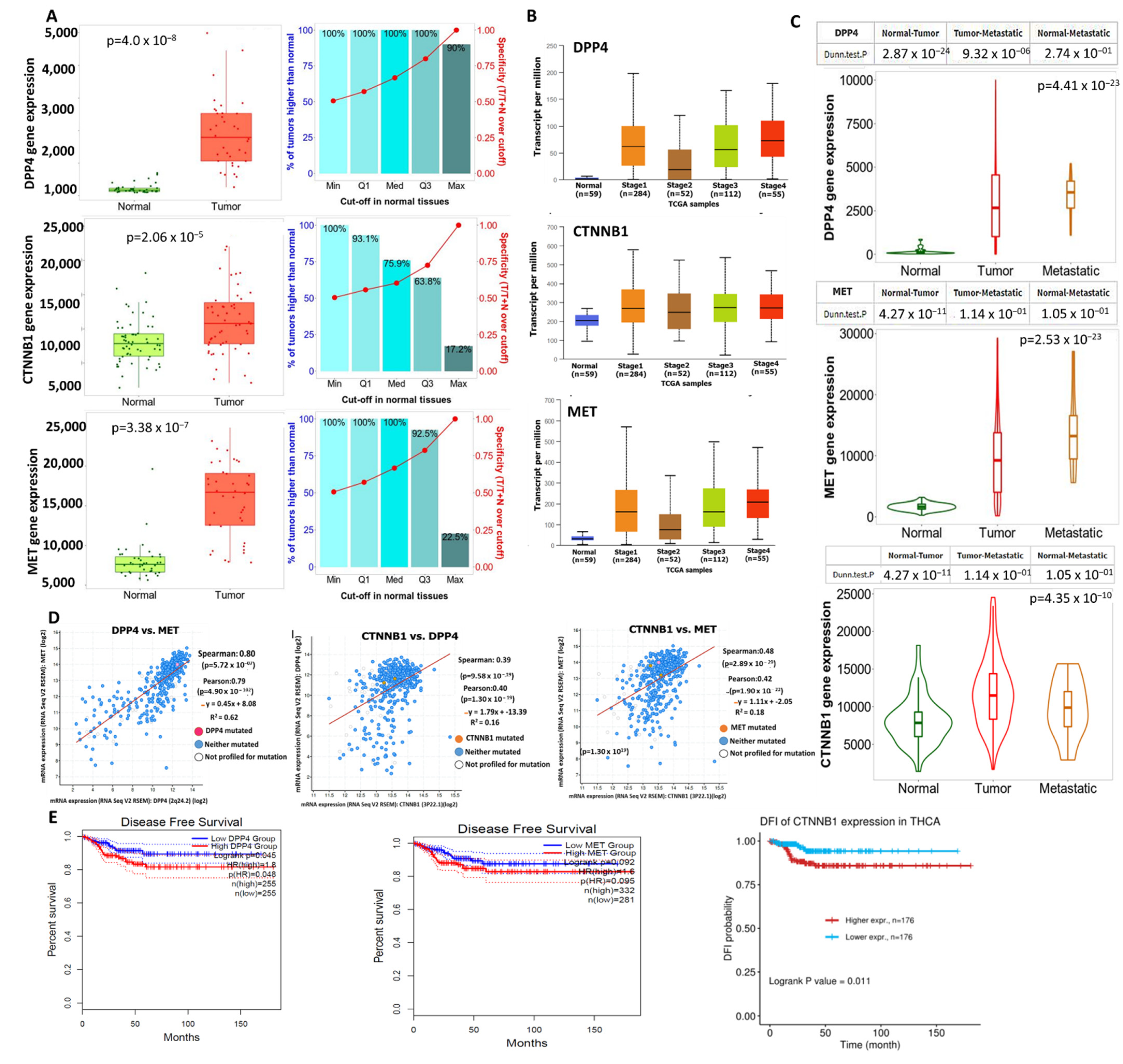
2.3. Comparisons of DPP4/CTNNB1/MET Expressions in Normal, Primary, and Metastatic Tumor of Thyroid Cancer Cohorts
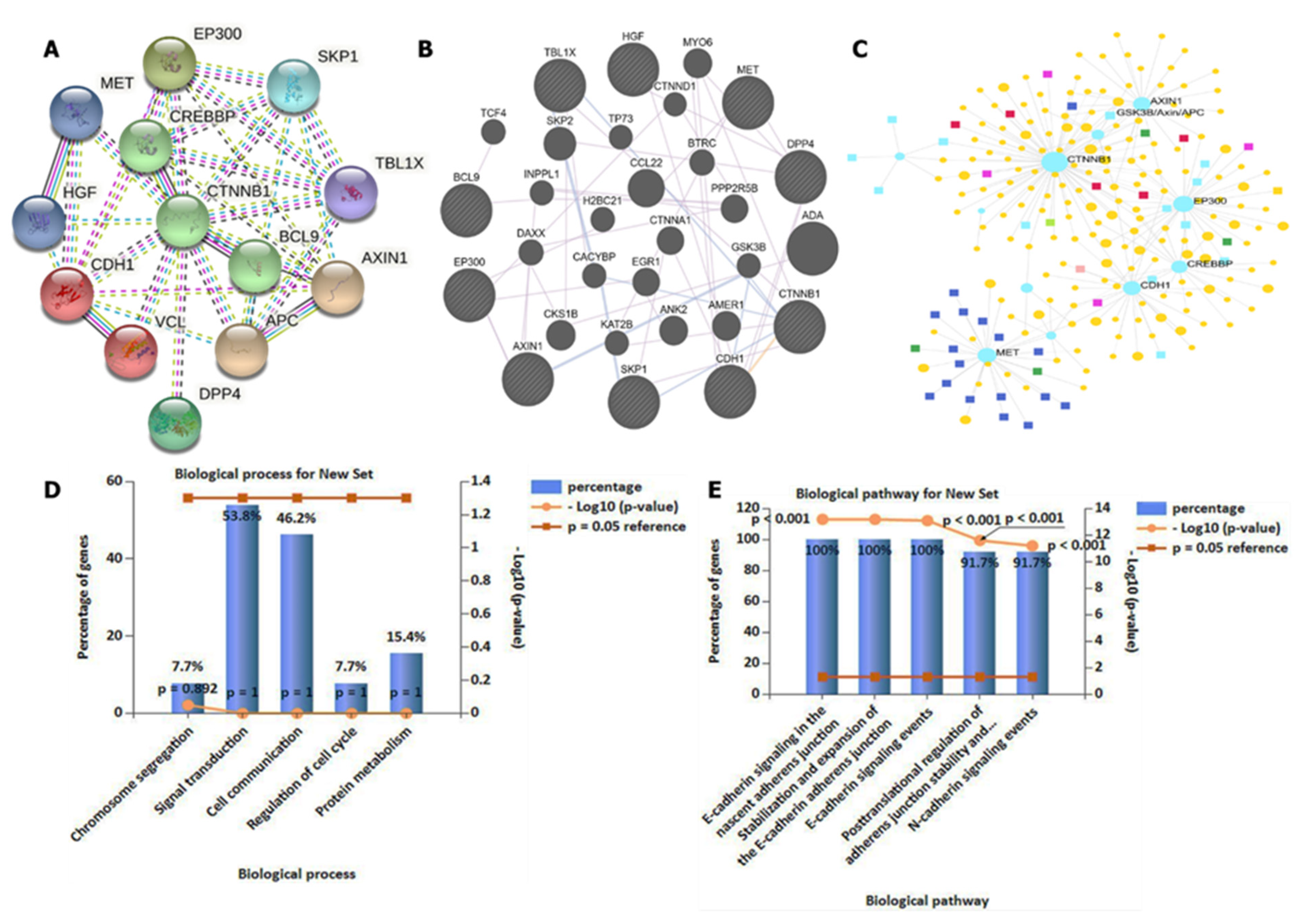
2.4. Interaction Network and Gene Enrichment Analysis
2.5. Analysis of Genomic Alterations and Mutations of the DPP4/CTNNB1/MET Oncogenes in THCA
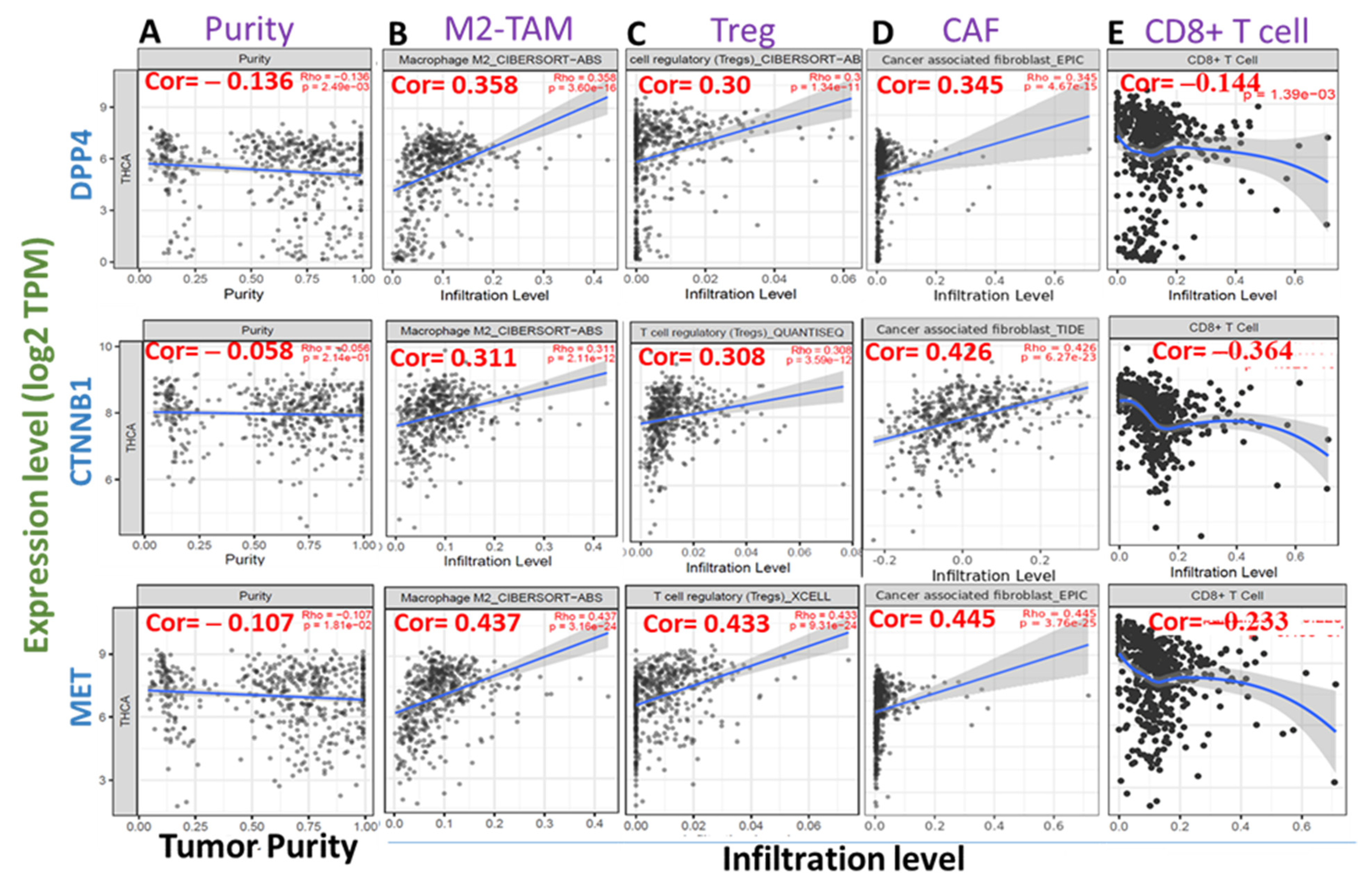
2.6. Correlations of DPP4/CTNNB1/MET Expressions and Tumor Infiltration Levels of Immune and Immunosuppressive Cells in THCA
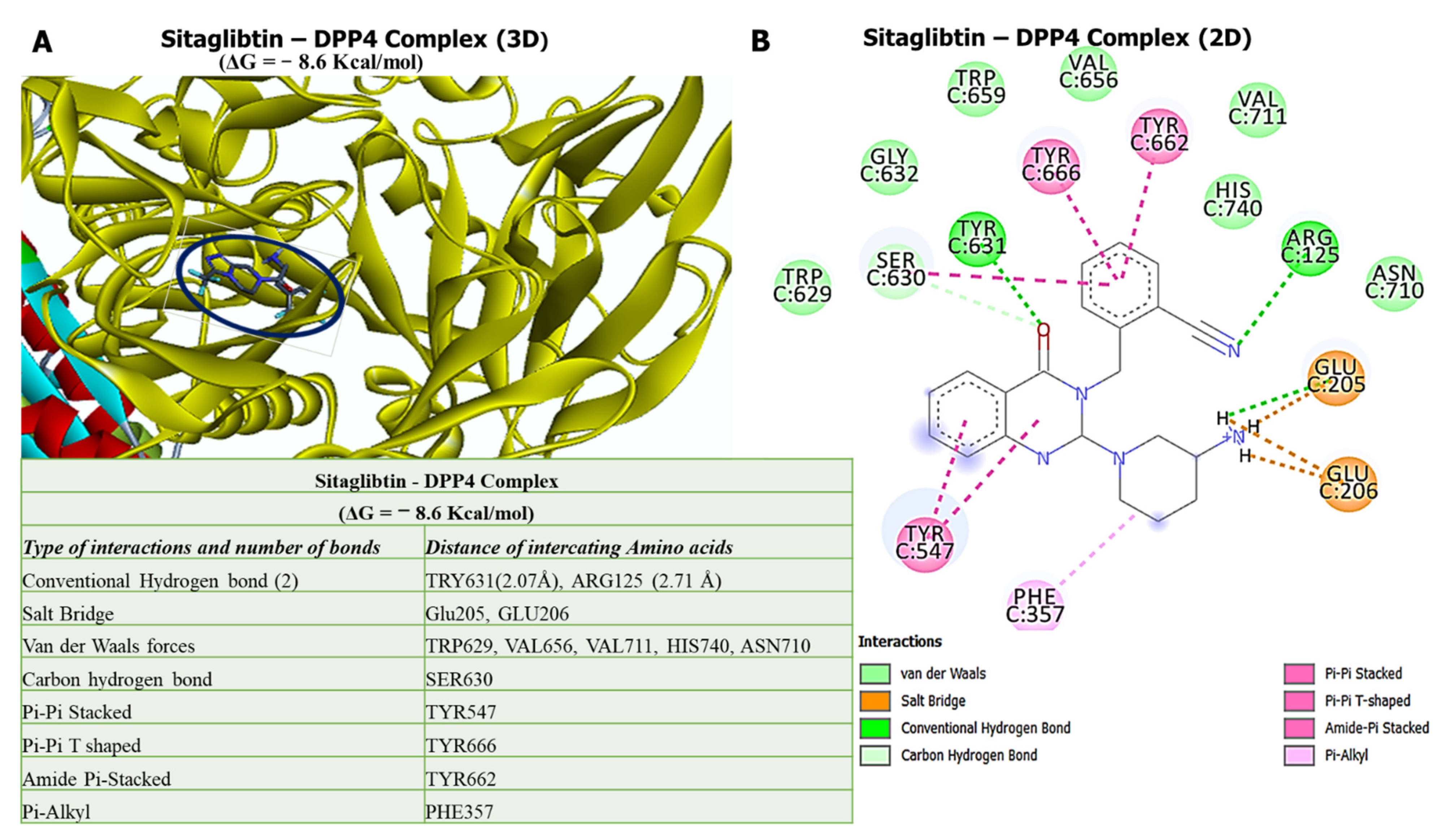
2.7. In Silico Molecular Docking of the DPP4/CTNNB1/MET Oncogenes with Sitagliptin
3. Results
3.1. Identification of Common Oncogenes in THCA
3.2. DPP4/CTNNB1/MET Expressions Are Associated with THCA Progression, Metastasis, and Worse Prognosis of THCA Cohorts
3.3. DPP4/CTNNB1/MET Genes Are Frequently Altered and Their Mutations Are Linked to Genetic Expressions in THCA
3.4. DPP4/CTNNB1/MET Genes Potentially Promote Tumor Growth by Interacting with Different Oncogenic Targets/Pathways
3.5. High Expression Levels of DPP4/CTNNB1/MET Are Associated with Immunosuppressive Phenotypes of THCA Tissues
3.6. Molecular Docking Reveals Higher Inhibitory Effects of Sitagliptin on the DPP4 Oncogene
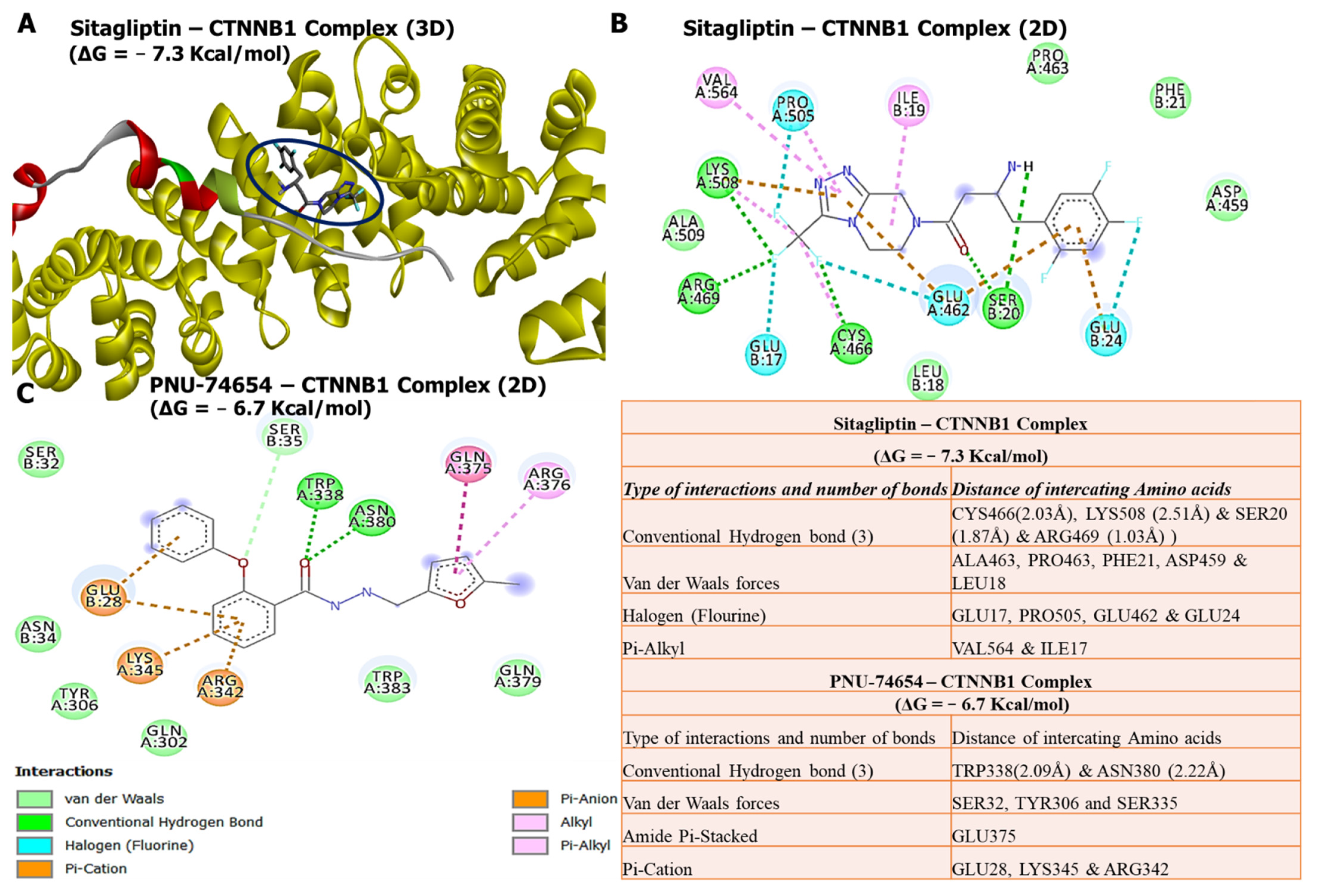
3.7. Molecular Docking Revealed Potential Inhibitory Effects of Sitagliptin on the CTNNB1 Oncogene
3.8. Molecular Docking Revealed Potential Inhibitory Effects of Sitagliptin on the MET Oncogene
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, Q.T.; Lee, E.J.; Huang, M.G.; Park, Y.I.; Khullar, A.; Plodkowski, R.A. Diagnosis and treatment of patients with thyroid cancer. Am. Health Drug Benefits 2015, 8, 30–40. [Google Scholar] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Malvezzi, M.; Bosetti, C.; Garavello, W.; Bertuccio, P.; Levi, F.; Negri, E. Thyroid cancer mortality and incidence: A global overview. Int. J. Cancer 2015, 136, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Roman, B.R.; Morris, L.G.; Davies, L. The thyroid cancer epidemic, 2017 perspective. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 332–336. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Fagin, J.A.; Wells, S.A., Jr. Biologic and clinical perspectives on thyroid cancer. N. Engl. J. Med. 2016, 375, 1054–1067. [Google Scholar] [CrossRef]
- Molinaro, E.; Romei, C.; Biagini, A.; Sabini, E.; Agate, L.; Mazzeo, S.; Materazzi, G.; Sellari-Franceschini, S.; Ribechini, A.; Torregrossa, L. Anaplastic thyroid carcinoma: From clinicopathology to genetics and advanced therapies. Nat. Rev. Endocrinol. 2017, 13, 644–660. [Google Scholar] [CrossRef]
