Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation
Abstract
Simple Summary
Abstract
1. Introduction
2. Factors Affecting Tomato Crop Cultivation and Its Nutritional Value
3. Tomato Constituents for Health
4. Health Effects
4.1. Tomatoes and Cancer Pathology
4.2. Tomato’s Specific Influence on Prostate Cancer
| Biological Property Studied | Type of Study (In Vitro/In Vivo) | Main Findings | References |
|---|---|---|---|
| Antioxidant and anticancer activity | In vitro study with human prostate cancer (PC-3) and human breast adenocarcinoma (MCF-7) cell lines. | Cell viability assay showed chemically induced lycopene oxidised products (1–50 µM) were a key component in cancer cell apoptosis. | [152] |
| In vitro study with HL-60 human promyelocytic leukaemia cells. | Products of lycopene oxidation, identified by spectral analyses, were added to HL-60 cell suspension as a 1% (v/v) concentration. This treatment was shown to induce apoptosis in leukaemia cells, shown using flow cytometry to evaluate the ratio of apoptotic cell death. | [151] | |
| Anti-angiogenic role in cancer cells | In vitro study testing human umbilical vein endothelial cells (HUVEC) and rat aortic rings. | Lycopene inhibited angiogenesis in HUVEC and rat aortic rings at physiologically relevant concentrations (1–2 μmol/L) when angiogenesis was analysed using phase-contrast microscopy. | [159] |
| In vitro and in vivo study testing human umbilical vein endothelial cells (HUVEC). | Lycopene (0, 1, 5, 10 µM) was shown to inhibit angiogenesis of HUVEC cells in vitro and in vivo by inhibiting MMP-2/uPA system through VEGFR2-mediated PI3K–Akt and ERK/p38 signalling pathways. Cell proliferation assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay, cell migration assessed with Millipore QCM™ Endothelial Migration Assay Kit. | [168] | |
| Longitudinal cohort study. | Lycopene used as a marker of tomato intake and higher intake inversely correlated with total, and the aggressive nature of prostate cancer. The reduced severity of cancer and lesser degree of angiogenesis were reported only in individuals who consumed a tomato-rich diet for a long time period but not in those whose intake recently increased. Tissue microarrays and immunohistochemistry were used to assess tumour biomarker expression. | [169] | |
| Modulation of molecular pathways in cancer cells | In vitro study with HT-29 human colon cancer cells. | Lycopene treatment (0, 2, 5, 10 µM) was shown to inhibit the PI3K–AKT signalling pathway in colon cancer cells, demonstrating its effects on tumour development via angiogenesis inhibition. Assessment of cell proliferation using MTT assay and gene expression investigated using transient transfection and luciferase reporter assays. | [157] |
| Ex vivo and in vivo study testing human umbilical vein endothelial cells (HUVEC) and rat aortic rings. | Lycopene (400 μg/mouse) reduced angiogenesis cell signalling through inhibition of the VEGF cell signalling pathway. Anti-angiogenic activity of lycopene confirmed by ex vivo rat aortic ring and in vivo chorioallantoic membrane assays. | [167] | |
| In vitro study with human prostate (PC-3) and breast (MDA-MB-231) cancer cell lines. | Lycopene (0.5–5 µM) inhibited different stages of the NF-κB cell signalling pathway in both cancer cell lines in vitro as seen in Western blots and NF-κB-responsive gene activation reporter assays. | [77] | |
| In vitro study in human gastric cancer (AGS) cells. | Lycopene at 0.3% was shown to induce apoptosis by inhibiting Wnt/β-catenin signalling, stopping the nuclear translocation of β-catenin and suppressing the expression of specific cell survival genes AGS cells. Cell viability, DNA fragmentation, and ROS concentrations were examined in these cells. | [150] | |
| Cytotoxicity and cancer cell growth | In vitro study testing human prostate epithelial cells (PrEC). | PrEC treated with lycopene (up to 5 μmol/L) showed no expression of cyclin D1 in vitro. This regulatory subunit of kinases essential to the cancer cell cycle, resulting in reduced cancer cell cycle progression. High-performance liquid chromatography (HPLC) analysis, a thymidine incorporation assay, and flow cytometry were carried out to assess the impact of lycopene. | [199] |
| In vitro study testing human prostate (PC-3) and breast (MDA-MB-231) cancer cell lines. | PC-3 and MDA-MB-231 cancer cell lines were tested in vitro in the absence and presence of lycopene at concentrations of 0.5–5 µM. MTS cell growth assays, Western blots, and NF-κB-responsive gene activation reporter assays showed that lycopene inhibits the NF-kB pathway at different stages in both cell lines. | [77] | |
| In vitro study treating Caco-2 colon cancer cells. | Treatment of Caco-2 colon cancer cells with 150 μmol/L dietary fibre ferulic acid delayed cell cycle progression in the S phase. Gene expression was analysed with cDNA microarray technique. | [115] | |
| Cancer cell apoptosis | In vitro study testing human prostate cells (PC-3). | Flow cytometry analysis showed 27–32% apoptosis in PC-3 when supplemented with (10–50 μM) β-carotene. | [174] |
| Gap junction communication in cancer cells | In vitro study with rat liver epithelial WB-F344 cells. | Incubation of WB-F344 cells with oxidation products of lycopene (0.2% v/v) improved the gap junction communication in dye transfer assay using microinjection of the fluorescent dye Lucifer Yellow CH. | [203] |
4.3. Cardioprotective Effects of Tomatoes
4.4. Neurodegenerative Disorders
4.5. Diabetes
4.6. Tomato Fruit for Skin Health
4.7. Tomatoes, Gut Microbiome, and Inflammation
4.8. Tomatoes and Exercise Recovery
4.9. Tomatoes and the Immune System
4.10. Tomatoes and Fertility
5. Detrimental Effects of Tomatoes
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhowmik, D.; Kumar, K.S.; Paswan, S.; Srivastava, S. Tomato-A Natural Medicine and Its Health Benefits. J. Pharmacogn. Phytochem. 2012, 1, 33–43. [Google Scholar]
- Toor, R.K.; Lister, C.E.; Savage, G.P. Antioxidant Activities of New Zealand-Grown Tomatoes. Int. J. Food Sci. Nutr. 2005, 56, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Borguini, R.G.; Ferraz Da Silva Torres, E.A. Tomatoes and Tomato Products as Dietary Sources of Antioxidants. Food Rev. Int. 2009, 25, 313–325. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Sanchez-Zapata, E.; Sayas-Barberá, E.; Sendra, E.; Pérez-Álvarez, J.A.; Fernández-López, J. Tomato and Tomato Byproducts. Human Health Benefits of Lycopene and Its Application to Meat Products: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1032–1049. [Google Scholar] [CrossRef]
- Rao, A.V.; Agarwal, S. Role of Lycopene as Antioxidant Carotenoid in the Prevention of Chronic Diseases: A Review. Nutr. Res. 1999, 19, 305–323. [Google Scholar] [CrossRef]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global Burden of Hypertension: Analysis of Worldwide Data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Agricultural Production. Available online: https://ourworldindata.org/agricultural-production (accessed on 5 January 2022).
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 5 January 2022).
- Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.; Cervilla, L.M.; Blasco, B.; Rios, J.J.; Rosales, M.A.; Romero, L.; Ruiz, J.M. Genotypic Differences in Some Physiological Parameters Symptomatic for Oxidative Stress under Moderate Drought in Tomato Plants. Plant Sci. 2010, 178, 30–40. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Valladares, F. Photosynthetic acclimation to simultaneous and interacting environmental stresses along natural light gradients: Optimality and constraints. Plant Biol. 2004, 6, 254–268. [Google Scholar] [CrossRef]
- Chand, J.B.; Hewa, G.; Hassanli, A.; Myers, B. Deficit Irrigation on Tomato Production in a Greenhouse Environment: A Review. J. Irrig. Drain. Eng. 2020, 147, 04020041. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Blasco, B.; Constán-Aguilar, C.; Romero, L.; Ruiz, J.M. Variation in the use efficiency of N under moderate water deficit in tomato plants (Solanum lycopersicum) differing in their tolerance to drought. Acta Physiol. Plant. 2011, 33, 1861–1865. [Google Scholar] [CrossRef]
- Singh, A.; Gulati, I.J.; Chopra, R. Effect of Various Fertigation Schedules and Organic Manures on Tomato (Lycopersicon Esculentum Mill.) Yield under Arid Condition. Bioscan 2013, 8, 1261–1264. [Google Scholar]
- Zhou, R.; Kong, L.; Yu, X.; Ottosen, C.-O.; Zhao, T.; Jiang, F.; Wu, Z. Oxidative damage and antioxidant mechanism in tomatoes responding to drought and heat stress. Acta Physiol. Plant. 2019, 41, 20. [Google Scholar] [CrossRef]
- Zhang, X.; Goatley, M.; Conner, J.; Wilkins, M.; Teshler, I.; Liu, J.; Fefer, M.; Ckurshumova, W. Copper Chlorophyllin Impacts on Growth and Drought Stress Tolerance of Tomato Plants. HortScience 2019, 54, 2195–2201. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, D.; Ervin, E.H.; Evanylo, G.K.; Cataldi, D.; Li, J. Biosolids Impact Antioxidant Metabolism Associated with Drought Tolerance in Tall Fescue. HortScience 2012, 47, 1550–1555. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The Roles of Reactive Oxygen Metabolism in Drought: Not So Cut and Dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef]
- Bian, S.; Jiang, Y. Reactive Oxygen Species, Antioxidant Enzyme Activities and Gene Expression Patterns in Leaves and Roots of Kentucky Bluegrass in Response to Drought Stress and Recovery. Sci. Hortic. 2009, 120, 264–270. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Tilahun, S.; Park, D.S.; Seo, M.H.; Jeong, C.S. Review on Factors Affecting the Quality and Antioxidant Properties of Tomatoes. Afr. J. Biotechnol. 2017, 16, 1678–1687. [Google Scholar] [CrossRef]
- Aherne, S.A.; Jiwan, M.A.; Daly, T.; O’brien, N.M. Geographical Location Has Greater Impact on Carotenoid Content and Bioaccessibility from Tomatoes than Variety. Plant Foods Hum. Nutr. 2009, 64, 250–256. [Google Scholar] [CrossRef]
- Kotíková, Z.; Lachman, J.; Hejtmánková, A.; Hejtmánková, K. Determination of antioxidant activity and antioxidant content in tomato varieties and evaluation of mutual interactions between antioxidants. Food Sci. Technol. 2011, 44, 1703–1710. [Google Scholar] [CrossRef]
- Abushita, A.A.; Daood, H.G.; Biacs, P.A. Change in Carotenoids and Antioxidant Vitamins in Tomato as a Function of Varietal and Technological Factors. J. Agric. Food Chem. 2000, 48, 2075–2081. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High Temperature Effects on Photosynthetic Activity of Two Tomato Cultivars with Different Heat Susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef]
- Berry, J.; Bjorkman, O. Photosynthetic Response and Adaptation to Temperature in Higher Plants. Annu. Rev. Plant Physiol. 2003, 31, 491–543. [Google Scholar] [CrossRef]
- Weis, E. Reversible Heat-Inactivation of the Calvin Cycle: A Possible Mechanism of the Temperature Regulation of Photosynthesis. Planta 1981, 151, 33–39. [Google Scholar] [CrossRef]
- Preedy, V. Tomatoes and Tomato Products: Nutritional, Medicinal and Therapeutic Properties; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- De Koning, A.N.M. The effect of temperature on fruit growth and fruit load of tomato. Acta Hortic. 1989, 248, 329–336. [Google Scholar] [CrossRef]
- Dorais, M.; Papadopoulos, A.P.; Gosselin, A. Greenhouse tomato fruit quality. In Horticultural Reviews; Janick, J., Ed.; John Wiley and Sons: New York, NY, USA, 2002; Volume 26, pp. 239–306. [Google Scholar]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of Environmental Factors and Agricultural Techniques on Antioxidant content of Tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Liptay, A.; Papadopoulos, A.P.; Bryan, H.H.; Gull, D. Ascorbic Acid Levels in Tomato (Lycopersicon Esculentum Mill.) at Low Temperatures. Agric. Biol. Chem. 1986, 50, 3185–3187. [Google Scholar] [CrossRef]
- Luthria, D.L.; Mukhopadhyay, S.; Krizek, D.T. Content of Total Phenolics and Phenolic Acids in Tomato (Lycopersicon Esculentum Mill.) Fruits as Influenced by Cultivar and Solar UV Radiation. J. Food Compos. Anal. 2006, 19, 771–777. [Google Scholar] [CrossRef]
- Giuffrida, F.; Consoli, S. Reusing Perlite Substrates in Soilless Cultivation: Analysis of Particle Size, Hydraulic Properties, and Solarization Effects. J. Irrig. Drain. Eng. 2015, 142, 04015047. [Google Scholar] [CrossRef]
- Wilson, G.C.S. Tomato production in different growing media. Acta Hortic. 1986, 178, 115–120. [Google Scholar] [CrossRef]
- Zhang, R.H.; Duan, Z.Q.; Li, Z.G. Use of Spent Mushroom Substrate as Growing Media for Tomato and Cucumber Seedlings. Pedosphere 2012, 22, 333–342. [Google Scholar] [CrossRef]
- Allaire, S.; Caron, J.; Ménard, C.; Dorais, M. Growing Media Varying in Particle Size and Shape for Greenhouse Tomato. Acta Hortic. 2004, 644, 307–311. [Google Scholar] [CrossRef]
- Xiong, J.; Tian, Y.; Wang, J.; Liu, W.; Chen, Q. Comparison of Coconut Coir, Rockwool, and Peat Cultivations for Tomato Production: Nutrient Balance, Plant Growth and Fruit Quality. Front. Plant Sci. 2017, 8, 1327. [Google Scholar] [CrossRef]
- Verma, S.; Sharma, A.; Kumar, R.; Kaur, C.; Arora, A.; Shah, R.; Nain, L. Improvement of Antioxidant and Defense Properties of Tomato (Var. Pusa Rohini) by Application of Bioaugmented Compost. Saudi J. Biol. Sci. 2015, 22, 256–264. [Google Scholar] [CrossRef]
- Verdoliva, S.G.; Gwyn-Jones, D.; Detheridge, A.; Robson, P. Controlled Comparisons between Soil and Hydroponic Systems Reveal Increased Water Use Efficiency and Higher Lycopene and β-Carotene Contents in Hydroponically Grown Tomatoes. Sci. Hortic. 2021, 279, 109896. [Google Scholar] [CrossRef]
