Simple Summary
Alzheimer’s disease (AD) is the most common neurodegenerative disease, intensifying impairments in cognition, behavior, and memory. Histopathological AD variations include extracellular senile plaques’ formation, tangling of intracellular neurofibrils, and synaptic and neuronal loss in the brain. Multiple evidence directly indicates that oxidative stress participates in an early phase of AD before cytopathology. Oxidative stress plays a crucial role in activating and causing various cell signaling pathways that result in lesion formations of toxic substances, which advances the disease. Antioxidants are widely preferred to combat oxidative stress, and those derived from natural sources, which are often incorporated into dietary habits, can play an important role in delaying the onset as well as reducing the progression of AD. However, this approach has not been extensively explored yet. Moreover, a combination of antioxidants in conjugation with a nutrient-rich diet might be more effective in tackling AD pathogenesis. Thus, considering the above-stated fact, this comprehensive review aims to elaborate the basics of AD and antioxidants, including the vitality of antioxidants in AD. Moreover, this review may help researchers to develop effectively and potentially improved antioxidant therapeutic strategies for this disease as it also deals with the clinical trials in the stated field.
Abstract
Alzheimer’s disease (AD) rate is accelerating with the increasing aging of the world’s population. The World Health Organization (WHO) stated AD as a global health priority. According to the WHO report, around 82 million people in 2030 and 152 million in 2050 will develop dementia (AD contributes 60% to 70% of cases), considering the current scenario. AD is the most common neurodegenerative disease, intensifying impairments in cognition, behavior, and memory. Histopathological AD variations include extracellular senile plaques’ formation, tangling of intracellular neurofibrils, and synaptic and neuronal loss in the brain. Multiple evidence directly indicates that oxidative stress participates in an early phase of AD before cytopathology. Moreover, oxidative stress is induced by almost all misfolded protein lumps like α-synuclein, amyloid-β, and others. Oxidative stress plays a crucial role in activating and causing various cell signaling pathways that result in lesion formations of toxic substances, which foster the development of the disease. Antioxidants are widely preferred to combat oxidative stress, and those derived from natural sources, which are often incorporated into dietary habits, can play an important role in delaying the onset as well as reducing the progression of AD. However, this approach has not been extensively explored yet. Moreover, there has been growing evidence that a combination of antioxidants in conjugation with a nutrient-rich diet might be more effective in tackling AD pathogenesis. Thus, considering the above-stated fact, this comprehensive review aims to elaborate the basics of AD and antioxidants, including the vitality of antioxidants in AD. Moreover, this review may help researchers to develop effectively and potentially improved antioxidant therapeutic strategies for this disease as it also deals with the clinical trials in the stated field.
1. Introduction
According to the World Health Organization (WHO), around 50 million individuals worldwide suffer from dementia, with roughly 10 million new cases occurring each year [1]. Alzheimer’s disease (AD) is the most frequent cause of dementia, accounting for 60 percent to 70 percent of all cases [1]. AD is an irreversible, progressive, and accelerating brain disorder that results in loss of memory, thinking capacity, and, seemingly, the loss of ability to complete a simple task [2]. Although AD has a multifactorial etiology, it is characterized histopathologically by the presence of intracellular neurofibrillary tangles (NFTs) and extracellular senile plaques. NFTs are generated by the hyperphosphorylation of tau, a microtubule-associated protein localized to the axons and associated with the proper functioning of the cytoskeletal. When hyperphosphorylated in AD, tau protein leads to neuron dystrophy, aberrant skeletal framework, injury to axonal transport, and disrupted cell functions [3,4]. Senile plaques are composed of amyloid-β (Aβ) peptides of different lengths and are particularly resistant to degradation, resulting from the sequential proteolytic cleavage of Aβ precursor protein (APP) by β- and γ-secretases. The hydrophilic portion of the Aβ42 peptide coordinately forms a bond with transition metal ions such as copper (II), resulting in abnormal yet stable neurotoxic aggregates of Aβ [5]. Furthermore, Aβ has been shown to have the ability to disturb calcium homeostasis in neurons by activating calcium channels in the intracellular and plasma membrane of neurons [6]. Moreover, Aβ causes lipid peroxidation by interacting with the lipid membrane depending on the 4-HNE (4-hydroxynonenal) induced oxidation of cysteine residue, which leads to the interaction of this lipid peroxidation product with the membrane proteins specific to the brain and disrupts their structure and functionality. Such an escalation can be detrimental to the metabolic proteins of the brain. This activity of Aβ suggests the association between oxidative stress and Aβ deposition [7]. NFTs and senile plaques accelerate the neuroinflammatory responses, determine cytoskeletal stresses, and promote neuronal dysfunction [8,9,10]. Hence, one can easily infer that oxidative stress is crucial in inducing or activating the signaling pathways, leading to AD development. Although multiple approaches for the treatment of AD have been studied, at present, only a few drugs have been FDA approved for therapeutic applications (Table 1), so there is scope for novel therapeutic approaches in this regard. Several research studies have been conducted highlighting the effect of antioxidants in AD (Figure 1). Therefore, this review discusses the role of antioxidants and their related therapy for AD.

Table 1.
FDA approved drugs for the treatment of AD (Adapted from Medications for Memory Loss|Alzheimer’s Association).
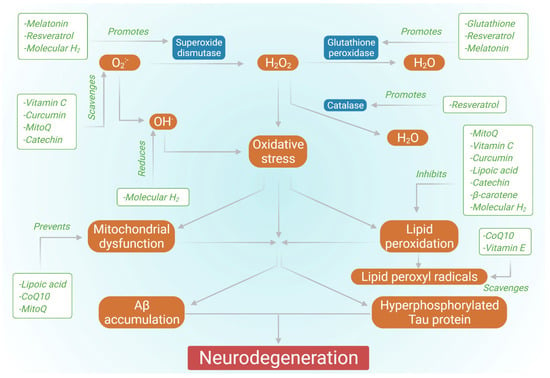
Figure 1.
Effect of various antioxidants in countering AD.
2. Oxidative Stress and Alzheimer’s Disease
Oxidative stress is defined as an imbalance between oxidants and antioxidants that causes a rise in oxidant levels [11,12,13,14]. It is well recognized as one of the clinical markers of AD; nevertheless, it is still unclear whether oxidative stress is a cause or a consequence of the process that occurs in AD patients’ brains. Reactive oxygen species (ROS) and Aβ are the major mediators of oxidative stress. The increase in the level of non-enzymatic glycation of cellular proteins, lipoproteins, and nucleic acids results from increased glucose levels [15]. These products are known as advanced glycation end products (AGEs) [16]. The administration of a diet rich in AGEs in the mouse hippocampus results in oxidative damage to the vasculature, increments in the level of Aβ, and memory impairments [17]. The receptor for AGEs is RAGE (receptor for advanced glycation end-products), a pattern recognition receptor that may bind massive ligands produced from the damaged cellular environment [18] and Aβ [19]. The NADPH oxidase (NOX) is activated by RAGE’s strong interactions with its ligands, resulting in increased ROS production [20]. AGER (gene encoding polymorphism in RAGE) has been linked to a hereditary predisposition to AD [17].
There are several pieces of evidence like APP23 mice carrying APP KM670/671NL mutation, which proves the early involvement of oxidative stress even before the deposition of Aβ in AD, [21], where triple transgenic mice carrying PS1 M146 V, Tau P301L, and APP KM670/671NL mutations were studied [22]. In the oxidative condition, Aβ generation may also be affected by the PS1/γ-secretase complex. It has been reported that 4-hydroxynonenal (4-HNE) or 4, 4-dithiodipyridine (DTDP) induces the pathogenic shift in the arrangement of PS1 subdomains within the γ-secretase complex resulting in enhancement of the aggregation and generation of Aβ species [23,24,25].
Furthermore, it has been demonstrated in recent research that not only may ROS regulate Aβ secretion/production, but that Aβ can also encourage the excessive development of ROS [26]. Overproduction of Aβ (as a result of APP overexpression) lowers the respiratory control ratio (RCR) and ATP production. It increases the production of ROS in HEK293 cells [27], implying the existence of a positive feedback loop between Aβ and ROS. ROS causes cPLA2 (calcium-dependent phospholipase A2) to be activated downstream, as well as phospholipid membrane disturbances, arachidonic acid release, and kinase activation [28,29,30], which are interlinked with the cause of AD.
The induction of oxidative stress by Aβ/APP has been reported in several studies [31]. For instance, APP KM670/671 NL and APP V717F mutation in the mouse model reported elevated lipid and protein oxidation markers like 3-NT (3-nitrotyrosine), 4-HNE, and others. Notably, the oxidative damage appears to become pronounced following the interaction of the sulfur-free radical with methionine 35 in the Aβ peptide [32,33].
3. Oxidative Stress Biomarkers in Blood Cells
Biomarkers detection helps in the early identification of AD. Brain imaging markers currently in use are neither cost-effective nor readily available [34]. However, blood-based markers can nail this as they are cost-effective, easily identifiable, and detectable. Moreover, detecting a blood-based biomarker can be repeated, and it is easily applicable in an aging population. Recently, depending upon oxidative stress, blood-based markers, most prominently cerebrospinal fluid (CSF) neurofilament light protein (NFL), plasma phospho tau (P-tau), total-tau (T-tau), and CSF Aβ42 are used to identify early AD [35]. Several pieces of the research reported that the imbalance in antioxidant defense and oxidative stress arising in the brain is reflected in the blood, which can further be easily accessed and used for early AD diagnosis [34,36,37,38]. In comparison to controls, ROS levels were higher in lymphocytes and platelets [39,40]. Protein carbonyls, 3-NT, NOS-2 (nitric oxide synthase 2), 4HNE, and other oxidative stress indicators appear to correlate with AD [41,42]. Furthermore, some investigations have explicitly linked oxidative stress to the early overproduction of Aβ [43,44,45]. Again, oxidative damage can be seen in proteins, lipids, and nucleic acids [46,47]. An increase in the levels of 8-hydroxy-2-deoxyguanosine (8-OHdG) and 8-hydroxyguanosine (8OHD), which are primarily localized in Aβ plaques, assesses oxidation in DNA and RNA [44]. Thus, these studies support the hypothesis that oxidative damage to lipids, nucleic acids, and proteins is a systematic series of events in peripheral cells in the blood.
4. Antioxidants
Antioxidants are compounds that can lower the harmful effects of oxidative stress. Antioxidants effectively decrease the rate of oxidation stress even in mild concentrations [48]. Based on the mechanism of action, antioxidants are mainly classified into two categories: (1) primary antioxidants and (2) secondary antioxidants. The former is responsible for scavenging free radicals and inhibiting the chain reaction resulting in oxidative stress. The latter undergo oxidation to decompose hydroperoxides into stable forms, thus exhibiting a synergistic effect with primary antioxidants. Secondary antioxidants are mainly involved in the regeneration of antioxidants, deactivation of metals, and reduction of singlet oxygen. As oxidative stress plays a vital role in AD likewise, antioxidants have been beneficial for AD [49]. An intricate natural antioxidant system in our body protects and prevents us from the damage caused by pro-oxidants [50]. Several reports show that various dietary sources can act as antioxidants [51,52,53]. Thus, the antioxidants system is classified into two types: (1) endogenous system and (2) exogenous system. Our body makes endogenous antioxidants, but humans procure exogenous antioxidants through diet. Glutathione peroxidase, catalase (CAT), glutathione reductase, superoxide dismutase (SOD), and others are directly involved in eliminating ROS. Nevertheless, cellular compounds like NADPH, vitamin C, mannitol, bilirubin, GSH, β-carotene, and others can be significant antioxidants.