- Knauf, J.A.; Ma, X.; Smith, E.P.; Zhang, L.; Mitsutake, N.; Liao, X.-H.; Refetoff, S.; Nikiforov, Y.E.; Fagin, J.A. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005, 65, 4238–4245. [Google Scholar] [CrossRef]
- Brito, J.P.; Morris, J.C.; Montori, V.M. Thyroid cancer: Zealous imaging has increased detection and treatment of low risk tumours. BMJ 2013, 347, f4706. [Google Scholar] [CrossRef]
- Udelsman, R.; Zhang, Y. The epidemic of thyroid cancer in the United States: The role of endocrinologists and ultrasounds. Thyroid 2014, 24, 472–479. [Google Scholar] [CrossRef]
- Furuya-Kanamori, L.; Bell, K.J.; Clark, J.; Glasziou, P. Prevalence of differentiated thyroid cancer in autopsy studies over six decades: A meta-analysis. J. Clin. Oncol. 2016, 34, 3672–3679. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.S.; Welch, H.G. South Korea’s thyroid-cancer “epidemic”—Turning the tide. N. Engl. J. Med. 2015, 373, 2389–2390. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, C.M.; Schneider, A.; Brenner, A.V. Chapter 44: Thyroid cancer. In Cancer Epidemiology and Prevention, 4th ed.; Schottenfeld, D., Fraumeni, J., Eds.; Oxford University Press: New York, NY, USA, 2016; pp. 839–860. [Google Scholar]
- Wei, W.J.; Zhang, G.Q.; Luo, Q.Y. Postsurgical Management of Differentiated Thyroid Cancer in China. Trends Endocrinol. Metab. 2018, 29, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Nikiforova, M.N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011, 7, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Long, K.L.; Grubbs, E.G. Carcinoma of the thyroid gland and neoplasms of the parathyroid glands. In The MD Anderson Surgical Oncology Handbook, 6th ed.; Wolters Kluwer Health Adis (ESP): London, UK, 2018; pp. 463–491. [Google Scholar]
- Ho, A.S.; Luu, M.; Barrios, L.; Chen, I.; Melany, M.; Ali, N.; Patio, C.; Chen, Y.; Bose, S.; Fan, X.; et al. Incidence and Mortality Risk Spectrum Across Aggressive Variants of Papillary Thyroid Carcinoma. JAMA Oncol. 2020, 6, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Kakudo, K.; Jung, C.K. Updates in the Pathologic Classification of Thyroid Neoplasms: A Review of the World Health Organization Classification. Endocrinol. Metab. 2020, 35, 696–715. [Google Scholar] [CrossRef]
- WHO. WHO Classification of Tumours of Endocrine Organs; IARC: Lion, France, 2017. [Google Scholar]
- Nath, M.C.; Erickson, L.A. Aggressive Variants of Papillary Thyroid Carcinoma: Hobnail, Tall Cell, Columnar, and Solid. Adv. Anat. Pathol. 2018, 25, 172–179. [Google Scholar] [CrossRef]
- Cavaco, D.; Martins, A.F.; Cabrera, R.; Vilar, H.; Leite, V. Diffuse sclerosing variant of papillary thyroid carcinoma: Outcomes of 33 cases. Eur. Thyroid. J. 2022, 11, e210020. [Google Scholar] [CrossRef]