- Sumalan, R.M.; Ciulca, S.I.; Poiana, M.A.; Moigradean, D.; Radulov, I.; Negrea, M.; Crisan, M.E.; Copolovici, L.; Sumalan, R.L. The Antioxidant Profile Evaluation of Some Tomato Landraces with Soil Salinity Tolerance Correlated with High Nutraceuticaland Functional Value. Agronomy 2020, 10, 500. [Google Scholar] [CrossRef]
- Caron, J.; Heinse, R.; Charpentier, S. Organic Materials Used in Agriculture, Horticulture, Reconstructed Soils, and Fil-Tering Applications. Vadose Zone J. 2015, 14, 1–6. [Google Scholar] [CrossRef]
- Margenot, A.J.; Griffin, D.E.; Alves, B.S.Q.; Rippner, D.A.; Li, C.; Parikh, S.J. Substitution of Peat Moss with Softwood Biochar for Soil-Free Marigold Growth. Ind. Crops Prod. 2018, 112, 160–169. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing Sustainability of Growing Media Constituents and Stand-Alone Substrates in Soilless Culture Systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable Sources of Plant Biostimulation: Microalgae as a Sustainable Means to Improve Crop Performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef]
- Mohd Hanafi, F.H.; Rezania, S.; Mat Taib, S.; Din, M.F.; Yamauchi, M.; Sakamoto, M.; Hara, H.; Park, J.; Ebrahimi, S.S. Environmentally Sustainable Applications of Agro-Based Spent Mushroom Substrate (SMS): An Overview. J. Mater. Cycles Waste Manag. 2018, 20, 1383–1396. [Google Scholar] [CrossRef]
- Kraska, T.; Kleinschmidt, B.; Weinand, J.; Pude, R. Cascading Use of Miscanthus as Growing Substrate in Soilless Culti-Vation of Vegetables (Tomatoes, Cucumbers) and Subsequent Direct Combustion. Sci. Hortic. 2018, 235, 205–213. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive Peptides from Marine Processing Waste and Shellfish: A Review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- De Tender, C.; Vandecasteele, B.; Verstraeten, B.; Ommeslag, S.; De Meyer, T.; De Visscher, J.; Dawyndt, P.; Clement, L.; Kyndt, T.; Debode, J. Chitin in Strawberry Cultivation: Foliar Growth and Defense Response Promotion, but Reduced Fruit Yield and Disease Resistance by Nutrient Imbalances. Mol. Plant Microbe Interact. 2021, 34, 227–239. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Amery, F.; Ommeslag, S.; Vanhoutte, K.; Visser, R.; Robbens, J.; De Tender, C.; Debode, J. Chemically versus Thermally Processed Brown Shrimp Shells or Chinese Mitten Crab as a Source of Chitin, Nutrients or Salts and as Microbial Stimulant in Soilless Strawberry Cultivation. Sci. Total Environ. 2021, 771, 145263. [Google Scholar] [CrossRef]
- De Tender, C.; Measure, B.; Van Der Jeugt, F.; Haegeman, A.; Ruttink, T.; Vandecasteele, B.; Dawyndt, P.; Debode, J.; Kuramae, E.E. Peat Substrate Amended with Chitin Modulates the N-Cycle, Siderophore and Chitinase Responses in the Lettuce Rhizobiome. Sci. Rep. 2019, 9, 9890. [Google Scholar] [CrossRef]
- Muymas, P.; Pichyangkura, R.; Wiriyakitnateekul, W.; Wangsomboondee, T.; Chadchawan, S.; Seraypheap, K. Effects of Chitin-Rich Residues on Growth and Postharvest Quality of Lettuce. Biol. Agric. Hortic. 2015, 31, 108–117. [Google Scholar] [CrossRef]
- Walker, R.; Morris, S.; Brown, P.; Gracie, A. Evaluation of Potential for Chitosan to Enhance Plant Defence; RIRDC Publication: Canberra, Australia, 2004. [Google Scholar]
- Kader, A.; Stevens, A.; Albright-Holton, M.; Morris, L.; Algazi, M. Effect of Fruit Ripeness When Picked on Flavor and Composition in Fresh Market Tomatoes. J. Am. Soc. Hort. Sci. 1977, 102, 724–731. [Google Scholar]
- Giovanelli, G.; Lavelli, V.; Peri, C.; Nobili, S. Variation in Antioxidant Components of Tomato during Vine and Post-Harvest Ripening. J. Sci. Food. Agric. 1999, 79, 1583–1588. [Google Scholar] [CrossRef]
- Vaughan, J.G.; Geissler, C. The New Oxford Book of Food Plants; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Rančić, D.; Quarrie, S.P.; Pećinar, I. Anatomy of Tomato Fruit and Fruit Pedicel during Fruit Development. Microsc. Sci. Technol. Appl. Educ. 2010, 2, 851–861. [Google Scholar]
- Hunt, G. Phenolic Constituents of Tomato Fruit Cuticles. Phytochemistry 1980, 19, 1415–1419. [Google Scholar] [CrossRef]
- Yeats, T.H.; Buda, G.J.; Wang, Z.; Chehanovsky, N.; Moyle, L.C.; Jetter, R.; Schaffer, A.A.; Rose, J.K.C. The Fruit Cuticles of Wild Tomato Species Exhibit Architectural and Chemical Diversity, Providing a New Model for Studying the Evolution of Cuticle Function. Plant J. 2012, 69, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Muños, S.; Ranc, N.; Botton, E.; Bérard, A.; Rolland, S.; Duffé, P.; Carretero, Y.; Le Paslier, M.C.; Delalande, C.; Bouzayen, M.; et al. Increase in Tomato Locule Number Is Controlled by Two Single-Nucleotide Polymorphisms Located near WUSCHEL. Plant Physiol. 2011, 156, 2244–2254. [Google Scholar] [CrossRef] [PubMed]
- Gi Gillaspy, G.; Ben-David, H.; Gruissem, W. Fruits: A Developmental Perspective. Plant Cell 1993, 5, 1439–1451. [Google Scholar] [CrossRef]
- Takeda, S.; Miyasaka, K.; Shimoda, H. Lycoperoside H, a Steroidal Alkaloid Saponin in Tomato Seeds, Ameliorates Atopic Dermatitis-like Symptoms in IL-33 Transgenic Mice. J. Food Biochem. 2021, 45, e13877. [Google Scholar] [CrossRef]
- Boulanger, N.; Lipsker, D. Protection Contre Les Piqûres de Tiques. Ann. Dermatol. Venereol. 2015, 142, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Moretti, C.L.; Sargent, S.A.; Huber, D.J.; Calbo, A.G.; Puschmann, R. Chemical Composition and Physical Properties of Pericarp, Locule, and Placental Tissues of Tomatoes with Internal Bruising. J. Am. Soc. Hortic. Sci. 1998, 123, 656–660. [Google Scholar] [CrossRef]
- Ruan, Q.Y.; Zheng, X.Q.; Chen, B.L.; Xiao, Y.; Peng, X.X.; Leung, D.W.M.; Liu, E.-E. Determination of Total Oxalate Contents of a Great Variety of Foods Commonly Available in Southern China Using an Oxalate Oxidase Prepared from Wheat Bran. J. Food Compos. Anal. 2013, 32, 6–11. [Google Scholar] [CrossRef]
- Chakraborty, N.; Ghosh, R.; Ghosh, S.; Narula, K.; Tayal, R.; Datta, A.; Chakraborty, S. Reduction of Oxalate Levels in Tomato Fruit and Consequent Metabolic Remodeling Following Overexpression of a Fungal Oxalate Decarboxylase. Plant Physiol. 2013, 162, 364–378. [Google Scholar] [CrossRef]
- Chai, W.; Liebman, M. Effect of Different Cooking Methods on Vegetable Oxalate Content. J. Agric. Food Chem. 2005, 53, 3027–3030. [Google Scholar] [CrossRef]
- Frusciante, L.; Carli, P.; Ercolano, M.R.; Pernice, R.; Di Matteo, A.; Fogliano, V.; Pellegrini, N. Antioxidant Nutritional Quality of Tomato. Mol. Nutr. Food Res. 2007, 51, 609–617. [Google Scholar] [CrossRef]
- NHS. Overview—Vitamins and Minerals. Available online: https://www.nhs.uk/conditions/vitamins-and-minerals/ (accessed on 18 December 2020).
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods 2020, 10, 45. [Google Scholar] [CrossRef]
- PubChem. Compound Summary for CID 6419725, Alpha-Carotene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/alpha-Carotene (accessed on 5 January 2022).
- Huang, Y.; Chen, H.; Su, Y.; Liu, H.; Hu, J.; Hong, K. Increased Blood Alpha-Carotene, All-Trans-Beta-Carotene and Lycopene Levels Are Associated with Beneficial Changes in Heart Rate Variability: A CVD-Stratified Analysis in an Adult Population-Based Study. Nutr. J. 2021, 20, 43. [Google Scholar] [CrossRef]
- Middha, P.; Weinstein, S.J.; Männistö, S.; Albanes, D.; Mondul, A.M. β-Carotene Supplementation and Lung Cancer In-Cidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: The Role of Tar and Nicotine. Nicotine Tob. Res. 2019, 21, 1045–1050. [Google Scholar] [CrossRef]
- Cho, K.S.; Shin, M.; Kim, S.; Lee, S.B. In Recent Advances in Studies on the Therapeutic Potential of Dietary Carotenoids in Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 4120458. [Google Scholar] [CrossRef]
- Park, H.A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-Apoptotic Effects of Carotenoids in Neurodegeneration. Molecules 2020, 25, 3453. [Google Scholar] [CrossRef]
- PubChem. Compound Summary for CID 446925, Lycopene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lycopene (accessed on 5 January 2022).
- Assar, E.A.; Vidalle, M.C.; Chopra, M.; Hafizi, S. Lycopene Acts through Inhibition of IκB Kinase to Suppress NF-κB Signaling in Human Prostate and Breast Cancer Cells. Tumor Biol. 2016, 37, 9375–9385. [Google Scholar] [CrossRef]
- Saini, R.K.; Rengasamy, K.R.R.; Mahomoodally, F.M.; Keum, Y.S. Protective Effects of Lycopene in Cancer, Cardio-Vascular, and Neurodegenerative Diseases: An Update on Epidemiological and Mechanistic Perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef]
- Miller, E.C.; Giovannucci, E.; Erdman, J.W., Jr.; Bahnson, R.; Schwartz, S.J.; Clinton, S.K. Tomato Products, Lycopene, and Prostate Cancer Risk. Urol. Clin. 2002, 29, 83–93. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, B.; Bai, Y.; Miao, T.; Rui, L.; Zhang, H.; Xia, B.; Li, Y.; Gao, S.; Wang, X.D.; et al. Lycopene in Protection against Obesity and Diabetes: A Mechanistic Review. Pharmacol. Res. 2020, 159, 104966. [Google Scholar] [CrossRef] [PubMed]
- Ratto, F.; Franchini, F.; Musicco, M.; Caruso, G.; Santo, S.G. A Narrative Review on the Potential of Tomato and Lycopene for the Prevention of Alzheimer’s Disease and Other Dementias. Crit. Rev. Food. Sci. Nutr. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.K.; Chopra, K. Lycopene Abrogates Aβ(1–42)-Mediated Neuroinflammatory Cascade in an Experimental Model of Alzheimer’s Disease. J. Nutr. Biochem. 2015, 26, 736–744. [Google Scholar] [CrossRef]
- Fukushi, Y.; Mariya, Y.; Yamada, K.; Yoshida, K.; Sasa, A.; Saito, H.; Hirai, A.; Suzuki, S.; Aizawa, K.; Suganuma, H.; et al. Tomato Juice Consumption Could Improve Breast Skin Adverse Effects of Radiotherapy in Breast Cancer Patients. In Vivo 2020, 34, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Prev ention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y.B. Oxidative Stress and Autophagy in Skin Aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Oakley, A.M.; Badri, T.; Harris, B.W. Photosensitivity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Madia, V.N.; Vita, D.; Ialongo, D.; Tudino, V.; Leo, A.; Scipione, L.; Santo, R.; Costi, R.; Messore, A. Recent Advances in Recovery of Lycopene from Tomato Waste: A Potent Antioxidant with Endless Benefits. Molecules 2021, 26, 4495. [Google Scholar] [CrossRef] [PubMed]
- Tanambell, H.; Bishop, K.S.; Quek, S.Y. Tangerine Tomatoes: Origin, Biochemistry, Potential Health Benefits and Future Prospects. Crit. Rev. Food Sci. Nutr. 2021, 61, 2237–2248. [Google Scholar] [CrossRef]
- Khachik, F.; Carvalho, L.; Bernstein, P.S.; Muir, G.J.; Zhao, D.Y.; Katz, N.B. Chemistry, Distribution, and Metabolism of Tomato Carotenoids and Their Impact on Human Health. Exp. Biol. Med. 2002, 227, 845–851. [Google Scholar] [CrossRef]
- PubChem. Compound Summary for CID 5280789, Neurosporene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Neurosporene (accessed on 5 January 2022).
- Ramaprasad, E.V.V.; Sasikala, C.; Ramana, C.V. Neurosporene Is the Major Carotenoid Accumulated by Rhodobacter Viridis JA737. Biotechnol. Lett. 2013, 35, 1093–1097. [Google Scholar] [CrossRef]
- PubChem. Compound Summary for CID 5280784, Phytoene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Phytoene (accessed on 5 January 2022).
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef]
- PubChem. Compound Summary for CID 6436722, Phytofluene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Phytofluene (accessed on 5 January 2022).
- Friedman, M.A. Cardioprotective, and Other Health Benefits of Tomato Compounds Lycopene, α-Tomatine, and Tomatidine in Pure Form and in Fresh and Processed Tomatoes. J. Agric. Food. Chem. 2013, 61, 9534–9550. [Google Scholar] [CrossRef]
- PubChem. Compound Summary for CID 28523, Tomatine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tomatine (accessed on 5 January 2022).
- Kim, S.P.; Nam, S.H.; Friedman, M. The Tomato Glycoalkaloid α-Tomatine Induces Caspase-Independent Cell Death in Mouse Colon Cancer CT-26 Cells and Transplanted Tumors in Mice. J. Agric. Food. Chem. 2015, 63, 1142–1150. [Google Scholar] [CrossRef]
- Heal, K.G.; Taylor-Robinson, A.W. Tomatine Adjuvantation of Protective Immunity to a Major Pre-Erythrocytic Vaccine Candidate of Malaria Is Mediated via CD8+ T Cell Release of IFN-γ. J. Biomed. Biotechnol. 2010, 2010, 834326. [Google Scholar] [CrossRef]
- PubChem. Compound Summary for CID 10887728, Esculeoside, A. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Esculeoside-A (accessed on 5 January 2022).
- Yang, Z.; Zhang, L.; Liu, J.; Lu, F.; Wang, L.; Chen, Y.; Li, D. Hypoglycemic Effects of Esculeoside A Are Mediated via Activation of AMPK and Upregulation of IRS-1. BMC Complement. Altern. Med. 2019, 19, 136. [Google Scholar] [CrossRef]
- Takeda, S.; Miyasaka, K.; Shrestha, S.; Manse, Y.; Morikawa, T.; Shimoda, H.L.H.; Saponin, T.S. Improves Epidermal Dehydration by Increasing Ceramide in the Stratum Corneum and Steroidal Anti-Inflammatory Effect. Molecules 2021, 26, 5860. [Google Scholar] [CrossRef]
- Martínez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic Compounds, Lycopene and Antioxidant Activity in Commercial Varieties of Tomato (Lycopersicum Esculentum). J. Sci. Food. Agri. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- PubChem. Compound Summary for CID 5280863, Kaempferol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Kaempferol (accessed on 5 January 2022).
- PubChem. Compound Summary for CID 5280343, Quercetin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Quercetin (accessed on 5 January 2022).
- PubChem. Compound Summary for CID 932, Naringenin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Naringenin (accessed on 5 January 2022).
