Sex-Specific and Long-Term Impacts of Early-Life Venlafaxine Exposure in Zebrafish
Abstract
:Simple Summary
Abstract
1. Introduction
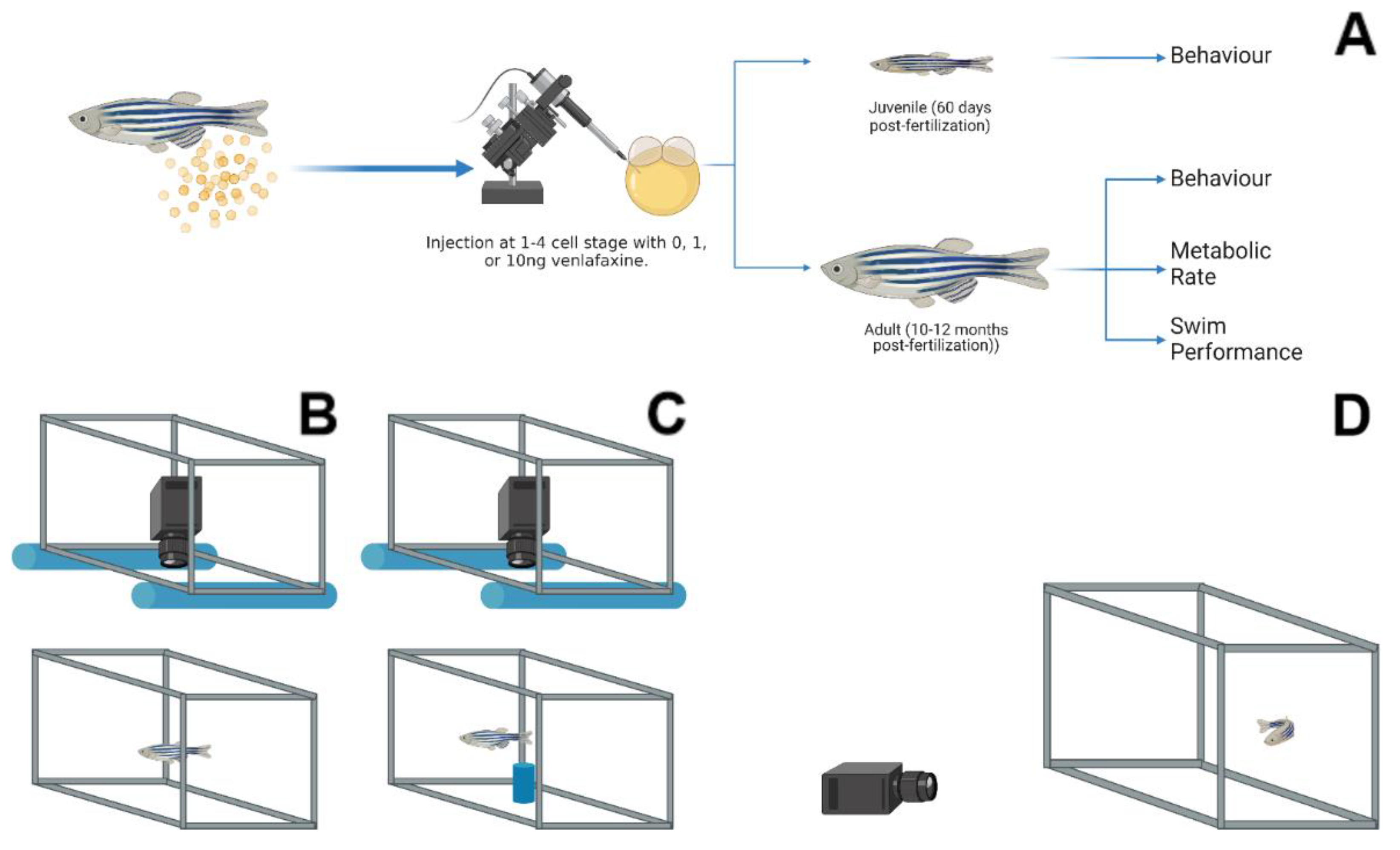
2. Materials and Methods
2.1. Fish Husbandry and Microinjection
2.2. Metabolism and Ucrit
2.3. Behaviour
2.4. Statistics
3. Results
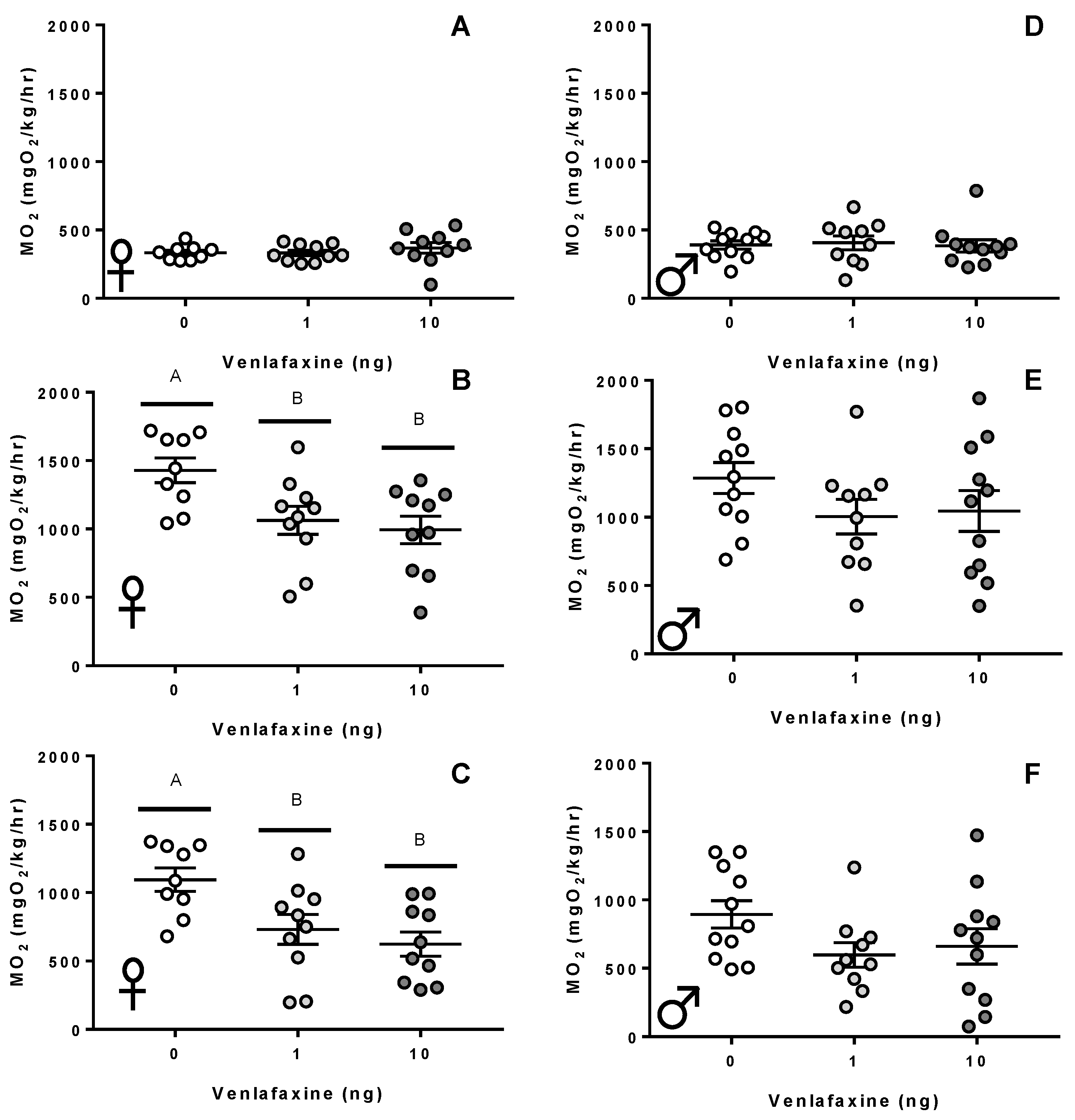
3.1. Metabolism and Swimming Performance
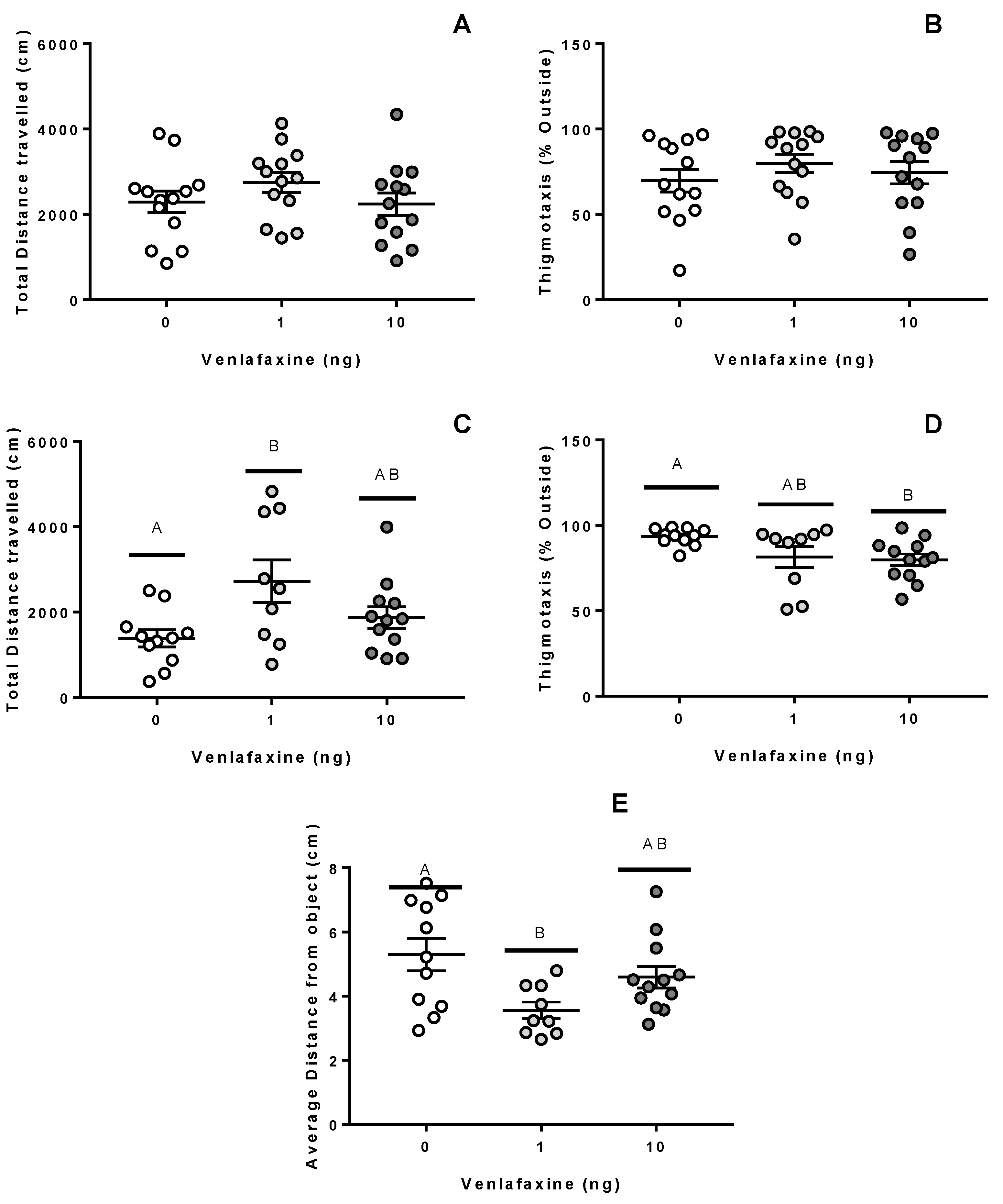
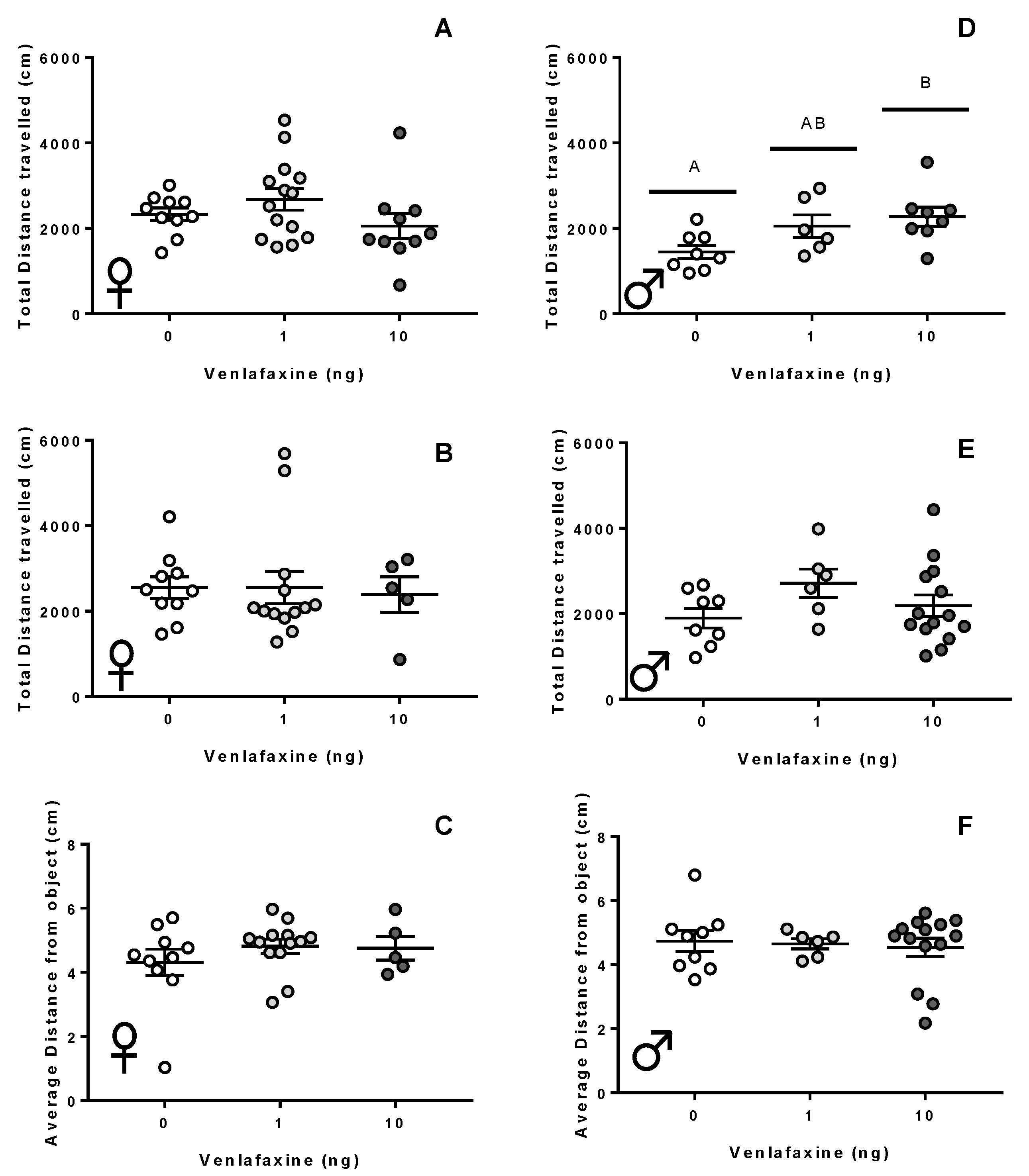
3.2. Behaviour
3.2.1. Juvenile Stage
3.2.2. Adult Stage
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harvey, A.T.; Rudolph, R.L.; Preskorn, S.H. Evidence of the dual mechanisms of action of venlafaxine. Arch. Gen. Psychiatry 2000, 57, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.D.; Chu, S.; Judt, C.; Li, H.; Oakes, K.D.; Servos, M.R.; Andrews, D.M. Antidepressants and their metabolites in municipal wastewater, and downstream exposure in an urban watershed. Environ. Toxicol. Chem. 2010, 29, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Rúa-Gómez, P.C.; Püttmann, W. Occurrence and removal of lidocaine, tramadol, venlafaxine, and their metabolites in German wastewater treatment plants. Environ. Sci. Pollut. Res. 2012, 19, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Schlüsener, M.P.; Hardenbicker, P.; Nilson, E.; Schulz, M.; Viergutz, C.; Ternes, T.A. Occurrence of venlafaxine, other antidepressants and selected metabolites in the Rhine catchment in the face of climate change. Environ. Pollut. 2015, 196, 247–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabicova, K.; Lindberg, R.H.; Ostman, M.; Grabic, R.; Randak, T.; Larsson, D.G.J.; Fick, J. Tissue-specific bioconcentration of antidepressants in fish exposed to effluent from a municipal sewage treatment plant. Sci. Total Environ. 2014, 488–489, 46–50. [Google Scholar] [CrossRef]