- Roman, S.; Sosa, J.A. Aggressive variants of papillary thyroid cancer. Curr. Opin. Oncol. 2013, 25, 33–38. [Google Scholar] [CrossRef]
- Besic, N.; Auersperg, M.; Dremelj, M.; Vidergar-Kralj, B.; Gazic, B. Neoadjuvant chemotherapy in 16 patients with locally advanced papillary thyroid carcinoma. Thyroid 2013, 23, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, R.; Adamo, L.; Iannolo, G.; Vicari, L.; Giuffrida, D.; Eramo, A.; Gulisano, M.; Memeo, L.; Conticello, C. Resistance of papillary thyroid cancer stem cells to chemotherapy. Oncol. Lett. 2016, 12, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B.; Cabanillas, M.E.; Lazar, A.J.; Williams, M.D.; Sanders, D.L.; Ilagan, J.L.; Nolop, K.; Lee, R.J.; Sherman, S.I. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring BRAF(V600E) mutation. Thyroid 2013, 23, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Noels, H.; Theelen, W.; Sternkopf, M.; Jankowski, V.; Moellmann, J.; Kraemer, S.; Lehrke, M.; Marx, N.; Martin, L.; Marx, G.; et al. Reduced post-operative DPP4 activity associated with worse patient outcome after cardiac surgery. Sci. Rep. 2018, 8, 11820. [Google Scholar] [CrossRef]
- Javidroozi, M.; Zucker, S.; Chen, W.T. Plasma seprase and DPP4 levels as markers of disease and prognosis in cancer. Dis. Markers 2012, 32, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Wang, T.Y.; Liu, C.L.; Chien, M.N.; Chen, M.J.; Hsu, Y.C.; Leung, C.H.; Cheng, S.P. Dipeptidyl Peptidase IV as a Prognostic Marker and Therapeutic Target in Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2017, 102, 2930–2940. [Google Scholar] [CrossRef]
- Kotani, T.; Aratake, Y.; Ogata, Y.; Umeki, K.; Araki, Y.; Hirai, K.; Kuma, K.; Ohtaki, S. Expression of dipeptidyl aminopeptidase IV activity in thyroid carcinoma. Cancer Lett. 1991, 57, 203–208. [Google Scholar] [CrossRef]
- Aratake, Y.; Kotani, T.; Tamura, K.; Araki, Y.; Kuribayashi, T.; Konoe, K.; Ohtaki, S. Dipeptidyl aminopeptidase IV staining of cytologic preparations to distinguish benign from malignant thyroid diseases. Am. J. Clin. Pathol. 1991, 96, 306–310. [Google Scholar] [CrossRef][Green Version]
- Nouraee, N.; Van Roosbroeck, K.; Vasei, M.; Semnani, S.; Samaei, N.M.; Naghshvar, F.; Omidi, A.A.; Calin, G.A.; Mowla, S.J. Expression, tissue distribution and function of miR-21 in esophageal squamous cell carcinoma. PLoS ONE 2013, 8, e73009. [Google Scholar] [CrossRef]
- Pang, R.; Law, W.L.; Chu, A.C.; Poon, J.T.; Lam, C.S.; Chow, A.K.; Ng, L.; Cheung, L.W.; Lan, X.R.; Lan, H.Y.; et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell 2010, 6, 603–615. [Google Scholar] [CrossRef]
