Hydrological Season Can Have Unexpectedly Insignificant Influences on the Elevational Patterns of Functional Diversity of Riverine Macroinvertebrates
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
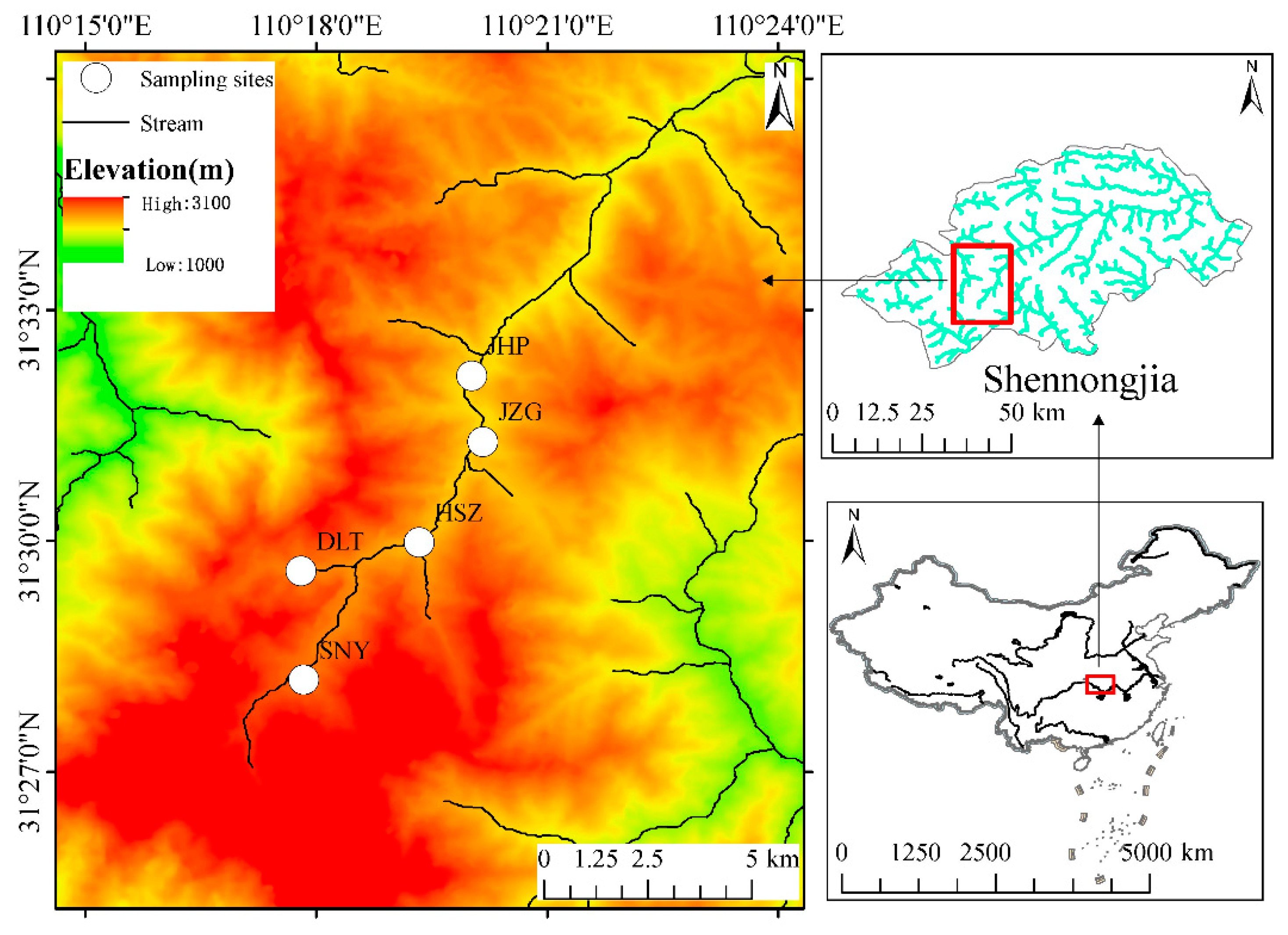
2.1. Study Area
2.2. Macroinvertebrates’ Sampling and Identification
2.3. Environmental Variables
2.4. Biodiversity Indices
2.5. Generalized Additive Model
3. Results
3.1. Assemblages of Macroinvertebrates
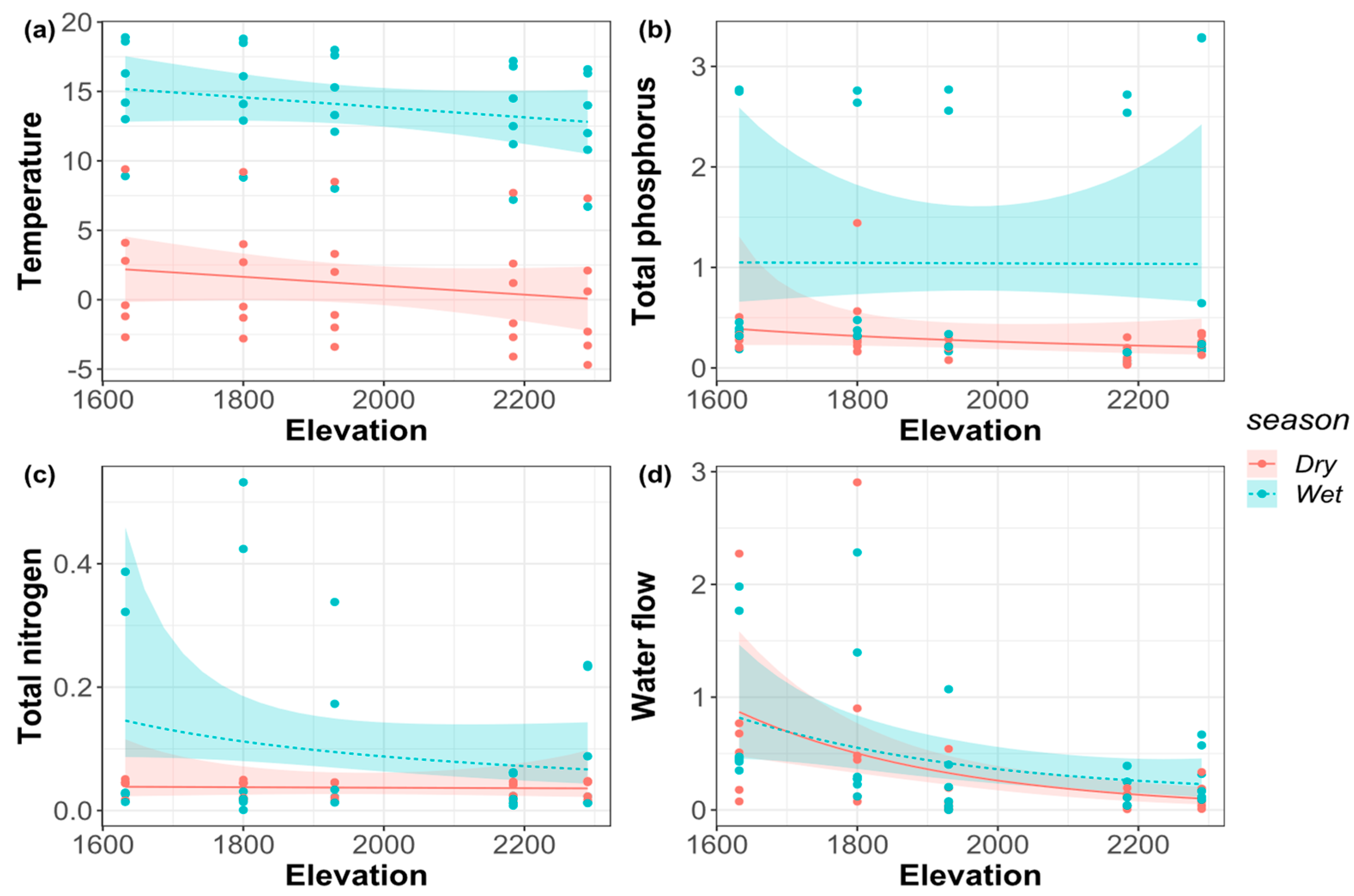
3.2. Environmental Factors along the Elevational Gradient
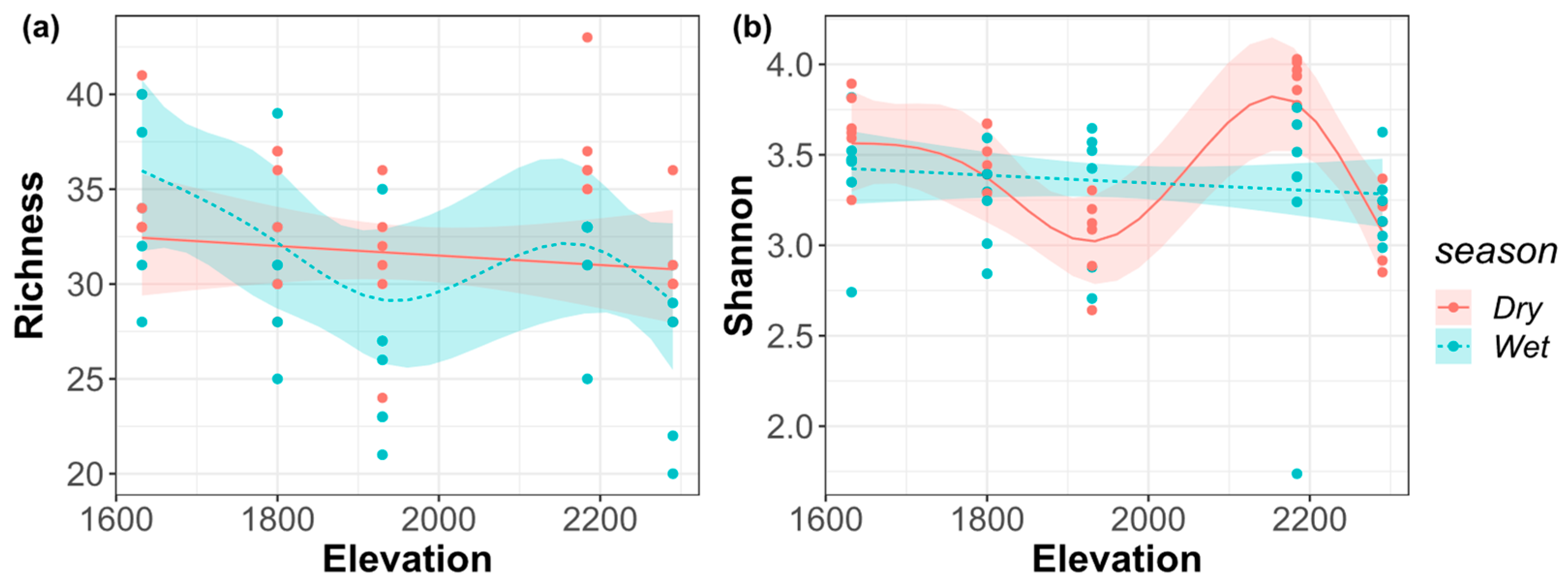
3.3. Taxonomic Diversity along the Elevational Gradient
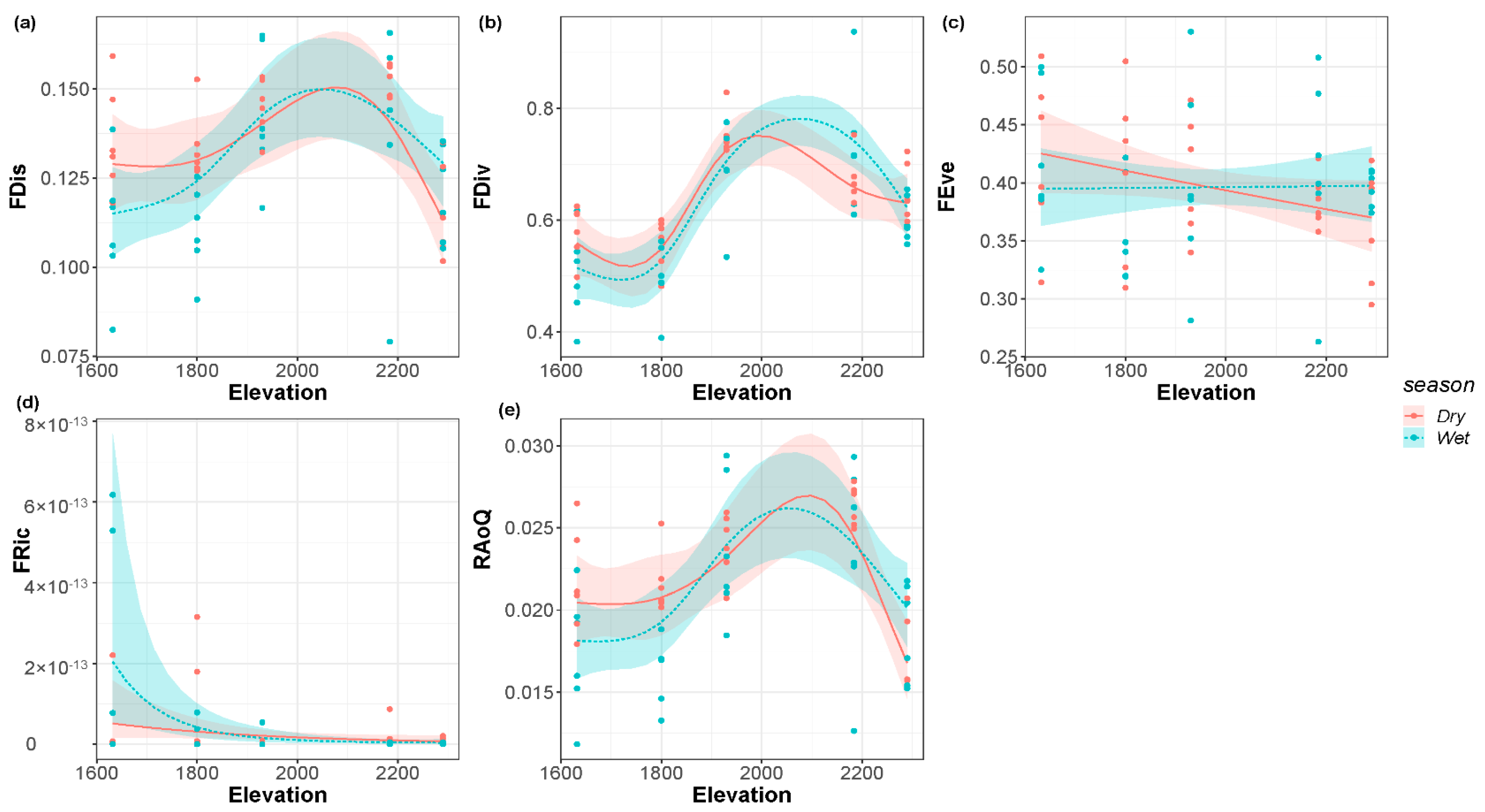
3.4. Functional Diversity along the Elevational Gradient
4. Discussion
4.1. Elevational Patterns of Functional Diversity
4.2. Seasonal Influences
4.3. Functional Diversity Indices
4.4. Unconsidered Aspects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lomolino, M.V. Elevation gradients of species-density: Historical and prospective views. Glob. Ecol. Biogeogr. 2001, 10, 3–13. [Google Scholar] [CrossRef]
- Sundqvist, M.K.; Sanders, N.J.; Wardle, D.A. Community and Ecosystem Responses to Elevational Gradients: Processes, Mechanisms, and Insights for Global Change. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 261–280. [Google Scholar] [CrossRef] [Green Version]
- Szewczyk, T.; McCain, C.M. A Systematic Review of Global Drivers of Ant Elevational Diversity. PLoS ONE 2016, 11, e0155404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Liu, Y. Climate stability is more important than water–energy variables in shaping the elevational variation in species richness. Ecol. Evol. 2018, 8, 6872–6879. [Google Scholar] [CrossRef] [PubMed]
- McCain, C.M.; Grytnes, J. Elevational Gradients in Species Richness; John Wiley & Sons, Ltd.: Chichester, UK, 2010. [Google Scholar]
- Butterfield, B.J.; Suding, K. Single-trait functional indices outperform multi-trait indices in linking environmental gradients and ecosystem services in a complex landscape. J. Ecol. 2012, 101, 9–17. [Google Scholar] [CrossRef]
- McGill, B.J.; Enquist, B.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.; Finlay, K.; Beisner, B.E. Functional diversity of crustacean zooplankton communities: Towards a trait-based classification. Freshw. Biol. 2007, 52, 796–813. [Google Scholar] [CrossRef]
