Vascular Accesses in Cardiac Stimulation and Electrophysiology: An Italian Survey Promoted by AIAC (Italian Association of Arrhythmias and Cardiac Pacing)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
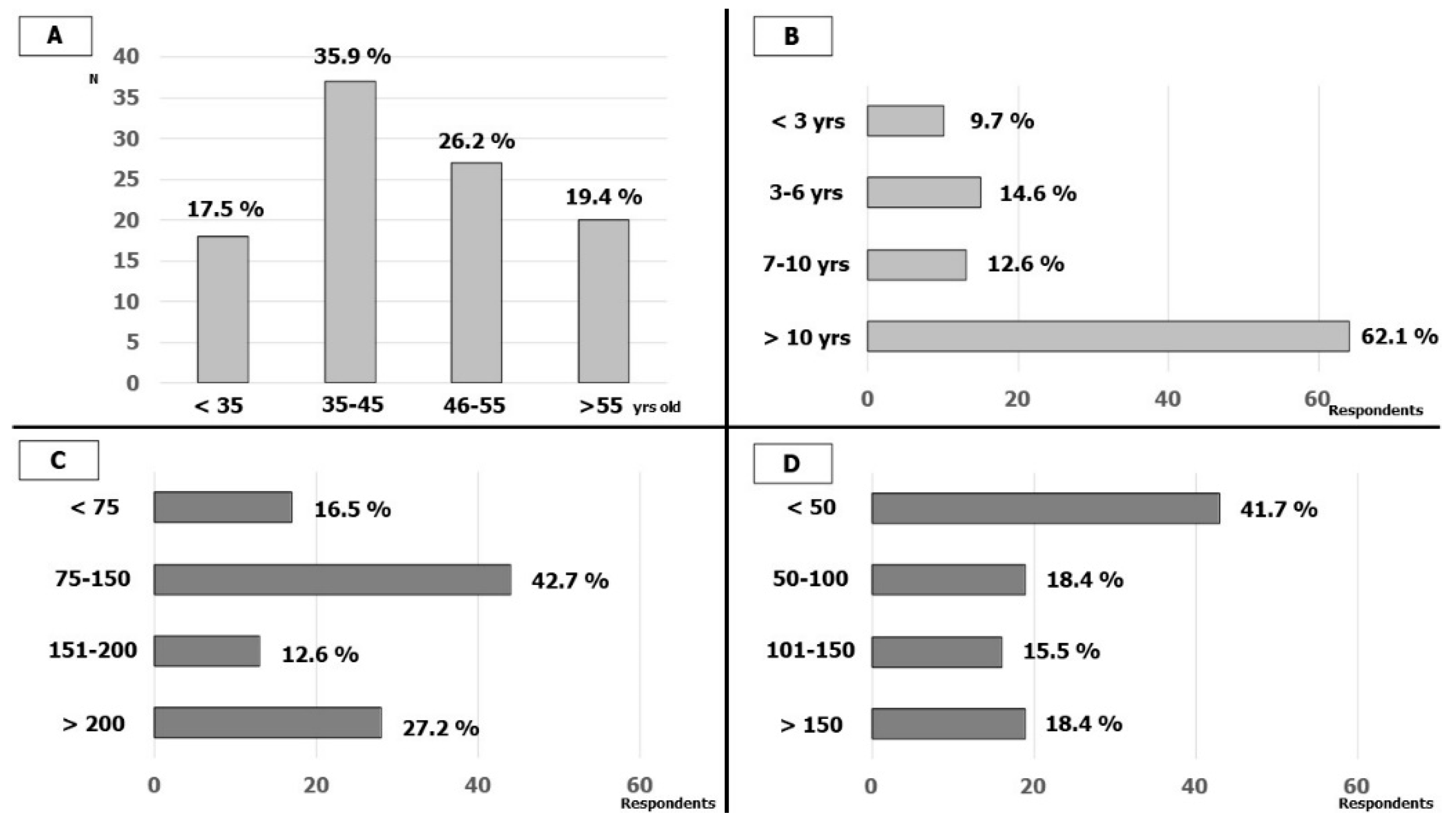
3.1. Respondents’ Characteristics
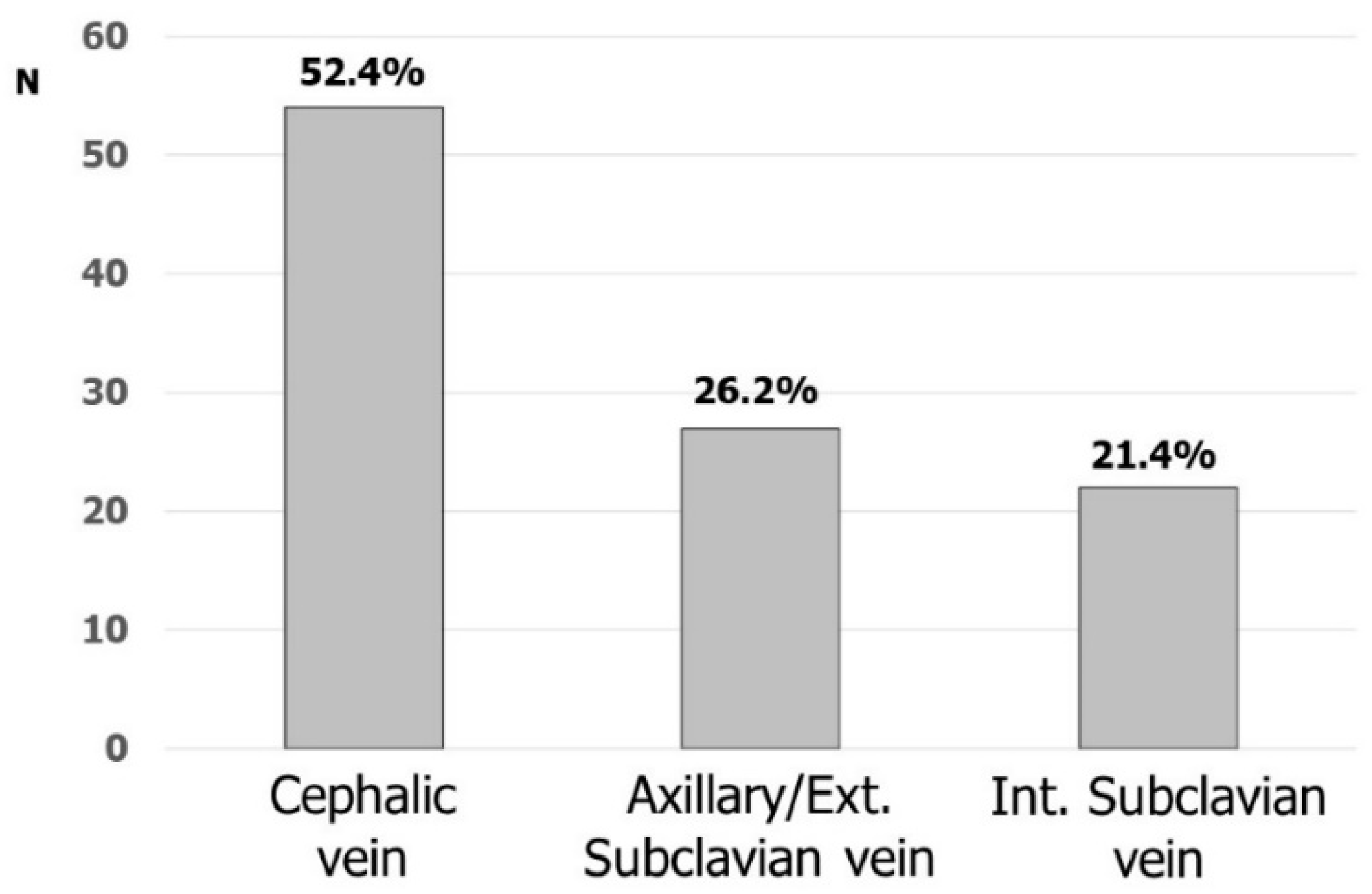
3.2. Vascular Accesses in Cardiac Stimulation
3.3. Vascular Accesses in Electrophysiology
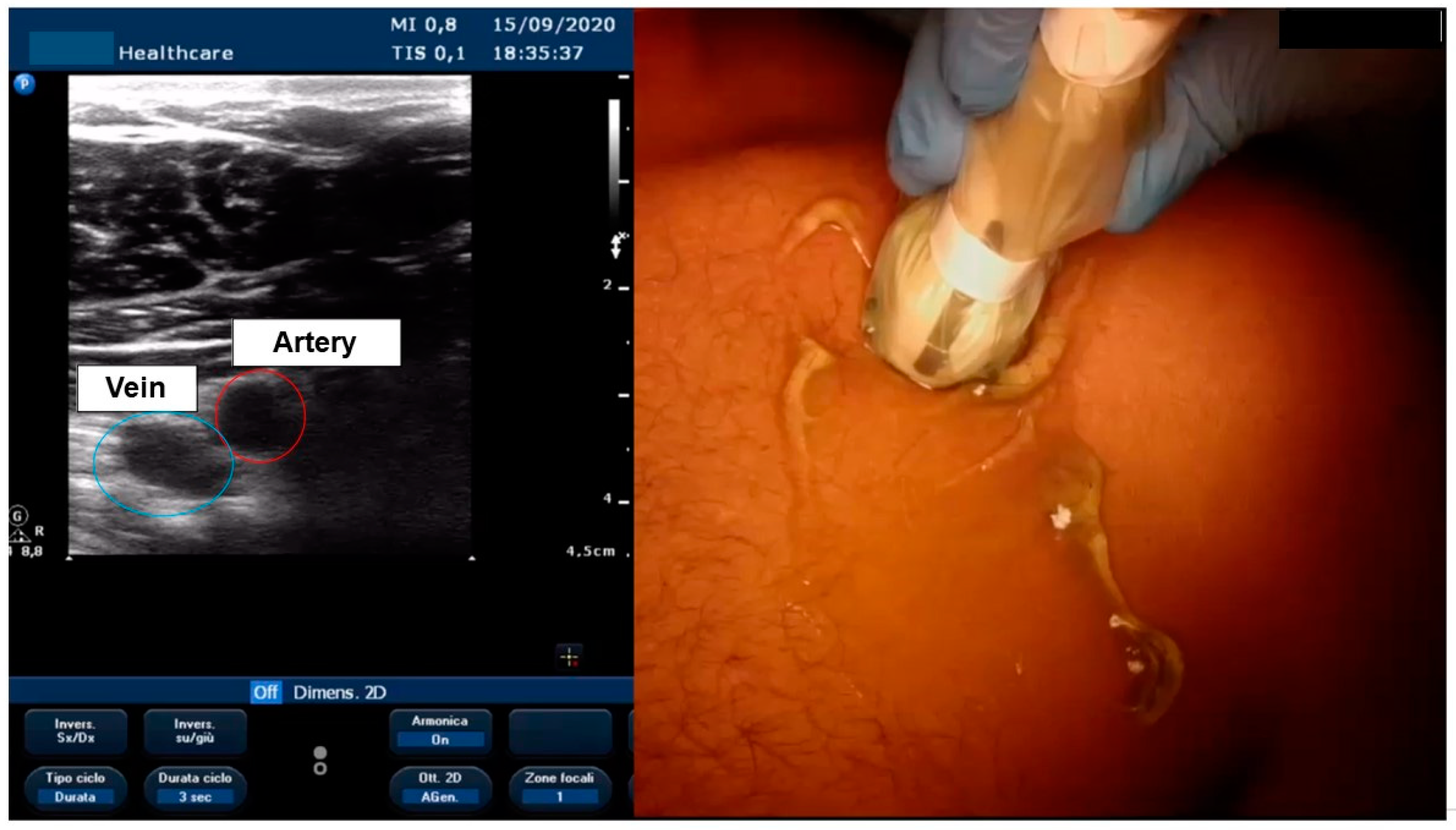
3.4. Ultrasound-Guided Vascular Access
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Burri, H.; Starck, C.; Auricchio, A.; Biffi, M.; Burri, M.; D’Avila, A.L.R.; Deharo, J.C.; Glikson, M.; Israel, C.; Lau, C.A.R.; et al. EHRA expert consensus statement and practical guide on optimal implantation technique for conventional pacemakers and implantable cardioverter-defibrillators: Endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), and the Latin-American Heart Rhythm Society (LAHRS). Europace 2021, 23, 983–1008. [Google Scholar] [CrossRef] [PubMed]
- Bongiorni, M.G.; Proclemer, A.; Dobreanu, D.; Marinskis, G.; Pison, L.; Blomstrom-Lundqvist, C.; Scientific Initiative Committee, E.H.R.A. Preferred tools and techniques for implantation of cardiac electronic devices in Europe: Results of the European Heart Rhythm Association survey. Europace 2013, 15, 1664–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atti, V.; Turagam, M.K.; Garg, J.; Koerber, S.; Angirekula, A.; Gopinathannair, R.; Natale, A.; Lakkireddy, D. Subclavian and Axillary Vein Access Versus Cephalic Vein Cutdown for Cardiac Implantable Electronic Device Implantation: A Meta-Analysis. JACC Clin. Electrophysiol. 