Antioxidants with potential therapeutic applications against AD are shown in Table 2.

Table 2.
List of antioxidants with potential therapeutic effects against AD.
5. Role of Antioxidant-Rich Diet in Alzheimer’s Disease
It is well established that diet affects both mental and physical health. Food sources have a significant role in treating AD, as reported in many studies [88,89,90]. Vitamin C, E, carotenoids, flavonoids, polyphenols, and others are included in natural dietary antioxidants [91]. Moreover, it is suggested by literature and clinical reports that a proper diet including vitamins, proteins, and minerals will surely complement the medicine for the treatment of AD [89,90,91]. Apple cider has been reported to increase the activity of SOD, CAT, and glutathione peroxidase (GPx) to reduce lipid peroxidation [92,93].
Dietary potassium helps reduce ROS, alters the Aβ aggregation pattern, and helps in improving cognitive abilities [94]. Furthermore, increasing dietary potassium (to the optimum concentration) benefits individuals by preventing or delaying age and diet-related neurodegenerative diseases [95]. Garlic and its components show an admirable effect on brain function and neuronal physiology, leading to pharmacotherapy for AD [96]. Citrus fruits containing flavanone glycoside may be responsible for the conformational change of the beta-amyloid precursor protein cleaving enzyme 1 [97]. It has been found that supplementation of soaked almonds in the AD animal models of C57Bl/6 mice and adults of Sprague–Dawley rats, after overnight fasting, enhances memory due to the enrichment of vitamin E [97]. Despite under-conducted research, the cross-sectional studies of the overall role of diet and its patterns in AD are still questionable. Thus, research in this area is also needed. Moreover, people’s apprehension of the quantity and quality of individual bioactive components present in food items is insufficient for significant neuroprotection. Different classes of antioxidants with potential therapeutic applications against AD are displayed in Figure 2.
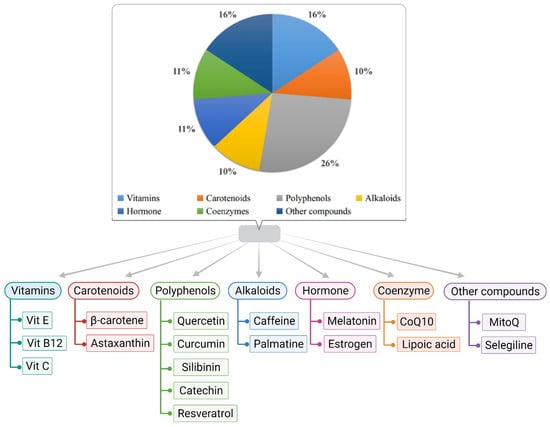
Figure 2.
Various classes of antioxidants are documented for having the potential to counter AD.
6. Role of Antioxidants in Alzheimer’s Disease
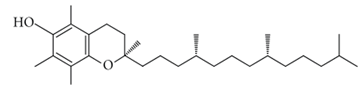
6.1. Vitamin E
Vitamin E is the most promising antioxidant for peroxyl radicals [98]. It can act on lipid-soluble membrane lipoproteins and low-density lipoproteins [99]. It has the potential to inhibit and delay neuronal death caused by inflammation. Moreover, it eliminates free radicals present in the red blood cell membrane and inhibits the spread of lipoperoxidation [100]. Furthermore, α-tocopherol is the most abundant form of vitamin E with high bioavailability in human tissue [101,102]. Vitamin E can be helpful in overcoming the increased expression of alpha-tocopherol transfer protein (α-TTP) in the patient’s brain suffering from AD [103]. A meta-analysis report of AD patients shows a reduced level of vitamin E in the blood plasma [104]. In one of the clinical trials, vitamin E and Ginkgo biloba extract were potentially significant in improving cognitive function of the brain [11]. Additionally, in another meta-analysis, it has been reported that a low concentration of serum vitamin E is associated with AD [105]. Moreover, substantial evidence suggested that vitamin E successfully suppresses tau-induced neurotoxicity in Drosophila [106,107,108]. In one of the recent studies, it is proposed that vitamin E has significantly reduced oxidative and nitrosative damage in AD [109]. However, the positive effect being evaluated of vitamin E in AD is still in the ongoing phases of various clinical trials.
6.2. Glutathione
Glutathione also plays a significant role in protein and DNA synthesis, cell cycle regulation, and storage and transport of cysteine. It has the potential to scavenge lipid peroxidation products like acrolein, 4-hydroxy-2-nonenal (HNE), and others [110]. It is used to maintain the thiol redox of cells, detox electrophiles, and metals, and protect from oxidative stress. It also can form metal complexes that reduce the toxicity of the metals and facilitate their further excretion from the body [110,111,112]. Recently, it was reviewed that cholesterol-mediated depletion of mitochondrial glutathione is linked with increased Aβ-induced oxidative stress in mitochondria [113]. The introduction of glutathione ethyl ester in transgenic mice featuring a high expression of sterol regulatory element-binding protein-2 (SREBP-2) has been shown to prevent neuroinflammation and neuronal damage [114]. Further, one recent study reveals the redox pathway of glutathione antioxidant responsible for regulating mitochondrial dynamics in axons [115]. However, the mechanistic overview of the exclusive role of glutathione in AD is still unclear.
6.3. Molecular Hydrogen
Molecular hydrogen is also an antioxidant that can modulate the Keap1-Nrf2-ARE signaling pathway and reduce inflammation [116]. It has a potential role in the selective reduction of hydroxyl radicals involved in the demolishing of proteins, nucleic acid and leads to lipid peroxidation, which is also a reported feature in AD [117]. It has been reported that molecular hydrogen administration increases short-lived Drosophila’s survival and life span [118]. At the same time, it is found that the hydrogen-rich water causes the increment in the level of glutathione and SOD [52]. Having both an indirect and direct role, the application of molecular hydrogen shows satisfying results for AD. However, more human trials are required for solid suggestions and recommendations.
6.4. Monoamine Oxidase-b Inhibitor
Monoamine oxidase catalyzes the oxidative deamination of xenobiotic and biogenic amines. In peripheral tissue and the central nervous system, they play an important role in the metabolism and control of vasoactive and neuroactive amines. In cerebral blood arteries, a monoamine oxidase-b inhibitor can rapidly produce the vasodilator nitric oxide [119]. By blocking oxidative deamination, it shields the vascular endothelium from the effects of Aβ and improves the survival and function of nigral neurons [120,121]. It is also reported to decrease the progression of AD by reducing neuronal damage [122]. L-deprenyl, a monoamine oxidase-b inhibitor, enhances nitric oxide production accompanied by vasodilation; however, the study also suggests that L-deprenyl may involve other pathways for its effectivity [119].
6.5. Melatonin
Melatonin, a mammalian hormone synthesized in the pineal gland, can scavenge oxygen and nitrogen-based reactants. It performs by stimulating and promoting the activity and expression of NO synthase, SOD, and GPx [123]. It has a significant role in reducing oxidative damage of cells [124]. In recent literature, it has been reported that antioxidant melatonin can mitigate tau hyperphosphorylation [125,126,127,128,129] and inhibit the toxicity induced by Aβ [130].
6.6. Ascorbyl Palmitate
It is a lipid-soluble form of vitamin C. It maintains all the vitamin C activity without creating problems associated with ascorbic acids, such as less recycling capacity of α-tocopherol in the lipid bilayer, reduced viability in-vivo, and others [122]. Additionally, it is reported that the demand for vitamin C can be better fulfilled with lipophilic form rather than hydrophilic form [131]. Ascorbyl palmitate can successfully cross the blood-brain barrier (BBB) [132] and is reported for its significant role in treating AD [133]. As ascorbyl palmitate resides in the cell membrane, it can accelerate the production of vitamin E. However, the protective role of vitamin C is still in debate as it is not yet clear whether vitamin C is acting alone or in combination for treating AD.
6.7. Curcumin
Multiple desirable features reside in curcumin for a neuroprotective drug, including antioxidant, anti-protein aggregates, and anti-inflammatory activities [134]. It has been studied that curcumin reduces inflammation, oxidative damage, and cognitive deficits in rats where Aβ toxicity has affected their central nervous system. Curcumin possesses substantial free radical scavenging properties, whereby it targets NO-based radicals to scavenge them, which helps inhibit lipid peroxidation [135]. Curcumin has also been reported to bind with metal ions, which prevents them from causing aggregation of Aβ and reduces oxidative stress [136]. Moreover, curcumin was also found to restore glutathione levels in brain tissue and reduce oxidized proteins in mice models with AD [137,138]. However, in one of the clinical trials, curcumin’s beneficial effect in AD couldn’t be determined; this may be due to highly poor pharmacokinetics and pharmacodynamics properties [139].
6.8. Coenzyme Q and SK-PC-B70M
Coenzyme Q is currently studied for its role in Parkinson’s disease and amyotrophic lateral sclerosis [106]. Moreover, it helps in the generation of ATP. It is the only lipid synthesized directly within the body and can maintain a redox function [140]. Coenzyme Q has the potential to neutralize free radicals and stabilize the optimal functioning of the cell membrane. The contribution of coenzyme Q in AD treatment must be explored as there is a high possibility that it might play an influential, protective, and preventing role in AD. SK-PC-B70M, an oleanolic-glycoside saponin enriched fraction, is derived from Pulsatilla Korean. Currently, it has been reported for its neuroprotective activity against the cytotoxicity effect induced by Aβ in SK-N-SH [141].
6.9. Estrogen, Astaxanthin, and Quercetin
Estrogen protects neurons against the toxicity of Aβ by acting as an antioxidant [142]. It appears to have a neuroprotective effect [52] without improving function or cognition in people with AD [142]. Astaxanthin is a powerful carotenoid that can prevent apoptosis, oxidative stress, inflammation, memory loss, and protect against Aβ’s neurotoxic effects [94,142,143,144]. Quercetin is the most prominent and significant dietary antioxidant effective on health as it protects against severe diseases like lung cancer, cardiovascular disease, osteoporosis, and others [145]. There are ongoing clinical trials for estimating its accurate effect on AD [146].
6.10. Lipoic Acid
The medicinal antioxidant lipoic acid (α-lipoic acid) is found in the mitochondria. Pyruvate dehydrogenase and α-ketoglutarate dehydrogenase both use it as a cofactor. However, it is also involved in the recycling of other antioxidants such as vitamin C and E, as well as glutathione, in order to boost ACh production [53]. Lipoic acid is also implicated in some redox-active chelating metals, which helps to prevent lipid peroxidation from building up [147]. When used in combination with acetylcarnitine, lipoic acid was found to protect neuronal cells through cell-signaling pathways, including specific extracellular kinase pathways, mainly the Ras-MAPK pathway that were dysregulated in AD [148]. Studies undertaken on the brain of control and AD mouse models showed that lipoic acid reduced the expression of F2 isoprostanes and neuroprostanes, which are oxidative stress markers [149]. Lipoic acid also induces the transcription factor Nrf2, which regulates a number of different antioxidant enzymes involved in protection from oxidative stress [150]. Lipoic acid improved memory and reversed oxidative stress indices in the senescence-accelerated mouse-prone 8 (SAMP8) models [151]. Lipoic acid is a potent antioxidant as it can traverse the BBB, making it ideal for therapeutic applications in AD [152].