- Liu, L.; Yan, M.; Zhao, F.; Li, J.; Ge, C.; Geng, Q.; Zhu, M.; Sun, L.; He, X.; Li, J. CD26/dipeptidyl peptidase IV contributes to tumor metastasis in human lung adenocarcinoma. Bangladesh J. Pharmacol. 2013, 8, 198–206. [Google Scholar] [CrossRef][Green Version]
- Jang, J.; Haberecker, M.; Curioni, A.; Janker, F.; Soltermann, A.; Gil-Bazo, I.; Hwang, I.; Kwon, K.; Weder, W.; Jungraithmayr, W. EP1.03–33 CD26/DPP4 as a Novel Prognostic Marker for Lung Adenocarcinoma. J. Thorac. Oncol. 2019, 14, S965. [Google Scholar] [CrossRef]
- Lu, Z.; Qi, L.; Bo, X.J.; Liu, G.D.; Wang, J.M.; Li, G. Expression of CD26 and CXCR4 in prostate carcinoma and its relationship with clinical parameters. J. Res. Med. 2013, 18, 647–652. [Google Scholar]
- Kretzschmar, K.; Weber, C.; Driskell, R.R.; Calonje, E.; Watt, F.M. Compartmentalized Epidermal Activation of β-Catenin Differentially Affects Lineage Reprogramming and Underlies Tumor Heterogeneity. Cell Rep. 2016, 14, 269–281. [Google Scholar] [CrossRef]
- Dong, C.; Yang, H.; Wang, Y.; Yan, X.; Li, D.; Cao, Z.; Ning, Y.; Zhang, C. Anagliptin stimulates osteoblastic cell differentiation and mineralization. Biomed. Pharmacother. 2020, 129, 109796. [Google Scholar] [CrossRef]
- Ren, X.; Zhu, R.; Liu, G.; Xue, F.; Wang, Y.; Xu, J.; Zhang, W.; Yu, W.; Li, R. Effect of sitagliptin on tubulointerstitial Wnt/β-catenin signalling in diabetic nephropathy. Nephrology 2019, 24, 1189–1197. [Google Scholar] [CrossRef]
- Garcia, C.; Buffet, C.; El Khattabi, L.; Rizk-Rabin, M.; Perlemoine, K.; Ragazzon, B.; Bertherat, J.; Cormier, F.; Groussin, L. MET overexpression and activation favors invasiveness in a model of anaplastic thyroid cancer. Oncotarget 2019, 10, 2320–2334. [Google Scholar] [CrossRef]
- Trovato, M.; Campennì, A.; Giovinazzo, S.; Siracusa, M.; Ruggeri, R.M. Hepatocyte Growth Factor/C-Met Axis in Thyroid Cancer: From Diagnostic Biomarker to Therapeutic Target. Biomark. Insights 2017, 12, 1177271917701126. [Google Scholar] [CrossRef]
- Mineo, R.; Costantino, A.; Frasca, F.; Sciacca, L.; Russo, S.; Vigneri, R.; Belfiore, A. Activation of the hepatocyte growth factor (HGF)-Met system in papillary thyroid cancer: Biological effects of HGF in thyroid cancer cells depend on Met expression levels. Endocrinology 2004, 145, 4355–4365. [Google Scholar] [CrossRef]
- Di Renzo, M.F.; Olivero, M.; Ferro, S.; Prat, M.; Bongarzone, I.; Pilotti, S.; Belfiore, A.; Costantino, A.; Vigneri, R.; Pierotti, M.A.; et al. Overexpression of the c-MET/HGF receptor gene in human thyroid carcinomas. Oncogene 1992, 7, 2549–2553. [Google Scholar] [PubMed]