- Jiang, W.X.; He, F.Z.; Cai, Q.H. Spatial distribution patterns of trait composition and functional diversity of aquatic insects in the Xiangxi River system. Acta Ecol. Sin. 2017, 37, 1861–1870. [Google Scholar]
- Tilman, D.; Naeem, S.; Knops, J.; Reich, P.; Siemann, E.; Wedin, D.; Ritchie, M.; Lawton, J. Biodiversity and Ecosystem Properties. Science 1997, 278, 1865–1869. [Google Scholar] [CrossRef]
- Petchey, O.L.; Hector, A.; Gaston, K.J. How do different measures of functional diversity perform? Ecology 2004, 85, 847–857. [Google Scholar] [CrossRef]
- Verberk, W.; Van Noordwijk, C.G.E.; Hildrew, A.G. Delivering on a promise: Integrating species traits to transform descriptive community ecology into a predictive science. Freshw. Sci. 2013, 32, 531–547. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Kelt, D.A.; Sun, Z.; Liu, H.; Hu, L.-J.; Ren, H.; Wen, J. Global variation in elevational diversity patterns. Sci. Rep. 2013, 3, 3007. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, R.H.; MacArthur, R.H. Geographical Ecology: Patterns in the Distribution of Species; Princeton University Press: Princeton, NJ, USA, 1972; pp. VII–XVIII, 1–269. [Google Scholar]
- Stevens, G.C. The Elevational Gradient in Altitudinal Range: An Extension of Rapoport’s Latitudinal Rule to Altitude. Am. Nat. 1992, 140, 893–911. [Google Scholar] [CrossRef] [PubMed]
- Quintero, I.; Jetz, W. Global elevational diversity and diversification of birds. Nature 2018, 555, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Liang, W.; Shi, Y.; Lin, X.; Zhang, H.; Wu, X.; Xie, G.; Chain, P.; Grogan, P.; Chu, H. Contrasting elevational diversity patterns between eukaryotic soil microbes and plants. Ecology 2014, 95, 3190–3202. [Google Scholar] [CrossRef] [Green Version]
- Füreder, L.; Ettinger, R.; Boggero, A.; Thaler, B.; Thies, H. Macroinvertebrate Diversity in Alpine Lakes: Effects of Altitude and Catchment Properties. Hydrobiologia 2006, 562, 123–144. [Google Scholar] [CrossRef]
- De Mendoza, G.; Traunspurger, W.; Palomo, A.; Catalan, J. Nematode distributions as spatial null models for macroinvertebrate species richness across environmental gradients: A case from mountain lakes. Ecol. Evol. 2017, 7, 3016–3028. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, D. Contrasting patterns in local and zonal family richness of stream invertebrates along an Andean altitudinal gradient. Freshw. Biol. 2004, 49, 1293–1305. [Google Scholar] [CrossRef]
