Atrial High-Rate Episodes Detected by Cardiac Implantable Electronic Devices: Dynamic Changes in Episodes and Predictors of Incident Atrial Fibrillation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
Statistical Analysis
3. Results
3.1. Study and Population Characteristics
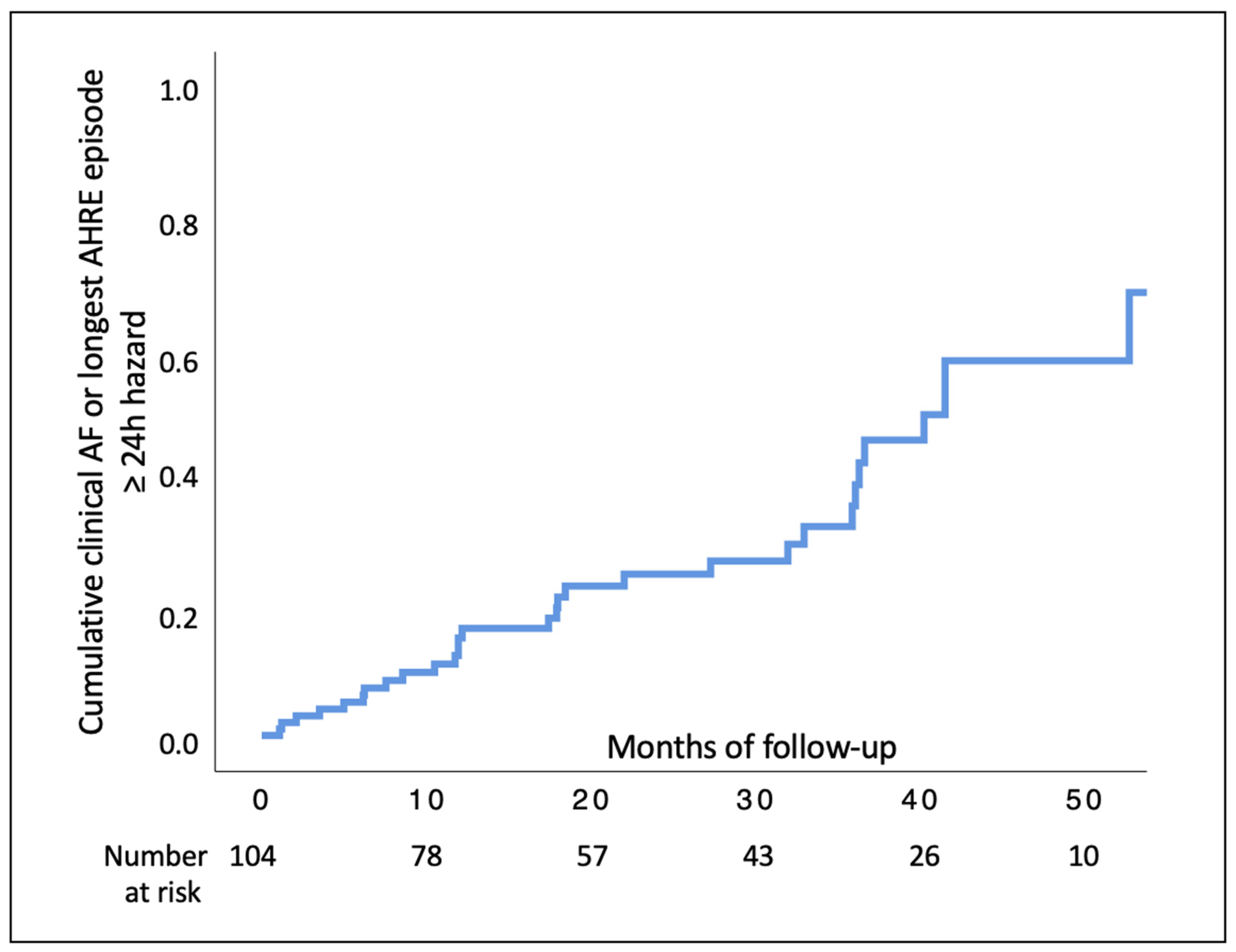
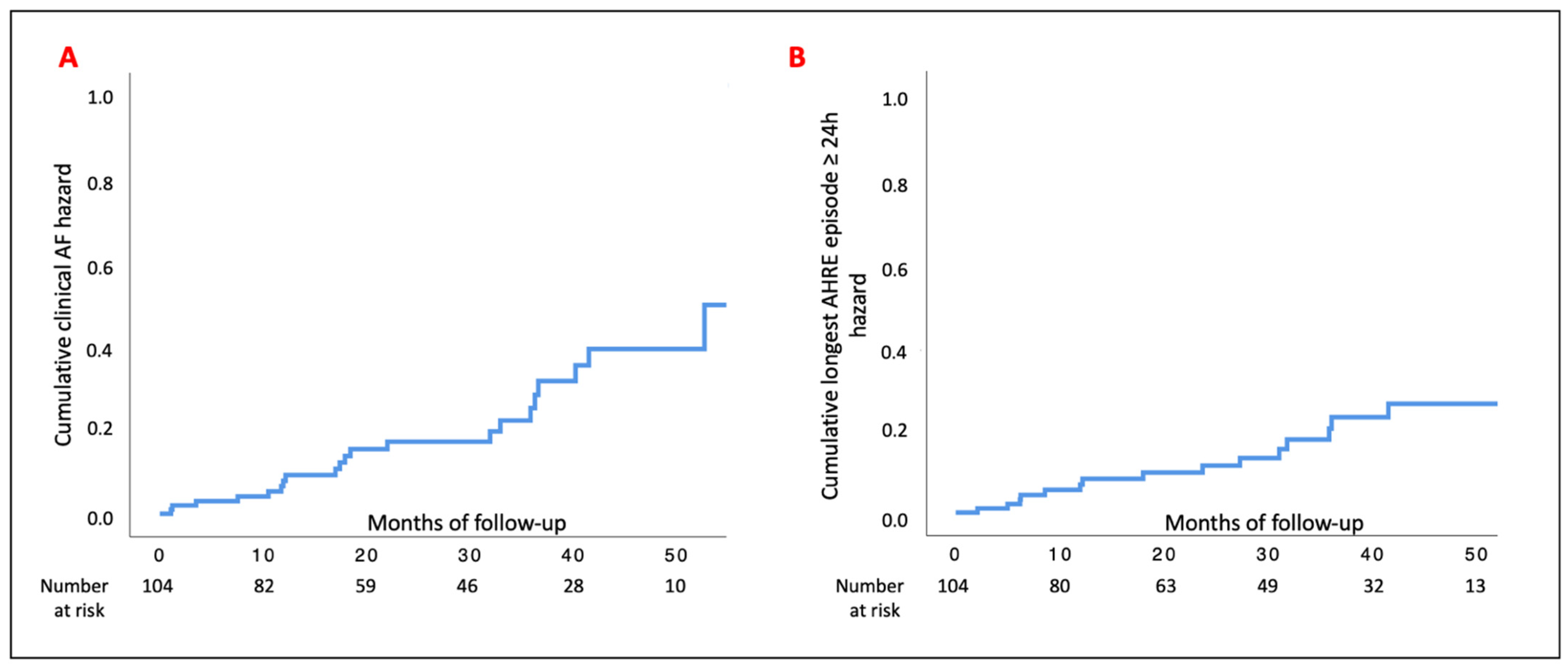
3.2. Study Outcomes
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Vitolo, M.; Imberti, J.F.; Potpara, T.S.; Lip, G.Y.H. What do we do about atrial high rate episodes? Eur. Heart J. Suppl. 2020, 22, O42–O52. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Glotzer, T.V.; Ziegler, P.D.; De Melis, M.; Stefano, L.M.D.S.; Sepsi, M.; Landolina, M.; Lunati, M.; Lewalter, T.; Camm, A.J. Detection of new atrial fibrillation in patients with cardiac implanted electronic devices and factors associated with transition to higher device-detected atrial fibrillation burden. Heart Rhythm 2018, 15, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Diederichsen, S.Z.; Haugan, K.J.; Brandes, A.; Lanng, M.B.; Graff, C.; Krieger, D.; Kronborg, C.; Holst, A.G.; Køber, L.; Højberg, S.; et al. Natural History of Subclinical Atrial Fibrillation Detected by Implanted Loop Recorders. J. Am. Coll. Cardiol. 2019, 74, 2771–2781. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Healey, J.S.; Crijns, H.J.G.M.; Wang, J.; Hohnloser, S.H.; Gold, M.R.; Capucci, A.; Lau, C.P.; Morillo, C.A.; Hobbelt, A.H.; et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur. Heart J. 2017, 38, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Sagris, D.; Georgiopoulos, G.; Pateras, K.; Perlepe, K.; Korompoki, E.; Milionis, H.; Tsiachris, D.; Chan, C.; Lip, G.Y.H.; Ntaios, G. Atrial High-Rate Episode Duration Thresholds and Thromboembolic Risk: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e022487. [Google Scholar] [CrossRef] [PubMed]
- Healey, J.S.; Connolly, S.J.; Gold, M.R.; Israel, C.W.; Van Gelder, I.C.; Capucci, A.; Lau, C.P.; Fain, E.; Yang, S.; Bailleul, C.; et al. Subclinical atrial fibrillation and the risk of stroke. N. Engl. J. Med. 2012, 366, 120–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitolo, M.; Imberti, J.F.; Maisano, A.; Albini, A.; Bonini, N.; Valenti, A.C.; Malavasi, V.L.; Proietti, M.; Healey, J.S.; Lip, G.Y.; et al. Device-detected atrial high rate episodes and the risk of stroke/thrombo-embolism and atrial fibrillation incidence: A systematic review and meta-analysis. Eur. J. Intern. Med. 2021, 92, 100–106. [Google Scholar] [CrossRef]