- Shi, G.J.; Li, Y.; Cao, Q.H.; Wu, H.X.; Tang, X.Y.; Gao, X.H.; Yu, J.Q.; Chen, Z.; Yang, Y. In Vitro and in Vivo Evidence That Quercetin Protects against Diabetes and Its Complications: A Systematic Review of the Literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef]
- Tsuhako, R.; Yoshida, H.; Sugita, C.; Kurokawa, M. Naringenin Suppresses Neutrophil Infiltration into Adipose Tissue in High-Fat Diet-Induced Obese Mice. J. Nat. Med. 2020, 74, 229–237. [Google Scholar] [CrossRef]
- Hou, D.D.; Zhang, W.; Gao, Y.L.; Sun, Y.; Wang, H.X.; Qi, R.Q.; Chen, H.D.; Gao, X.-H. Anti-Inflammatory Effects of Quercetin in a Mouse Model of MC903-Induced Atopic Dermatitis. Int. Immunopharmacol. 2019, 74, 105676. [Google Scholar] [CrossRef]
- Rahul; Siddique, Y.H. Neurodegenerative Diseases and Flavonoids: Special Reference to Kaempferol. CNS Neurol. Disord. Drug Targets 2021, 20, 327–342. [Google Scholar] [CrossRef]
- PubChem. Compound Summary for CID 689043, Caffeic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Caffeic-acid (accessed on 5 January 2022).
- PubChem. Compound Summary for CID 637542, 4-Hydroxycinnamic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4-Hydroxycinnamic-acid (accessed on 5 January 2022).
- PubChem. Compound Summary for CID 445858, Ferulic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ferulic-acid (accessed on 5 January 2022).
- Zheng, Y.; You, X.; Guan, S.; Huang, J.; Wang, L.; Zhang, J.; Wu, J. Poly (Ferulic Acid) with an Anticancer Effect as a Drug Nanocarrier for Enhanced Colon Cancer Therapy. Adv. Funct. Mater. 2019, 29, 1808646. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Ferulic Acid: Pharmacological and Toxicological Aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef]
- Janicke, B.; Hegardt, C.; Krogh, M.; Önning, G.; Åkesson, B.; Cirenajwis, H.; Oredsson, S. The Antiproliferative Effect Of Dietary Fiber Phenolic Compounds Ferulic Acid and P-Coumaric Acid on the Cell Cycle of Caco-2 Cells. Nutr. Cancer 2011, 63, 611–622. [Google Scholar] [CrossRef]
- Ragab, A.S.; Fleet, J.; Jankowski, B.; Park, J.H.; Bobzin, S.C. Detection and Quantitation of Resveratrol in Tomato Fruit (Lycopersicon Esculentum Mill.). J. Agric. Food Chem. 2006, 54, 7175–7179. [Google Scholar] [CrossRef]
- PubChem. Compound Summary for CID 445154, Resveratrol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Resveratrol (accessed on 5 January 2022).
- Higdon, J.; Drake, V.J. An Evidence-Based Approach to Phytochemicals and Other Dietary Factors, 2nd ed.; Thieme Publishing Group: Stuttgart, Germany, 2012. [Google Scholar]
- Farinetti, A.; Zurlo, V.; Manenti, A.; Coppi, F.; Mattioli, A.V. Mediterranean Diet and Colorectal Cancer: A Systematic Review. Nutrition 2017, 43, 83–88. [Google Scholar] [CrossRef]
- Ghaiad, H.R.; Nooh, M.M.; El-Sawalhi, M.M.; Shaheen, A.A. Resveratrol Promotes Remyelination in Cuprizone Model of Multiple Sclerosis: Biochemical and Histological Study. Mol. Neurobiol. 2017, 54, 3219–3229. [Google Scholar] [CrossRef]
- Szulc-Musioł, B.; Sarecka-Hujar, B. The Use of Micro- and Nanocarriers for Resveratrol Delivery into and across the Skin in Different Skin Diseases—A Literature Review. Pharmaceutics 2021, 13, 451. [Google Scholar] [CrossRef]
- Pyo, I.S.; Yun, S.; Yoon, Y.E.; Choi, J.-W.; Lee, S.-J. Mechanisms of Aging and the Preventive Effects of Resveratrol on Age-Related Diseases. Molecules 2020, 25, 4649. [Google Scholar] [CrossRef] [PubMed]
- Mes, P.J.; Boches, P.; Myers, J.R.; Durst, R. Characterization of Tomatoes Expressing Anthocyanin in the Fruit. J. Am. Soc. Hortic. Sci. 2008, 133, 262–269. [Google Scholar] [CrossRef]
- PubChem. Compound Summary for CID 68245, Delphinidin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Delphinidin (accessed on 5 January 2022).
- Su, X.; Xu, J.; Rhodes, D.; Shen, Y.; Song, W.; Katz, B.; Tomich, J.; Wang, W. Identification and quantification of anthocyanins in transgenic purple tomato. Food Chem. 2016, 202, 184–188. [Google Scholar] [CrossRef]
- PubChem. Compound Summary for CID 441774, Petunidin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Petunidin (accessed on 5 January 2022).
- Gonzali, S.; Perata, P. Anthocyanins from Purple Tomatoes as Novel Antioxidants to Promote Human Health. Antioxidants 2020, 9, 1017. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Butelli, E.; Santis, S.D.; Cavalcanti, E.; Hill, L.; Angelis, M.D.; Giovinazzo, G.; Chieppa, M.; Martin, C.; Santino, A. Combined Dietary Anthocyanins, Flavonols, and Stilbenoids Alleviate Inflammatory Bowel Disease Symptoms in Mice. Front. Nutr. 2018, 4, 75. [Google Scholar] [CrossRef]
- Liso, M.; Santis, S.D.; Scarano, A.; Verna, G.; Dicarlo, M.; Galleggiante, V.; Campiglia, P.; Mastronardi, M.; Lippolis, A.; Vacca, M.; et al. A Bronze-Tomato Enriched Diet Affects the Intestinal Microbiome under Homeostatic and Inflammatory Conditions. Nutrients 2018, 10, 1862. [Google Scholar] [CrossRef]
- Upadhyaya, P.; Tyagi, K.; Sarma, S.; Tamboli, V.; Sreelakshmi, Y.; Sharma, R. Natural Variation in Folate Levels among Tomato (Solanum Lycopersicum) Accessions. Food. Chem. 2017, 217, 610–619. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Chavarro, J.E. Diet and Fertility: A Review. Am. J. Obstet. Gynecol. 2018, 218, 379–389. [Google Scholar] [CrossRef]
- PubChem. Compound Summary for CID 54670067, Ascorbic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ascorbic-acid (accessed on 5 January 2022).
- Jacobs, E.J.; Connell, C.J.; McCullough, M.L.; Chao, A.; Jonas, C.R.; Rodriguez, C.; Calle, E.E.; Thun, M.J. Vitamin C, Vitamin E, and Multivitamin Supplement Use and Stomach Cancer Mortality in the Cancer Prevention Study II Cohort. Cancer Epidemiol. Biomark. Prev. 2002, 11, 35–41. [Google Scholar]
- Upritchard, J.E.; Sutherland, W.H.; Mann, J.I. Effect of Supplementation with Tomato Juice, Vitamin E, and Vitamin C on LDL Oxidation and Products of Inflammatory Activity in Type 2 Diabetes. Diabetes Care 2000, 23, 733–738. [Google Scholar] [CrossRef]
- Pullar, J.; Carr, A.; Vissers, M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Steiling, H.; Longet, K.; Moodycliffe, A.; Mansourian, R.; Bertschy, E.; Smola, H.; Mauch, C.; Williamson, G. Sodi-Um-Dependent Vitamin C Transporter Isoforms in Skin: Distribution, Kinetics, and Effect of UVB-Induced Oxidative Stress. Free Radic. Biol. Med. 2007, 43, 752–762. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Compound Summary for CID 14985, Vitamin, E. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Vitamin-E (accessed on 5 January 2022).
- Fuhrman, B.; Ben-Yaish, L.; Attias, J.; Hayek, T.; Aviram, M. Tomato Lycopene and Beta-Carotene Inhibit Low Density Lipoprotein Oxidation and This Effect Depends on the Lipoprotein Vitamin E Content. Nutr. Metab. Cardiovasc. Dis. 1997, 7, 433–444. [Google Scholar]
- Sabetian, S.; Jahromi, B.N.; Vakili, S.; Forouhari, S.; Alipour, S. The Effect of Oral Vitamin E on Semen Parameters and IVF Outcome: A Double-Blinded Randomized Placebo-Controlled Clinical Trial. BioMed Res. Int. 2021, 2021, 5588275. [Google Scholar] [CrossRef] [PubMed]
- Jie, K.S.; Bots, M.L.; Vermeer, C.; Witteman, J.C.; Grobbee, D.E. Vitamin K intake and osteocalcin levels in women with and without aortic atherosclerosis: A population-based study. Atherosclerosis 1995, 116, 117–123. [Google Scholar] [CrossRef]
- Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2018; Available online: https://gco.iarc.fr/today (accessed on 5 January 2022).
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef]
- Capurso, A.; Crepaldi, G.; Capurso, C. The Mediterranean Diet: A Pathway to Successful Aging. Aging Clin. Exp. Res. 2020, 32, 1187–1188. [Google Scholar] [CrossRef]
- Moran, N.E.; Cichon, M.J.; Riedl, K.M.; Grainger, E.M.; Schwartz, S.J.; Novotny, J.A.; Erdman, J.W.; Clinton, S.K. Compartmental and Noncompartmental Modeling of 13C-Lycopene Absorption, Isomerization, and Distribution Kinetics in Healthy Adults. Am. J. Clin. Nutr. 2015, 102, 1436–1449. [Google Scholar] [CrossRef]
- Giovannucci, E.T. Tomato-Based Products, Lycopene, and Cancer: Review of the Epidemiologic Literature. J. Natl. Cancer Inst. 1999, 91, 317–331. [Google Scholar] [CrossRef]
- Giovannucci, E. A Review of Epidemiologic Studies of Tomatoes, Lycopene, and Prostate Cancer. Exp. Biol. Med. 2002, 227, 852–859. [Google Scholar] [CrossRef]
- Yang, T.; Yang, X.; Wang, X.; Wang, Y.; Song, Z. The Role of Tomato Products and Lycopene in the Prevention of Gastric Cancer: A Meta-Analysis of Epidemiologic Studies. Med. Hypotheses 2013, 80, 383–388. [Google Scholar] [CrossRef]
- Wei, M.Y.; Giovannucci, E.L.L. Tomato Products, and Prostate Cancer Incidence: A Review and Reassessment in the PSA Screening Era. J. Oncol. 2012, 2012, 271063. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.H.; Lim, J.W.; Kim, H. Lycopene Induces Apoptosis by Inhibiting Nuclear Translocation of β-Catenin in Gastric Cancer Cells. J. Physiol. Pharmacol. 2019, 70, 605–611. [Google Scholar] [CrossRef]
- Zhang, H. A Novel Cleavage Product Formed by Autoxidation of Lycopene Induces Apoptosis in HL-60 Cells. Free Radic. Biol. Med. 2003, 35, 1653–1663. [Google Scholar] [CrossRef]
- Arathi, B.P.; Sowmya, P.R.R.; Kuriakose, G.C.; Vijay, K.; Baskaran, V.; Jayabaskaran, C.; Lakshminarayana, R. Enhanced Cytotoxic and Apoptosis Inducing Activity of Lycopene Oxidation Products in Different Cancer Cell Lines. Food Chem. Toxicol. 2016, 97, 265–276. [Google Scholar] [CrossRef]
- Przybylska, S. Lycopene–a Bioactive Carotenoid Offering Multiple Health Benefits: A Review. Int. J. Food Sci. Technol. 2020, 55, 11–32. [Google Scholar] [CrossRef]
- Peyrat, J.P.; Bonneterre, J.; Hecquet, B.; Vennin, P.; Louchez, M.M.; Fournier, C.; Lefebvre, J.; Demaille, A. Plasma Insulin-like Growth Factor-1 (IGF-1) Concentrations in Human Breast Cancer. Eur. J. Cancer 1993, 29, 492–497. [Google Scholar] [CrossRef]
- Vivanco, I.; Sawyers, C.L. The Phosphatidylinositol 3-Kinase–AKT Pathway in Human Cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef]
- Majumder, P.K.; Sellers, W.R. Akt-Regulated Pathways in Prostate Cancer. Oncogene 2005, 24, 7465–7474. [Google Scholar] [CrossRef]
- Tang, F.Y.; Shih, C.J.; Cheng, L.H.; Ho, H.J.; Chen, H.J. Lycopene Inhibits Growth of Human Colon Cancer Cells via Suppression of the Akt Signaling Pathway. Mol. Nutr. Food Res. 2008, 52, 646–654. [Google Scholar] [CrossRef]
- Preet, R.; Mohapatra, P.; Das, D.; Satapathy, S.R.; Choudhuri, T.; Wyatt, M.D.; Kundu, C.N. Lycopene Synergistically Enhances Quinacrine Action to Inhibit Wnt-TCF Signaling in Breast Cancer Cells through APC. Carcinogenesis 2012, 34, 277–286. [Google Scholar] [CrossRef]
- Elgass, S.; Cooper, A.; Chopra, M. Lycopene Inhibits Angiogenesis in Human Umbilical Vein Endothelial Cells and Rat Aortic Rings. Br. J. Nutr. 2011, 108, 431–439. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Breemen, R.B.; Pajkovic, N. Multitargeted Therapy of Cancer by Lycopene. Cancer Lett. 2008, 269, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Dan, H.; Wu, R.; Meng, W.; Liu, N.; Jin, X.; Zhou, M.; Zeng, X.; Zhou, G.; Chen, Q. Lycopene: Features and Potential Significance in the Oral Cancer and Precancerous Lesions. J. Oral Pathol. Med. 2011, 40, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, V.K.L. Lycopene in Cancer Therapy. J. Pharm. Bioallied Sci. 2016, 8, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Phytochemicals in Cancer Prevention and Therapy: Truth or Dare? Toxins 2010, 2, 517–551. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Imrhan, V. Tomatoes versus Lycopene in Oxidative Stress and Carcinogenesis: Conclusions from Clinical Trials. Eur. J. Clin. Nutr. 2007, 61, 295–303. [Google Scholar] [CrossRef]
- Kim, S.J.; Nara, E.; Kobayashi, H.; Terao, J.; Nagao, A. Formation of Cleavage Products by Autoxidation of Lycopene. Lipids 2001, 36, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P.; Russell, R.M.; Krinsky, N.I. In Vitro Inhibition of Proliferation of Estrogen-Dependent and Estro-Gen-Independent Human Breast Cancer Cells Treated with Carotenoids or Retinoids. J. Nutr. 2001, 131, 1574–1580. [Google Scholar] [CrossRef]
- Chen, M.L.; Lin, Y.H.; Yang, C.M.; Hu, M.L. Lycopene Inhibits Angiogenesis Both in Vitro and in Vivo by Inhibiting MMP-2/UPA System through VEGFR2-Mediated PI3K-Akt and ERK/P38 Signaling Pathways. Mol. Nutr. Food Res. 2012, 56, 889–899. [Google Scholar] [CrossRef]
- Şahin, M.; Şahin, E.; Gümüşlü, S. Effects of Lycopene and Apigenin on Human Umbilical Vein Endothelial Cells in Vitro under Angiogenic Stimulation. Acta Histochem. 2012, 114, 94–100. [Google Scholar] [CrossRef]
- Zu, K.; Mucci, L.; Rosner, B.A.; Clinton, S.K.; Loda, M.; Stampfer, M.J.; Giovannucci, E. Dietary Lycopene, Angiogenesis, and Prostate Cancer: A Prospective Study in the Prostate-Specific Antigen Era. J. Natl. Cancer Inst. 2014, 106, 430. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Orhan, C.; Tuzcu, M.; Tastan, H.; Bilir, B.; Sahin, N.; Oner, D.A.; Kucuk, O. Tomato Powder Modulates NF-κB, MTOR, and Nrf2 Pathways during Aging in Healthy Rats. J. Aging Res. 2019, 2019, 1643243. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, K.; Kowshik, J.; Kishore, T.K.; Baba, A.B.; Nagini, S. Astaxanthin Inhibits NF-κB and Wnt/β-Catenin Signaling Pathways via Inactivation of Erk/MAPK and PI3K/Akt to Induce Intrinsic Apoptosis in a Hamster Model of Oral Cancer. Biochim. Biophys. Acta Gen. 2013, 1830, 4433–4444. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K. Potential Targets for Prevention of Colorectal Cancer: A Focus on PI3K/Akt/MTOR and Wnt Pathways. Asian Pac. J. Cancer Prev. 2013, 14, 2201–2205. [Google Scholar] [CrossRef]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-κB in Development and Progression of Human Cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Jayappriyan, K.R.; Rajkumar, R.; Venkatakrishnan, V.; Nagaraj, S.; Rengasamy, R. In Vitro Anticancer Activity of Natural β-Carotene from Dunaliella Salina EU5891199 in PC-3 Cells. Biomed. Prev. Nutr. 2013, 3, 99–105. [Google Scholar] [CrossRef]
- Chew, B.P.; Park, J.S.; Wong, M.W.; Wong, T.S. A Comparison of the Anticancer Activities of Dietary Beta-Carotene, Canthaxanthin and Astaxanthin in Mice in Vivo. Anticancer Res. 1999, 19, 1849–1853. [Google Scholar]
- Pezzuto, J.M. Plant-Derived Anticancer Agents. Biochem. Pharmacol. 1997, 53, 121–133. [Google Scholar] [CrossRef]
- Sierra, R.; Chinnock, A.; Ohshima, H.; Pignatelli, B.; Malaveille, C.; Gamboa, C.; Teuchmann, S.; Muñoz, N.; Bartsch, H. In Vivo Nitrosoproline Formation and Other Risk Factors in Costa Rican Children from High- and Low-Risk Areas for Gastric Cancer. Cancer Epidemiol. Biomark. Prev. 1993, 2, 563–568. [Google Scholar]
- Levine, M.; Padayatty, S.J.; Espey, M.G. Vitamin C: A Concentration-Function Approach Yields Pharmacology and Therapeutic Discoveries. Adv. Nutr. 2011, 2, 78–88. [Google Scholar] [CrossRef]
- Del Valle, M.; Cámara, M.; Torija, M.-E. Chemical Characterization of Tomato Pomace. J. Sci. Food Agric. 2006, 86, 1232–1236. [Google Scholar] [CrossRef]
- Bultman, S.J. Interplay between Diet, Gut Microbiota, Epigenetic Events, and Colorectal Cancer. Mol. Nutr. Food Res. 2017, 61, 1500902. [Google Scholar] [CrossRef]
- McNabney, S.M.; Henagan, T.M. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef]
- Lycopene Supplements. Available online: https://www.rogelcancercenter.org/support/symptoms-and-side-effects/cancer-nutrition-services/nutrition-and-prevention/are-lycopene-supplements-a-good-idea-for-you (accessed on 24 August 2021).