- Castro, D.M.P.; Callisto, M.; Solar, R.R.C.; Macedo, D.R.; Fernandes, G.W. Beta diversity of aquatic invertebrates increases along an altitudinal gradient in a Neotropical mountain. Biotropica 2019, 51, 399–411. [Google Scholar] [CrossRef]
- Jacobsen, D.; Milner, A.M.; Brown, L.; Dangles, O. Biodiversity under threat in glacier-fed river systems. Nat. Clim. Chang. 2012, 2, 361–364. [Google Scholar] [CrossRef]
- Teshome, M.; Asfaw, Z.; Mohammed, M. Pattern of functional diversity along the elevation gradient in the dry evergreen Afromontane forest of Hararghe Highland, Southeast Ethiopia. Biosyst. Divers. 2020, 28, 257–264. [Google Scholar] [CrossRef]
- De Bello, F.; Lavorel, S.; Lavergne, S.; Albert, C.H.; Boulangeat, I.; Mazel, F.; Thuiller, W. Hierarchical effects of environmental filters on the functional structure of plant communities: A case study in the French Alps. Ecography 2012, 36, 393–402. [Google Scholar] [CrossRef]
- Swenson, N.G.; Anglada-Cordero, P.; Barone, J.A. Deterministic tropical tree community turnover: Evidence from patterns of functional beta diversity along an elevational gradient. Proc. R. Soc. B Boil. Sci. 2011, 278, 877–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.-T.; Li, M.; Nie, E. Pattern of functional diversity along an altitudinal gradient in the Baihua Mountain Reserve of Beijing, China. Braz. J. Bot. 2014, 37, 37–45. [Google Scholar] [CrossRef]
- Lafferty, M.H. Changes in Taxonomic and Functional Diversity of Aquatic Macroinvertebrates Along a Gradient of Stream Size and Flow Stability in the Northeastern Colorado Rocky Mountains. Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2018. [Google Scholar]
- Thakur, D.; Chawla, A. Functional diversity along elevational gradients in the high altitude vegetation of the western Himalaya. Biodivers. Conserv. 2019, 28, 1977–1996. [Google Scholar] [CrossRef]
- Gazol, A.; Moiseev, P.; Camarero, J.J. Changes in plant taxonomic and functional diversity patterns following treeline advances in the South Urals. Plant Ecol. Divers. 2017, 10, 283–292. [Google Scholar] [CrossRef]