- Boriani, G.; Proietti, M.; Laroche, C.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.; Dan, G.A.; Kalarus, Z.; Tavazzi, L.; et al. Association between antithrombotic treatment and outcomes at 1-year follow-up in patients with atrial fibrillation: The EORP-AF General Long-Term Registry. Europace 2019, 21, 1013–1022. [Google Scholar] [CrossRef]
- Burdett, P.; Lip, G.Y.H. Atrial Fibrillation in the United Kingdom: Predicting Costs of an Emerging Epidemic Recognising and Forecasting the Cost Drivers of Atrial Fibrillation-related costs. Eur. Heart J. Qual. Care Clin. Outcomes 2020. [Google Scholar] [CrossRef]
- Chao, T.F.; Joung, B.; Takahashi, Y.; Lim, T.W.; Choi, E.K.; Chan, Y.H.; Guo, Y.; Sriratanasathavorn, C.; Oh, S.; Okumura, K.; et al. 2021 Focused Update Consensus Guidelines of the Asia Pacific Heart Rhythm Society on Stroke Prevention in Atrial Fibrillation: Executive Summary. Thromb. Haemost. 2021, 122, 20–47. [Google Scholar] [CrossRef] [PubMed]
- Romiti, G.F.; Pastori, D.; Rivera-Caravaca, J.M.; Ding, W.Y.; Gue, Y.X.; Menichelli, D.; Gumprecht, J.; Koziel, M.; Yang, P.S.; Guo, Y.; et al. Adherence to the ‘Atrial Fibrillation Better Care’ (ABC) Pathway in Patients with Atrial Fibrillation. Thromb. Haemost. 2021, 122, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H. The ABC pathway: An integrated approach to improve AF management. Nat. Rev. Cardiol. 2017, 14, 627–628. [Google Scholar] [CrossRef] [PubMed]
- Bayés de Luna, A.; Platonov, P.; Cosio, F.G.; Cygankiewicz, I.; Pastore, C.; Baranowski, R.; Bayés-Genis, A.; Guindo, J.; Viñolas, X.; Garcia-Niebla, J.; et al. Interatrial blocks. A separate entity from left atrial enlargement: A consensus report. J. Electrocardiol. 2012, 45, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.A.; Conen, D.; Van Gelder, I.C.; McIntyre, W.F.; Crijns, H.J.; Wang, J.; Gold, M.R.; Hohnloser, S.H.; Lau, C.P.; Capucci, A.; et al. Progression of Device-Detected Subclinical Atrial Fibrillation and the Risk of Heart Failure. J. Am. Coll. Cardiol. 2018, 71, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Sgreccia, D.; Manicardi, M.; Malavasi, V.L.; Vitolo, M.; Valenti, A.C.; Proietti, M.; Lip, G.Y.H.; Boriani, G. Comparing Outcomes in Asymptomatic and Symptomatic Atrial Fibrillation: A Systematic Review and Meta-Analysis of 81,462 Patients. J. Clin. Med. 2021, 10, 3979. [Google Scholar] [CrossRef] [PubMed]
- Imberti, J.F.; Tosetti, A.; Mei, D.A.; Maisano, A.; Boriani, G. Remote monitoring and telemedicine in heart failure: Implementation and benefits. Curr. Cardiol. Rep. 2021, 23, 55. [Google Scholar] [CrossRef]