2020, 6, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Benz, A.P.; Vamos, M.; Erath, J.W.; Hohnloser, S.H. Cephalic vs. subclavian lead implantation in cardiac implantable electronic devices: A systematic review and meta-analysis. Europace 2019, 21, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Magney, J.E.; Flynn, D.M.; Parsons, J.A.; Staplin, D.H.; Chin-Purcell, M.V.; Milstein, S.; Hunter, D.W. Anatomical mechanisms explaining damage to pacemaker leads, defibrillator leads, and failure of central venous catheters adjacent to the sternoclavicular joint. Pacing Clin. Electrophysiol. PACE 1993, 16, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Ramza, B.M.; Brinker, J.; Atiga, W.; Donahue, K.; Nsah, E.; Taylor, E.; Halperin, H.; Lawrence, J.H.; Tomaselli, G.; et al. Prospective randomized comparison of the safety and effectiveness of placement of endocardial pacemaker and defibrillator leads using the extrathoracic subclavian vein guided by contrast venography versus the cephalic approach. Pacing Clin. Electrophysiol. PACE 2001, 24, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Neri, R.; Cesario, A.S.; Baragli, D.; Monti, F.; Danisi, N.; Glaciale, G.; Gambelli, G. Permanent pacing lead insertion through the cephalic vein using an hydrophilic guidewire. Pacing Clin. Electrophysiol. PACE 2003, 26, 2313–2314. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.Y.; Kwong, N.P.; Cheong, A.P. Venous access and long-term pacemaker lead failure: Comparing contrast-guided axillary vein puncture with subclavian puncture and cephalic cutdown. Europace 2017, 19, 1193–1197. [Google Scholar] [CrossRef]