- Datry, T.; Bonada, N.; Heino, J. Towards understanding the organisation of metacommunities in highly dynamic ecological systems. Oikos 2015, 125, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Bloch, C.P.; Higgins, C.L.; Willig, M.R. Effects of large-scale disturbance on metacommunity structure of terrestrial gastropods: Temporal trends in nestedness. Oikos 2007, 116, 395–406. [Google Scholar] [CrossRef]
- Dudgeon, D. Large-Scale Hydrological Changes in Tropical Asia: Prospects for Riverine Biodiversity. BioScience 2000, 50, 793–806. [Google Scholar] [CrossRef]
- Quinn, J.M.; Cooper, A.B.; Davies-Colley, R.J.; Rutherford, J.C.; Williamson, R.B. Land use effects on habitat, water quality, periphyton, and benthic invertebrates in Waikato, New Zealand, hill-country streams. N. Z. J. Mar. Freshw. Res. 1997, 31, 579–597. [Google Scholar] [CrossRef]
- Reyjol, Y.; Lim, P.; Dauba, F.; Baran, P.; Belaud, A. Role of temperature and flow regulation on the Salmoniform—Cypriniform transition. Archiv. Hydrobiol. 2001, 152, 567–582. [Google Scholar] [CrossRef]
- Covich, A.P.; Palmer, M.A.; Crowl, T.A. The role of benthic invertebrate species in freshwater ecosystems—Zoobenthic species influence energy flows and nutrient cycling. Bioscience 1999, 49, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Mermillod-Blondin, F. The functional significance of bioturbation and biodeposition on biogeochemical processes at the water–sediment interface in freshwater and marine ecosystems. J. N. Am. Benthol. Soc. 2011, 30, 770–778. [Google Scholar] [CrossRef] [Green Version]
- Dohet, A.; Hlúbiková, D.; Wetzel, C.E.; L’Hoste, L.; Iffly, J.F.; Hoffmann, L.; Ector, L. Influence of thermal regime and land use on benthic invertebrate communities inhabiting headwater streams exposed to contrasted shading. Sci. Total Environ. 2014, 505, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.Q. Scientific Investigation of Shennongjia Nature Reserve; China Forestry Publishing: Beijing, China, 1999. [Google Scholar]
- Zhang, Y.; Cong, J.; Lu, H.; Li, G.; Xue, Y.; Deng, Y.; Li, H.; Zhou, J.; Li, D. Soil bacterial diversity patterns and drivers along an elevational gradient on Shennongjia Mountain, China. Microb. Biotechnol. 2015, 8, 739–746. [Google Scholar] [CrossRef] [Green Version]
- Hui, Y.; Zhang, X.H.; Chen, Z.J. Present situation and strategy about the natural environment of the Xiangxi river basin. Resour. Environ. Yangtza Basin 2000, 9, 27–33. [Google Scholar]