- Christophersen, I.E.; Yin, X.; Larson, M.G.; Lubitz, S.A.; Magnani, J.W.; McManus, D.D.; Ellinor, P.T.; Benjamin, E.J. A comparison of the CHARGE-AF and the CHA2DS2-VASc risk scores for prediction of atrial fibrillation in the Framingham Heart Study. Am. Heart J. 2016, 178, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Vitali, F.; Serenelli, M.; Airaksinen, J.; Pavasini, R.; Tomaszuk-Kazberuk, A.; Mlodawska, E.; Jaakkola, S.; Balla, C.; Falsetti, L.; Tarquinio, N.; et al. CHA2DS2-VASc score predicts atrial fibrillation recurrence after cardioversion: Systematic review and individual patient pooled meta-analysis. Clin. Cardiol. 2019, 42, 358–364. [Google Scholar] [CrossRef]
- Sgreccia, D.; Mauro, E.; Vitolo, M.; Manicardi, M.; Valenti, A.C.; Imberti, J.F.; Ziacchi, M.; Boriani, G. Implantable cardioverter defibrillators and devices for cardiac resynchronization therapy: What perspective for patients’ apps combined with remote monitoring? Expert Rev. Med. Devices 2022. [Google Scholar] [CrossRef]
- Boriani, G.; Glotzer, T.V.; Santini, M.; West, T.M.; De Melis, M.; Sepsi, M.; Gasparini, M.; Lewalter, T.; Camm, J.A.; Singer, D.E. Device-detected atrial fibrillation and risk for stroke: An analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur. Heart J. 2014, 35, 508–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glotzer, T.V.; Daoud, E.G.; Wyse, D.G.; Singer, D.E.; Ezekowitz, M.D.; Hilker, C.; Miller, C.; Qi, D.; Ziegler, P.D. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: The TRENDS study. Circ. Arrhythm Electrophysiol. 2009, 2, 474–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zecchin, M.; Torre, M.; Carrani, E.; Sampaolo, L.; Ciminello, E.; Ortis, B.; Ricci, R.; Proclemer, A.; Sinagra, G.; Boriani, G. Seventeen-year trend (2001–2017) in pacemaker and implantable cardioverter-defibrillator utilization based on hospital discharge database data: An analysis by age groups. Eur. J. Intern. Med. 2021, 84, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Mairesse, G.H.; Braunschweig, F.; Klersy, K.; Cowie, M.R.; Leyva, F. Implementation and reimbursement of remote monitoring for cardiac implantable electronic devices in Europe: A survey from the health economics committee of the European Heart Rhythm Association. Europace 2015, 17, 814–818. [Google Scholar] [CrossRef]
- Boriani, G. Remote monitoring of cardiac implantable electrical devices in Europe: Quo vadis? Europace 2015, 17, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Palmisano, P.; Guerra, F.; Bertini, M.; Zanotto, G.; Lavalle, C.; Notarstefano, P.; Accogli, M.; Bisignani, G.; Forleo, G.B.; et al. Impact of COVID-19 pandemic on the clinical activities related to arrhythmias and electrophysiology in Italy: Results of a survey promoted by AIAC (Italian Association of Arrhythmology and Cardiac Pacing). Intern. Emerg. Med. 2020, 15, 1445–1456. [Google Scholar] [CrossRef]