- Rowles, J.L.; Ranard, K.M.; Applegate, C.C.; Jeon, S.; An, R.; Erdman, J.W. Processed and Raw Tomato Consumption and Risk of Prostate Cancer: A Systematic Review and Dose–Response Meta-Analysis. Prostate Cancer Prostatic Dis. 2018, 21, 319–336. [Google Scholar] [CrossRef]
- Liu, A.; Pajkovic, N.; Pang, Y.; Zhu, D.; Calamini, B.; Mesecar, A.L.; Breemen, R.B. Absorption and Subcellular Localization of Lycopene in Human Prostate Cancer Cells. Mol. Cancer Ther. 2006, 5, 2879–2885. [Google Scholar] [CrossRef]
- Giovannucci, E.B.; Ascherio, A.J.; Rimm, E.A.; Stampfer, M.C.; Colditz, G.; Willett, W. Intake of Carotenoids and Retinol in Relation to Risk of Prostate Cancer. J. Natl. Cancer Inst. 1995, 87, 1767–1776. [Google Scholar] [CrossRef]
- Mayne, S.T.; Cartmel, B.; Silva, F.; Kim, C.S.; Fallon, B.G.; Briskin, K.; Zheng, T.; Baum, M.; Shor-Posner, G.; Goodwin, W.J. Plasma Lycopene Concentrations in Humans Are Determined by Lycopene Intake, Plasma Cholesterol Concentrations and Selected Demographic Factors. J. Nutr. 1999, 129, 849–854. [Google Scholar] [CrossRef]
- Bowen, P.; Chen, L.; Stacewicz-Sapuntzakis, M.; Duncan, C.; Sharifi, R.; Ghosh, L.; Kim, H.S.; Christov-Tzelkov, K.; Breemen, R.V. Tomato sauce supplementation and prostate cancer: Lycopene accumulation and modulation of biomarkers of carcinogenesis. Exp. Biol. Med. 2002, 227, 886–893. [Google Scholar] [CrossRef]
- Chan, J.M.; Stampfer, M.J.; Giovannucci, E.; Gann, P.H.; Ma, J.; Wilkinson, P.; Hennekens, C.H.; Pollak, M. Plasma Insulin-Like Growth Factor-I and Prostate Cancer Risk: A Prospective Study. Science 1998, 279, 563–566. [Google Scholar] [CrossRef]
- Mucci, L.; Tamimi, R.; Lagiou, P.; Trichopoulou, A.; Benetou, V.; Spanos, E.; Trichopoulos, D. Are Dietary Influences on the Risk of Prostate Cancer Mediated through the Insulin-like Growth Factor System? BJU Int. 2001, 87, 814–820. [Google Scholar] [CrossRef]
- Gunnell, D.; Oliver, S.E.; Peters, T.J.; Donovan, J.L.; Persad, R.; Maynard, M.; Gillatt, D.; Pearce, A.; Hamdy, F.C.; Neal, D.E.; et al. Are Diet–Prostate Cancer Associations Mediated by the IGF Axis? A Cross-Sectional Analysis of Diet, IGF-1 and IGFBP-3 in Healthy Middle-Aged Men. Br. J. Cancer 2003, 88, 1682–1686. [Google Scholar] [CrossRef]
- Ivanov, N.I.; Cowell, S.P.; Brown, P.; Rennie, P.S.; Guns, E.S.; Cox, M.E. Lycopene Differentially Induces Quiescence and Apoptosis in Androgen-Responsive and -Independent Prostate Cancer Cell Lines. Clin. Nutr. 2007, 26, 252–263. [Google Scholar] [CrossRef]
- Chan, J.M.; Weinberg, V.; Magbanua, M.J.; Sosa, E.; Simko, J.; Shinohara, K.; Federman, S.; Mattie, M.; Hughes-Fulford, M.; Haqq, C.; et al. Nutritional Supplements, COX-2 and IGF-1 Expression in Men on Active Surveillance for Prostate Cancer. Cancer Causes Control 2011, 22, 141–150. [Google Scholar] [CrossRef]
- Graydon, R.; Gilchrist, S.E.C.M.; Young, I.S.; Obermüller-Jevic, U.; Hasselwander, O.; Woodside, J.V. Effect of Lycopene Supplementation on Insulin-like Growth Factor-1 and Insulin-like Growth Factor Binding Protein-3: A Double-Blind, Placebo-Controlled Trial. Eur. J. Clin. Nutr. 2007, 61, 1196–1200. [Google Scholar] [CrossRef]
- Applegate, C.; Rowles, J.; Erdman, J. Can Lycopene Impact the Androgen Axis in Prostate Cancer?: A Systematic Review of Cell Culture and Animal Studies. Nutrients 2019, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Mohler, J.L.; Gregory, C.W.; Ford, O.H.; Kim, D.; Weaver, C.M.; Petrusz, P.; Wilson, E.M.; French, F.S. The Androgen Axis in Recurrent Prostate Cancer. Clin. Cancer Res. 2004, 10, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Pezaro, C.; Woo, H.H.; Davis, I.D. Prostate Cancer: Measuring PSA. Intern. Med. J. 2014, 44, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Obermüller-Jevic Ute, C.; Olano-Martin, E.; Corbacho, A.M.; Eiserich, J.P.; Vliet, A.V.D.; Valacchi, G.; Cross, C.E.; Packer, L. Lycopene Inhibits the Growth of Normal Human Prostate Epithelial Cells in Vitro. J. Nutr. 2003, 133, 3356–3360. [Google Scholar] [CrossRef][Green Version]
- Wertz, K.; Siler, U.; Goralczyk, R. Lycopene: Modes of Action to Promote Prostate Health. Arch Biochem. Biophys. 2004, 430, 127–134. [Google Scholar] [CrossRef]
- Nahum, A.; Zeller, L.; Danilenko, M.; Prall, O.W.J.; Watts, C.K.W.; Sutherland, R.L.; Levy, J.; Sharoni, Y. Lycopene Inhibition of IGF-Induced Cancer Cell Growth Depends on the Level of Cyclin D1. Eur. J. Nutr. 2006, 45, 275–282. [Google Scholar] [CrossRef]
- Nahum, A.; Hirsch, K.; Danilenko, M.; Watts, C.K.; Prall, O.W.; Levy, J.; Sharoni, Y. Lycopene Inhibition of Cell Cycle Progression in Breast and Endometrial Cancer Cells Is Associated with Reduction in Cyclin D Levels and Retention of P27Kip1 in the Cyclin E–Cdk2 Complexes. Oncogene 2001, 20, 3428–3436. [Google Scholar] [CrossRef]
- Habermann, H.; Ray, V.; Habermann, W.; Prins, G.S. Alterations In Gap Junction Protein Expression In Human Benign Prostatic Hyperplasia And Prostate Cancer. J. Urol. 2002, 167, 655–660. [Google Scholar] [CrossRef]
- Mirahmadi, M.; Azimi-Hashemi, S.; Saburi, E.; Kamali, H.; Pishbin, M.; Hadizadeh, F. Potential Inhibitory Effect of Lycopene on Prostate Cancer. Biomed. Pharmacother. 2020, 129, 110459. [Google Scholar] [CrossRef]
- Aust, O.; Ale-Agha, N.; Zhang, L.; Wollersen, H.; Sies, H.; Stahl, W. Lycopene Oxidation Product Enhances Gap Junctional Communication. Food Chem. Toxicol. 2003, 41, 1399–1407. [Google Scholar] [CrossRef]
- Song, B.; Liu, K.; Gao, Y.; Zhao, L.; Fang, H.; Li, Y.; Pei, L.; Xu, Y. Lycopene and Risk of Cardiovascular Diseases: A Me-Ta-Analysis of Observational Studies. Mol. Nutr. Food Res. 2017, 61, 1601009. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.W.; Siervo, M.; Lara, J. Lycopene and Tomato and Risk of Cardiovascular Diseases: A Systematic Review and Meta-Analysis of Epidemiological Evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 141–158. [Google Scholar] [CrossRef]
- Valderas-Martinez, P.; Chiva-Blanch, G.; Casas, R.; Arranz, S.; Martínez-Huélamo, M.; Urpi-Sarda, M.; Torrado, X.; Co-rella, D.; Lamuela-Raventós, R.; Estruch, R. Tomato Sauce Enriched with Olive Oil Exerts Greater Effects on Cardiovascular Disease Risk Factors than Raw Tomato and Tomato Sauce: A Randomized Trial. Nutrients 2016, 8, 170. [Google Scholar] [CrossRef]
- Duran, E.K.; Aday, A.W.; Cook, N.R.; Buring, J.E.; Ridker, P.M.; Pradhan, A.D. Triglyceride-Rich Lipoprotein Cholesterol, Small Dense LDL Cholesterol, and Incident Cardiovascular Disease. J. Am. Coll. Cardiol. 2020, 75, 2122–2135. [Google Scholar] [CrossRef]
- Griffin, B.A.; Freeman, D.J.; Tait, G.W.; Thomson, J.; Caslake, M.J.; Packard, C.J.; Shepherd, J. Role of Plasma Triglyceride in the Regulation of Plasma Low Density Lipoprotein (LDL) Subfractions: Relative Contribution of Small, Dense LDL to Coronary Heart Disease Risk. Atherosclerosis 1994, 106, 241–253. [Google Scholar] [CrossRef]
- Silaste, M.-L.; Alfthan, G.; Aro, A.; Kesäniemi, Y.A.; Hörkkö, S. Tomato juice decreases LDL cholesterol levels and increases LDL resistance to oxidation. Br. J. Nutr. 2007, 98, 1251–1258. [Google Scholar] [CrossRef]
- Ferro, Y.; Mazza, E.; Angotti, E.; Pujia, R.; Mirarchi, A.; Salvati, M.A.; Terracciano, R.; Savino, R.; Romeo, S.; Scuteri, A.; et al. Effect of a Novel Functional Tomato Sauce (OsteoCol) from Vine-Ripened Tomatoes on Serum Lipids in Individuals with Common Hypercholesterolemia: Tomato Sauce and Hypercholesterolemia. J. Transl. Med. 2021, 19, 19. [Google Scholar] [CrossRef]
- Engelhard, Y.N.; Gazer, B.; Paran, E. Natural antioxidants from tomato extract reduce blood pressure in patients with grade-1 hypertension: A double-blind, placebo-controlled pilot study. Am. Heart J. 2006, 151, 100.e6. [Google Scholar] [CrossRef]
- Curtis, D.R.; Johnston, G.A. Amino acid transmitters in the mammalian central nervous system. Ergeb. Physiol. 1974, 69, 97–188. [Google Scholar] [CrossRef]
- Yoshimura, M.; Toyoshi, T.; Sano, A.; Izumi, T.; Fujii, T.; Konishi, C.; Inai, S.; Matsukura, C.; Fukuda, N.; Ezura, H.; et al. Antihypertensive Effect of a γ-Aminobutyric Acid Rich Tomato Cultivar ‘DG03-9’ in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2010, 58, 615–619. [Google Scholar] [CrossRef]
- Nonaka, S.; Arai, C.; Takayama, M.; Matsukura, C.; Ezura, H. Efficient increase of ɣ-aminobutyric acid (GABA) content in tomato fruits by targeted mutagenesis. Sci. Rep. 2017, 7, 7057. [Google Scholar] [CrossRef]
- Matsubara, F.; Ueno, H.; Tadano, K.; Suyama, T.; Imaizumi, K.; Suzuki, T.; Magata, K.; Kikuchi, N.; Muneyuki, K.; Nakamichi, N.; et al. Effects of GABA Supplementation on Blood Pressure and Safety in Adults with Mild Hypertension. Jpn. Pharmacol. Ther. 2002, 30, 963–972. [Google Scholar]
- Saito, T.; Matsukura, C.; Sugiyama, M.; Watahiki, A.; Ohshima, I.; Iijima, Y.; Konishi, C.; Fujii, T.; Inai, S.; Fukuda, N.; et al. Screening for γ-Aminobutyric Acid (GABA)-Rich Tomato Varieties. J. Jpn. Soc. Hortic. Sci. 2008, 77, 242–250. [Google Scholar] [CrossRef]
- Paran, E.; Novack, V.; Engelhard, Y.N.; Hazan-Halevy, I. The Effects of Natural Antioxidants from Tomato Extract in Treated but Uncontrolled Hypertensive Patients. Cardiovasc. Drugs Ther. 2009, 23, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Witztum, J.L. Oxidized Low-Density Lipoprotein and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2311–2316. [Google Scholar] [CrossRef] [PubMed]
- Berliner, J.A.; Navab, M.; Fogelman, A.M.; Frank, J.S.; Demer, L.L.; Edwards, P.A.; Watson, A.D.; Lusis, A.J. Atherosclerosis: Basic Mechanisms: Oxidation, Inflammation, and Genetics. Circulation 1995, 91, 2488–2496. [Google Scholar] [CrossRef] [PubMed]
- Chopra, M.; O’Neill, M.E.; Keogh, N.; Wortley, G.; Southon, S.; Thurnham, D.I. Influence of Increased Fruit and Vegetable Intake on Plasma and Lipoprotein Carotenoids and LDL Oxidation in Smokers and Nonsmokers. Clin. Chem. 2000, 46, 1818–1829. [Google Scholar] [CrossRef]
- Alarcón, M.; Toro, C.; Palomo, I. The Role of Platelets in the Pathophysiology of Atherosclerosis (Review). Mol. Med. Rep. 2008, 1, 179–184. [Google Scholar] [CrossRef][Green Version]
- Palomo, I.; Fuentes, E.; Padró, T.; Badimon, L. Platelets and Atherogenesis: Platelet Anti-Aggregation Activity and Endothelial Protection from Tomatoes (Solanum Lycopersicum, L.). Exp. Ther. Med. 2012, 3, 577–584. [Google Scholar] [CrossRef]
- Tsoupras, A.; Zabetakis, I.; Lordan, R. Platelet Aggregometry Assay for Evaluating the Effects of Platelet Agonists and An-Tiplatelet Compounds on Platelet Function in Vitro. MethodsX 2019, 6, 63–70. [Google Scholar] [CrossRef]
- O’Kennedy, N.; Crosbie, L.; Whelan, S.; Luther, V.; Horgan, G.; Broom, J.I.; Webb, D.J.; Duttaroy, A.K. Effects of Tomato Extract on Platelet Function: A Double-Blinded Crossover Study in Healthy Humans. Am. J. Clin. Nutr. 2006, 84, 561–569. [Google Scholar] [CrossRef]
- Dutta-Roy, A.K.; Crosbie, L.; Gordon, M.J. Effects of Tomato Extract on Human Platelet Aggregation in Vitro. Platelets 2001, 12, 218–227. [Google Scholar] [CrossRef]
- Lazarus, S.A.; Garg, M.L. Tomato Extract Inhibits Human Platelet Aggregation in Vitro without Increasing Basal CAMP Levels. Int. J. Food Sci. Nutr. 2004, 55, 249–256. [Google Scholar] [CrossRef]
- Wald, N.J. A Strategy to Reduce Cardiovascular Disease by More than 80%. BMJ 2003, 326, 1419. [Google Scholar] [CrossRef]
- Hu, F.B.; Rimm, E.B.; Stampfer, M.J.; Ascherio, A.; Spiegelman, D.; Willett, W.C. Prospective Study of Major Dietary Patterns and Risk of Coronary Heart Disease in Men. Am. J. Clin. Nutr. 2000, 72, 912–921. [Google Scholar] [CrossRef]