- Jiang, W.X. Impact of Human Activities on Macroinvertebrate Communities in Xiangxi River; Institute of hydrobiology, Chinese Academy of Sciences: Wuhan, China, 2008. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5–6. Available online: https://CRAN.R-project.org/package=vegan (accessed on 17 January 2020).
- R Development Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Poff, N.L.; Olden, J.D.; Vieira, N.K.M.; Finn, D.S.; Simmons, M.P.; Kondratieff, B.C. Functional trait niches of North American lotic insects: Traits-based ecological applications in light of phylogenetic relationships. J. N. Am. Benthol. Soc. 2006, 25, 730–755. [Google Scholar] [CrossRef] [Green Version]
- Usseglio-Polatera, P.; Bournaud, M.; Richoux, P.; Tachet, H. Biological and ecological traits of benthic freshwater macroinvertebrates: Relationships and definition of groups with similar traits. Freshw. Biol. 2000, 43, 175–205. [Google Scholar] [CrossRef]
- Ding, N.; Yang, W.; Zhou, Y.; González-Bergonzoni, I.; Zhang, J.; Chen, K.; Vidal, N.; Jeppesen, E.; Liu, Z.; Wang, B. Different responses of functional traits and diversity of stream macroinvertebrates to environmental and spatial factors in the Xishuangbanna watershed of the upper Mekong River Basin, China. Sci. Total Environ. 2017, 574, 288–299. [Google Scholar] [CrossRef]
- Colzani, E.; Siqueira, T.; Suriano, M.T.; Roque, F.O. Responses of Aquatic Insect Functional Diversity to Landscape Changes in Atlantic Forest. Biotropica 2013, 45, 343–350. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Shimatani, K. On the measurement of species diversity incorporating species differences. Oikos 2001, 93, 135–147. [Google Scholar] [CrossRef]
- De Bello, F.; Lepš, J.; Sebastià, M.-T. Variations in species and functional plant diversity along climatic and grazing gradients. Ecography 2006, 29, 801–810. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P.; Shipley, B. FD: Measuring functional diversity (FD) from multiple traits, and other tools for functional ecology. 2014; R Package Version 1.0-12. [Google Scholar]
- Ouyang, F.; Ge, F. Nonlinear analysis of insect population dynamics based on generalized additive models and statistical computing using R. Chin. J. Appl. Entomol. 2013, 50, 1170–1177. [Google Scholar]
- Anderson-Cook, C.M. Generalized additive models: An introduction with r. J. Am. Stat. Assoc. 2007, 102, 760–761. [Google Scholar] [CrossRef]