- Grandinetti, M.; Di Molfetta, A.; Graziani, F.; Delogu, A.B.; Lillo, R.; Perri, G.; Pavone, N.; Bruno, P.; Aspromonte, N.; Amodeo, A.; et al. Telemedicine for adult congenital heart disease patients during the first wave of COVID-19 era: A single center experience. J. Cardiovasc. Med. 2021, 22, 706–710. [Google Scholar] [CrossRef]
- Maines, M.; Palmisano, P.; Del Greco, M.; Melissano, D.; De Bonis, S.; Baccillieri, S.; Zanotto, G.; D’Onofrio, A.; Ricci, R.P.; De Ponti, R.; et al. Impact of COVID-19 Pandemic on Remote Monitoring of Cardiac Implantable Electronic Devices in Italy: Results of a Survey Promoted by AIAC (Italian Association of Arrhythmology and Cardiac Pacing). J. Clin. Med. 2021, 10, 4086. [Google Scholar] [CrossRef]
- Freedman, B.; Boriani, G.; Glotzer, T.V.; Healey, J.S.; Kirchhof, P.; Potpara, T.S. Management of atrial high-rate episodes detected by cardiac implanted electronic devices. Nat. Rev. Cardiol. 2017, 14, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.D.; Alings, M.; Connolly, S.J.; Beresh, H.; Granger, C.B.; Mazuecos, J.B.; Boriani, G.; Nielsen, J.C.; Conen, D.; Hohnloser, S.H.; et al. Rationale and design of the Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA) trial. Am. Heart J. 2017, 189, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Blank, B.F.; Calvert, M.; Camm, A.J.; Chlouverakis, G.; Diener, H.C.; Goette, A.; Huening, A.; Lip, G.Y.H.; Simantirakis, E.; et al. Probing oral anticoagulation in patients with atrial high rate episodes: Rationale and design of the Non-vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes (NOAH-AFNET 6) trial. Am. Heart J. 2017, 190, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.T.; Bersohn, M.M.; Waldo, A.L.; Wathen, M.S.; Choucair, W.K.; Lip, G.Y.; Ip, J.; Holcomb, R.; Akar, J.G.; Halperin, J.L.; et al. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur. Heart J. 2015, 36, 1660–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daoud, E.G.; Glotzer, T.V.; Wyse, D.G.; Ezekowitz, M.D.; Hilker, C.; Koehler, J.; Ziegler, P.D.; Investigators, T. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: A subgroup analysis of TRENDS. Heart Rhythm 2011, 8, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace 2016, 18, 1455–1490. [Google Scholar] [CrossRef] [PubMed]
- Allessie, M.A. Atrial electrophysiologic remodeling: Another vicious circle? J. Cardiovasc. Electrophysiol. 1998, 9, 1378–1393. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Imberti, J.F.; Vitolo, M. The challenge to improve knowledge on the interplay between subclinical atrial fibrillation, atrial cardiomyopathy, and atrial remodeling. J. Cardiovasc. Electrophysiol. 2021, 32, 1364–1366. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Vitolo, M.; Diemberger, I.; Proietti, M.; Valenti, A.C.; Malavasi, V.L.; Lip, G.Y.H. Optimizing indices of atrial fibrillation susceptibility and burden to evaluate atrial fibrillation severity, risk and outcomes. Cardiovasc. Res. 2021, 117, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sohns, C.; Marrouche, N.F. Atrial fibrillation and cardiac fibrosis. Eur. Heart J. 2020, 41, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Malavasi, V.L.; Fantecchi, E.; Tordoni, V.; Melara, L.; Barbieri, A.; Vitolo, M.; Lip, G.Y.H.; Boriani, G. Atrial fibrillation pattern and factors affecting the progression to permanent atrial fibrillation. Intern. Emerg. Med. 2021, 16, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Doundoulakis, I.; Tsiachris, D.; Gatzoulis, K.A.; Stefanadis, C.; Tsioufis, K. Atrial high rate episodes as a marker of atrial cardiomyopathy: In the quest of the Holy Grail. Eur. J. Intern. Med. 2021, 93, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Yang, P.S.; Jang, E.; Yu, H.T.; Kim, T.H.; Uhm, J.S.; Kim, J.Y.; Sung, J.H.; Pak, H.N.; Lee, M.H.; et al. Improved Population-Based Clinical Outcomes of Patients with Atrial Fibrillation by Compliance with the Simple ABC (Atrial Fibrillation Better Care) Pathway for Integrated Care Management: A Nationwide Cohort Study. Thromb. Haemost. 2019, 119, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Romiti, G.F.; Olshansky, B.; Lane, D.A.; Lip, G.Y.H. Improved Outcomes by Integrated Care of Anticoagulated Patients with Atrial Fibrillation Using the Simple ABC (Atrial Fibrillation Better Care) Pathway. Am. J. Med. 2018, 131, 1359–1366.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proietti, M.; Vitolo, M.; Lip, G.Y.H. Integrated care and outcomes in patients with atrial fibrillation and comorbidities. Eur. J. Clin. Investig. 2021, 51, e13498. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lane, D.A.; Wang, L.; Zhang, H.; Wang, H.; Zhang, W.; Wen, J.; Xing, Y.; Wu, F.; Xia, Y.; et al. Mobile Health Technology to Improve Care for Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 75, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guo, J.; Shi, X.; Yao, Y.; Sun, Y.; Xia, Y.; Yu, B.; Liu, T.; Chen, Y.; Lip, G.Y.H.; et al. Mobile health technology-supported atrial fibrillation screening and integrated care: A report from the mAFA-II trial Long-term Extension Cohort. Eur. J. Intern. Med. 2020, 82, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Imberti, J.F.; Kotalczyk, A.; Wang, Y.; Lip, G.Y.H.; Investigators, C.R. 4S-AF scheme and ABC pathway guided management improves outcomes in atrial fibrillation patients. Eur. J. Clin. Investig. 2022, e13751. [Google Scholar] [CrossRef] [PubMed]
| N (%) (n = 104) | |
|---|---|