- Torres-Urrutia, C.; Guzmán, L.; Schmeda-Hirschmann, G.; Moore-Carrasco, R.; Alarcón, M.; Astudillo, L.; Gutierrez, M.; Carrasco, G.; Yuri, J.A.; Aranda, E.; et al. Antiplatelet, Anticoagulant, and Fibrinolytic Activity in Vitro of Extracts from Selected Fruits and Vegetables. Blood Coagul. Fibrinolysis 2011, 22, 197–205. [Google Scholar] [CrossRef]
- Yamamoto, J.; Taka, T.; Yamada, K.; Ijiri, Y.; Murakami, M.; Hirata, Y.; Naemura, A.; Hashimoto, M.; Yamashita, T.; Oiwa, K.; et al. Tomatoes Have Natural Anti-Thrombotic Effects. Br. J. Nutr. 2003, 90, 1031–1038. [Google Scholar] [CrossRef]
- Fuentes, E.J.; Astudillo, L.A.; Gutiérrez, M.I.; Contreras, S.O.; Bustamante, L.O.; Rubio, P.I.; Moore-Carrasco, R.; Alarcón, M.A.; Fuentes, J.A.; González, D.E.; et al. Fractions of Aqueous and Methanolic Extracts from Tomato (Solanum Lycopersicum L.) Present Platelet Antiaggregant Activity. Blood Coagul. Fibrinolysis 2012, 23, 109–117. [Google Scholar] [CrossRef]
- O’Kennedy, N.; Crosbie, L.; Lieshout, M.V.; Broom, J.I.; Webb, D.J.; Duttaroy, A.K. Effects of Antiplatelet Components of Tomato Extract on Platelet Function in Vitro and Ex Vivo: A Time-Course Cannulation Study in Healthy Humans. Am. J. Clin. Nutr. 2006, 84, 570–579. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X.-G.; Liu, L.; Zhang, Q.-L.; Ding, S.-L.; Chen, Y.; Wang, J.-Y.; Wang, L.; Liang, R.-X.; Liao, F.-L.; et al. Effects of Water-Soluble Tomato Concentrate on Platelet Aggregation. World J. Tradit. Chin. Med. 2019, 5, 260. [Google Scholar] [CrossRef]
- Rosenberg, N.; Yatuv, R.; Sobolev, V.; Peretz, H.; Zivelin, A.; Seligsohn, U. Major Mutations in Calf-1 and Calf-2 Domains of Glycoprotein IIb in Patients with Glanzmann Thrombasthenia Enable GPIIb/IIIa Complex Formation, but Impair Its Transport from the Endoplasmic Reticulum to the Golgi Apparatus. Blood 2003, 101, 4808–4815. [Google Scholar] [CrossRef][Green Version]
- Calvete, J.J. Clues for Understanding the Structure and Function of a Prototypic Human Integrin: The Platelet Glyco-Protein IIb/IIIa Complex. Thromb. Haemost. 1994, 72, 1–15. [Google Scholar] [CrossRef]
- Badimon, L.; Vilahur, G. Enfermedad aterotrombótica coronaria: Avances en el tratamiento antiplaquetario. Rev. Española Cardiol. 2008, 61, 501–513. [Google Scholar] [CrossRef][Green Version]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and Atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Hyson, D.; Rutledge, J.C.; Berglund, L. Postprandial Lipemia and Cardiovascular Disease. Curr. Atheroscler. Rep. 2003, 5, 437–444. [Google Scholar] [CrossRef]
- Arvind, A.; Osganian, S.A.; Cohen, D.E.; Corey, K.E. Lipid and Lipoprotein Metabolism in Liver Disease. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Brand, K.; Page, S.; Rogler, G.; Bartsch, A.; Brandl, R.; Knuechel, R.; Page, M.; Kaltschmidt, C.; Baeuerle, P.A.; Neumeier, D. Activated Transcription Factor Nuclear Factor-Kappa B is Present in the Atherosclerotic Lesion. J. Clin. Investig. 1996, 97, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Hajra, L.; Evans, A.I.; Chen, M.; Hyduk, S.J.; Collins, T.; Cybulsky, M.I. The NF-Kappa B Signal Transduction Pathway in Aortic Endothelial Cells Is Primed for Activation in Regions Predisposed to Atherosclerotic Lesion Formation. Proc. Natl. Acad. Sci. USA 2000, 97, 9052–9057. [Google Scholar] [CrossRef] [PubMed]
- Collins, T. Endothelial Nuclear Factor-Kappa B and the Initiation of the Atherosclerotic Lesion. Lab. Investig. 1993, 68, 499–508. [Google Scholar] [PubMed]
- Stangl, V.; Kuhn, C.; Hentschel, S.; Jochmann, N.; Jacob, C.; Böhm, V.; Fröhlich, K.; Müller, L.; Gericke, C.; Lorenz, M. Lack of Effects of Tomato Products on Endothelial Function in Human Subjects: Results of a Randomised, Placebo-Controlled Cross-over Study. Br. J. Nutr. 2011, 105, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Dalbeni, A.; Treggiari, D.; Tagetti, A.; Bevilaqua, M.; Bonafini, S.; Montagnana, M.; Scaturro, G.; Minuz, P.; Fava, C. Positive Effects of Tomato Paste on Vascular Function After a Fat Meal in Male Healthy Subjects. Nutrients 2018, 10, 1310. [Google Scholar] [CrossRef]
- Rosato, V.; Temple, N.J.; La Vecchia, C.; Castellan, G.; Tavani, A.; Guercio, V. Mediterranean Diet and Cardiovascular Disease: A Systematic Review and Meta-Analysis of Observational Studies. Eur. J. Nutr. 2019, 58, 173–191. [Google Scholar] [CrossRef]
- Devi, S.; Kumar, V.; Singh, S.K.; Dubey, A.K.; Kim, J.-J. Flavonoids: Potential Candidates for the Treatment of Neurodegenerative Disorders. Biomedicines 2021, 9, 99. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Karimi, G.; Iranshahi, M. Carotenoids in the Treatment of Diabetes Mellitus and Its Complications: A Mechanistic Review. Biomed. Pharmacother. 2017, 91, 31–42. [Google Scholar] [CrossRef]
- Karppi, J.; Laukkanen, J.A.; Sivenius, J.; Ronkainen, K.; Kurl, S. Serum Lycopene Decreases the Risk of Stroke in Men. A Population-Based Follow-up Study. Neurology 2012, 79, 1540–1547. [Google Scholar] [CrossRef]
- Li, X.; Xu, J. Dietary and Circulating Lycopene and Stroke Risk: A Meta-Analysis of Prospective Studies. Sci. Rep. 2014, 4, 5031. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Thukham-mee, W.; Muchimapura, S.; Tong-Un, T.; Wannanon, P.T.; Food, P.Y. Tomato, a Potential Yin Food, Protects against Stroke. Chin. Med. 2012, 3, 144–150. [Google Scholar] [CrossRef][Green Version]
- Hwang, S.; Lim, J.W.; Kim, H. Inhibitory Effect of Lycopene on Amyloid-Beta-Induced Apoptosis in Neuronal Cells. Nutrients 2017, 9, 883. [Google Scholar] [CrossRef]
- Prakash, A.; Kumar, A. Implicating the Role of Lycopene in Restoration of Mitochondrial Enzymes and BDNF Levels in Beta-Amyloid Induced Alzheimer’s Disease. Eur. J. Pharmacol. 2014, 741, 104–111. [Google Scholar] [CrossRef]
- Yu, L.; Wang, W.; Pang, W.; Xiao, Z.; Jiang, Y.; Hong, Y. Dietary Lycopene Supplementation Improves Cognitive Per-Formances in Tau Transgenic Mice Expressing P301L Mutation via Inhibiting Oxidative Stress and Tau Hyperphosphor-Ylation. J. Alzheimer’s Dis. 2017, 57, 475–482. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, H.; Wang, J.; Liu, P.; Tan, X.; Ren, B.; Liu, Z.; Liu, X. Lycopene Supplementation Attenuates Oxidative Stress, Neuroinflammation, and Cognitive Impairment in Aged CD-1 Mice. J. Agric. Food Chem. 2018, 66, 3127–3136. [Google Scholar] [CrossRef]
- Zhu, N.W.; Yin, X.L.; Lin, R.; Fan, X.L.; Chen, S.J.; Zhu, Y.M.; Zhao, X.Z. Possible Mechanisms of Lycopene Amelioration of Learning and Memory Impairment in Rats with Vascular Dementia. Neural Regen. Res. 2020, 15, 332–341. [Google Scholar] [CrossRef]
- Rinaldi, P.; Polidori, M.C.; Metastasio, A.; Mariani, E.; Mattioli, P.; Cherubini, A.; Catani, M.; Cecchetti, R.; Senin, U.; Mecocci, P. Plasma Antioxidants Are Similarly Depleted in Mild Cognitive Impairment and in Alzheimer’s Disease. Neuro-Biol. Aging 2003, 24, 915–919. [Google Scholar] [CrossRef]
- Mullan, K.; Williams, M.A.; Cardwell, C.R.; McGuinness, B.; Passmore, P.; Silvestri, G.; Woodside, J.V.; McKay, G.J. Serum Concentrations of Vitamin E and Carotenoids Are Altered in Alzheimer’s Disease: A Case-Control Study. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 432–439. [Google Scholar] [CrossRef]
- Mullan, K.; Cardwell, C.R.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Plasma Antioxidant Status in Patients with Alzheimer’s Disease and Cognitively Intact Elderly: A Meta-Analysis of Case-Control Studies. J. Alzheimer’s Dis. 2018, 62, 305–317. [Google Scholar] [CrossRef]
- Praticò, D.; Lee, V.M.-Y.; Trojanowski, J.Q.; Rokach, J.; Fitzgerald, G.A. Increased F2-Isoprostanes in Alzheimer’s Disease: Evidence for Enhanced Lipid Peroxidation in Vivo. FASEB J. 1998, 12, 1777–1783. [Google Scholar] [CrossRef]
- Montine, T.J.; Peskind, E.R.; Quinn, J.F.; Wilson, A.M.; Montine, K.S.; Galasko, D. Increased Cerebrospinal Fluid F2-Isoprostanes Are Associated with Aging and Latent Alzheimer’s Disease as Identified by Biomarkers. Neuromol. Med. 2011, 13, 37–43. [Google Scholar] [CrossRef]
- Praticò, D.; Clark, C.M.; Liun, F.; Lee, V.Y.; Trojanowski, J.Q. Increase of Brain Oxidative Stress in Mild Cognitive Im-Pairment: A Possible Predictor of Alzheimer Disease. Arch. Neurol. 2002, 59, 972–976. [Google Scholar] [CrossRef]
- Devore, E.E.; Kang, J.H.; Stampfer, M.J.; Grodstein, F. The Association of Antioxidants and Cognition in the Nurses’ Health Study. Am. J. Epidemiol. 2013, 177, 33–41. [Google Scholar] [CrossRef]
- Koch, M.; Furtado, J.D.; Cronjé, H.T.; DeKosky, S.T.; Fitzpatrick, A.L.; Lopez, O.L.; Kuller, L.H.; Mukamal, K.J.; Jensen, M.K. Plasma Antioxidants and Risk of Dementia in Older Adults. Transit. Res. Clin. Interv. 2021, 7, e12208. [Google Scholar] [CrossRef]
- Suganuma, H.; Hirano, T.; Arimoto, Y.; Inakuma, T. Effect of tomato intake on striatal monoamine level in a mouse model of experimental Parkinson’s disease. J. Nutr. Sci. Vitaminol. 2002, 48, 251–254. [Google Scholar] [CrossRef]
- Asokan, P.; Janakiraman, U.; Manivasagam, T.; Thenmozhi, A.J. Neuroprotective Effect of Lycopene against MPTP Induced Experimental Parkinson’s Disease in Mice. Neurosci. Lett. 2015, 599, 12–15. [Google Scholar] [CrossRef]
- De Nuccio, F.; Cianciulli, A.; Porro, C.; Kashyrina, M.; Ruggiero, M.; Calvello, R.; Miraglia, A.; Nicolardi, G.; Lofrumento, D.D.; Panaro, M.A. Inflammatory Response Modulation by Vitamin C in an MPTP Mouse Model of Parkinson’s Disease. Biology 2021, 10, 1155. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Will, J.C.; Bowman, B.A.; Narayan, K.M. Diabetes Mellitus and Serum Carotenoids: Findings from the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 1999, 149, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Polidori, M.C.; Mecocci, P.; Stahl, W.; Parente, B.; Cecchetti, R.; Cherubini, A.; Cao, P.; Sies, H.; Senin, U. Plasma Levels of Lipophilic Antioxidants in Very Old Patients with Type 2 Diabetes. Diabetes Metab. Res. Rev. 2000, 16, 15–19. [Google Scholar] [CrossRef]
- Bates, C.J.; Lean, M.E.; Mansoor, M.A. Nutrient Intakes; Biochemical and Risk Indices Associated with Type 2 Diabetes and Glycosylated Haemoglobin, in the British National Diet and Nutrition Survey of People Aged 65 Years and Over. Diabet. Med. 2004, 21, 677–684. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Pradhan, A.D.; Manson, J.E.; Buring, J.E.; Gaziano, J.M.; Sesso, H.D. Plasma Lycopene, Other Carotenoids, and the Risk of Type 2 Diabetes in Women. Am. J. Epidemiol. 2006, 164, 576–585. [Google Scholar] [CrossRef]
- Butkowski, E.G.; Jelinek, H.F. Hyperglycaemia, Oxidative Stress and Inflammation Markers. Redox Rep. 2017, 22, 257–264. [Google Scholar] [CrossRef]
- Karahan, F.; Dede, S.; Ceylan, E. The Effect of Lycopene Treatment on Oxidative DNA Damage of Experimental Diabetic Rats. Open Clin. Biochem. J. 2018, 8, 1–6. [Google Scholar] [CrossRef]
- Yin, Y.; Zheng, Z.; Jiang, Z. Effects of Lycopene on Metabolism of Glycolipid in Type 2 Diabetic Rats. Biomed. Pharmacother. 2019, 109, 2070–2077. [Google Scholar] [CrossRef]
- Leh, H.E.; Mohd Sopian, M.; Abu Bakar, M.H.; Lee, L.K. The Role of Lycopene for the Amelioration of Glycaemic Status and Peripheral Antioxidant Capacity among the Type II Diabetes Mellitus Patients: A Case-Control Study. Ann. Med. 2021, 53, 1059–1065. [Google Scholar] [CrossRef]
- Figueiredo, I.D.; Lima, T.F.O.; Inácio, M.D.; Costa, M.C.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Lycopene Improves the Metformin Effects on Glycemic Control and Decreases Biomarkers of Glycoxidative Stress in Diabetic Rats. Diabetes Metab. Syndr. Obes. 2020, 13, 3117–3135. [Google Scholar] [CrossRef]
- Shidfar, F.; Froghifar, N.; Vafa, M.; Rajab, A.; Hosseini, S.; Shidfar, S.; Gohari, M. The Effects of Tomato Consumption on Serum Glucose, Apolipoprotein B, Apolipoprotein A-I, Homocysteine and Blood Pressure in Type 2 Diabetic Patients. Int. J. Food Sci. Nutr. 2011, 62, 289–294. [Google Scholar] [CrossRef]