- Thompson, W.A.; Vijayan, M.M. Environmental levels of venlafaxine impact larval behavioural performance in fathead minnows. Chemosphere 2020, 259, 127437. [Google Scholar] [CrossRef]
- Thompson, W.A.; Arnold, V.I.; Vijayan, M.M. Venlafaxine in Embryos Stimulates Neurogenesis and Disrupts Larval Behavior in Zebrafish. Environ. Sci. Technol. 2017, 51, 12889–12897. [Google Scholar] [CrossRef] [PubMed]
- Sehonova, P.; Hodkovicova, N.; Urbanova, M.; Örn, S.; Blahova, J.; Svobodova, Z.; Faldyna, M.; Chloupek, P.; Briedikova, K.; Carlsson, G. Effects of antidepressants with different modes of action on early life stages of fish and amphibians. Environ. Pollut. 2019, 254, 112999. [Google Scholar] [CrossRef]
- Thompson, W.A.; Vijayan, M.M. Zygotic Venlafaxine Exposure Impacts Behavioral Programming by Disrupting Brain Serotonin in Zebrafish. Environ. Sci. Technol. 2020, 54, 14578–14588. [Google Scholar] [CrossRef]
- Thompson, W.A.; Vijayan, M.M. Zygotic exposure to venlafaxine disrupts cortisol stress axis activity in multiple generations of zebrafish. Environ. Pollut. 2021, 274, 116535. [Google Scholar] [CrossRef]
- Rodrigues, P.; Cunha, V.; Oliva-teles, L.; Ferreira, M.; Guimarães, L. Norfluoxetine and venlafaxine in zebrafish larvae: Single and combined toxicity of two pharmaceutical products relevant for risk assessment. J. Hazard. Mater. 2020, 400, 123171. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.A.; Shvartsburd, Z.; Vijayan, M.M. The antidepressant venlafaxine perturbs cardiac development and function in larval zebrafish. Aquat. Toxicol. 2022, 242, 106041. [Google Scholar] [CrossRef]