| Age * | 79.7 (74.0–84.2) |
| Female sex | 35 (33.7) |
| Heart failure | 27 (26.0) |
| Diabetes | 36 (34.6) |
| Stroke | 8 (7.7) |
| CAD | 31 (29.8) |
| PAD | 16 (15.4) |
| Hypertension | 87 (83.7) |
| CKD (n = 86) | 40 (46.5) |
| CHA2DS2-VASc * | 4 (3–5) |
| Total IAB (n = 102) | 7 (6.9) |
| Class I/III AADs (n = 95) | 3 (3.2) |
| Beta-blockers (n = 96) | 55 (57.3) |
| ACEi/ARB/ARNI (n = 96) | 55 (57.3) |
| MRA (n = 96) | 13 (12.5) |
| Statin (n = 96) | 52 (54.2) |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | p-Value | CI | HR | p-Value | CI | |
| Age | 1.00 | 0.88 | 0.96–1.05 | |||
| Age ≥ 80 years | 1.23 | 0.56 | 0.61–2.50 | |||
| Female sex | 0.74 | 0.45 | 0.35–1.61 | |||
| CHA2DS2-VASc | 1.41 | <0.01 | 1.10–1.81 | 1.40 | 0.01 | 1.07–1.83 |
| CKD | 1.24 | 0.57 | 0.59–2.57 | |||
| Pacing mode at implant | ||||||
| DDD | Ref. | |||||
| VVI | 1.10 | 0.92 | 0.14–8.56 | |||
| AAI | 1.48 | 0.53 | 0.43–5.06 | |||
| VDD | 0.86 | 0.76 | 0.32–2.30 | |||
| Total IAB | 1.91 | 0.24 | 0.66–5.53 | |||
| Longest AHRE episode at enrollment | ||||||
| 5–59 min | Ref. | Ref. | ||||
| 1 h–11 h 59 min | 1.74 | 0.18 | 0.78–3.88 | 1.52 | 0.31 | 0.68–3.43 |
| 12 h–23 h 59 min | 7.94 | <0.01 | 2.27–27.79 | 8.15 | <0.01 | 2.32–28.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imberti, J.F.; Bonini, N.; Tosetti, A.; Mei, D.A.; Gerra, L.; Malavasi, V.L.; Mazza, A.; Lip, G.Y.H.; Boriani, G. Atrial High-Rate Episodes Detected by Cardiac Implantable Electronic Devices: Dynamic Changes in Episodes and Predictors of Incident Atrial Fibrillation. Biology 2022, 11, 443. https://doi.org/10.3390/biology11030443
Imberti JF, Bonini N, Tosetti A, Mei DA, Gerra L, Malavasi VL, Mazza A, Lip GYH, Boriani G. Atrial High-Rate Episodes Detected by Cardiac Implantable Electronic Devices: Dynamic Changes in Episodes and Predictors of Incident Atrial Fibrillation. Biology. 2022; 11(3):443. https://doi.org/10.3390/biology11030443
Chicago/Turabian StyleImberti, Jacopo Francesco, Niccolò Bonini, Alberto Tosetti, Davide Antonio Mei, Luigi Gerra, Vincenzo Livio Malavasi, Andrea Mazza, Gregory Y. H. Lip, and Giuseppe Boriani. 2022. "Atrial High-Rate Episodes Detected by Cardiac Implantable Electronic Devices: Dynamic Changes in Episodes and Predictors of Incident Atrial Fibrillation" Biology 11, no. 3: 443. https://doi.org/10.3390/biology11030443
APA StyleImberti, J. F., Bonini, N., Tosetti, A., Mei, D. A., Gerra, L., Malavasi, V. L., Mazza, A., Lip, G. Y. H., & Boriani, G. (2022). Atrial High-Rate Episodes Detected by Cardiac Implantable Electronic Devices: Dynamic Changes in Episodes and Predictors of Incident Atrial Fibrillation. Biology, 11(3), 443. https://doi.org/10.3390/biology11030443