- Yang, F.; Kulbak, G. A new trick to a routine procedure: Taking the fear out of the axillary vein stick using the 35 degrees caudal view. Europace 2015, 17, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Kotter, J.; Lolay, G.; Charnigo, R.; Leung, S.; McKibbin, C.; Sousa, M.; Jimenez, L.; Gurley, J.; Biase, L.D.; Natale, A.; et al. Predictors, Morbidity, and Costs Associated with Pneumothorax during Electronic Cardiac Device Implantation. Pacing Clin. Electrophysiol. PACE 2016, 39, 985–991. [Google Scholar] [CrossRef]
- Tagliari, A.P.; Kochi, A.N.; Mastella, B.; Saadi, R.P.; di Leoni Ferrari, A.; Saadi, E.K.; Polanczyk, C.A. Axillary vein puncture guided by ultrasound vs cephalic vein dissection in pacemaker and defibrillator implant: A multicenter randomized clinical trial. Heart Rhythm 2020, 17, 1554–1560. [Google Scholar] [CrossRef]
- Squara, F.; Tomi, J.; Scarlatti, D.; Theodore, G.; Moceri, P.; Ferrari, E. Self-taught axillary vein access without venography for pacemaker implantation: Prospective randomized comparison with the cephalic vein access. Europace 2017, 19, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Noori, V.J.; Eldrup-Jorgensen, J. A systematic review of vascular closure devices for femoral artery puncture sites. J. Vasc. Surg. 2018, 68, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.; Andras, A.; Colgan, F.; Jackson, R. Vascular closure devices for femoral arterial puncture site haemostasis. Cochrane Database Syst. Rev. 2016, 3, CD009541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Zou, J.; Ma, H.; Jiao, Y.; Yang, H.; Zhang, X.; Miao, Y. Network