- Thompson, W.A.; Vijayan, M.M. Venlafaxine deposition in the zygote disrupts the endocrine control of growth in juvenile zebrafish. Environ. Res. 2021, 202, 111665. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.R.; Sloman, K.A. The effects of environmental pollutants on complex fish behaviour: Integrating behavioural and physiological indicators of toxicity. Aquat. Toxicol. 2004, 68, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Mok, E.Y.M.; Munro, A.D. Effects of dopaminergic drugs on locomotor activity in teleost fish of the genus Oreochromis (Cichlidae): Involvement of the telencephalon. Physiol. Behav. 1998, 64, 227–234. [Google Scholar] [CrossRef]
- Kawashima, T.; Zwart, M.F.; Yang, C.; Mensh, B.D.; Ahrens, M.B.; Kawashima, T.; Zwart, M.F.; Yang, C.; Mensh, B.D.; Ahrens, M.B. The Serotonergic System Tracks the Outcomes of Actions to Mediate Short-Term Motor Learning Article The Serotonergic System Tracks the Outcomes of Actions to Mediate Short-Term Motor Learning. Cell 2016, 167, 933–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansai, S.; Hosokawa, H.; Maegawa, S.; Naruse, K.; Washio, Y.; Sato, K.; Kinoshita, M. Deficiency of serotonin in raphe neurons and altered behavioral responses in tryptophan hydroxylase 2-knockout medaka (Oryzias latipes). Zebrafish 2017, 14, 495–507. [Google Scholar] [CrossRef]
- Fuiman, L.A.; Cowan, J.H. Behavior and recruitment success in fish larvae: Repeatability and covariation of survival skills. Ecology 2003, 84, 53–67. [Google Scholar] [CrossRef]
- Fuiman, L.A.; Cowan, J.H.; Smith, M.E.; O’Neal, J.P. Behavior and recruitment success in fish larvae: Variation with growth rate and the batch effect. Can. J. Fish. Aquat. Sci. 2005, 62, 1337–1349. [Google Scholar] [CrossRef]