- Wang, J.; Soininen, J.; Zhang, Y.; Wang, B.; Yang, X.; Shen, J. Contrasting patterns in elevational diversity between microorganisms and macroorganisms. J. Biogeogr. 2010, 38, 595–603. [Google Scholar] [CrossRef]
- Villéger, S.; Miranda, J.R.; Hernández, D.F.; Mouillot, D. Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol. Appl. 2010, 20, 1512–1522. [Google Scholar] [CrossRef]
- Jacobsen, D. Low oxygen pressure as a driving factor for the altitudinal decline in taxon richness of stream macroinvertebrates. Oecologia 2007, 154, 795–807. [Google Scholar] [CrossRef]
- Wohl, E. Human impacts to mountain streams. Geomorphology 2006, 79, 217–248. [Google Scholar] [CrossRef] [Green Version]
- Colwell, R.K.; Rahbek, C.; Gotelli, N.J. The mid-domain effect and species richness patterns: What have we learned so far? Am. Nat. 2004, 163, E1–E23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Čiamporová-Zaťovičová, Z.; Hamerlík, L.; Šporka, F.; Bitušík, P. Littoral benthic macroinvertebrates of alpine lakes (Tatra Mts) along an altitudinal gradient: A basis for climate change assessment. Hydrobiologia 2010, 648, 19–34. [Google Scholar] [CrossRef]
- Bozinovic, F.; Calosi, P.; Spicer, J.I. Physiological Correlates of Geographic Range in Animals. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 155–179. [Google Scholar] [CrossRef] [Green Version]
- Rahbek, C. The elevational gradient of species richness: A uniform pattern? Ecography 1995, 18, 200–205. [Google Scholar] [CrossRef]
- Broxton, P.D.; Troch, P.A.; Lyon, S.W. On the role of aspect to quantify water transit times in small mountainous catchments. Water Resour. Res. 2009, 45. [Google Scholar] [CrossRef] [Green Version]
- Geroy, I.J.; Gribb, M.M.; Marshall, H.-P.; Chandler, D.; Benner, S.G.; McNamara, J.P. Aspect influences on soil water retention and storage. Hydrol. Process. 2011, 25, 3836–3842. [Google Scholar] [CrossRef]
- Caissie, D. The thermal regime of rivers: A review. Freshw. Biol. 2006, 51, 1389–1406. [Google Scholar] [CrossRef]
- Malard, F.; Uehlinger, U.; Zah, R.; Tockner, K. Flood-pulse and riverscape dynamics in a braided glacial river. Ecology 2006, 87, 704–716. [Google Scholar] [CrossRef] [Green Version]