Meta-analysis of Randomized Trials on the Safety of Vascular Closure Devices for Femoral Arterial Puncture Site Haemostasis. Sci. Rep. 2015, 5, 13761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz-Schupke, S.; Helde, S.; Gewalt, S.; Ibrahim, T.; Linhardt, M.; Haas, K.; Hoppe, K.; Bottiger, C.; Groha, P.; Bradaric, C.; et al. Comparison of vascular closure devices vs manual compression after femoral artery puncture: The ISAR-CLOSURE randomized clinical trial. Jama 2014, 312, 1981–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resnic, F.S.; Majithia, A.; Marinac-Dabic, D.; Robbins, S.; Ssemaganda, H.; Hewitt, K.; Ponirakis, A.; Loyo-Berrios, N.; Moussa, I.; Drozda, J.; et al. Registry-Based Prospective, Active Surveillance of Medical-Device Safety. N. Engl. J. Med. 2017, 376, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Farooq, V.; Goedhart, D.; Ludman, P.; de Belder, M.A.; Harcombe, A.; El-Omar, M.; British Cardiovascular Intervention Society; National Institute for Cardiovascular Outcomes Research. Relationship between Femoral Vascular Closure Devices and Short-Term Mortality From 271 845 Percutaneous Coronary Intervention Procedures Performed in the United Kingdom between 2006 and 2011: A Propensity Score-Corrected Analysis From the British Cardiovascular Intervention Society. Circ. Cardiovasc. Interv. 2016, 9, e003560. [Google Scholar] [CrossRef] [PubMed]