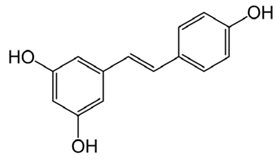
6.11. Resveratrol
Resveratrol (3, 5, 4′-trihydroxy-trans-stilbene) is a polyphenolic compound found in a number of plants, like red grapes, blueberries, dark chocolate, and peanut butter. Resveratrol has been reported to possess antioxidant properties and was found to diminish malondialdehyde and nitrite levels and restore glutathione levels [84]. Studies in a number of cell lines expressing mutant AβPP695 reported that resveratrol exhibited anti-amyloidogenic activity through reduction in secreted intracellular Aβ peptide levels [153]. Levels of intracellular antioxidant enzymes SOD, CAT, GPx, and HO-1 were increased by resveratrol while simultaneously reducing lipid peroxidation [79]. Another essential function of resveratrol was diminishing ROS production in brain tissue by preventing disruption in the mitochondrial membrane potential [154]. The binding of metal ions to Aβ and NFTs enhances their aggregation and increases ROS production. Resveratrol counteracts this through dysregulation of the metal ion balance [84]. Along with antioxidant properties, resveratrol has been reported to promote an anti-inflammatory response, reduce levels of tau protein phosphorylation and increase the activity of SIRT-1 [154]. This makes resveratrol an interesting natural antioxidant in combating AD pathogenesis.
6.12. MitoQ
MitoQ is an antioxidant that targets the mitochondria in AD. MitoQ is made by adding the lipophilic triphenylphosphonium (TPP+) cation to ubiquinone, a component of the mitochondrial electron transport chain, via a ten-carbon chain [155]. TPP+ facilitates entry of ubiquinone into the mitochondrial matrix, where the complex II reduces ubiquinone to ubiquinol, the active antioxidant form, decreasing lipid peroxidation, which reduces oxidative damage [156]. MitoQ is able to traverse the BBB rapidly and has been found to accumulate several hundred folds in the mitochondrial membrane. The uptake of MitoQ in the mitochondria is driven by the high membrane potential of the inner mitochondrial membrane [157]. MitoQ has been found to reduce free radicals and oxidative damage while helping to regulate mitochondrial functions of the cells [158]. MitoQ was found to lower Aβ peptide levels, minimize synaptic loss and astrogliosis and improve cognitive functions in AD mouse model studies wherein the administration of MitoQ was initiated at a young age [155,159]. MitoQ was also reported to enhance neurite outgrowth in neurons and protection against Aβ peptide toxicity in cells of AD mouse models [160].
6.13. Catechins
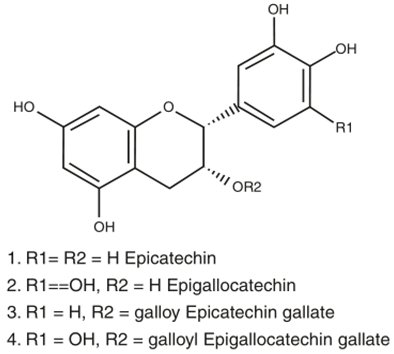
Catechins are the bioactive components found in tea—most abundant in green tea (green tea catechins or GTC)—which includes four different types of catechins: viz. epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC), and epigallocatechin gallate (EGCG) [153]. Catechins exhibit antioxidative effects by scavenging ROS and chelating metal ions like copper, iron, and zinc, thereby reducing their accumulation in the brain of AD patients [86]. EGCG was reported to reduce caspase levels and oxidative stress along with reducing lipid peroxidation in the hippocampus of the rat model [161]. A long-term study on male Wistar rats revealed that the administration of 0.5% GTC in water resulted in counteracting Aβ-induced cognitive impairment, along with reduced levels of plasma lipid peroxide and ROS levels [162]. In addition toantioxidant properties, catechins were also reported to exhibit anti-inflammatory properties, along with inhibition of acetylcholinesterase (AChE) activity. At the same time, EGCG was found to directly interact with Aβ peptides and prevent the formation of aggregates [86,163,164]. Furthermore, catechins are BBB permeable, as found in rodent models, making them a potential therapeutic candidate for AD treatment [86].
6.14. Silibinin
Silibinin, an antioxidant flavonolignan obtained from Silybum marianum, can boost the amount of newly formed microglia, astrocytes, neurons, and neural precursor cells in the brain [165]. In one study, silibinin was found to be a dual inhibitor of AChE and Aβ peptide aggregation, implying a therapeutic method for treating Alzheimer’s disease [165]. It can potentially prevent the injuries caused by Aβ1-42-indued oxidative stress by lowering the production of H2O2 in Aβ1-42-stressed neurons [166]. Another study reported that streptozotocin-induced tau hyperphosphorylation (ser404) in the hippocampus was substantially reduced by silibinin [167]. Though these results indicate that silibinin may be a novel therapeutic agent for treating AD, no clinical trials are on board.
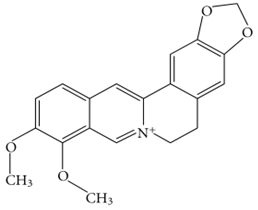
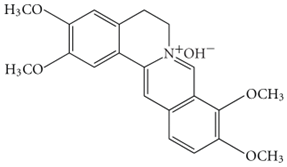
6.15. Palmatine
Palmatine, an isoquinoline alkaloid, acts against Aβ induced neurotoxicity [168]. It is reported that palmatine activated the Nfr2 knockdown and AMPK pathway [168]. It is reported for having anti-inflammatory, antioxidative and antiproliferative effects [169]. Another study reported the combined impact of palmatine and berberine on the inhibition of AChE [169,170]. Though it is reported in several in-silico and in-vivo studies, there is still a massive absence of its proper application in AD. Moreover, the mode of action underlying their neuroprotective effect is poorly characterized in vivo.
6.16. Serotonin
Serotonin, an indoleamine neurotransmitter, can disassemble performed Aβ fibrils [171]. Ample evidence reflects that a combination of disturbances in serotonergic and cholinergic function may possess a vital role in cognitive impairment in AD [172]. In one study, it is indicated that alterations of the serotonergic system contribute to neuropsychiatric symptoms in AD as their results suggest that a decline in neurons expressing 5-HT2A plays a role in the etiopathology of neuropsychiatric symptoms in AD [173]. Furthermore, while many of these compounds will likely be used as adjuvant therapy in the treatment of AD symptoms, there are currently just a few pharmacological entities with activity against serotonin receptors that have the potential to slow the illness’s progression.
6.17. Gintonin
Gintonin, a glycol-lipoprotein, can help in maintaining the integrity of BBB [174]. It can suppress the activated inflammatory mediators and microglial cells in the brains of Aβ-injected mice [175]. Recent findings suggest that treatment with gintonin in AD results in improved synaptic and memory functions in the brain [176]. It reflects an emerging role as a modulator of neurogenesis and synaptic transmission, and it has the potential to regulate autophagy in primary cortical astrocytes [176,177]. Moreover, as a novel agonist of lysophosphatidic acid receptors, gintonin regulated several GPCR, including GPR55 and GPR40 [177]. Nevertheless, further exploration is still required to understand gintonin’s underlying mode of action in AD.
7. Role of Other Nutrients in Alzheimer’s Disease
Apart from antioxidant activities, natural products have exhibited other vital properties to combat AD progression through anti-inflammatory response, prevention of Aβ aggregation, accumulation of tau protein, and the promotion of cholinergic signaling [178]. Alkaloids, such as cryptolepine and tetrandrine, have been reported to be involved in the inhibition of NF-κB, thereby acting as anti-inflammatory agents [179]. Flavonoids, owing to their characteristic property of inhibiting inflammatory response, have shown potential for working against AD progression [180]. Studies in animal models of AD have reported terpenoids, such as artemisinin, parthenolide, and carnosol can inhibit NF-κB and p38 MAPK pathways [181,182,183]. Ginsenoside Rg1, a compound obtained from the roots of the Ginseng plant, has been reported to cause a significant drop in levels of Aβ peptide levels in AD mice [184]. Natural plant products like crocin, α-cyperone, chrysophanol, and aloe-emodin have been found to exhibit properties that inhibit tau protein formation and reduce AD progression [185,186,187]. Caffeine, one of the most widely consumed alkaloids, has been found to inhibit Aβ deposition in vitro [188]. It was also found to reduce ROS production and enhance SOD levels in human neuroblastoma cells cultured with Aβ [189]. Caffeine has also been shown to exhibit anti-neuroinflammatory properties as well as decreasing tau protein phosphorylation in the hippocampus [190]. In low to moderate doses, caffeine inhibits AChE, thereby improving cognitive actions and reducing the progression of AD [191]. Eugenol, found in cloves, has been reported to reduce amyloid plagues and increase memory in rat models induced with Aβ peptides [192]. Dietary patterns have also been found to impact the onset and progression of AD. A Western diet characterized by higher meat intake was associated with an increased risk of AD [193]. In contrast to this, the Mediterranean diet, characterized by higher consumption of fruits, vegetables, and fish with lower meat intake, was found to reduce the risk of AD in the population [194].
8. Limitations and Future Perspectives
Antioxidants have been shown to be effective against AD. The clinical trials for evaluating the therapeutic potential for several antioxidants in AD are shown in Table 3. But the availability of data related to pre-clinical studies falls short to justify their widespread application [195]. Antioxidants have been shown to lower the damage by oxidative stress in the brain though limited human trials and make it difficult to conclude accordingly. The research interventions for AD should mainly focus on the patient’s lifestyle, keeping in mind their cognitive status. The application of antioxidants and their effect could be monitored in AD patients through the nutrient-rich diet they are being advised for intake [196]. A holistic approach towards the treatment of AD has become the need of the hour. AD treatment needs to be addressed at the right time to minimize the chances of failure. Genomic sequencing commenced by the National Institutes of Health (NIH) in 2012 has opened up new avenues in developing a more contemporary and specialized treatment for AD [197]. Inflammatory responses have been an innate part of AD pathogenesis that needs to be addressed with much-advanced technology as soon as possible. ROS poses a significant threat to neurons. Oxidative damage is a prominent pathological symbol for AD. Antioxidants have ROS scavenging ability, making them a feasible candidate in the fight against AD [198]. Nonetheless, inconclusive results from human trials make it difficult for the physician to recommend AD treatment. The synergistic administration of various antioxidants could counter oxidative stress with much efficiency. The concentration of antioxidants administered should be taken care of since high doses could disrupt the normal physiological process where ROS plays a prominent role. Extensive studies should be carried upon placing a closer look at toxicity, bioavailability issues, and long-term exposure of antioxidants in AD patients [199]. The most important aspects to look for while carrying out antioxidant-based research for AD are the time-span of consumption and the age group of people above 60 for the administration of antioxidants. The onset of dementia might get initiated even before the appearance of definitive symptoms [200]. The requirement of biomarker identification and neuropathological assessment has become a must for diagnosing and providing the antioxidant at the required time-span before the onset of AD [201]. Limitations of antioxidants, such as lower bioavailability of polyphenols, resveratrol, and others require proper research interventions [202]. Mixing polyphenols with juice or extracts from fruits could be looked for extensively, in order to mediate the shortcomings of polyphenol therapy for AD [203]. The viable method of administering antioxidants needs to be addressed with further pharmacological studies since some patients may not be comfortable with tablets, and some may not be with extracts. Thus, the viable mode needs to be addressed according to the patient’s needs [204]. Additionally, the intervention of nanotechnology could be crucial towards the enhancement of bioavailability and moving past the BBB, which is another shortcoming of antioxidants being administered conventionally [205]. The role of antioxidants could be vital in the battle against a plethora of neurodegenerative diseases. Therefore, extensive human trials are required to test the efficacy of the antioxidant in AD patients.