- Levin, E.D.; Bencan, Z.; Cerutti, D.T. Anxiolytic effects of nicotine in zebrafish. Physiol. Behav. 2007, 90, 54–58. [Google Scholar] [CrossRef]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, A.M.; Kalueff, A.V. The developing utility of zebrafish models for cognitive enhancers research. Curr. Neuropharmacol. 2012, 10, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Maximino, C.; da Silva, A.W.B.; Gouveia, A.; Herculano, A.M. Pharmacological analysis of zebrafish (Danio rerio) scototaxis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 624–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maximino, C.; de Brito, T.M.; da Silva Batista, A.W.; Herculano, A.M.; Morato, S.; Gouveia, A. Measuring anxiety in zebrafish: A critical review. Behav. Brain Res. 2010, 214, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Genz, J.; Jyde, M.B.; Svendsen, J.C.; Steffensen, J.F.; Ramløv, H. Excess post-hypoxic oxygen consumption is independent from lactate accumulation in two cyprinid fishes. Comp. Biochem. Physiol.-A Mol. Integr. Physiol. 2013, 165, 54–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakamatsu, Y.; Ogino, K.; Hirata, H. Swimming capability of zebrafish is governed by water temperature, caudal fin length and genetic background. Sci. Rep. 2019, 9, 16307. [Google Scholar] [CrossRef] [PubMed]
- Tierney, K.B. Behavioural assessments of neurotoxic effects and neurodegeneration in zebrafish. Biochim. Biophys. Acta-Mol. Basis Dis. 2011, 1812, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Farrell, A.P.; Hinch, S.G.; Cooke, S.J.; Patterson, D.A.; Crossin, G.T.; Lapointe, M.; Mathes, M.T. Pacific salmon in hot water: Applying aerobic scope models and biotelemetry to predict the success of spawning migrations. Physiol. Biochem. Zool. 2008, 81, 697–708. [Google Scholar] [CrossRef] [Green Version]
- Perrot-Minnot, M.J.; Banchetry, L.; Cézilly, F. Anxiety-like behaviour increases safety from fish predation in an amphipod crustacea. R. Soc. Open Sci. 2017, 4, 171558. [Google Scholar] [CrossRef] [Green Version]