- Bose, K.S.; Agrawal, B.K. Effect of long-term supplementation of tomatoes (cooked) on levels of antioxidant enzymes, lipid peroxidation rate, lipid profile and glycated haemoglobin in Type 2 diabetes mellitus. West Indian Med. J. 2006, 55, 274–278. [Google Scholar] [CrossRef]
- Zidani, S.; Benakmoum, A.; Ammouche, A.; Benali, Y.; Bouhadef, A.; Abbeddou, S. Effect of Dry Tomato Peel Supple-Mentation on Glucose Tolerance, Insulin Resistance, and Hepatic Markers in Mice Fed High-Saturated-Fat/High-Cholesterol Diets. J. Nutr. Biochem. 2017, 40, 164–171. [Google Scholar] [CrossRef]
- Gao, Q.; Zhong, C.; Zhou, X.; Chen, R.; Xiong, T.; Hong, M.; Li, Q.; Kong, M.; Han, W.; Sun, G.; et al. The Association between Intake of Dietary Lycopene and Other Carotenoids and Gestational Diabetes Mellitus Risk during Mid-Trimester: A Cross-Sectional Study. Br. J. Nutr. 2019, 121, 1405–1412. [Google Scholar] [CrossRef]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C.D. Lifestyle, and the Risk of Type 2 Diabetes Mellitus in Women. N. Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef]
- Mayor, S. Healthy diet markedly Reduces Type 2 Diabetes risk in ethnic minority women, Study Finds. BMJ 2015, 350, 263. [Google Scholar] [CrossRef] [PubMed]
- Groten, K.; Marini, A.; Grether-Beck, S.; Jaenicke, T.; Ibbotson, S.H.; Moseley, H.; Ferguson, J.; Krutmann, J. Tomato Phytonutrients Balance UV Response: Results from a Double-Blind, Randomized, Placebo-Controlled Study. Skin Pharmacol. Physiol. 2019, 32, 101–108. [Google Scholar] [CrossRef]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Stahl, W.; Krutmann, J. Molecular Evidence That Oral Supplementation with Lycopene or Lutein Protects Human Skin against Ultraviolet Radiation: Results from a Double-Blinded, Place-Bo-Controlled, Crossover Study. Br. J. Dermatol. 2017, 176, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Saewan, N.; Jimtaisong, A. Natural Products as Photoprotection. J. Cosmet. Dermatol. 2015, 14, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W. Mutations of the BRAF Gene in Human Cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic Effects of Ultraviolet Radiation on the Skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef]
- Modenese, A.; Korpinen, L.; Gobba, F. Solar Radiation Exposure and Outdoor Work: An Underestimated Occupational Risk. Int. J. Environ. Res. Public Health 2018, 15, 2063. [Google Scholar] [CrossRef]
- Aust, O.; Stahl, W.; Sies, H.; Tronnier, H.; Heinrich, U. Supplementation with Tomato-Based Products Increases Lycopene, Phytofluene, and Phytoene Levels in Human Serum and Protects Against UV-Light-Induced Erythema. Int. J. Vitam. Nutr. Res. 2005, 75, 54–60. [Google Scholar] [CrossRef]
- Baswan, S.M.; Marini, A.; Klosner, A.E.; Jaenicke, T.; Leverett, J.; Murray, M.; Gellenbeck, K.W.; Krutmann, J. Orally Administered Mixed Carotenoids Protect Human Skin against Ultraviolet A-induced Skin Pigmentation: A Double-blind, Placebo-controlled, Randomized Clinical Trial. Photodermatol. Photoimmunol. Photomed. 2020, 36, 219–225. [Google Scholar] [CrossRef]
- Calniquer, G.; Khanin, M.; Ovadia, H.; Linnewiel-Hermoni, K.; Stepensky, D.; Trachtenberg, A.; Sedlov, T.; Braverman, O.; Levy, J.; Sharoni, Y. Combined Effects of Carotenoids and Polyphenols in Balancing the Response of Skin Cells to UV Irradiation. Molecules 2021, 26, 1931. [Google Scholar] [CrossRef]
- Katta, R.; Kramer, M.J. Skin and Diet: An Update on the Role of Dietary Change as a Treatment Strategy for Skin Disease. Skin Ther. Lett. 2018, 23, 1–5. [Google Scholar]
- Tito, A.; Carola, A.; Bimonte, M.; Barbulova, A.; Arciello, S.; Laurentiis, F.; Monoli, I.; Hill, J.; Gibertoni, S.; Colucci, G.; et al. A Tomato Stem Cell Extract, Containing Antioxidant Compounds and Metal Chelating Factors, Protects Skin Cells from Heavy Metal-Induced Damages. Int. J. Cosmet. Sci. 2011, 33, 543–552. [Google Scholar] [CrossRef]
- Maarouf, M.; Hendricks, A.J.; Shi, V.Y. Bathing Additives for Atopic Dermatitis—A Systematic Review. Dermatitis 2019, 30, 191–197. [Google Scholar] [CrossRef]
- Ligęza, M.; Wyglądacz, D.; Tobiasz, A.; Jaworecka, K.; Reich, A. Natural Cold Pressed Oils as Cosmetic Products. Fam. Med. Prim. Care Rev. 2016, 4, 443–447. [Google Scholar] [CrossRef]
- Rizwan, M.; Rodriguez-Blanco, I.; Harbottle, A.; Birch-Machin, M.A.; Watson, R.E.B.; Rhodes, L.E. Tomato Paste Rich in Lycopene Protects against Cutaneous Photodamage in Humans in Vivo: A Randomized Controlled Trial. Br. J. Dermatol. 2011, 164, 154–162. [Google Scholar] [CrossRef]
- Motwani, M.S.; Khan, K.; Pai, A.; Joshi, R. Efficacy of a Collagen Hydrolysate and Antioxidants-containing Nutraceutical on Metrics of Skin Health in Indian Women. J. Cosmet. Dermatol. 2020, 19, 3371–3382. [Google Scholar] [CrossRef]
- Future Market Insights. Tomato Seed Oil Sales in Asia Pacific to Increase at 7.3% by 2031, Increasing Demand in China, South Korea to Continue Supporting Sales through 2031. Available online: https://www.prnewswire.co.uk/news-releases/tomato-seed-oil-sales-in-asia-pacific-to-increase-at-7-3-by-2031-increasing-demand-in-china-south-korea-to-continue-supporting-sales-through-2031-future-market-insights-837364039.html (accessed on 8 December 2021).
- Vaughn, A.R.; Foolad, N.; Maarouf, M.; Tran, K.A.; Shi, V.Y. Micronutrients in Atopic Dermatitis: A Systematic Review. J. Altern. Complement. Med. 2019, 25, 567–577. [Google Scholar] [CrossRef]
- Dos Santos, Z.M.Q.; Dos Santos, M.Q.; Zancanaro, V.; Bellaver, E.H.; Nardi, G.M.; Gelinski, J.M.L.; Locatelli, C. Topical Application of Phenolic Compounds Suppresses Propionibacterium Acnes-Induced Inflammatory Responses in Mice with Ear Edema. Naunyn-Schmiedebergs Arch. Pharmacol. 2019, 392, 529–540. [Google Scholar] [CrossRef]
- Kurokawa, I.; Nakase, K. Recent Advances in Understanding and Managing Acne. F1000Research 2020, 9, 792. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Chaidez, C.; Ornelas-Paz, J.; López-Mata, M.A.; Márquez-Ríos, E.; Estrada, M.I. Chemical Constitution and Effect of Extracts of Tomato Plants Byproducts on the Enteric Viral Surrogates. Int. J. Environ. Health Res. 2015, 25, 299–311. [Google Scholar] [CrossRef]
- Simonetti, O.; Bacchetti, T.; Ferretti, G.; Molinelli, E.; Rizzetto, G.; Bellachioma, L.; Offidani, A. Oxidative Stress and Alterations of Paraoxonases in Atopic Dermatitis. Antioxidants 2021, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Sapuntsova, S.G.; Lebed’ko, O.A.; Shchetkina, M.V.; Fleyshman, M.Y.; Kozulin, E.A.; Timoshin, S.S. Status of Free-Radical Oxidation and Proliferation Processes in Patients with Atopic Dermatitis and Lichen Planus. Bull. Exp. Biol. Med. 2011, 150, 690–692. [Google Scholar] [CrossRef]
- Paul, B.; Barnes, S.; Demark-Wahnefried, W.; Morrow, C.; Salvador, C.; Skibola, C.; Tollefsbol, T.O. Influences of Diet and the Gut Microbiome on Epigenetic Modulation in Cancer and Other Diseases. Clin. Epigenet. 2015, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Moco, S.; Martin, F.P.; Rezzi, S. Metabolomics View on Gut Microbiome Modulation by Polyphenol-Rich Foods. J. Proteome Res. 2012, 11, 4781–4790. [Google Scholar] [CrossRef] [PubMed]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The Gut Microbiome in Health and in Disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut Microbiome–Mediated Bile Acid Metabolism Regulates Liver Cancer via NKT Cells. Science 2018, 360, eaan5931. [Google Scholar] [CrossRef]
- Xia, H.; Liu, C.; Li, C.-C.; Fu, M.; Takahashi, S.; Hu, K.-Q.; Aizawa, K.; Hiroyuki, S.; Wu, G.; Zhao, L.; et al. Dietary Tomato Powder Inhibits High-Fat Diet–Promoted Hepatocellular Carcinoma with Alteration of Gut Microbiota in Mice Lacking Carotenoid Cleavage Enzymes. Cancer Prev. Res. 2018, 11, 797–810. [Google Scholar] [CrossRef]
- Li, C.C.; Liu, C.; Fu, M.; Hu, K.Q.; Aizawa, K.; Takahashi, S.; Hiroyuki, S.; Cheng, J.; von Lintig, J.; Wang, X.D. Tomato Powder Inhibits Hepatic Steatosis and Inflammation Potentially Through Restoring SIRT1 Activity and Adiponectin Function Independent of Carotenoid Cleavage Enzymes in Mice. Mol. Nutr. Food Res. 2018, 62, 1700738. [Google Scholar] [CrossRef]
- Wigg, A.J.; Roberts-Thomson, I.C.; Dymock, R.B.; McCarthy, P.J.; Grose, R.H.; Cummins, A.G. The Role of Small Intestinal Bacterial Overgrowth, Intestinal Permeability, Endotoxaemia, and Tumour Necrosis Factor Alpha in the Pathogenesis of Non-Alcoholic Steatohepatitis. Gut 2001, 48, 206–211. [Google Scholar] [CrossRef]
- De Souza, H.S.P.; Fiocchi, C. Immunopathogenesis of IBD: Current State of the Art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef]
- Khavari, A.P.; Haghdoost, S. Effects of Tomato Juice Intake on Salivary 8-Oxo-DG Levels as Oxidative Stress Biomarker after Extensive Physical Exercise. Oxid. Med. Cell. Longev. 2020, 2020, 8948723. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-Radical Scavenging Capacity and Reducing Power of Wild Edible Mushrooms from Northeast Portugal: Individual Cap and Stipe Activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- Lee, J.; Koo, N.; Min, D.B. Reactive Oxygen Species, Aging, and Antioxidative Nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. Comptes Rendus Biol. 2008, 331, 865–873. [Google Scholar] [CrossRef]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and Human Health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Wang, X.-D. Lycopene Metabolism and Its Biological Significance. Am. J. Clin. Nutr. 2012, 96, 1214S–1222S. [Google Scholar] [CrossRef]
- Nieman, D.C.; Capps, C.L.; Capps, C.R.; Shue, Z.L.; Mcbride, J.E. Effect of 4-Week Ingestion of Tomato-Based Carotenoids on Exercise-Induced Inflammation, Muscle Damage, and Oxidative Stress in Endurance Runners. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 266–273. [Google Scholar] [CrossRef]
- Tsitsimpikou, C.; Kioukia-Fougia, N.; Tsarouhas, K.; Stamatopoulos, P.; Rentoukas, E.; Koudounakos, A.; Papalexis, P.; Liesivuori, J.; Jamurtas, A. Administration of Tomato Juice Ameliorates Lactate Dehydrogenase and Creatinine Kinase Responses to Anaerobic Training. Food Chem. Toxicol. 2013, 61, 9–13. [Google Scholar] [CrossRef]
- Harms-Ringdahl, M.; Jenssen, D.; Haghdoost, S. Tomato Juice Intake Suppressed Serum Concentration of 8-OxodG after Extensive Physical Activity. Nutr. J. 2012, 11, 29. [Google Scholar] [CrossRef]
- Watzl, B.; Bub, A.; Briviba, K.; Rechkemmer, G. Supplementation of a Low-Carotenoid Diet with Tomato or Carrot Juice Modulates Immune Functions in Healthy Men. Ann. Nutr. Metab. 2003, 47, 255–261. [Google Scholar] [CrossRef]
- Riso, P.; Visiolo, F.; Erba, D.; Testolin, G.; Porrini, M. Lycopene and Vitamin C Concentrations Increase in Plasma and Lymphocytes after Tomato Intake. Effects on Cellular Antioxidant Protection. Eur. J. Clin. Nutr. 2004, 58, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Van Gorkom, G.N.; Klein Wolterink, R.G.; Van Elssen, C.H.; Wieten, L.; Germeraad, W.T.; Bos, G.M. Influence of Vitamin C on Lymphocytes: An Overview. Antioxidants 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Von Boehmer, H.V. Positive Selection of Lymphocytes. Cell 1994, 76, 219–228. [Google Scholar] [CrossRef]
- Trinchieri, G. Biology of Natural Killer Cells. Adv. Immunol. 1989, 47, 187–376. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, S.; Okishima, N.; Sumida, S.; Okamura, K.; Doi, T.; Kishino, Y. β-Carotene Supplementation Enhances Lym-Phocyte Proliferation with Mitogens in Human Peripheral Blood Lymphocytes. Nutr. Res. 1996, 16, 211–218. [Google Scholar] [CrossRef]