Table 3.
Completed clinical trials conducted for antioxidants relevant to AD (“Completed” status here means the study has ended, and participants are no longer being examined or treated (that is, the last participant’s last visit has occurred).
9. Conclusions
With a long asymptomatic period, AD is a chronic neurodegenerative condition. Multiple literature and evidence infer that oxidative damage or stress plays a significant role in the pathogenesis of AD through various mechanisms and pathways. Thus, new treatment strategies are required to either prevent or reduce oxidative damage and may provide therapeutic efficacy against AD. Natural bioactive, often incorporated into the diet, can become a widely adopted approach to avoid the onset of AD. Moreover, this approach can be conjugated with approved drugs for patients with progressive AD. The integrated system of antioxidants with multiple drugs may provide higher effectiveness. Some antioxidants have proven positive effectors on AD, but some still need attention and work. Moreover, there is limited data on the role of antioxidants in AD from human clinical trials and epidemiological studies. Additionally, some antioxidants show significant effects on an animal model but exhibit diminished efficacy on humans during clinical trials. Due to this, there is a lot of skepticism about the success of antioxidant therapy for AD. It is quite necessary to explore a more definitive and precise approach integrated with antioxidants for lowering or inhibiting the progression of AD. The link between inflammation and AD is unavoidable, so antioxidants’ integrated role in decreasing inflammation must be considered. Thus, further advanced studies and human clinical trials are necessary to determine and estimate the antioxidants potential for AD.
Author Contributions
Conceptualization, S.K.J. and N.K.J.; writing—original draft preparation, P.P., R.D., A.B. and R.S.; figures, N.K.J.; writing—review and editing, D.K., A.K.J. and C.V.; supervision, S.K.J. and N.K.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Graphical abstract was created with BioRender.com (accessed on 26 December 2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Dementia. 21 September 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 22 December 2021).
- National Institute on Aging. Alzheimer’s Disease Fact Sheet. 22 May 2019. Available online: https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet (accessed on 21 December 2021).
- Sanabria-Castro, A.; Alvarado-Echeverría, I.; Monge-Bonilla, C. Molecular pathogenesis of Alzheimer’s disease: An update. Ann. Neurosci. 2017, 24, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.A.; Parker, T.D.; Cash, D.M.; Macpherson, K.; Donnachie, E.; Murray-Smith, H.; Barnes, A.; Barker, S.; Beasley, D.G.; Bras, J.; et al. Study protocol: Insight 46—A neuroscience sub-study of the MRC National Survey of Health and Development. BMC Neurol. 2017, 17, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, M.; Sharma, A.K. Cu and Zn interactions with Aβ peptides: Consequence of coordination on aggregation and formation of neurotoxic soluble Aβ oligomers. Metallomics 2019, 11, 64–84. [Google Scholar] [CrossRef]
- Korol, T.Y.; Kostyuk, E.P.; Kostyuk, P.G. Disruption of calcium homeostasis in Alzheimer’s disease. Neurophysiology 2008, 40, 385–392. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Smith, M.A.; Peggy, L.; Richey, H.; Sayre, L.M.; Beckman, J.S.; Perry, G. Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J. Neurosci. 1997, 17, 2653–2657. [Google Scholar] [CrossRef] [Green Version]
- Kalaria, R.N. Microglia and Alzheimer’s disease. Curr. Opin. Hematol. 1999, 6, 15. [Google Scholar] [CrossRef]
- Hempen, B.; Brion, J.-P. Reduction of acetylated α-tubulin immunoreactivity in neurofibrillary tangle-bearing neurons in Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 1996, 55, 964–972. [Google Scholar] [CrossRef] [Green Version]
- Kandiah, N.; Ong, P.A.; Yuda, T.; Ng, L.; Mamun, K.; Merchant, R.A.; Chen, C.; Dominguez, J.; Marasigan, S.; Ampil, E.; et al. Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: Expert consensus on the use of Ginkgo biloba extract, EGb 761®. CNS Neurosci. Ther. 2019, 25, 288–298. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Domenico, S.; Boulestin, H.; Campbell, F.M.; Williams, L.M. The role of dietary advanced glycation end products in metabolic dysfunction. Mol. Nutr. Food Res. 2021, 65, 1900934. [Google Scholar]
- Wiramon, R.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar]
- Irit, L.; Ricny, J.; Atrakchi-Baranes, D.; Shemesh, C.; Kravitz, E.; Liraz-Zaltsman, S.; Maksin-Matveev, A.; Cooper, I.; Leibowitz, A.; Uribarri, J.; et al. High dietary advanced glycation end products are associated with poorer spatial learning and accelerated Aβ deposition in an Alzheimer mouse model. Aging Cell 2016, 15, 309–316. [Google Scholar]
- Piras, S.; Furfaro, A.L.; Domenicotti, C.; Traverso, N.; Marinari, U.M.; Pronzato, M.A.; Nitti, M. RAGE expression and ROS generation in neurons: Differentiation versus damage. Oxidative Med. Cell. Longev. 2016, 2016, 9348651. [Google Scholar] [CrossRef] [Green Version]
- Leclerc, E.; Sturchler, E.; Vetter, S.W. The S100B/RAGE axis in Alzheimer’s disease. Cardiovasc. Psychiatry Neurol. 2010, 2010, 539581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Zhang, H.; Schmidt, A.M.; Zhang, C. AGE/RAGE produces endothelial dysfunction in coronary arterioles in type 2 diabetic mice. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H491–H498. [Google Scholar] [CrossRef] [Green Version]
- Resende, R.; Moreira, P.I.; Proença, T.; Deshpande, A.; Busciglio, J.; Pereira, C.; Oliveira, C.R. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic. Biol. Med. 2008, 44, 2051–2057. [Google Scholar] [CrossRef] [Green Version]
- Hartl, D.; Schuldt, V.; Forler, S.; Zabel, C.; Klose, J.; Rohe, M. Presymptomatic alterations in energy metabolism and oxidative stress in the APP23 mouse model of Alzheimer disease. J. Proteome Res. 2012, 11, 3295–3304. [Google Scholar] [CrossRef]
- Arimon, M.; Takeda, S.; Post, K.L.; Svirsky, S.; Hyman, B.T.; Berezovska, O. Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol. Dis. 2015, 84, 109–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoltowska, M.K.; Berezovska, O. Dynamic nature of presenilin1/γ-secretase: Implication for Alzheimer’s disease pathogenesis. Mol. Neurobiol. 2018, 55, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Gwon, A.-R.; Park, J.-S.; Arumugam, T.V.; Kwon, Y.-K.; Chan, S.L.; Kim, S.H.; Baik, S.H.; Yang, S.; Yun, Y.K.; Choi, Y.; et al. Oxidative lipid modification of nicastrin enhances amyloidogenic γ-secretase activity in Alzheimer’s disease. Aging Cell 2012, 11, 559–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, C.; Chan, H.; Ashok, I.; Krishnamoorthi, S.K.; Li, M.; Li, H.; Wong, M.S. Amyloid-β oligomer targeted theranostic probes for in vivo NIR imaging and inhibition of self-aggregation and amyloid-β induced ROS generation. Talanta 2021, 224, 121830. [Google Scholar] [CrossRef] [PubMed]
- Leuner, K.; Schütt, T.; Kurz, C.; Eckert, S.H.; Schiller, C.; Occhipinti, A.; Mai, S.; Jendrach, M.; Eckert, G.P.; Kruse, S.E.; et al. Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation. Antioxid. Redox Signal. 2012, 16, 1421–1433. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Cui, J.; Lee, J.C.M.; Ding, S.; Chalimoniuk, M.; Simonyi, A.; Sun, A.Y.; Gu, Z.; Weisman, G.A.; Wood, W.G.; et al. Prolonged exposure of cortical neurons to oligomeric amyloid-β impairs NMDA receptor function via NADPH oxidase-mediated ROS production: Protective effect of green tea (-)-epigallocatechin-3-gallate. ASN Neuro 2011, 3, AN20100025. [Google Scholar]
- Shelat Phullara, B.; Chalimoniuk, M.; Wang, J.; Strosznajder, J.B.; Lee, J.C.; Sun, A.Y.; Simonyi, A.; Sun, G.Y. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J. Neurochem. 2008, 106, 45–55. [Google Scholar] [CrossRef]
- Faridis, S.; Chang, A.; Hernandez, C.; Pautler, R.G.; Sweatt, J.D.; Klann, E. NADPH oxidase mediates β-amyloid peptide-induced activation of ERK in hippocampal organotypic cultures. Mol. Brain 2009, 2, 1–10. [Google Scholar]
- Bilkei-Gorzo, A. Genetic mouse models of brain ageing and Alzheimer’s disease. Pharmacol. Ther. 2014, 142, 244–257. [Google Scholar] [CrossRef]
- Robinson, R.A.; Lange, M.B.; Sultana, R.; Galvan, V.; Fombonne, J.; Gorostiza, O.; Bredesen, D.E. Differential expression and redox proteomics analyses of an Alzheimer disease transgenic mouse model: Effects of the amyloid-β peptide of amyloid precursor protein. Neuroscience 2011, 177, 207–222. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Galvan, V.; Lange, M.B.; Tang, H.; Sowell, R.A.; Spilman, P.; Fombonne, J.; Gorostiza, O.; Zhang, J.; Sultana, R.; et al. In vivo oxidative stress in brain of Alzheimer disease transgenic mice: Requirement for methionine 35 in amyloid β-peptide of APP. Free Radic. Biol. Med. 2010, 48, 136–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojsiat, J.; Laskowska-Kaszub, K.; Mietelska-Porowska, A.; Wojda, U. Search for Alzheimer’s disease biomarkers in blood cells: Hypotheses-driven approach. Biomark. Med. 2017, 11, 917–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angiulli, F.; Conti, E.; Zoia, C.P.; da Re, F.; Appollonio, I.; Ferrarese, C.; Tremolizzo, L. Blood-based biomarkers of neuroinflammation in Alzheimer’s disease: A central role for periphery? Diagnostics 2021, 11, 1525. [Google Scholar] [CrossRef] [PubMed]
- Wojda, U. Alzheimer’s disease lymphocytes: Potential for biomarkers? Biomark. Med. 2016, 10, 1–4. [Google Scholar] [CrossRef]
- Wojsiat, J.; Prandelli, C.; Laskowska-Kaszub, K.; Martín-Requero, A.; Wojda, U. Oxidative stress and aberrant cell cycle in Alzheimer’s disease lymphocytes: Diagnostic prospects. J. Alzheimer’s Dis. 2015, 46, 329–350. [Google Scholar] [CrossRef]
- Khan, T.K.; Alkon, D.L. Peripheral biomarkers of Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 44, 729–744. [Google Scholar] [CrossRef] [Green Version]
- Leutner, S.; Schindowski, K.; Frölich, L.; Maurer, K.; Kratzsch, T.; Eckert, A.; Müller, W.E. Enhanced ROS-generation in lymphocytes from Alzheimer’s patients. Pharmacopsychiatry 2005, 38, 312–315. [Google Scholar] [CrossRef] [Green Version]
- Vignini, A.; Nanetti, L.; Moroni, C.; Tanase, L.; Bartolini, M.; Luzzi, S.; Mazzanti, L. Modifications of platelet from Alzheimer disease patients: A possible relation between membrane properties and NO metabolites. Neurobiol. Aging 2007, 28, 987–994. [Google Scholar] [CrossRef]
- Calabrese, V.; Sultana, R.; Scapagnini, G.; Guagliano, E.; Sapienza, M.; Bella, R.; Kanski, J.; Pennisi, G.; Mancuso, C.; Stella, A.M.G.; et al. Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer’s disease. Antioxid. Redox Signal. 2006, 8, 1975–1986. [Google Scholar] [CrossRef] [Green Version]
- Skoumalova, A.; Ivica, J.; Šantorová, P.; Topinkova, E.; Wilhelm, J. The lipid peroxidation products as possible markers of Alzheimer’s disease in blood. Exp. Gerontol. 2011, 46, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Buizza, L.; Cenini, G.; Lanni, C.; Ferrari-Toninelli, G.; Prandelli, C.; Govoni, S.; Szybinska, A. Conformational altered p53 as an early marker of oxidative stress in Alzheimer’s disease. PLoS ONE 2012, 7, e29789. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Perry, G.; Aliev, G.; Hirai, K.; Takeda, A.; Balraj, E.K.; Jones, P.K.; Ghanbari, H.; Wataya, T.; Shimohama, S.; et al. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001, 60, 759–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunomura, A.; Castellani, R.J.; Zhu, X.; Moreira, P.I.; Perry, G.; Smith, M.A. Involvement of oxidative stress in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006, 65, 631–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadioglu, E.; Sardas, S.; Aslan, S.; Isik, E.; Karakaya, A.E. Detection of oxidative DNA damage in lymphocytes of patients with Alzheimer’s disease. Biomarkers 2004, 9, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Mórocz, M.; Kálmán, J.; Juhász, A.; Sinkó, I.; McGlynn, A.P.; Downes, C.S.; Janka, Z.; Raskó, I. Elevated levels of oxidative DNA damage in lymphocytes from patients with Alzheimer’s disease. Neurobiol. Aging 2002, 23, S0197–S4580. [Google Scholar]
- Velagapudi, R.; El-Bakoush, A.; Olajide, O.A. Activation of nrf2 pathway contributes to neuroprotection by the dietary flavonoid tiliroside. Mol. Neurobiol. 2018, 55, 8103–8123. [Google Scholar] [CrossRef] [Green Version]
- Irshad, M.; Chaudhuri, P.S. Oxidant-Antioxidant System: Role and Significance in Human Body. 2002. Available online: http://nopr.niscair.res.in/handle/123456789/23569 (accessed on 15 December 2021).