- Hulthén, K.; Chapman, B.B.; Nilsson, P.A.; Hansson, L.A.; Skov, C.; Brodersen, J.; Vinterstare, J.; Brönmark, C. A predation cost to bold fish in the wild. Sci. Rep. 2017, 7, 3–7. [Google Scholar] [CrossRef]
- Herculano, A.M.; Maximino, C. Serotonergic modulation of zebrafish behavior: Towards a paradox. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 55, 50–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connors, K.A.; Valenti, T.W.; Lawless, K.; Sackerman, J.; Onaivi, E.S.; Brooks, B.W.; Gould, G.G. Similar anxiolytic effects of agonists targeting serotonin 5-HT1A or cannabinoid CB receptors on zebrafish behavior in novel environments. Aquat. Toxicol. 2014, 151, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallinen, V.; Sundvik, M.; Reenilä, I.; Peitsaro, N.; Khrustalyov, D.; Anichtchik, O.; Toleikyte, G.; Kaslin, J.; Panula, P. Hyperserotonergic phenotype after monoamine oxidase inhibition in larval zebrafish. J. Neurochem. 2009, 109, 403–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, A.M.; Cachat, J.; Gaikwad, S.; Robinson, K.S.L.; Gebhardt, M.; Kalueff, A.V. Perspectives on experimental models of serotonin syndrome in zebrafish. Neurochem. Int. 2013, 62, 893–902. [Google Scholar] [CrossRef]
- Nishizawa, S.; Benkelfat, C.; Young, S.N.; Leyton, M.; Mzengeza, S.; De Montigny, C.; Blier, P.; Diksic, M. Differences between males and females in rates of serotonin synthesis in human brain. Proc. Natl. Acad. Sci. USA 1997, 94, 5308–5313. [Google Scholar] [CrossRef] [Green Version]
- Luo, T.; Sakai, Y.; Wagner, E.; Drager, U.C. Reduced Metabolites Mediate Neuroprotective Effects of Progesterone in the Adult Rat Hippocampus. J. Neurobiol. 2006, 66, 677–686. [Google Scholar] [CrossRef]