- Van Poppel, G.V.; Spanhaak, S.; Ockhuizen, T. Effect of β-carotene on immunological indexes in healthy male smokers. Am. J. Clin. Nutr. 1993, 57, 402–407. [Google Scholar] [CrossRef]
- Niu, X.; Sang, H.; Wang, J. Naringenin Attenuates Experimental Autoimmune Encephalomyelitis by Protecting the Intact of Blood-Brain Barrier and Controlling Inflammatory Cell Migration. J. Nutr. Biochem. 2021, 89, 108560. [Google Scholar] [CrossRef]
- Niu, X.; Wu, C.; Li, M.; Zhao, Q.; Meydani, S.N.; Wang, J.; Wu, D. Naringenin Is an Inhibitor of T Cell Effector Functions. J. Nutr. Biochem. 2018, 58, 71–79. [Google Scholar] [CrossRef]
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simões, S. Lycopene in Human Health. LWT 2020, 127, 109323. [Google Scholar] [CrossRef]
- Kun, Y.; Lule, U.S.; Xiao-Lin, D. Lycopene: Its Properties and Relationship to Human Health. Food Rev. Int. 2006, 22, 309–333. [Google Scholar] [CrossRef]
- Campos, K.K.D.; Araújo, G.R.; Martins, T.L.; Bandeira, A.C.B.; Costa, G.D.P.; Talvani, A.; Garcia, C.C.M.; Oliveira, L.A.M.; Costa, D.C.; Bezerra, F.S. The Antioxidant and Anti-Inflammatory Properties of Lycopene in Mice Lungs Exposed to Cigarette Smoke. J. Nutr. Biochem. 2017, 48, 9–20. [Google Scholar] [CrossRef]
- Palozza, P.; Catalano, A.; Simone, R.; Cittadini, A. Lycopene as a Guardian of Redox Signalling. Acta Biochim. Pol. 2012, 59, 21–25. [Google Scholar] [CrossRef]
- Lederer, J.A.; Rodrick, M.L.; Mannick, J.A. The Effects Of Injury On The Adaptive Immune Response. Shock 1999, 11, 153–159. [Google Scholar] [CrossRef]
- Massa, S.; Paolini, F.; Marino, C.; Franconi, R.; Venuti, A. Bioproduction of a Therapeutic Vaccine Against Human Papillomavirus in Tomato Hairy Root Cultures. Front. Plant Sci. 2019, 10, 452. [Google Scholar] [CrossRef]
- Sohrab, S.S. An Edible Vaccine Development for Coronavirus Disease 2019: The Concept. Clin. Exp. Vaccine Res. 2020, 9, 164–168. [Google Scholar] [CrossRef]
- Shchelkunov, S.N.; Salyaev, R.K.; Pozdnyakov, S.G.; Rekoslavskaya, N.I.; Nesterov, A.E.; Ryzhova, T.S.; Sumtsova, V.M.; Pakova, N.V.; Mishutina, U.O.; Kopytina, T.V.; et al. Immunogenicity of a Novel, Bivalent, Plant-Based Oral Vaccine against Hepatitis B and Human Immunodeficiency Viruses. Biotechnol. Lett. 2006, 28, 959–967. [Google Scholar] [CrossRef]
- Davod, J.; Fatemeh, D.N.; Honari, H.; Hosseini, R. Constructing and transient expression of a gene cassette containing edible vaccine elements and shigellosis, anthrax and cholera recombinant antigens in tomato. Mol. Biol. Rep. 2018, 45, 2237–2246. [Google Scholar] [CrossRef]
- Pantazica, A.M.M.; Cucos, L.M.; Stavaru, C.; Clarke, J.L.; Branza-Nichita, N. Challenges and Prospects of Plant-Derived Oral Vaccines against Hepatitis B and C Viruses. Plants 2021, 10, 2037. [Google Scholar] [CrossRef]
- Salyaev, R.K.; Rekoslavskaya, N.I.; Shchelkunov, S.N.; Stolbikov, A.S.; Hammond, R.V. Study of the Mucosal Immune Response Duration in Mice after Administration of a Candidate Edible Vaccine Based on Transgenic Tomato Plants Carrying the TBI-HBS Gene. Dokl. Biochem. Biophys. 2009, 428, 232–234. [Google Scholar] [CrossRef]
- Tvrdá, E.; Benko, F.; Slanina, T.; Plessis, S.S. The Role of Selected Natural Biomolecules in Sperm Production and Functionality. Molecules 2021, 26, 5196. [Google Scholar] [CrossRef]
- Ye, R.-J.; Yang, J.-M.; Hai, D.-M.; Liu, N.; Ma, L.; Lan, X.-B.; Niu, J.-G.; Zheng, P.; Yu, J.-Q. Interplay between Male Reproductive System Dysfunction and the Therapeutic Effect of Flavonoids. Fitoterapia 2020, 147, 104756. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A.; Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant Supplements and Semen Parameters: An Evidence Based Review. Int. J. Reprod. Biomed. 2016, 14, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.; Go, A.; Kim, D.B.; Park, M.; Jeon, H.W.; Kim, B. Role of Antioxidant Natural Products in Management of Infertility: A Review of Their Medicinal Potential. Antioxidants 2020, 9, 957. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Leisegang, K.; Majzoub, A.; Henkel, R.; Finelli, R.; Panner Selvam, M.K.; Tadros, N.; Parekh, N.; Ko, E.Y.; Cho, C.-L.; et al. Utility of Antioxidants in the Treatment of Male Infertility: Clinical Guidelines Based on a Systematic Review and Analysis of Evidence. World J. Men’s Health 2021, 39, 233. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Braga, P.C.; Martins, A.D.; Silva, B.M.; Alves, M.G.; Oliveira, P.F. Antioxidants Present in Reproductive Tract Fluids and Their Relevance for Fertility. Antioxidants 2021, 10, 1441. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Panchal, H.; Saraf, P. Ameliorating Effects of Natural Antioxidant Compounds on Female Infertility: A Review. Reprod. Sci. 2021, 28, 1227–1256. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, Y.; Feng, Y.; Zhang, H.; Li, L.; Shen, W.; Zhao, M.; Min, L. β-Carotene Improves Oocyte Development and Maturation under Oxidative Stress in Vitro. In Vitro Cell. Dev. Biol. Anim. 2019, 55, 548–558. [Google Scholar] [CrossRef]
- Akbaribazm, M.; Goodarzi, N.; Rahimi, M. Female Infertility and Herbal Medicine: An Overview of the New Findings. Food Sci. Nutr. 2021, 9, 5869–5882. [Google Scholar] [CrossRef]
- De Luca, M.N.; Colone, M.; Gambioli, R.; Stringaro, A.; Unfer, V. Oxidative Stress and Male Fertility: Role of Antioxidants and Inositols. Antioxidants 2021, 10, 1283. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef]
- Goyal, A.; Chopra, M.; Lwaleed, B.; Birch, B.; Cooper, A. The Effects of Dietary Lycopene Supplementation on Human Seminal Plasma. BJU Int. 2007, 99, 1456–1460. [Google Scholar] [CrossRef]
- Greish, S.M.; Abdel Kader, G.S.; Abdelaziz, E.Z.; Eltamany, D.A.; Sallam, H.S.; Abogresha, N.M. Lycopene Is Superior to Moringa in Improving Fertility Markers in Diet-Induced Obesity Male Rats. Saudi J. Biol. Sci. 2021, 28, 2956–2963. [Google Scholar] [CrossRef]
- Williams, E.A.; Parker, M.; Robinson, A.; Pitt, S.; Pacey, A.A. A Randomized Placebo-Controlled Trial to Investigate the Effect of Lactolycopene on Semen Quality in Healthy Males. Eur. J. Nutr. 2020, 59, 825–833. [Google Scholar] [CrossRef]
- Zhai, L.; Tang, Z. Lycopene Improves Sperm Quality: A Promising Nutrient for the Treatment of Male Infertility. Phytother. Res. 2020, 34, 1187–1188. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Aizawa, K.; Mieno, M.; Karamatsu, M.; Hirano, Y.; Furui, K.; Miyashita, T.; Yamazaki, K.; Inakuma, T.; Sato, I. The Effects of Tomato Juice on Male Infertility. Asia Pac. J. Clin. Nutr. 2017, 26, 65–71. [Google Scholar] [CrossRef]
- Stewart, A.J.; Bozonnet, S.; Mullen, W.; Jenkins, G.I.; Lean, M.E.; Crozier, A. Occurrence of Flavonols in Tomatoes and Tomato-Based Products. J. Agric. Food Chem. 2000, 48, 2663–2669. [Google Scholar] [CrossRef]
- Aitken, R.J.; Muscio, L.; Whiting, S.; Connaughton, H.S.; Fraser, B.A.; Nixon, B.; Smith, N.D.; De Iuliis, G.N. Analysis of the Effects of Polyphenols on Human Spermatozoa Reveals Unexpected Impacts on Mitochondrial Membrane Potential, Oxidative Stress and DNA Integrity; Implications for Assisted Reproductive Technology. Biochem. Pharmacol. 2016, 121, 78–96. [Google Scholar] [CrossRef]
- Tvrdá, E.; Debacker, M.; Ďuračka, M.; Kováč, J.; Bučko, O. Quercetin and Naringenin Provide Functional and Antioxidant Protection to Stored Boar Semen. Animals 2020, 10, 1930. [Google Scholar] [CrossRef]
- Sahin, Z.; Ozkaya, A.; Cuce, G.; Uckun, M.; Yologlu, E. Investigation of the Effect of Naringenin on Oxidative Stress-Related Alterations in Testis of Hydrogen Peroxide-Administered Rats. J. Biochem. Mol. Toxicol. 2017, 31, e21928. [Google Scholar] [CrossRef]
- Moretti, E.; Mazzi, L.; Terzuoli, G.; Bonechi, C.; Iacoponi, F.; Martini, S.; Rossi, C.; Collodel, G. Effect of Quercetin, Rutin, Naringenin and Epicatechin on Lipid Peroxidation Induced in Human Sperm. Reprod. Toxicol. 2012, 34, 651–657. [Google Scholar] [CrossRef]
- Greco, E.; Iacobelli, M.; Rienzi, L.; Ubaldi, F.; Ferrero, S.; Tesarik, J. Reduction of the Incidence of Sperm DNA Fragmentation by Oral Antioxidant Treatment. J. Androl. 2005, 26, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Nalluswami, K.; Snider, C.; Perch, M.; Balasegaram, M.; Burmeister, D.; Lockett, J.; Sandt, C.; Hoekstra, R.M.; Montgomery, S. Outbreak of Salmonella Braenderup Infections Associated with Roma Tomatoes, Northeastern United States, 2004: A Useful Method for Subtyping Exposures in Field Investigations. Epidemiol. Infect. 2007, 135, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Behravesh, C.B.; Blaney, D.; Medus, C.; Bidol, S.A.; Phan, Q.; Soliva, S.; Daly, E.R.; Smith, K.; Miller, B.; Taylor, T.; et al. Multistate Outbreak of Salmonella Serotype Typhimurium Infections Associated with Consumption of Restaurant Tomatoes, USA, 2006: Hypothesis Generation through Case Exposures in Multiple Restaurant Clusters. Epidemiol. Infect. 2012, 140, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Gallot, C.; Grout, L.; Roque-Afonso, A.-M.; Couturier, E.; Carrillo-Santisteve, P.; Pouey, J.; Letort, M.-J.; Hoppe, S.; Capdepon, P.; Saint-Martin, S. Hepatitis A Associated with Semidried Tomatoes, France 2010. Emerg. Infect. Dis. 2011, 17, 566–567. [Google Scholar] [CrossRef]
- Carvalho, C.; Thomas, H.; Balogun, K.; Tedder, R.; Pebody, R.; Ramsay, M.; Ngui, S. A Possible Outbreak of Hepatitis A Associated with Semi-Dried Tomatoes, England, July–November 2011. Eurosurveillance 2012, 17, 20083. [Google Scholar] [CrossRef]
- Mollers, M.; Boxman, I.L.A.; Vennema, H.; Slegers-Fitz-James, I.A.; Brandwagt, D.; Friesema, I.H.; Batstra, J.S.; Wierik, M.J.M. Successful Use of Advertisement Pictures to Assist Recall in a Food-Borne Hepatitis A Outbreak in The Netherlands, 2017. Food Environ. Virol. 2018, 10, 272–277. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial Effects and Potential Risks of Tomato Consumption for Human Health: An Overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef]
- Hlihor, R.-M.; Pogăcean, M.O.; Rosca, M.; Cozma, P.; Gavrilescu, M. Modelling the Behavior of Pesticide Residues in Tomatoes and Their Associated Long-Term Exposure Risks. J. Environ. Manag. 2019, 233, 523–529. [Google Scholar] [CrossRef]
- Wesselink, A.K.; Hatch, E.E.; Rothman, K.J.; Willis, S.K.; Orta, O.R.; Wise, L.A. Pesticide Residue Intake from Fruits and Vegetables and Fecundability in a North American Preconception Cohort Study. Environ. Int. 2020, 139, 105693. [Google Scholar] [CrossRef]
- Grochowska-Niedworok, E.; Nieć, J.; Baranowska, R. Assessment of Cadmium and Lead Content in Tomatoes and Tomato Products. Rocz. Państw. Zakł. Hig. 2020, 71, 313–319. [Google Scholar] [CrossRef]
- Romero-Estévez, D.; Yánez-Jácome, G.S.; Simbaña-Farinango, K.; Vélez-Terreros, P.Y.; Navarrete, H. Determination of Cadmium and Lead in Tomato (Solanum Lycopersicum) and Lettuce (Lactuca Sativa) Consumed in Quito, Ecuador. Toxicol. Rep. 2020, 7, 893–899. [Google Scholar] [CrossRef]
- Piscitelli, C.; Lavorgna, M.; Prisco, R.; Coppola, E.; Grilli, E.; Russo, C.; Isidori, M. Tomato Plants (Solanum Lycopersicum L.) Grown in Experimental Contaminated Soil: Bioconcentration of Potentially Toxic Elements and Free Radical Scavenging Evaluation. PLoS ONE 2020, 15, e0237031. [Google Scholar] [CrossRef]
- Donnan, E.J.; Fielding, J.E.; Gregory, J.E.; Lalor, K.; Rowe, S.; Goldsmith, P.; Antoniou, M.; Fullerton, K.E.; Knope, K.; Copland, J.G. A Multistate Outbreak of Hepatitis A Associated With Semidried Tomatoes in Australia, 2009. Clin. Infect. Dis. 2012, 54, 775–781. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Tierney, A.C.; Rumble, C.E.; Billings, L.M.; George, E.S. Effect of Dietary and Supplemental Lycopene on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. Adv. Nutr. 2020, 11, 1453–1488. [Google Scholar] [CrossRef]