- Li, F.Q. The Community Characteristics of Benthic Macroinvertebrates in East Asian Monsoon River and Its Response to Land-Use Change and Climate Change—A Case Study of Xiangxi River Watershed; Institute of hydrobiology, Chinese Academy of Sciences: Wuhan, China, 2010. [Google Scholar]
- Füreder, L.; Wallinger, M.; Burger, R. Longitudinal and seasonal pattern of insect emergence in alpine streams. Aquat. Ecol. 2005, 39, 67–78. [Google Scholar] [CrossRef]
- Mouton, T.L.; Tonkin, J.D.; Stephenson, F.; Verburg, P.; Floury, M. Increasing climate-driven taxonomic homogenization but functional differentiation among river macroinvertebrate assemblages. Glob. Chang. Biol. 2020, 26, 6904–6915. [Google Scholar] [CrossRef] [PubMed]
- Erica, E.S.; Florencia, F.C.; Susana, L.S.; Todd, W. Seasonal influence and local factors affecting macroinvertebrate structure in a high-altitude Andean stream. J. Mt. Sci. 2020, 17, 1374–1386. [Google Scholar]
- Laiolo, P.; Pato, J.; Obeso, J.R. Ecological and evolutionary drivers of the elevational gradient of diversity. Ecol. Lett. 2018, 21, 1022–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, J.M.; Humpesch, U.H.; Macan, T.T. Larvae of the British Ephemeroptera: A Key with Ecological Notes; Scientific Publication Freshwater Biological Association: Ambleside, UK, 1988. [Google Scholar]
- Kishi, D.; Murakami, M.; Nakano, S.; Maekawa, K. Water temperature determines strength of top-down control in a stream food web. Freshw. Biol. 2005, 50, 1315–1322. [Google Scholar] [CrossRef]
- Lepori, F.; Palm, D.; Malmqvist, B. Effects of stream restoration on ecosystem functioning: Detritus retentiveness and decomposition. J. Appl. Ecol. 2005, 42, 228–238. [Google Scholar] [CrossRef]
- Tonkin, J.D.; Altermatt, F.; Finn, D.S.; Heino, J.; Olden, J.D.; Pauls, S.U.; Lytle, D.A. The role of dispersal in river network metacommunities: Patterns, processes, and pathways. Freshw. Biol. 2017, 63, 141–163. [Google Scholar] [CrossRef] [Green Version]
- Heino, J. The importance of metacommunity ecology for environmental assessment research in the freshwater realm. Biol. Rev. 2012, 88, 166–178. [Google Scholar] [CrossRef]
- McGill, B.J. Matters of Scale. Science 2010, 328, 575–576. [Google Scholar] [CrossRef] [Green Version]
- McGill, B.J.; Dornelas, M.; Gotelli, N.J.; Magurran, A. Fifteen forms of biodiversity trend in the Anthropocene. Trends Ecol. Evol. 2015, 30, 104–113. [Google Scholar] [CrossRef]
| Variable | Distribution | Data Type and Range |