- Lesko, E.; Majka, M. The biological role of HGF-MET axis in tumor growth and development of metastasis. Front. Biosci. 2008, 13, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Monga, S.P.; Mars, W.M.; Pediaditakis, P.; Bell, A.; Mulé, K.; Bowen, W.C.; Wang, X.; Zarnegar, R.; Michalopoulos, G.K. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res. 2002, 62, 2064–2071. [Google Scholar] [PubMed]
- Tward, A.D.; Jones, K.D.; Yant, S.; Cheung, S.T.; Fan, S.T.; Chen, X.; Kay, M.A.; Wang, R.; Bishop, J.M. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc. Natl. Acad. Sci. USA 2007, 104, 14771–14776. [Google Scholar] [CrossRef]
- Sastre-Perona, A.; Santisteban, P. Role of the wnt pathway in thyroid cancer. Front. Endocrinol. 2012, 3, 31. [Google Scholar] [CrossRef]
- Rezk, S.; Brynes, R.K.; Nelson, V.; Thein, M.; Patwardhan, N.; Fischer, A.; Khan, A. beta-Catenin expression in thyroid follicular lesions: Potential role in nuclear envelope changes in papillary carcinomas. Endocr. Pathol. 2004, 15, 329–337. [Google Scholar] [CrossRef]
- Buchanan, S.G.; Hendle, J.; Lee, P.S.; Smith, C.R.; Bounaud, P.Y.; Jessen, K.A.; Tang, C.M.; Huser, N.H.; Felce, J.D.; Froning, K.J.; et al. SGX523 is an exquisitely selective, ATP-competitive inhibitor of the MET receptor tyrosine kinase with antitumor activity in vivo. Mol. Cancer 2009, 8, 3181–3190. [Google Scholar] [CrossRef]
- Amritha, C.A.; Kumaravelu, P.; Chellathai, D.D. Evaluation of Anti Cancer Effects of DPP-4 Inhibitors in Colon Cancer- An Invitro Study. J. Clin. Diagn. Res. 2015, 9, Fc14–Fc16. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Bartha, Á.; Győrffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.; Rodriguez, H.; Lopes, C.; Zuberi, K.; Montojo, J.; Bader, G.D.; Morris, Q. GeneMANIA update 2018. Nucleic Acids Res. 2018, 46, W60–W64. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Soufan, O.; Ewald, J.; Hancock, R.E.W.; Basu, N.; Xia, J. NetworkAnalyst 3.0: A visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019, 47, W234–W241. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.; Keerthikumar, S.; Ang, C.S.; Gangoda, L.; Quek, C.Y.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A.; et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef] [PubMed]
- Juneja, M.; Kobelt, D.; Walther, W.; Voss, C.; Smith, J.; Specker, E.; Neuenschwander, M.; Gohlke, B.-O.; Dahlmann, M.; Radetzki, S.; et al. Statin and rottlerin small-molecule inhibitors restrict colon cancer progression and metastasis via MACC1. PLoS Biol. 2017, 15, e2000784. [Google Scholar] [CrossRef]