- Hill, M.F. Emerging role for antioxidant therapy in protection against diabetic cardiac complications: Experimental and clinical evidence for utilization of classic and new antioxidants. Curr. Cardiol. Rev. 2008, 4, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, A.; Chatterjee, A.; Ghosal, S.; Bhattacharya, S.K. Antioxidant Activity of Active Tannoid Principles of Emblica Officinalis (Amla). 1999. Available online: http://nopr.niscair.res.in/handle/123456789/19103 (accessed on 13 December 2021).
- Veurink, G.; Perry, G.; Singh, S.K. Role of antioxidants and a nutrient rich diet in Alzheimer’s disease. Open Biol. 2020, 10, 200084. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X. Antioxidant therapies for Alzheimer’s disease. Oxidative Med. Cell. Longev. 2012, 2012, 472932. [Google Scholar] [CrossRef] [Green Version]
- Suárez, S.; Mu, T.; Sun, H.; Añón, M.C. Antioxidant activity, nutritional, and phenolic composition of sweet potato leaves as affected by harvesting period. Int. J. Food Prop. 2020, 23, 178–188. [Google Scholar] [CrossRef]
- Orsavová, J.; Hlaváčová, I.; Mlček, J.; Snopek, L.; Mišurcová, L. Contribution of phenolic compounds, ascorbic acid and vitamin E to antioxidant activity of currant (Ribes L.) and gooseberry (Ribes uva-crispa L.) fruits. Food Chem. 2019, 284, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Lopa, S.S.; Al-Amin, M.; Hasan, M.; Ahammed, M.; Islam, K.M.; Alam, A.H.M.; Tanaka, T.; Sadik, M. Phytochemical analysis and cholinesterase inhibitory and antioxidant activities of Enhydra fluctuans relevant in the management of Alzheimer’s disease. Int. J. Food Sci. 2021, 2021, 8862025. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.E.; Ahmed, S.A.; Amer, S.A.; Al-Gabri, N.A.; Ahmed, A.I.; Abdel-Warith, A.W.A.; Younis, E.S.M.; Metwally, A.E. Influence of vitamin C feed supplementation on the growth, antioxidant activity, immune status, tissue histomorphology, and disease resistance in Nile tilapia, Oreochromis niloticus. Aquac. Rep. 2020, 18, 100545. [Google Scholar] [CrossRef]
- Ouknin, M.; Aghraz, A.; Chibane, M.; Boumezzourh, A.; Costa, J.; Majidi, L. Enzyme inhibitory, antioxidant activity and phytochemical analysis of essential oil from cultivated Rosmarinus officinalis. J. Food Meas. Charact. 2021, 15, 3782–3790. [Google Scholar] [CrossRef]
- Boccardi, V.; Arosio, B.; Cari, L.; Bastiani, P.; Scamosci, M.; Casati, M.; Ferri, E.; Bertagnoli, L.; Ciccone, S.; Rossi, P.D.; et al. Beta-carotene, telomerase activity and Alzheimer’s disease in old age subjects. Eur. J. Nutr. 2019, 59, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Mielech, A.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. Vitamins in Alzheimer’s disease—Review of the latest reports. Nutritients 2020, 12, 3458. [Google Scholar] [CrossRef] [PubMed]
- Alam, J. Vitamins: A nutritional intervention to modulate the Alzheimer’s disease progression. Nutr. Neurosci. 2020, 1–18. [Google Scholar] [CrossRef]
- Gavazza, M.; Marmunti, M.; Leaden, P.; Zeinsteger, P.; Palacios, A. Alpha-lipoic acid: Antioxidant activity against non-enzymatic peroxidation of rat kidney and liver mitochondria. J. Clin. Biomed. Investig. 2021, 1, 1–4. [Google Scholar] [CrossRef]
- Pei, X.; Hu, F.; Luo, F.; Huang, X.; Li, X.; Xing, S.; Long, D. The neuroprotective effects of alpha-lipoic acid on an experimental model of Alzheimer’s disease in PC12 cells. J. Appl. Toxicol. 2021, 42, 284–295. [Google Scholar] [CrossRef]
- Wear, D.; Vegh, C.; Sandhu, J.K.; Sikorska, M.; Cohen, J.; Pandey, S. Ubisol-Q10, a nanomicellar and water-dispersible formulation of coenzyme-Q10 as a potential treatment for Alzheimer’s and Parkinson’s disease. Antioxidants 2021, 10, 764. [Google Scholar] [CrossRef]
- Attia, H.; Albuhayri, S.; Alaraidh, S.; Alotaibi, A.; Yacoub, H.; Mohamad, R.; Al-Amin, M. Biotin, coenzyme Q10, and their combination ameliorate aluminium chloride-induced Alzheimer’s disease via attenuating neuroinflammation and improving brain insulin signaling. J. Biochem. Mol. Toxicol. 2020, 34, e22519. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.F.; Chang, W.H.; Black, R.M.; Liu, J.R.; Sompol, P.; Chen, Y.; Wei, H.; Zhao, Q.; Cheng, I.H. Crude caffeine reduces memory impairment and amyloid β1–42 levels in an Alzheimer’s mouse model. Food Chem. 2012, 135, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Londzin, P.; Zamora, M.; Kąkol, B.; Taborek, A.; Folwarczna, J. Potential of caffeine in Alzheimer’s disease—A review of experimental studies. Nutritients 2021, 13, 537. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, C.; Zhang, D.; Yuan, M.; Chen, C.-H.; Li, M. Synergic effects of berberine and curcumin on improving cognitive function in an Alzheimer’s disease mouse model. Neurochem. Res. 2020, 45, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Rubab, M.; Tyagi, A.; Oh, D.-H. Curcumin and its derivatives as theranostic agents in Alzheimer’s disease: The implication of nanotechnology. Int. J. Mol. Sci. 2021, 22, 196. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H. Berberine neuroprotection and antioxidant activity. Oxidative stress diet. Antioxid. Neurol. Dis. 2020, 199–216. [Google Scholar] [CrossRef]
- Tarabasz, D.; Kukula-Koch, W. Palmatine: A review of pharmacological properties and pharmacokinetics. Phyther. Res. 2020, 34, 33–50. [Google Scholar] [CrossRef]
- Chaves, S.K.M.; Afzal, M.I.; Islam, M.T.; Hameed, A.; Da Mata, A.M.O.F.; da Silva Araújo, L.; Ali, S.W.; Rolim, H.M.L.; De Medeiros, M.D.G.F.; Costa, E.V.; et al. Palmatine antioxidant and anti-acetylcholinesterase activities: A pre-clinical assessment. Cell. Mol. Biol. 2020, 66, 54–59. [Google Scholar] [CrossRef]
- Qajari, N.M.; Khonakdar-Tarsi, A. Short communication silibinin and neurological diseases. J. Neurobiol. Physiol. 2021, 3, 8–9. [Google Scholar]
- Bai, D.; Jin, G.; Yin, S.; Zou, D.; Zhu, Q.; Yang, Z.; Liu, X.; Ren, L.; Sun, Y.; Gan, S. Antioxidative and anti-apoptotic roles of silibinin in reversing learning and memory deficits in APP/PS1 mice. Neurochem. Res. 2017, 42, 3439–3445. [Google Scholar] [CrossRef]
- Zhao, L.J.; Liu, W.; Xiong, S.H.; Tang, J.; Lou, Z.H.; Xie, M.X.; Xia, B.H.; Lin, L.M.; Liao, D.F. Determination of total flavonoids contents and antioxidant activity of Ginkgo biloba leaf by near-infrared reflectance method. Int. J. Anal. Chem. 2018, 2018, 8195784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and antioxidant effect of Ginkgo biloba extract against AD and other neurological disorders. Neurotherapeuthics 2019, 16, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Pachón-Angona, I.; Refouvelet, B.; Andrýs, R.; Martin, H.; Luzet, V.; Iriepa, I.; Moraleda, I.; Diez-Iriepa, D.; Oset-Gasque, M.J.; Marco-Contelles, J.; et al. Donepezil + chromone + melatonin hybrids as promising agents for Alzheimer’s disease therapy. J. Enzym. Inh. Med. Chem. 2019, 34, 479–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.F.; Wang, N.; Chen, R.; Li, S.; Roy, J.; Uddin, M.G.; Li, Z.; Lim, L.W.; Song, Y.Q. Exploring the multifunctional role of melatonin in regulating autophagy and sleep to mitigate Alzheimer’s disease neuropathology. Ageing Res. Rev. 2021, 67, 101304. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Yan, Y.; He, X.Y.; Yang, H.; Liang, B.; Wang, J.; He, Y.; Ding, Y.; Yu, H. Effects of resveratrol on the mechanisms of antioxidants and estrogen in Alzheimer’s disease. BioMed. Res. Int. 2019, 2019, 8983752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guglielmotto, M.; Manassero, G.; Vasciaveo, V.; Venezia, M.; Tabaton, M.; Tamagno, E. Estrogens inhibit amyloid-β-mediated paired helical filament-like conformation of tau through antioxidant activity and miRNA 218 regulation in hTau mice. J. Alzheimer’s Dis. 2020, 77, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Guglielmotto, M. Estrogens still represent an attractive therapeutic approach for Alzheimer’s disease. Neural Regen. Res. 2022, 17, 93. [Google Scholar] [CrossRef]
- Feizipour, S.; Sobhani, S.; Mehrafza, S.; Gholami, M.; Motaghinejad, M.; Motevalian, M.; Safari, S.; Davoudizadeh, R. Selegiline acts as neuroprotective agent against methamphetamine-prompted mood and cognitive related behavior and neurotoxicity in rats: Involvement of CREB/BDNF and Akt/GSK3 signal pathways. Iran. J. Basic Med. Sci. 2020, 23, 606. [Google Scholar] [CrossRef]
- Cai, M.; Yang, E.J. Effect of combined electroacupuncture and selegiline treatment in Alzheimer’s disease: An animal model. Front. Pharmacol. 2020, 11, 2048. [Google Scholar] [CrossRef]
