Cognitive Impairment in Heart Failure—A Review
Abstract
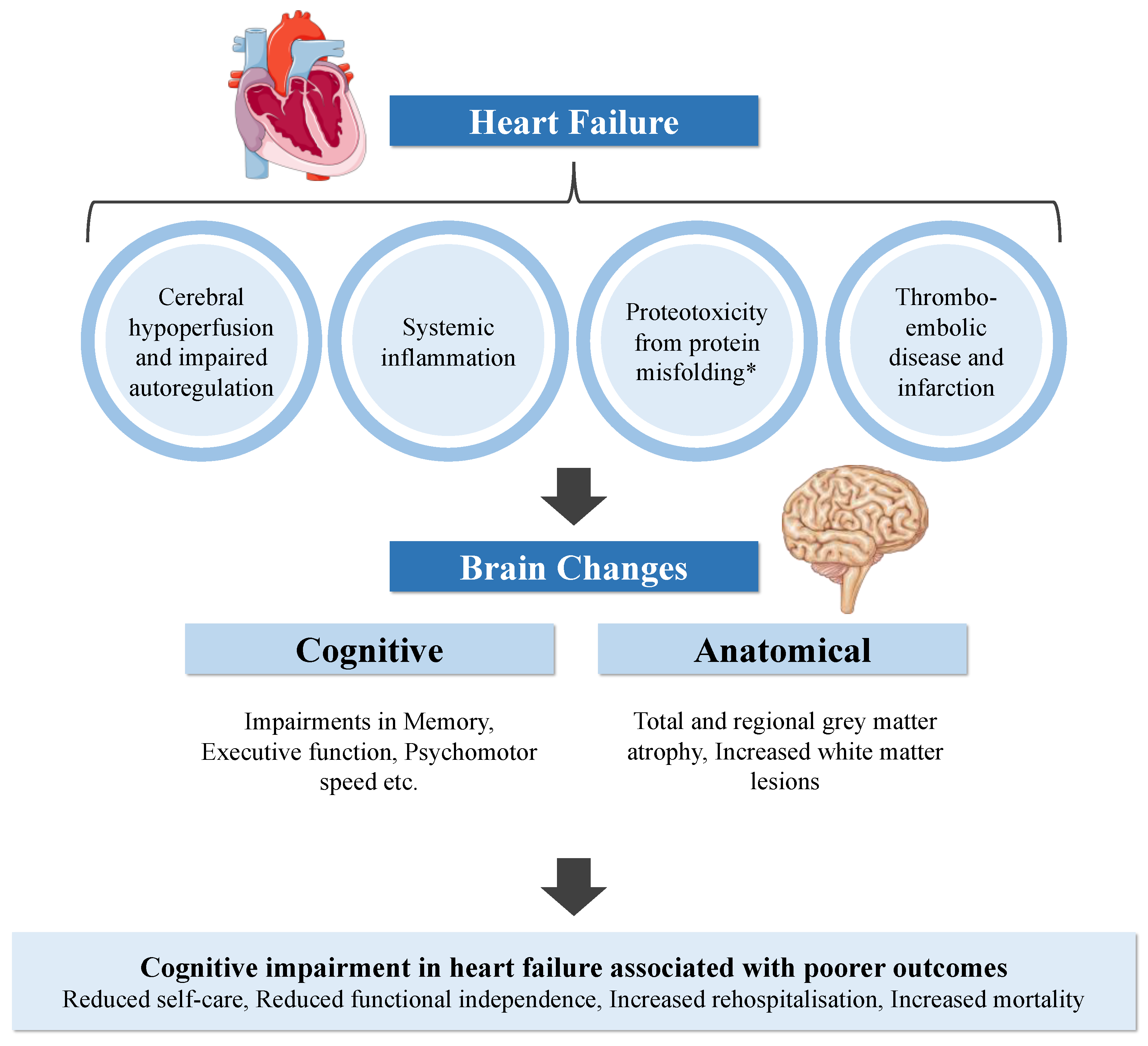
Simple Summary
Abstract
1. Introduction
2. Methods
3. Epidemiology of HF and Prevalence of CI
4. Cognitive Changes in HF
4.1. HF Severity and Degree of CI
4.2. The Impact of Ejection Fraction on CI
4.3. Potential Confounders in the Association between HF and CI
5. Anatomical Brain Changes
5.1. Grey Matter Atrophy
5.2. White Matter Lesions
6. Proposed Aetiologies of CI in HF
6.1. Cerebral Hypoperfusion and Impaired Autoregulation
6.2. Systemic Inflammation
6.3. Proteotoxicity
6.4. Thromboembolic Disease and Cerebral Infarction
7. Impact of CI on Prognosis in HF
8. Screening for CI in Patients with HF
9. Impact of HF Therapies on CI
10. Future Challenges
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gallagher, R.; Sullivan, A.; Burke, R.; Hales, S.; Gillies, G.; Cameron, J.; Saliba, B.; Tofler, G. Mild Cognitive Impairment, Screening, and Patient Perceptions in Heart Failure Patients. J. Card. Fail. 2013, 19, 641–646. [Google Scholar] [CrossRef]
- Vogels, R.L.C.; Oosterman, J.M.; Van Harten, B.; Scheltens, P.; Van Der Flier, W.M.; Schroeder-Tanka, J.M.; Weinstein, H.C. Profile of Cognitive Impairment in Chronic Heart Failure. J. Am. Geriatr. Soc. 2007, 55, 1764–1770. [Google Scholar] [CrossRef]
- Zuccala, G.; Onder, G.; Pedone, C.; Carosella, L.; Pahor, M.; Bernabei, R.; Cocchi, A. Hypotension and cognitive impairment: Selective association in patients with heart failure. Neurology 2001, 57, 1986–1992. [Google Scholar] [CrossRef]
- González-Moneo, M.J.; Sánchez-Benavides, G.; Rotellar, J.M.V.; Cladellas, M.; Bruguera, J.; Quiñones-Ubeda, S.; Enjuanes, C.; Peña-Casanova, J.; Comín-Colet, J. Ischemic aetiology, self-reported frailty, and gender with respect to cognitive impairment in chronic heart failure patients. BMC Cardiovasc. Disord. 2016, 16, 163. [Google Scholar] [CrossRef]
- Levin, S.N.; Hajduk, A.M.; McManus, D.D.; Darling, C.E.; Gurwitz, J.H.; Spencer, F.A.; Goldberg, R.J.; Saczynski, J.S. Cognitive status in patients hospitalized with acute decompensated heart failure. Am. Hear. J. 2014, 168, 917–923. [Google Scholar] [CrossRef]
- Dodson, J.A.; Truong, T.-T.N.; Towle, V.R.; Kerins, G.; Chaudhry, S.I. Cognitive Impairment in Older Adults with Heart Failure: Prevalence, Documentation, and Impact on Outcomes. Am. J. Med. 2013, 126, 120–126. [Google Scholar] [CrossRef]
- Vellone, E.; Chialà, O.; Boyne, J.; Klompstra, L.; Evangelista, L.S.; Back, M.; Ben Gal, T.; Mårtensson, J.; Strömberg, A.; Jaarsma, T. Cognitive impairment in patients with heart failure: An international study. ESC Hear. Fail. 2019, 7, 47–54. [Google Scholar] [CrossRef]