- Lawal, B.; Lin, L.-C.; Lee, J.-C.; Chen, J.-H.; Bekaii-Saab, T.S.; Wu, A.T.H.; Ho, C.-L. Multi-Omics Data Analysis of Gene Expressions and Alterations, Cancer-Associated Fibroblast and Immune Infiltrations, Reveals the Onco-Immune Prognostic Relevance of STAT3/CDK2/4/6 in Human Malignancies. Cancers 2021, 13, 954. [Google Scholar] [CrossRef]
- Lawal, B.; Tseng, S.-H.; Olugbodi, J.O.; Iamsaard, S.; Ilesanmi, O.B.; Mahmoud, M.H.; Ahmed, S.H.; Batiha, G.E.-S.; Wu, A.T.H. Pan-Cancer Analysis of Immune Complement Signature C3/C5/C3AR1/C5AR1 in Association with Tumor Immune Evasion and Therapy Resistance. Cancers 2021, 13, 4124. [Google Scholar] [CrossRef]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Lin, K.-C.; Lawal, B.; Wu, A.T.H.; Wu, C.-Z. MXD3 as an onco-immunological biomarker encompassing the tumor microenvironment, disease staging, prognoses, and therapeutic responses in multiple cancer types. Comput. Struct. Biotechnol. J. 2021, 19, 4970–4983. [Google Scholar] [CrossRef]
- Wu, A.T.H.; Lawal, B.; Tzeng, Y.-M.; Shih, C.-C.; Shih, C.-M. Identification of a Novel Theranostic Signature of Metabolic and Immune-Inflammatory Dysregulation in Myocardial Infarction, and the Potential Therapeutic Properties of Ovatodiolide, a Diterpenoid Derivative. Int. J. Mol. Sci. 2022, 23, 1281. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.T.H.; Lawal, B.; Wei, L.; Wen, Y.-T.; Tzeng, D.T.W.; Lo, W.-C. Multiomics Identification of Potential Targets for Alzheimer Disease and Antrocin as a Therapeutic Candidate. Pharmaceutics 2021, 13, 1555. [Google Scholar] [CrossRef] [PubMed]
- Goodsell, D.S.; Sanner, M.F.; Olson, A.J.; Forli, S. The AutoDock suite at 30. Protein Sci. 2021, 30, 31–43. [Google Scholar] [CrossRef]
- Mokgautsi, N.; Wen, Y.-T.; Lawal, B.; Khedkar, H.; Sumitra, M.R.; Wu, A.T.; Huang, H.-S. An integrated bioinformatics study of a novel niclosamide derivative, nsc765689, a potential gsk3β/β-catenin/stat3/cd44 suppressor with anti-glioblastoma properties. Int. J. Mol. Sci. 2021, 22, 2464. [Google Scholar] [CrossRef] [PubMed]
- Lawal, B.; Liu, Y.-L.; Mokgautsi, N.; Khedkar, H.; Sumitra, M.R.; Wu, A.T.H.; Huang, H.-S. Pharmacoinformatics and Preclinical Studies of NSC765690 and NSC765599, Potential STAT3/CDK2/4/6 Inhibitors with Antitumor Activities against NCI60 Human Tumor Cell Lines. Biomedicines 2021, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Raman, E.P.; Paul, T.J.; Hayes, R.L.; Brooks, C.L., 3rd. Automated, Accurate, and Scalable Relative Protein-Ligand Binding Free-Energy Calculations Using Lambda Dynamics. J. Chem. Theory Comput. 2020, 16, 7895–7914. [Google Scholar] [CrossRef]
- Huang, Y.; Prasad, M.; Lemon, W.J.; Hampel, H.; Wright, F.A.; Kornacker, K.; LiVolsi, V.; Frankel, W.; Kloos, R.T.; Eng, C.; et al. Gene expression in papillary thyroid carcinoma reveals highly consistent profiles. Proc. Natl. Acad. Sci. USA 2001, 98, 15044–15049. [Google Scholar] [CrossRef]