- Fuentes, E.; Trostchansky, A.; Reguengo, L.M.; Junior, M.; Palomo, I. Antiplatelet Effects of Bioactive Compounds Present in Tomato Pomace. Curr. Drug Targets 2021, 22, 1716–1724. [Google Scholar] [CrossRef]
- Mozos, I.; Stoian, D.; Caraba, A.; Malainer, C.; Horbańczuk, J.O.; Atanasov, A.G. Lycopene and Vascular Health. Front. Pharmacol. 2018, 9, 251. [Google Scholar] [CrossRef]
- Sawardekar, S.; Patel, T.; Uchil, D. Comparative Evaluation of Antiplatelet Effect of Lycopene with Aspirin and the Effect of Their Combination on Platelet Aggregation: An in Vitro Study. Indian J. Pharmacol. 2016, 48, 26. [Google Scholar] [CrossRef]
- Harvie, M. Nutritional Supplements and Cancer: Potential Benefits and Proven Harms. Am. Soc. Clin. Oncol. Educ. B 2014, 34, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.; Coates, P.; Smith, M. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef]
- Fortmann, S.P.; Burda, B.U.; Senger, C.A.; Lin, J.S.; Whitlock, E.P. Vitamin and Mineral Supplements in the Primary Prevention of Cardiovascular Disease and Cancer: An Updated Systematic Evidence Review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 17, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Eun, J.-B. Flavonoids in Fruits and Vegetables after Thermal and Nonthermal Processing: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 3159–3188. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Kikuzaki, H.; Ueno, H. Effects of Cooking Methods on Free Amino Acid Contents in Vegetables. J. Nutr. Sci. Vitaminol. 2019, 65, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef]
- Jacob, K.; Garcia-Alonso, F.J.; Ros, G. Periago MJ Stability of Carotenoids, Phenolic Compounds, Ascorbic Acid and Antioxidant Capacity of Tomatoes during Thermal Processing. Arch. Latinoam. Nutr. 2010, 60, 192–198. [Google Scholar]
- Yang, C.; Jiang, X.; Ma, L.; Xiong, W.; Zhang, S.; Zhang, J.; Zhang, L. Carotenoid Composition and Antioxidant Activities of Chinese Orange-colored Tomato Cultivars and the Effects of Thermal Processing on the Bioactive Components. J. Food Sci. 2021, 86, 1751–1765. [Google Scholar] [CrossRef]
- Zhong, S.; Vendrell-Pacheco, M.; Heskitt, B.; Chitchumroonchokchai, C.; Failla, M.; Sastry, S.K.; Francis, D.M.; Martin-Belloso, O.; Elez-Martínez, P.; Kopec, R.E. Novel Processing Technologies as Compared to Thermal Treatment on the Bioaccessibility and Caco-2 Cell Uptake of Carotenoids from Tomato and Kale-Based Juices. J. Agric. Food Chem. 2019, 67, 10185–10194. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; Mckenna, B.A.; Lombi, E. Soil and the Intensification of Agriculture for Global Food Security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Dalal, R.C.; Finn, D.; Menzies, N.W. Global Changes in Soil Stocks of Carbon, Nitrogen, Phosphorus, and Sulphur as Influenced by Long-term Agricultural Production. Glob. Change Biol. 2017, 23, 2509–2519. [Google Scholar] [CrossRef]
- Campestrini, L.H.; Melo, P.S.; Peres, L.E.P.; Calhelha, R.C.; Ferreira, I.C.F.R.; Alencar, S.M. A New Variety of Purple Tomato as a Rich Source of Bioactive Carotenoids and Its Potential Health Benefits. Heliyon 2019, 5, e02831. [Google Scholar] [CrossRef]
- Gramazio, P.; Takayama, M.; Ezura, H. Challenges and Prospects of New Plant Breeding Techniques for GABA Improvement in Crops: Tomato as an Example. Front. Plant Sci. 2020, 11, 577980. [Google Scholar] [CrossRef]
- Delaney, B.; Goodman, R.E.; Ladics, G.S. Food and Feed Safety of Genetically Engineered Food Crops. Toxicol. Sci. 2018, 162, 361–371. [Google Scholar] [CrossRef]
- Sun, S.; Wang, X.; Wang, K.; Cui, X. Dissection of Complex Traits of Tomato in the Post-Genome Era. Theor. Appl. Genet. 2019, 133, 1763–1776. [Google Scholar] [CrossRef]
| (a) | Subclass and Chemical Structure | Role in Diseases | |||
| Carotenoids (tetraterpenoids) | Carotenes | α-Carotene [70,71]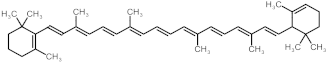 | Cardiovascular [72] | ||
β-Carotene [70] | Cardiovascular [72] Cancer [73] Neurodegenerative [74,75] | ||||
Lycopene [70,76]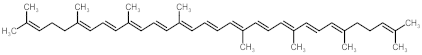 | Cancer [77,78,79] Diabetes [80] Cardiovascular [72,81,82] Neurodegenerative [74,75] Skin [83,84,85,86,87] | ||||
Neurosporene [88,89,90]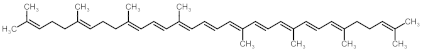 | Skin [91] | ||||
Phytoene [89,92]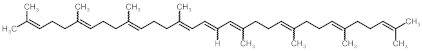 | Skin [93] | ||||
Phytofluene [89,94] | Skin [93] | ||||
| Glycoalkaloids | Saponins | Tomatine [95,96]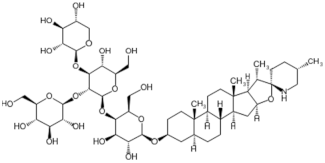 | Cancer [97] Cardiovascular [95] Malaria [98] | ||
Esculeoside A [99,100]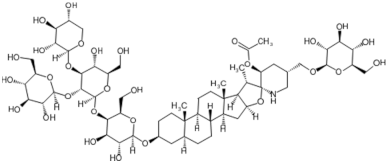 | Diabetes [100] | ||||
Lycoperoside H [62,101]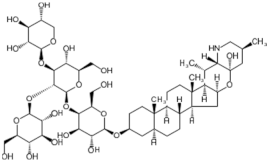 | Skin [62] Inflammation [101] | ||||
| (b) | |||||
| Polyphenols | Flavonoids | Kaempferol [102,103]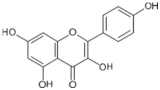 | Quercetin [102,104]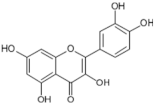 | Naringenin [102,105]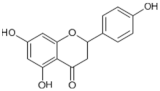 | Diabetes [106,107] Skin [108] Neurodegenerative [109] |
| Phenolic acids | Caffeic acid [102,110]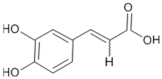 | p-Coumaric acid [102,111] | Ferulic acid [102,112]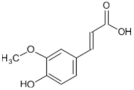 | Cancer [113,114,115] | |
| Stillbenoids | Resveratrol [116,117] | Cancer [118,119] Diabetes [118] Cardiovascular [118] Neurodegenerative [120] Skin [121,122] | |||
| Anthocyanins | Delphinidin [123,124]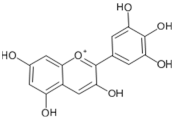 | Petunidin [123,125,126]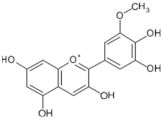 | Cancer [127] Cardiovascular [127,128] Neurodegenerative [128] Skin [127] IBD [127,129,130] | ||
| Vitamins | B | Folate (vitamin B9) [70,131]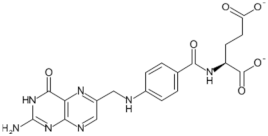 | Infertility [132] | ||
| C | Ascorbic acid [70,133]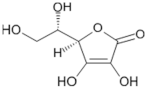 | Cancer [134] Diabetes [135] Skin [136,137] | |||
| E | α-Tocopherol [70,138] | Cancer [134] Diabetes [135] Cardiovascular [139] Infertility [140] | |||
| K | Phylloquinone (vitamin K1) [70] | Atherosclerosis [141] | |||
| Condition | Study Type | Main Findings | References |
|---|---|---|---|
| Hypertension | Double-blind placebo study of grade 1 hypertension patients. | Subjects were administered one Lyc-O-Mato tablet (250 mg capsule consists of 15 mg lycopene (6%), beta carotene (0.15%), phytoene, and phytofluene (1%); and 5 mg vitamin E (2%), phospholipids (15%), and phytosterol (0.6%), suspended in tomato oleoresin oil) per day over an 8-week treatment period. Systolic and diastolic blood pressure was found to decrease following this short-term treatment. | [212] |
| Atherosclerosis | Randomised, double-blinded, placebo-controlled cross-over study with 90 human subjects selected for normal platelet function. Ex vivo platelet aggregation induced by ADP and collagen were measured. | Study suggested administration of a standardised tomato extract drink (200 mL per day) may have a role in atherosclerosis prevention by reducing platelet activation 3 h after consumption. Significant reductions in ex vivo platelet aggregation were observed 3 h after tomato extract drink consumption. | [225] |
| Inflammation | Open, prospective, randomised, cross-over, controlled feeding trial. | Participants received either 7 g of raw tomato, 3.5 g of tomato sauce, or 3.5 g of tomato sauce with refined olive oil and 0.25 g of sugar dissolved in water (each per kg of body weight) on four different days with a month interval between each.The day of intervention, blood samples were collected in ethylenediaminetetraacetic acid (EDTA) tubes at baseline (0 h) and 6 h after the supplementation. Results of blood parameter analysis suggested that reduced inflammation was due to decreased LDL-cholesterol levels after tomato supplementation. | [207] |
| Atopic dermatitis | In vitro study. | Atopic dermatitis is linked to an overproduction of ROS and a decrease in antioxidant defence, as seen in skin biopsy specimens treated with thymodressin (0.1%, 1 mL over 30 days). Tomatoes are a natural source of antioxidants that protect skin cells against ROS. | [304] |
| Liver inflammatory disease | In vivo study with 49 mice. | Mice supplemented with tomato powder (238.8 mg of lycopene, 10.9 mg of β-carotene, and a trace amount of phytoene per 100 g) in the amount 41.9 g/kg diet. Tomato powder was shown to have a significant increase in microbiome diversity and richness, leading to a reduced production of hepatotoxic compounds such as lipopolysaccharides. | [277] |
| Neurodegenerative disorders | In vitro study testing human neuroblastoma SH-SY5Y cells. | Cells treated with lycopene (0.2 or 0.5 µM) were monitored for ROS levels, apoptosis, NF-κB activation and Nucling expression, cell viability, mitochondrial membrane potential, and oxygen consumption rate. Lycopene treatment reduced apoptosis by decreasing ROS and inhibited mitochondrial dysfunction and NF-κB target gene Nucling expression in cells. | [252] |
| Diabetes | In vivo study with 16 mice. | Esculeoside A (main saponin compound in tomatoes) was administered to the mice for 56 days (100 mg/kg). Examination of blood and liver biochemical parameters and liver insulin signalling-related protein expression showed that esculeoside A reduced fasting blood glucose levels and improved glucose tolerance, suggesting it can be a functional supplement for diabetes treatment. | [99] |
| Exercise-induced physiological stress | Randomised control trial on 15 anaerobically trained athletes of similar age and BMI. Randomised control trial on 15 healthy and untrained participants. | Biochemical evaluation suggested that athlete’s replacement of “usual carbohydrate supplementation beverage” with 100 g tomato juice led to a significant decrease in compounds LDH and CPK related to muscle damage due to anaerobic exercise. Participants engaged in 20 min of aerobic exercise on bicycle after receiving 150 mL of tomato juice for 5 weeks, followed by 5 weeks without tomato juice and tomato juice again for another 5 weeks. The blood samples were collected before and after each intervention, showing that tomato juice intake significantly suppressed 8-oxodG levels produced by aerobic exercise. | [319] [320] |
| Pathogenic infection | Blinded, randomised, cross-over study. | Enhanced lytic activity of natural killer cells and lymphocyte proliferation observed in human subjects supplemented with 330 mL/day tomato or carrot juice (both high in β-carotene). | [322] |
| OS and infertility | Randomised experimental study on male infertility patients with poor sperm concentration. | OS, caused by a build-up of ROS, is a main mediator of female infertility. Antioxidants found in tomatoes reduce cellular ROS content. Daily consumption of “one can” of tomato juice (containing 30 g lycopene) was shown to improve sperm motility after seminal parameters were measured every 6 weeks during tomato juice consumption period. | [357] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation. Biology 2022, 11, 239. https://doi.org/10.3390/biology11020239
Collins EJ, Bowyer C, Tsouza A, Chopra M. Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation. Biology. 2022; 11(2):239. https://doi.org/10.3390/biology11020239
Chicago/Turabian StyleCollins, Edward J., Cressida Bowyer, Audrey Tsouza, and Mridula Chopra. 2022. "Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation" Biology 11, no. 2: 239. https://doi.org/10.3390/biology11020239
APA StyleCollins, E. J., Bowyer, C., Tsouza, A., & Chopra, M. (2022). Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation. Biology, 11(2), 239. https://doi.org/10.3390/biology11020239