- Maekawa, F.; Tsukahara, S.; Kawashima, T.; Nohara, K.; Ohki-Hamazaki, H. The mechanisms underlying sexual differentiation of behavior and physiology in mammals and birds: Relative contributions of sex steroids and sex chromosomes. Front. Neurosci. 2014, 8, 242. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, M.; Carlsson, A. A regional study of sex differences in rat brain serotonin. Prog. Neuropsychopharmacol. Biol. Psychiatry 1988, 12, 53–61. [Google Scholar] [CrossRef]
- Linder, A.E.; Davis, R.P.; Burnett, R.; Watts, S.W. Function of the Serotonin Transporter in Vasculature of the Female Rat: Comparison with the male. Clin. Exp. Pharmacol. Physiol. 2011, 38, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Songtachalert, T.; Roomruangwong, C.; Carvalho, A.F.; Bourin, M.; Maes, M. Anxiety Disorders: Sex Differences in Serotonin and Tryptophan Metabolism. Curr. Top. Med. Chem. 2018, 18, 1704–1715. [Google Scholar] [CrossRef]
- Olivier, B.; Chan, J.S.; Snoeren, E.M.; Olivier, J.D.A.; Veening, J.G.; Vinkers, C.H.; Waldinger, M.D.; Oosting, R.S. Differences in Sexual Behaviour in Male and Female Rodents: Role of Serotonin. In Biological Basis of Sex Differences in Psychopharmacology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 8, ISBN 9783642200052. [Google Scholar]
- Telgkamp, P.; Combs, N.; Smith, G.T. Serotonin in a Diencephalic Nucleus Controlling Communication in an Electric Fish: Sexual Dimorphism and Relationship to Inidcators of Dominance. Dev. Neurobiol. 2007, 67, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Mennigen, J.A.; Martyniuk, C.J.; Crump, K.; Xiong, H.; Zhao, E.; Popesku, J.; Anisman, H.; Cossins, A.R.; Xia, X.; Trudeau, V.L. Effects of fluoxetine on the reproductive axis of female goldfish (Carassius auratus). Physiol. Genom. 