- Petrakis, D.; Vassilopoulou, L.; Mamoulakis, C.; Psycharakis, C.; Anifantaki, A.; Sifakis, S.; Docea, A.O.; Tsiaoussis, J.; Makrigiannakis, A.; Tsatsakis, A.M. Endocrine Disruptors Leading to Obesity and Related Diseases. Int. J. Environ. Res. Public Health 2017, 14, 1282. [Google Scholar] [CrossRef]
- Takano, T. Natural history of thyroid cancer [Review]. Endocr. J. 2017, 64, 237–244. [Google Scholar] [CrossRef]
- Schlumberger, M.; Parmentier, C.; Delisle, M.J.; Couette, J.E.; Droz, J.P.; Sarrazin, D. Combination therapy for anaplastic giant cell thyroid carcinoma. Cancer 1991, 67, 564–566. [Google Scholar] [CrossRef]
- Tennvall, J.; Lundell, G.; Wahlberg, P.; Bergenfelz, A.; Grimelius, L.; Akerman, M.; Hjelm Skog, A.L.; Wallin, G. Anaplastic thyroid carcinoma: Three protocols combining doxorubicin, hyperfractionated radiotherapy and surgery. Br. J. Cancer 2002, 86, 1848–1853. [Google Scholar] [CrossRef] [PubMed]
- Ancker, O.V.; Krüger, M.; Wehland, M.; Infanger, M.; Grimm, D. Multikinase Inhibitor Treatment in Thyroid Cancer. Int. J. Mol. Sci. 2019, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.J.; Oucharek, J.; Learoyd, D.; Sidhu, S.B. Standard and emerging therapies for metastatic differentiated thyroid cancer. Oncologist 2010, 15, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Kapiteijn, E.; Schneider, T.C.; Morreau, H.; Gelderblom, H.; Nortier, J.W.R.; Smit, J.W.A. New treatment modalities in advanced thyroid cancer. Ann. Oncol. 2012, 23, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Wang, Q.; Wang, K.; Wang, F. Mesenchymal stem cells-originated exosomal microRNA-152 impairs proliferation, invasion and migration of thyroid carcinoma cells by interacting with DPP4. J. Endocrinol. Investig. 2020, 43, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Sitagliptin use and thyroid cancer risk in patients with type 2 diabetes. Oncotarget 2016, 7, 24871–24879. [Google Scholar] [CrossRef]
- Chen, J.-H.; Wu, A.T.H.; Lawal, B.; Tzeng, D.T.W.; Lee, J.-C.; Ho, C.-L.; Chao, T.-Y. Identification of Cancer Hub Gene Signatures Associated with Immune-Suppressive Tumor Microenvironment and Ovatodiolide as a Potential Cancer Immunotherapeutic Agent. Cancers 2021, 13, 3847. [Google Scholar] [CrossRef]