- Hajduk, A.M.; Lemon, S.C.; McManus, D.D.; Lessard, D.M.; Gurwitz, J.H.; Spencer, F.A.; Goldberg, R.J.; Saczynski, J.S. Cog-nitive impairment and self-care in heart failure. Clin. Epidemiol. 2013, 5, 407–416. [Google Scholar] [CrossRef]
- Connors, E.J.; Hauson, A.O.; Barlet, B.D.; Sarkissians, S.; Stelmach, N.P.; Walker, A.D.; Nemanim, N.M.; Greenwood, K.L.; Chesher, N.J.; Wollman, S.C.; et al. Neuropsychological Assessment and Screening in Heart Failure: A Meta-Analysis and Systematic Review. Neuropsychol. Rev. 2021, 31, 312–330. [Google Scholar] [CrossRef]
- Alosco, M.L.; Spitznagel, M.B.; Raz, N.; Cohen, R.; Sweet, L.H.; Colbert, L.H.; Josephson, R.; Van Dulmen, M.; Hughes, J.; Rosneck, J.; et al. Executive dysfunction is independently associated with reduced functional independence in heart failure. J. Clin. Nurs. 2014, 23, 829–836. [Google Scholar] [CrossRef]
- Havakuk, O.; King, K.; Grazette, L.; Yoon, A.J.; Fong, M.; Bregman, N.; Elkayam, U.; Kloner, R.A. Heart Failure-Induced Brain Injury. J. Am. Coll. Cardiol. 2017, 69, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Holm, H.; Bachus, E.; Jujic, A.; Nilsson, E.D.; Wadström, B.; Molvin, J.; Minthon, L.; Fedorowski, A.; Nägga, K.; Magnusson, M. Cognitive test results are associated with mortality and rehospitalization in heart failure: Swedish prospective cohort study. ESC Hear. Fail. 2020, 7, 2948–2955. [Google Scholar] [CrossRef] [PubMed]
- Huijts, M.; Van Oostenbrugge, R.J.; Duits, A.; Burkard, T.; Muzzarelli, S.; Maeder, M.T.; Schindler, R.; Pfisterer, M.E.; Rocca, H.-P.B.-L. Cognitive impairment in heart failure: Results from the Trial of Intensified versus standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME-CHF) randomized trial. Eur. J. Hear. Fail. 2013, 15, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Christiansen, M.N.; Køber, L.; Weeke, P.; Vasan, R.S.; Jeppesen, J.L.; Smith, J.G.; Gislason, G.; Torp-Pedersen, C.; Andersson, C. Age-Specific Trends in Incidence, Mortality, and Comorbidities of Heart Failure in Denmark, 1995 to 2012. Circulation 2017, 135, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.P.; Garrido, G.J.; Etherton-Beer, C.; Lautenschlager, N.T.; Arnolda, L.; Flicker, L. Cognitive and brain changes associated with ischaemic heart disease and heart failure. Eur. Hear. J. 2012, 33, 1769–1776. [Google Scholar] [CrossRef]
- Vogels, R.L.C.; Scheltens, P.; Schroeder-Tanka, J.M.; Weinstein, H.C. Cognitive impairment in heart failure: A systematic review of the literature. Eur. J. Hear. Fail. 2007, 9, 440–449. [Google Scholar] [CrossRef]