- Ketterle, J.; Rittger, H.; Helmig, I.; Klinghammer, L.; Zimmermann, S.; Hohenforst-Schmidt, W.; Brachmann, J.; Nef, H.; Achenbach, S.; Schlundt, C. Comparison of Exo-Seal((R)) and Angio-Seal ((R)) for arterial puncture site closure: A randomized, multicenter, single-blind trial. Herz 2015, 40, 809–816. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziacchi, M.; Placci, A.; Angeletti, A.; Quartieri, F.; Balla, C.; Virzi, S.; Bertini, M.; De Ponti, R.; Biffi, M.; Boriani, G.; et al. Vascular Accesses in Cardiac Stimulation and Electrophysiology: An Italian Survey Promoted by AIAC (Italian Association of Arrhythmias and Cardiac Pacing). Biology 2022, 11, 265. https://doi.org/10.3390/biology11020265
Ziacchi M, Placci A, Angeletti A, Quartieri F, Balla C, Virzi S, Bertini M, De Ponti R, Biffi M, Boriani G, et al. Vascular Accesses in Cardiac Stimulation and Electrophysiology: An Italian Survey Promoted by AIAC (Italian Association of Arrhythmias and Cardiac Pacing). Biology. 2022; 11(2):265. https://doi.org/10.3390/biology11020265
Chicago/Turabian StyleZiacchi, Matteo, Angelo Placci, Andrea Angeletti, Fabio Quartieri, Cristina Balla, Santo Virzi, Matteo Bertini, Roberto De Ponti, Mauro Biffi, Giuseppe Boriani, and et al. 2022. "Vascular Accesses in Cardiac Stimulation and Electrophysiology: An Italian Survey Promoted by AIAC (Italian Association of Arrhythmias and Cardiac Pacing)" Biology 11, no. 2: 265. https://doi.org/10.3390/biology11020265
APA StyleZiacchi, M., Placci, A., Angeletti, A., Quartieri, F., Balla, C., Virzi, S., Bertini, M., De Ponti, R., Biffi, M., Boriani, G., & for AIAC Ricerca Investigators’ Network. (2022). Vascular Accesses in Cardiac Stimulation and Electrophysiology: An Italian Survey Promoted by AIAC (Italian Association of Arrhythmias and Cardiac Pacing). Biology, 11(2), 265. https://doi.org/10.3390/biology11020265