- Lawal, B.; Lee, C.-Y.; Mokgautsi, N.; Sumitra, M.R.; Khedkar, H.; Wu, A.T.H.; Huang, H.-S. mTOR/EGFR/iNOS/MAP2K1/FGFR/TGFB1 Are Druggable Candidates for N-(2,4-Difluorophenyl)-2′,4′-Difluoro-4-Hydroxybiphenyl-3-Carboxamide (NSC765598), With Consequent Anticancer Implications. Front. Oncol. 2021, 11, 656738. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Lawal, B.; Hsiao, M.; Huang, T.-H.; Huang, C.-Y.F. Identification of NSP3 (SH2D3C) as a Prognostic Biomarker of Tumor Progression and Immune Evasion for Lung Cancer and Evaluation of Organosulfur Compounds from Allium sativum L. as Therapeutic Candidates. Biomedicines 2021, 9, 1582. [Google Scholar] [CrossRef]
- Lawal, B.; Wang, Y.-C.; Wu, A.T.H.; Huang, H.-S. Pro-Oncogenic c-Met/EGFR, Biomarker Signatures of the Tumor Microenvironment are Clinical and Therapy Response Prognosticators in Colorectal Cancer, and Therapeutic Targets of 3-Phenyl-2H-benzo[e][1,3]-Oxazine-2,4(3H)-Dione Derivatives. Front. Pharmacol. 2021, 12, 691234. [Google Scholar] [CrossRef]
- Lawal, B.; Kuo, Y.-C.; Tang, S.-L.; Liu, F.-C.; Wu, A.T.H.; Lin, H.-Y.; Huang, H.-S. Transcriptomic-Based Identification of the Immuno-Oncogenic Signature of Cholangiocarcinoma for HLC-018 Multi-Target Therapy Exploration. Cells 2021, 10, 2873. [Google Scholar] [CrossRef] [PubMed]
- Lawal, B.; Kuo, Y.-C.; Sumitra, M.R.; Wu, A.T.; Huang, H.-S. In vivo Pharmacokinetic and Anticancer Studies of HH-N25, a Selective Inhibitor of Topoisomerase I, and Hormonal Signaling for Treating Breast Cancer. J. Inflamm. Res. 2021, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.-Y.; Wu, A.T.H.; Batiha, G.E.-S.; Ho, C.-L.; Lee, J.-C.; Lukman, H.Y.; Alorabi, M.; AlRasheedi, A.N.; Chen, J.-H. Identification of DPP4/CTNNB1/MET as a Theranostic Signature of Thyroid Cancer and Evaluation of the Therapeutic Potential of Sitagliptin. Biology 2022, 11, 324. https://doi.org/10.3390/biology11020324
Cheng S-Y, Wu ATH, Batiha GE-S, Ho C-L, Lee J-C, Lukman HY, Alorabi M, AlRasheedi AN, Chen J-H. Identification of DPP4/CTNNB1/MET as a Theranostic Signature of Thyroid Cancer and Evaluation of the Therapeutic Potential of Sitagliptin. Biology. 2022; 11(2):324. https://doi.org/10.3390/biology11020324
Chicago/Turabian StyleCheng, Sheng-Yao, Alexander T. H. Wu, Gaber El-Saber Batiha, Ching-Liang Ho, Jih-Chin Lee, Halimat Yusuf Lukman, Mohammed Alorabi, Abdullah N. AlRasheedi, and Jia-Hong Chen. 2022. "Identification of DPP4/CTNNB1/MET as a Theranostic Signature of Thyroid Cancer and Evaluation of the Therapeutic Potential of Sitagliptin" Biology 11, no. 2: 324. https://doi.org/10.3390/biology11020324
APA StyleCheng, S.-Y., Wu, A. T. H., Batiha, G. E.-S., Ho, C.-L., Lee, J.-C., Lukman, H. Y., Alorabi, M., AlRasheedi, A. N., & Chen, J.-H. (2022). Identification of DPP4/CTNNB1/MET as a Theranostic Signature of Thyroid Cancer and Evaluation of the Therapeutic Potential of Sitagliptin. Biology, 11(2), 324. https://doi.org/10.3390/biology11020324