- Gomes, B.A.Q.; Silva, J.P.; Romeiro, C.F.; Dos Santos, S.M.; Rodrigues, C.A.; Goncalves, P.R.; Sakai, J.T.; Mendes, P.F.; Varela, E.L.; Monteiro, M.C. Neuroprotective mechanisms of resveratrol in Alzheimer’s disease: Role of SIRT1. Oxidative Med. Cell. Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Chang, J.H.; Barroso, E.; Espina, M.; Kühne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-drug loaded nanoparticles of epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J. Control. Release 2019, 301, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Ide, K.; Matsuoka, N.; Yamada, H.; Furushima, D.; Kawakami, K. Effects of tea catechins on Alzheimer’s disease: Recent updates and perspectives. Molecules 2018, 23, 2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olajide, O.A.; Bhatia, H.S.; de Oliveira, A.C.; Wright, C.W.; Fiebich, B.L. Inhibition of neuroinflammation in LPS-activated microglia by cryptolepine. Evid. Based Complementary Altern. Med. 2013, 2013, 459723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynish, W.; Andrieu, S.; Nourhashemi, F.; Vellas, B. Nutritional factors and Alzheimer’s disease. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M675–M680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, F.C.; Shukitt-Hale, B.; Joseph, J.A. Nutritional intervention in brain aging. In Inflammation in the Pathogenesis of Chronic Diseases; Springer: Dordrecht, The Netherlands, 2007; pp. 299–318. [Google Scholar] [CrossRef]
- Burgener, S.C.; Buettner, L.; Buckwalter, K.C.; Beattie, E.; Bossen, A.L.; Fick, D.M.; Schreiner, A. Evidence supporting nutritional interventions for persons in early stage Alzheimer’s disease (AD). J. Nutr. Health Aging 2008, 12, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, D.S.; Perry, G. The four pillars of Alzheimer’s prevention. In Cerebrum: The Dana Forum on Brain Science; Dana Foundation: New York, NY, USA, 2017; Volume 2017. [Google Scholar]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Antioxidant activities, phenolic profiles, and organic acid contents of fruit vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef] [Green Version]
- Nazıroğlu, M.; Güler, M.; Özgül, C.; Saydam, G.; Küçükayaz, M.; Sözbir, E. Apple cider vinegar modulates serum lipid profile, erythrocyte, kidney, and liver membrane oxidative stress in ovariectomized mice fed high cholesterol. J. Membr. Biol. 2014, 247, 667–673. [Google Scholar] [CrossRef]
- Frassetto, L.; Morris, R.C., Jr.; Sellmeyer, D.E.; Todd, K.; Sebastian, A. Diet, evolution and aging. Eur. J. Nutr. 2001, 40, 200–213. [Google Scholar] [CrossRef]
- Sebastian, A.; Frassetto, L.A.; Sellmeyer, D.E.; Morris, R.C., Jr. The evolution-informed optimal dietary potassium intake of human beings greatly exceeds current and recommended intakes. Semin. Nephrol. 2006, 26, 447–453. [Google Scholar] [CrossRef]
- Mathew, B.C.; Biju, R.S. Neuroprotective effects of garlic a review. Libyan J. Med. 2008, 3, 23–33. [Google Scholar]
- Arslan, J.; Jamshed, H.; Qureshi, H. Early detection and prevention of Alzheimer’s disease: Role of oxidative markers and natural antioxidants. Front. Aging Neurosci. 2020, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Lloret, A.; Esteve, D.; Monllor, P.; Cervera-Ferri, A.; Lloret, A. The effectiveness of vitamin E treatment in Alzheimer’s disease. Int. J. Mol. Sci. 2019, 20, 879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliev, G.; Priyadarshini, M.; Reddy, V.P.; Grieg, N.H.; Kaminsky, Y.; Cacabelos, R.; Zamyatnin, J. Oxidative stress mediated mitochondrial and vascular lesions as markers in the pathogenesis of Alzheimer disease. Curr. Med. Chem. 2014, 21, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.P.; de Castro, A.A.; Soares, F.V.; da Cunha, E.F.; Ramalho, T.C. Future therapeutic perspectives into the alzheimer’s disease targeting the oxidative stress hypothesis. Molecules 2019, 24, 4410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, Y.B.; Praticò, D. Vitamin E in aging, dementia, and Alzheimer’s disease. Biofactors 2012, 38, 90–97. [Google Scholar] [CrossRef]
- Rigotti, A. Absorption, transport, and tissue delivery of vitamin E. Mol. Asp. Med. 2007, 28, 423–436. [Google Scholar] [CrossRef]
- Zhang, S.M.; Hernan, M.A.; Chen, H.; Spiegelman, D.; Willett, W.C.; Ascherio, A. Intakes of vitamins E and C, carotenoids, vitamin supplements, and PD risk. Neurology 2002, 59, 1161–1169. [Google Scholar] [CrossRef]
- Fata, G.L.; Weber, P.; Mohajeri, M.H. Effects of vitamin E on cognitive performance during ageing and in Alzheimer’s disease. Nutrients 2014, 6, 5453–5472. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Chen, X.; Liu, Y.; Shu, Y.; Chen, T.; Xu, L.; Li, M.; Guan, X. Do low-serum vitamin E levels increase the risk of Alzheimer disease in older people? Evidence from a meta-analysis of case-control studies. Int. J. Geriatr. Psychiatry 2018, 33, e257–e263. [Google Scholar] [CrossRef]
- Marklund, S.L. Properties of extracellular superoxide dismutase from human lung. Biochem. J. 1984, 220, 269–272. [Google Scholar] [CrossRef]
- Cowan, C.M.; Sealey, M.A.; Mudher, A. Suppression of tau-induced phenotypes by vitamin E demonstrates the dissociation of oxidative stress and phosphorylation in mechanisms of tau toxicity. J. Neurochem. 2021, 157, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Iijima-Ando, K.; Iijima, K. Transgenic Drosophila models of Alzheimer’s disease and tauopathies Brain Struct. Funct. 2010, 214, 245–262. [Google Scholar] [CrossRef] [Green Version]
- Casati, M.; Boccardi, V.; Ferri, E.; Bertagnoli, L.; Bastiani, P.; Ciccone, S.; Mansi, M.; Scamosci, M.; Rossi, P.D.; Mecocci, P.; et al. Vitamin E and Alzheimer’s disease: The mediating role of cellular aging. Aging Clin. Exp. Res. 2020, 32, 459–464. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Castegna, A.; Pocernich, C.B.; Drake, J.; Scapagnini, G.; Calabrese, V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J. Nutr. Biochem. 2002, 13, 444–461. [Google Scholar] [CrossRef]
- Dringen, R.; Brandmann, M.; Hohnholt, M.C.; Blumrich, E.M. Glutathione-dependent detoxification processes in astrocytes. Neurochem. Res. 2015, 40, 2570–2582. [Google Scholar] [CrossRef]
- Sears, M.E. 2013 Chelation: Harnessing and enhancing heavy metal detoxification—A review. Sci. World J. 2013, 2013, 219840. [Google Scholar] [CrossRef] [Green Version]
- Montserrat, M.; de Gregorio, E.; de Dios, C.; Roca-Agujetas, V.; Cucarull, B.; Tutusaus, A.; Morales, A.; Colell, A. Mitochondrial glutathione: Recent insights and role in disease. Antioxidants 2020, 9, 909. [Google Scholar]
- Barbero-Camps, E.; Fernández, A.; Martínez, L.; Fernández-Checa, J.C.; Colell, A. APP/PS1 mice overexpressing SREBP-2 exhibit combined Aβ accumulation and tau pathology underlying Alzheimer’s disease. Hum. Mol. Genet. 2013, 22, 3460–3476. [Google Scholar] [CrossRef]
- Mariet, A.; Zou, F.; Chai, H.S.; Younkin, C.S.; Miles, R.; Nair, A.A.; Crook, J.E.; Pankratz, V.S.; Carrasquillo, M.M.; Rowley, C.N.; et al. Glutathione S-transferase omega genes in Alzheimer and Parkinson disease risk, age-at-diagnosis and brain gene expression: An association study with mechanistic implications. Mol. Neurodegener. 2012, 7, 1–12. [Google Scholar]
- Slezák, J.; Kura, B.; Frimmel, K.; Zálešák, M.; Ravingerová, T.; Viczenczová, C.; Tribulová, N. Preventive and therapeutic application of molecular hydrogen in situations with excessive production of free radicals. Physiol. Res. 2016, 65 (Suppl. S1), S11–S28. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Klichko, V.I.; Safonov, V.L.; Safonov, M.Y.; Radyuk, S.N. Supplementation with hydrogen-producing composition confers beneficial effects on physiology and life span in Drosophila. Heliyon 2019, 5, e01679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, T. Monoamine oxidase-B inhibitors in the treatment of Alzheimers disease. Neurobiol. Aging 2000, 21, 343–348. [Google Scholar] [CrossRef]
- Sano, M.; Ernesto, C.; Thomas, R.G.; Klauber, M.R.; Schafer, K.; Grundman, M.; Schneider, L.S. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. N. Engl. J. Med. 1997, 336, 1216–1222. [Google Scholar] [CrossRef] [Green Version]
- Tuppo, E.E.; Forman, L.J. Free radical oxidative damage and Alzheimer’s disease. J. Am. Osteopath. Assoc. 2001, 101 (Suppl. S121), 11S–15S. [Google Scholar]
- Ross, D.; Mendiratta, S.; Qu, Z.C.; Cobb, C.E.; May, J.M. Ascorbate 6-palmitate protects human erythrocytes from oxidative damage. Free Radic. Biol. Med. 1999, 26, 81–89. [Google Scholar] [CrossRef]
- Nishida, S. Metabolic effects of melatonin on odative stress and dbetes mellitus. Endocrine 2005, 27, 131–135. [Google Scholar] [CrossRef]
- Fusco, D.; Colloca, G.; Monaco, M.R.L.; Cesari, M. Effects of antioxidant supplementation on the aging process. Clin. Interv. Aging 2007, 2, 377. [Google Scholar]
- Deng, Y.Q.; Xu, G.G.; Duan, P.; Zhang, Q.; Wang, J.Z. Effects of melatonin on wortmannin-induced tau hyperphosphorylation. Acta Pharmacol. Sin. 2005, 26, 519–526. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.Z.; Wang, Z.F. Role of melatonin in Alzheimer-like neurodegeneration. Acta Pharmacol. Sin. 2006, 27, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.J.; Wang, J.Z. Alzheimer-like tau phosphorylation induced by wortmannin in vivo and its attenuation by melatonin. Acta Pharmacol. Sin. 2002, 23, 183. [Google Scholar]
- Wang, D.L.; Ling, Z.Q.; Cao, F.Y.; Zhu, L.Q.; Wang, J.Z. Melatonin attenuates isoproterenol-induced protein kinase A overactivation and tau hyperphosphorylation in rat brain. J. Pineal Res. 2004, 37, 11–16. [Google Scholar] [CrossRef]