|---|---|---|
| Air temperature | Gaussian | −INF~INF |
| Total phosphorus (TP); Total nitrogen (TN) | Gamma | 0~INF |
| Water flow; Functional richness (FRic); Functional evenness (FEve); Shannon–Wiener index | Tweedie | ≥0 |
| Functional divergence (FDiv) | Quasi-binomial | 0~1 |
| Functional dispersion (FDis); Rao’s Quadratic (RaoQ) | Beta | 0~1 |
| Species richness | Poisson | Positive integers |
| Season | Temperature | Total Phosphorus (TP) | Total Nitrogen (TN) | Flow | ||||
|---|---|---|---|---|---|---|---|---|
| p | Adjusted R2 | p | Adjusted R2 | p | Adjusted R2 | p | Adjusted R2 | |
| Dry season | 0.28 | 0.74 | 0.24 | 0.11 | 0.89 | 0.06 | <0.01 * | 0.13 |
| Wet season | 0.22 | 0.98 | 0.13 | 0.03 * | ||||
| Season | Richness | Shannon | ||
|---|---|---|---|---|
| p | Adjusted R2 | P | Adjusted R2 | |
| Dry season | 0.55 | 0.11 | <0.01 * | 0.29 |
| Wet season | 0.14 | 0.43 | ||
| Season | FDiv | FRic | RaoQ | FEve | FDis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| p | Adjusted R2 | p | Adjusted R2 | p | Adjusted R2 | p | Adjusted R2 | p | Adjusted R2 | |
| Dry season | <0.01 * | 0.58 | 0.06 | 0.21 | <0.01 * | 0.39 | 0.07 | 0.02 | <0.01 * | 0.30 |
| Wet season | <0.01 * | <0.01 * | <0.01 * | 0.93 | <0.01 * | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Q.; Chiu, M.-C.; Tan, L.; Cai, Q. Hydrological Season Can Have Unexpectedly Insignificant Influences on the Elevational Patterns of Functional Diversity of Riverine Macroinvertebrates. Biology 2022, 11, 208. https://doi.org/10.3390/biology11020208
Luo Q, Chiu M-C, Tan L, Cai Q. Hydrological Season Can Have Unexpectedly Insignificant Influences on the Elevational Patterns of Functional Diversity of Riverine Macroinvertebrates. Biology. 2022; 11(2):208. https://doi.org/10.3390/biology11020208
Chicago/Turabian StyleLuo, Qingyi, Ming-Chih Chiu, Lu Tan, and Qinghua Cai. 2022. "Hydrological Season Can Have Unexpectedly Insignificant Influences on the Elevational Patterns of Functional Diversity of Riverine Macroinvertebrates" Biology 11, no. 2: 208. https://doi.org/10.3390/biology11020208
APA StyleLuo, Q., Chiu, M.-C., Tan, L., & Cai, Q. (2022). Hydrological Season Can Have Unexpectedly Insignificant Influences on the Elevational Patterns of Functional Diversity of Riverine Macroinvertebrates. Biology, 11(2), 208. https://doi.org/10.3390/biology11020208