2008, 35, 273–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halsey, L.G.; Killen, S.S.; Clark, T.D.; Norin, T. Exploring key issues of aerobic scope interpretation in ectotherms: Absolute versus factorial. Rev. Fish Biol. Fish. 2018, 28, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Auer, S.K.; Salin, K.; Anderson, G.J.; Metcalfe, N.B. Aerobic scope explains individual variation in feeding capacity. Biol. Lett. 2015, 11, 10–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bangasser, D.A.; Wiersielis, K.R.; Khantsis, S.M. Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress. Brain Res. 2015, 1641, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Curtis, A.L.; Bethea, T.; Valentino, R.J. Sexually Dimorphic Responses of the Brain Norepinephrine System to Stress and Corticotropin-Releasing Factor. Neuropsychopharmacology 2006, 31, 544–554. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Sampson, M.M.; Senturk, D.; Andrews, A.M. Sex- and SERT-Mediated Differences in Stimulated Serotonin Revealed by Fast Microdialysis. ACS Chem. Neurosci. 2015, 6, 1487–1501. [Google Scholar] [CrossRef]
- Clark, T.D.; Ryan, T.; Ingram, B.A.; Woakes, A.J.; Butler, P.J.; Frappell, P.B. Factorial aerobic scope is independent of temperature and primarily modulated by heart rate in exercising Murray cod (Maccullochella peelii). Physiol. Biochem. Zool. 2005, 78, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Laughlin, M.; Roseguini, B. Mechanisms for exercise training-induced increases in skeletal muscle blood flow capacity: Differences with interval sprint training versus aerobic endurance training. J. Physiol. Pharmacol. 2008, 59, 71–88. [Google Scholar]
- Primmett, D.R.N.; Randall, D.J.; Mazeaud, M.; Boutilier, R.G. The role of catecholamines in erythrocyte pH regulation and oxygen transport in rainbow trout (Salmo gairdneri) during exercise. J. Exp. Biol. 1986, 122, 139–148. [Google Scholar] [CrossRef]