- Wang, X.C.; Zhang, J.; Yu, X.; Han, L.; Zhou, Z.T.; Zhang, Y.; Wang, J.Z. Prevention of isoproterenol-induced tau hyperphosphorylation by melatonin in the rat. Sheng Li Xue Bao Acta Physiol. Sin. 2005, 57, 7–12. [Google Scholar]
- Zhou, J.; Zhang, S.; Zhao, X.; Wei, T. Melatonin impairs NADPH oxidase assembly and decreases superoxide anion production in microglia exposed to amyloid-β1–42. J. Pineal Res. 2008, 45, 157–165. [Google Scholar] [CrossRef]
- Fakhri, S.; Yosifova Aneva, I.; Farzaei, M.H.; Sobarzo-Sánchez, E. The neuroprotective effects of astaxanthin: Therapeutic targets and clinical perspective. Moleluces 2019, 24, 2640. [Google Scholar] [CrossRef] [Green Version]
- Pokorski, M.; Marczak, M.; Dymecka, A.; Suchocki, P. Ascorbyl palmitate as a carrier of ascorbate into neural tissues. J. Biomed. Sci. 2003, 10, 193–198. [Google Scholar] [CrossRef]
- Frank, B.; Gupta, S. A review of antioxidants and Alzheimer’s disease. Ann. Clin. Psychiatry 2005, 17, 269–286. [Google Scholar] [CrossRef]
- Reddy, P.H.; Manczak, M.; Yin, X.; Grady, M.C.; Mitchell, A.; Tonk, S.; Kuruva, C.S.; Bhatti, J.S.; Kandimalla, R.; Vijayan, M.; et al. Protective effects of Indian spice curcumin against amyloid-β in Alzheimer’s disease. J. Alzheimers Dis. 2018, 61, 843–866. [Google Scholar] [CrossRef]
- Wei, Q.Y.; Chen, W.F.; Zhou, B.; Yang, L.; Liu, Z.L. Inhibition of lipid peroxidation and protein oxidation in rat liver mitochondria by curcumin and its analogues. Biochim. Biophys. Acta 2006, 1760, 70–77. [Google Scholar] [CrossRef]
- Baum, L.; Ng, A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J. Alzheimers Dis. 2004, 6, 367–377. [Google Scholar] [CrossRef]
- Nishinaka, T.; Ichijo, Y.; Ito, M.; Kimura, M.; Katsuyama, M.; Iwata, K.; Miura, T.; Terada, T.; Yabe-Nishimura, C. Curcumin activates human glutathione S-transferase P1 expression through antioxidant response element. Toxicol. Lett. 2007, 170, 238–247. [Google Scholar] [CrossRef]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef]
- Serafini, M.M.; Catanzaro, M.; Rosini, M.; Racchi, M.; Lanni, C. Curcumin in Alzheimer’s disease: Can we think to new strategies and perspectives for this molecule? Pharmacol. Res. 2017, 124, 146–155. [Google Scholar] [CrossRef]
- Baschiera, E.; Sorrentino, U.; Calderan, C.; Desbats, M.A.; Salviati, L. The multiple roles of coenzyme Q in cellular homeostasis and their relevance for the pathogenesis of coenzyme Q deficiency. Free Radic. Biol. Med. 2021, 166, 277–286. [Google Scholar] [CrossRef]
- Seo, J.S.; Kim, T.K.; Leem, Y.H.; Lee, K.W.; Park, S.K.; Baek, I.S.; Han, P.L. SK-PC-B70M confers anti-oxidant activity and reduces Aβ levels in the brain of Tg2576 mice. Brain Res. 2009, 1261, 100–108. [Google Scholar] [CrossRef]
- Mulnard, R.A.; Cotman, C.W.; Kawas, C.; van Dyck, C.H.; Sano, M.; Doody, R.; Grundman, M. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: A randomized controlled trial. JAMA 2000, 283, 1007–1015. [Google Scholar] [CrossRef]
- Trivic, T.; Vojnovic, M.; Drid, P.; Ostojic, S.M. Drinking hydrogen-rich water for 4 weeks positively affects serum antioxidant enzymes in healthy men: A pilot study. Curr. Top. Nutraceutical Res. 2017, 15, 45–48. [Google Scholar]
- Shah, M.; Mahfuzur, R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Gong, N.; Zhao, J.; Yu, Z.; Gu, F.; Chen, J.; Dong, W. Powerful beneficial effects of benfotiamine on cognitive impairment and β-amyloid deposition in amyloid precursor protein/presenilin-1 transgenic mice. Brain 2010, 133, 1342–1351. [Google Scholar] [CrossRef]
- Gonzales, M.M.; Garbarino, V.R.; Marques Zilli, E.; Petersen, R.C.; Kirkland, J.L.; Tchkonia, T.; Musi, N.; Seshadri, S.; Craft, S.; Miranda, E. Orr senolytic therapy to modulate the progression of Alzheimer’s disease (SToMP-AD): A pilot clinical trial. J. Prev. Alzheimer’s Dis. 2021, 1–8. [Google Scholar] [CrossRef]
- Siedlak, S.L.; Casadesus, G.; Webber, K.M.; Pappolla, M.A.; Atwood, C.S.; Smith, M.A.; Perry, G. Chronic antioxidant therapy reduces oxidative stress in a mouse model of Alzheimer’s disease. Free Radic. Res. 2009, 43, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Raina, A.K.; Perry, G.; Smith, M.A. Alzheimer’s disease: The two-hit hypothesis. Lancet Neurol. 2004, 3, 219–226. [Google Scholar] [CrossRef]
- Quinn, J.F.; Bussiere, J.R.; Hammond, R.S.; Montine, T.J.; Henson, E.; Jones, R.E.; Stackman, R.W., Jr. Chronic dietary alpha-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol. Aging 2007, 28, 213–225. [Google Scholar] [CrossRef]
- Sajjad, N.; Wani, A.; Hassan, S.; Ali, R.; Hamid, R.; Akbar, S.; Bhat, E. Interplay of antioxidants in Alzheimer’s disease. J. Transl. Sci. 2019, 5, 1–11. [Google Scholar]
- Farr, S.A.; Price, T.O.; Banks, W.A.; Ercal, N.; Morley, J.E. Effect of alpha-lipoic acid on memory, oxidation, and lifespan in SAMP8 mice. J. Alzheimer’s Dis. 2012, 32, 447–455. [Google Scholar] [CrossRef]
- Banks William, A.; Elizabeth, M. Rhea the blood–brain barrier, oxidative stress, and insulin resistance. Antioxidants 2021, 10, 1695. [Google Scholar] [CrossRef]
- Singh, S.K.; Srikrishna, S.; Castellani, R.J.; Perry, G. Antioxidants in the prevention and treatment of Alzheimer’s disease. In Nutritional Antioxidant Therapies: Treatments and Perspectives; Springer: Cham, Switzerland, 2017; pp. 523–553. [Google Scholar]
- Arbo, B.D.; André-Miral, C.; Nasre-Nasser, R.G.; Schimith, L.E.; Santos, M.G.; Costa-Silva, D.; Muccillo-Baisch, A.L.; Hort, M.A. Resveratrol Derivatives as Potential Treatments for Alzheimer’s and Parkinson’s Disease. Front. Aging Neurosci. 2020, 12, 103. [Google Scholar] [CrossRef]
- McManus, M.J.; Murphy, M.P.; Franklin, J.L. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2011, 31, 15703–15715. [Google Scholar] [CrossRef] [Green Version]
- James, A.M.; Sharpley, M.S.; Manas, A.R.; Frerman, F.E.; Hirst, J.; Smith, R.A.; Murphy, M.P. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J. Biol. Chem. 2007, 282, 14708–14718. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.P.; Smith, R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 629–656. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, D.; Whiteman, M.; Armstrong, J.S. Is antioxidant potential of the mitochondrial targeted ubiquinone derivative MitoQ conserved in cells lacking mtDNA? Antioxid. Redox Signal. 2008, 10, 651–660. [Google Scholar] [CrossRef]
- Ng, L.F.; Gruber, J.; Cheah, I.K.; Goo, C.K.; Cheong, W.F.; Shui, G.; Halliwell, B. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic. Biol. Med. 2014, 71, 390–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manczak, M.; Mao, P.; Calkins, M.J.; Cornea, A.; Reddy, A.P.; Murphy, M.P.; Szeto, H.H.; Park, B.; Reddy, P.H. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J. Alzheimer’s Dis. JAD 2010, 20 (Suppl. S2), S609–S631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.T.; Jung, C.H.; Lee, S.R.; Bae, J.H.; Baek, W.L.; Suh, M.H.; Park, J.; Park, C.W.; Suh, S.I. The green tea polyphenol (−)-epigallocatechin gallate attenuates β-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 2001, 70, 603–614. [Google Scholar] [CrossRef]
- Haque, A.M.; Hashimoto, M.; Katakura, M.; Hara, Y.; Shido, O. Green tea catechins prevent cognitive deficits caused by Abeta1-40 in rats. J. Nutr. Biochem. 2008, 19, 619–626. [Google Scholar] [CrossRef]
- Kaur, T.; Pathak, C.M.; Pandhi, P.; Khanduja, K.L. Effects of green tea extract on learning, memory, behavior and acetylcholinesterase activity in young and old male rats. Brain Cogn. 2008, 67, 25–30. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Hasegawa, H.; Okuda, S.; Muraki, T.; Uruno, T.; Kubota, K. Ameliorative effects of tea catechins on active oxygen-related nerve cell injuries. J. Pharmacol. Exp. Ther. 1995, 274, 602–608. [Google Scholar]
- Duan, S.; Guan, X.; Lin, R.; Liu, X.; Yan, Y.; Lin, R.; Zhang, T.; Chen, X.; Huang, J.; Sun, X.; et al. Silibinin inhibits acetylcholinesterase activity and amyloid β peptide aggregation: A dual-target drug for the treatment of Alzheimer’s disease. Neurobiol. Aging 2015, 36, 1792–1807. [Google Scholar] [CrossRef]
- Yin, F.; Liu, J.; Ji, X.; Wang, Y.; Zidichouski, J.; Zhang, J. Silibinin: A novel inhibitor of Aβ aggregation. Neurochem. Int. 2011, 58, 399–403. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Cui, L.; Liu, B.; Liu, W.; Hayashi, T.; Mizuno, K.; Hattori, S.; Ushiki-Kaku, Y.; Onodera, S.; Ikejima, T. Silibinin ameliorates STZ-induced impairment of memory and learning by up-regulating insulin signaling pathway and attenuating apoptosis. Physiol. Behav. 2020, 213, 112689. [Google Scholar] [CrossRef]
- Jia, W.; Su, Q.; Cheng, Q.; Peng, Q.; Qiao, A.; Luo, X.; Zhang, J.; Wang, Y. Neuroprotective Effects of Palmatine via the Enhancement of Antioxidant Defense and Small Heat Shock Protein Expression in Aβ-Transgenic Caenorhabditis elegans. Oxidative Med. Cell. Longev. 2021, 2021, 9966223. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Hong, J.; Hu, C.; Huang, C.; Gao, J.; Huang, J.; Wang, D.; Geng, Q.; Dong, Y. Palmatine protects against cerebral ischemia/reperfusion injury by activation of the AMPK/Nrf2 pathway. Oxidative Med. Cell. Longev. 2021, 2021, 6660193. [Google Scholar] [CrossRef]