- Hajduk, A.M.; Kiefe, C.I.; Person, S.D.; Gore, J.G.; Saczynski, J.S. Cognitive change in heart failure: A systematic review. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 451–460. [Google Scholar] [CrossRef]
- Frey, A.; Sell, R.; Homola, G.A.; Malsch, C.; Kraft, P.; Gunreben, I.; Morbach, C.; Alkonyi, B.; Schmid, E.; Colonna, I.; et al. Cognitive Deficits and Related Brain Lesions in Patients with Chronic Heart Failure. JACC Hear. Fail. 2018, 6, 583–592. [Google Scholar] [CrossRef]
- Pressler, S.J.; Subramanian, U.; Kareken, D.; Perkins, S.M.; Gradus-Pizlo, I.; Sauvé, M.J.; Ding, Y.; Kim, J.; Sloan, R.; Jaynes, H.; et al. Cognitive Deficits in Chronic Heart Failure. Nurs. Res. 2010, 59, 127–139. [Google Scholar] [CrossRef]
- Sterling, M.R.; Jannat-Khah, D.; Bryan, J.; Banerjee, S.; McClure, L.A.; Wadley, V.G.; Unverzagt, F.W.; Levitan, E.B.; Goyal, P.; Peterson, J.C.; et al. The Prevalence of Cognitive Impairment Among Adults with Incident Heart Failure: The “Reasons for Geographic and Racial Differences in Stroke” (REGARDS) Study. J. Card. Fail. 2019, 25, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.A.; Blades, N.J.; Chaudhry, S.I.; Dodson, J.A.; Longstreth, W.T.J.; Heckbert, S.R.; Psaty, B.M.; Arnold, A.M.; Dublin, S.; Sitlani, C.M.; et al. Long-Term Cognitive Decline After Newly Diagnosed Heart Failure: Longitudinal Analysis in the CHS (Cardiovascular Health Study). Circ. Heart Fail. 2018, 11, e004476. [Google Scholar] [CrossRef] [PubMed]
- Harkness, K.; Demers, C.; Heckman, G.A.; McKelvie, R.S. Screening for cognitive deficits using the Montreal cognitive as-sessment tool in outpatients >=65 years of age with heart failure. Am. J. Cardiol. 2011, 107, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Trojano, L.; Incalzi, R.A.; Acanfora, D.; Picone, C.; Mecocci, P.; Rengo, F. Cognitive impairment: A key feature of congestive heart failure in the elderly. J. Neurol. 2003, 250, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Kindermann, I.; Fischer, D.; Karbach, J.; Link, A.; Walenta, K.; Barth, C.; Ukena, C.; Mahfoud, F.; Kollner, V.; Kindermann, M.; et al. Cognitive function in patients with decompensated heart failure: The Cognitive Impairment in Heart Failure (CogImpair-HF) study. Eur. J. Heart Fail. 2012, 14, 404–413. [Google Scholar] [CrossRef]
- Wolters, F.J.; Segufa, R.A.; Darweesh, S.K.L.; Bos, D.; Ikram, M.A.; Sabayan, B.; Hofman, A.; Sedaghat, S. Coronary heart disease, heart failure, and the risk of dementia: A systematic review and meta-analysis. Alzheimers Dement. 2018, 14, 1493–1504. [Google Scholar] [CrossRef]
- Adelborg, K.; Horváth-Puhó, E.; Ording, A.; Pedersen, L.; Sørensen, H.T.; Henderson, V.W. Heart failure and risk of dementia: A Danish nationwide population-based cohort study. Eur. J. Hear. Fail. 2016, 19, 253–260. [Google Scholar] [CrossRef]