- Calderone, V.; Baragatti, B.; Breschi, M.C.; Nieri, P.; Martinotti, E. Hormonal influence on the release of endothelial nitric oxide: Gender-related dimorphic sensitivity of rat aorta for noradrenaline. J. Pharm. Pharmacol. 2002, 54, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Reidy, S.P.; Kerr, S.R.; Nelson, J.A. Aerobic and anaerobic swimming performance of individual atlantic cod. J. Exp. Biol. 2000, 203, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.J.; Eliason, E.J.; Zolderdo, A.J.; Lapointe, D.; Best, C.; Gilmour, K.M.; Cooke, S.J. Cortisol modulates metabolism and energy mobilization in wild-caught pumpkinseed (Lepomis gibbosus). Fish Physiol. Biochem. 2019, 45, 1813–1828. [Google Scholar] [CrossRef]
- Pfalzgraff, T.; Lund, I.; Skov, P.V. Prolonged cortisol elevation alters whole body and tissue metabolism in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol.-Part A Mol. Integr. Physiol. 2022, 263, 111098. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, W.A.; Shvartsburd, Z.; Vijayan, M.M. Sex-Specific and Long-Term Impacts of Early-Life Venlafaxine Exposure in Zebrafish. Biology 2022, 11, 250. https://doi.org/10.3390/biology11020250
Thompson WA, Shvartsburd Z, Vijayan MM. Sex-Specific and Long-Term Impacts of Early-Life Venlafaxine Exposure in Zebrafish. Biology. 2022; 11(2):250. https://doi.org/10.3390/biology11020250
Chicago/Turabian StyleThompson, William Andrew, Zachary Shvartsburd, and Mathilakath M. Vijayan. 2022. "Sex-Specific and Long-Term Impacts of Early-Life Venlafaxine Exposure in Zebrafish" Biology 11, no. 2: 250. https://doi.org/10.3390/biology11020250
APA StyleThompson, W. A., Shvartsburd, Z., & Vijayan, M. M. (2022). Sex-Specific and Long-Term Impacts of Early-Life Venlafaxine Exposure in Zebrafish. Biology, 11(2), 250. https://doi.org/10.3390/biology11020250