- Mak, S.; Luk, W.W.; Cui, W.; Hu, S.; Tsim, K.W.; Han, Y. Synergistic inhibition on acetylcholinesterase by the combination of berberine and palmatine originally isolated from Chinese medicinal herbs. J. Mol. Neurosci. 2014, 53, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhan, C.; Zou, Y.; Qian, Z.; Wei, G.; Zhang, Q. Serotonin and Melatonin Show Different Modes of Action on Aβ42 Protofibril Destabilization. ACS Chem. Neurosci. 2021, 12, 799–809. [Google Scholar] [CrossRef]
- Geldenhuys, W.J.; Van der Schyf, C.J. Role of serotonin in Alzheimer’s disease. CNS Drugs 2011, 25, 765–781. [Google Scholar] [CrossRef]
- Aaldijk, E.; Vermeiren, Y. The role of serotonin within the microbiota-gut-brain axis in the development of Alzheimer’s disease: A narrative review. Ageing Res. Rev. 2022, 75, 101556. [Google Scholar] [CrossRef]
- Ikram, M.; Jo, M.G.; Park, T.J.; Kim, M.W.; Khan, I.; Jo, M.H.; Kim, M.O. Oral administration of gintonin protects THE Brains of mice against Aβ-induced Alzheimer disease pathology: Antioxidant and anti-inflammatory effects. Oxidative Med. Cell. Longev. 2021, 2021, 6635552. [Google Scholar] [CrossRef]
- Jakaria, M.; Azam, S.; Go, E.A.; Uddin, M.S.; Jo, S.H.; Choi, D.K. Biological evidence of gintonin efficacy in memory disorders. Pharmacol. Res. 2021, 163, 105221. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, R.; Nam, S.M.; Kim, D.G.; Cho, I.H.; Kim, H.C.; Cho, Y.; Rhim, H.; Nah, S.Y. Ginseng gintonin, aging societies, and geriatric brain diseases. Integr. Med. Res. 2021, 10, 100450. [Google Scholar] [CrossRef]
- Jang, M.; Choi, S.H.; Choi, J.H.; Oh, J.; Lee, R.M.; Lee, N.E.; Cho, Y.J.; Rhim, H.; Kim, H.C.; Cho, I.H.; et al. Ginseng gintonin attenuates the disruptions of brain microvascular permeability and microvascular endothelium junctional proteins in an APPswe/PSEN-1 double-transgenic mouse model of Alzheimer’s disease. Exp. Ther. Med. 2021, 21, 1. [Google Scholar] [CrossRef]
- Firdaus, Z.; Tryambak, D. Singh an insight in pathophysiological mechanism of Alzheimer’s disease and its management using plant natural products. Mini Rev. Med. Chem. 2021, 21, 35–57. [Google Scholar] [CrossRef] [PubMed]
- He, F.Q.; Qiu, B.Y.; Li, T.K.; Xie, Q.; Cui, D.J.; Huang, X.L.; Gan, H.T. Tetrandrine suppresses amyloid-β-induced inflammatory cytokines by inhibiting NF-κB pathway in murine BV2 microglial cells. Int. Immunopharmacol. 2011, 11, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, S.U. Flavonoids for treatment of Alzheimer’s disease: An up to date review. EXCLI J. 2021, 20, 495–502. [Google Scholar]
- Wang, J.A.; Tong, M.L.; Zhao, B.; Zhu, G.; Xi, D.H.; Yang, J.P. Parthenolide ameliorates intracerebral hemorrhage-induced brain injury in rats. Phytother. Res. PTR 2020, 34, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Qiang, W.; Cai, W.; Yang, Q.; Yang, L.; Dai, Y.; Zhao, Z.; Yin, J.; Li, Y.; Li, Q.; Wang, Y.; et al. Artemisinin B improves learning and memory impairment in AD dementia mice by suppressing neuroinflammation. Neuroscience 2018, 395, 1–12. [Google Scholar] [CrossRef]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.E.; Nofal, S.; Abdel-Mottaleb, Y.; Badary, O.A. Thymoquinone alleviates the experimentally induced Alzheimer’s disease inflammation by modulation of TLRs signaling. Hum. Exp. Toxicol. 2018, 37, 1092–1104. [Google Scholar] [CrossRef]
- Fang, F.; Chen, X.; Huang, T.; Lue, L.F.; Luddy, J.S.; Yan, S.S. Multi-faced neuroprotective effects of Ginsenoside Rg1 in an Alzheimer mouse model. Biochim. Biophys. Acta 2012, 1822, 286–292. [Google Scholar] [CrossRef] [Green Version]
- Karakani, A.M.; Riazi, G.; Mahmood Ghaffari, S.; Ahmadian, S.; Mokhtari, F.; Jalili Firuzi, M.; Zahra Bathaie, S. Inhibitory effect of corcin on aggregation of 1N/4R human tau protein in vitro. Iran. J. Basic Med Sci. 2015, 18, 485–492. [Google Scholar]
- Azimi, A.; Ghaffari, S.M.; Riazi, G.H.; Arab, S.S.; Tavakol, M.M.; Pooyan, S. α-Cyperone of Cyperus rotundus is an effective candidate for reduction of inflammation by destabilization of microtubule fibers in brain. J. Ethnopharmacol. 2016, 194, 219–227. [Google Scholar] [CrossRef]
- Gong, H.; He, Z.; Peng, A.; Zhang, X.; Cheng, B.; Sun, Y.; Zheng, L.; Huang, K. Effects of several quinones on insulin aggregation. Sci. Rep. 2014, 4, 5648. [Google Scholar] [CrossRef] [Green Version]
- Keshavarz, M.; Farrokhi, M.R.; Amiri, A. Caffeine neuroprotective mechanism against β-amyloid neurotoxicity in SHSY5Y cell line: Involvement of adenosine, ryanodine, and N-methyl-D-aspartate receptors. Adv. Pharm. Bull. 2017, 7, 579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Alcântara Almeida, I.; Dorvigny, B.M.; Tavares, L.S.; Santana, L.N.; Lima-Filho, J.V. Anti-inflammatory activity of caffeine (1, 3, 7-trimethylxanthine) after experimental challenge with virulent Listeria monocytogenes in Swiss mice. Int. Immunopharmacol. 2021, 100, 108090. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.L.; Arantes, L.P.; da Silveira, T.L.; Zamberlan, D.C.; Cordeiro, L.M.; Obetine, F.B.B.; da Silva, A.F.; da Cruz, I.B.M.; Soares, F.A.A.; Riva de Oliveria, P. Ilex paraguariensis extract provides increased resistance against oxidative stress and protection against Amyloid beta-induced toxicity compared to caffeine in Caenorhabditis elegans. Nutr. Neurosci. 2021, 24, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Solfrizzi, V.; Barulli, M.R.; Bonfiglio, C.; Guerra, V.; Osella, A.; Seripa, D.; Sabbà, C.; Pilotto, A.; Logroscino, G. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: A systematic review. J. Nutr. Health Aging 2015, 19, 313–328. [Google Scholar] [CrossRef]
- Taheri, P.; Yaghmaei, P.; Tehrani, H.S.; Ebrahim-Habibi, A. Effects of eugenol on alzheimer’s disease-like manifestations in insulin-and Aβ-induced rat models. Neurophysiology 2019, 51, 114–119. [Google Scholar] [CrossRef]
- Studzinski, C.M.; Li, F.; Bruce-Keller, A.J.; Fernandez-Kim, S.O.; Zhang, L.; Weidner, A.M.; Markesbery, W.R.; Murphy, M.P.; Keller, J.N. Effects of short-term Western diet on cerebral oxidative stress and diabetes related factors in APP× PS1 knock-in mice. J. Neurochem. 2009, 108, 860–866. [Google Scholar] [CrossRef] [Green Version]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Manly, J.J.; Schupf, N.; Luchsinger, J.A. Mediterranean diet and mild cognitive impairment. Arch. Neurol. 2009, 66, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Vardarajan, B.N.; Barral, S.; Jaworski, J.; Beecham, G.W.; Blue, E.; Tosto, G.; Reyes-Dumeyer, D.; Medrano, M.; Lantigua, R.; Naj, A.; et al. Whole genome sequencing of Caribbean Hispanic families with late-onset Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2018, 5, 406–417. [Google Scholar] [CrossRef] [Green Version]
- Nair, S.; Rocha-Ferreira, E.; Fleiss, B.; Nijboer, C.H.; Gressens, P.; Mallard, C.; Hagberg, H. Neuroprotection offered by mesenchymal stem cells in perinatal brain injury: Role of mitochondria, inflammation, and reactive oxygen species. J. Neurochem. 2021, 158, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Se Thoe, E.; Fauzi, A.; Tang, Y.Q.; Chamyuang, S.; Chia, A.Y.Y. A review on advances of treatment modalities for Alzheimer’s disease. Life Sci. 2021, 276, 119129. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Terzo, S.; Mulè, F. Natural compounds as beneficial antioxidant agents in neurodegenerative disorders: A focus on Alzheimer’s disease. Antioxidants 2019, 8, 608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabel, M.; Nackenoff, A.; Kirsch, W.M.; Harrison, F.E.; Perry, G.; Schrag, M. Markers of oxidative damage to lipids, nucleic acids and proteins and antioxidant enzymes activities in Alzheimer’s disease brain: A meta-analysis in human pathological specimens. Free Radic. Biol. Med. 2018, 115, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Khan, H.; Khan, I.; Guo, O.; Sobarzo-Sánchez, E.; Rastrelli, L.; Kamal, M.A. An increasing role of polyphenols as novel therapeutics for Alzheimer’s: A review. Med. Chem. 2019, 16, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Pasinetti, G.M.; Wang, J.; Ho, L.; Zhao, W.; Dubner, L. Roles of resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 1202–1208. [Google Scholar] [CrossRef] [Green Version]
- Pinchuk, I.; Shoval, H.; Dotan, Y.; Lichtenberg, D. Evaluation of antioxidants: Scope, limitations and relevance of assays. Chem. Phys. Lipids 2012, 165, 638–647. [Google Scholar] [CrossRef]
- Martinelli, C.; Pucci, C.; Battaglini, M.; Marino, A.; Ciofani, G. Antioxidants and nanotechnology: Promises and limits of potentially disruptive approaches in the treatment of central nervous system diseases. Adv. Healthc. Mater. 2020, 9, 1901589. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).