- Qiu, C.; Winblad, B.; Marengoni, A.; Klarin, I.; Fastbom, J.; Fratiglioni, L. Heart failure and risk of dementia and Alzheimer disease: A population-based cohort study. Arch. Intern. Med. 2006, 166, 1003–1008. [Google Scholar] [CrossRef]
- Jefferson, A.L.; Beiser, A.S.; Himali, J.J.; Seshadri, S.; O’Donnell, C.J.; Manning, W.J.; Wolf, P.A.; Au, R.; Benjamin, E. P3-136: Low cardiac index is associated with incident dementia and Alzheimer disease: The Framingham Heart Study. Circulation 2015, 131, 1333–1339. [Google Scholar] [CrossRef]
- Hanon, O.; Vidal, J.-S.; de Groote, P.; Galinier, M.; Isnard, R.; Logeart, D.; Komajda, M. Prevalence of Memory Disorders in Ambulatory Patients Aged ≥70 Years with Chronic Heart Failure (from the EFICARE Study). Am. J. Cardiol. 2014, 113, 1205–1210. [Google Scholar] [CrossRef]
- Lee, T.C.; Qian, M.; Liu, Y.; Graham, S.; Mann, D.L.; Nakanishi, K.; Teerlink, J.R.; Lip, G.Y.H.; Freudenberger, R.S.; Sacco, R.L.; et al. Cognitive Decline Over Time in Patients with Systolic Heart Failure: Insights From WARCEF. JACC Heart Fail. 2019, 7, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Arslanian-Engoren, C.; Giordani, B.J.; Algase, D.; Schuh, A.; Lee, C.; Moser, D.K. Cognitive dysfunction in older adults hos-pitalized for acute heart failure. J. Card. Fail. 2014, 20, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Emery, A.; Wells, J.; Klaus, S.P.; Mather, M.; Pessoa, A.; Pendlebury, S.T. Underestimation of Cognitive Impairment in Older Inpatients by the Abbreviated Mental Test Score versus the Montreal Cognitive Assessment: Cross-Sectional Observational Study. Dement. Geriatr. Cogn. Disord. Extra 2020, 10, 205–215. [Google Scholar] [CrossRef]
- Hall, C. NT-ProBNP: The mechanism behind the marker. J. Card. Fail. 2005, 11, S81–S83. [Google Scholar] [CrossRef] [PubMed]
- Rørth, R.; Jhund, P.S.; Yilmaz, M.B.; Kristensen, S.L.; Welsh, P.; Desai, A.S.; Køber, L.; Prescott, M.F.; Rouleau, J.L.; Solomon, S.D. Comparison of BNP and NT-proBNP in Patients with Heart Failure and Reduced Ejection Fraction. Circ. Hear. Fail. 2020, 13, e006541. [Google Scholar] [CrossRef] [PubMed]
- Hurk, K.V.D.; Reijmer, Y.D.; Berg, E.V.D.; Alssema, M.; Nijpels, G.; Kostense, P.J.; Stehouwer, C.D.; Paulus, W.J.; Kamp, O.; Dekker, J.M.; et al. Heart failure and cognitive function in the general population: The Hoorn Study. Eur. J. Hear. Fail. 2011, 13, 1362–1369. [Google Scholar] [CrossRef]
- Park, C.M.; Williams, E.D.; Chaturvedi, N.; Tillin, T.; Stewart, R.J.; Richards, M.; Shibata, D.; Mayet, J.; Hughes, A.D. Associ-ations Between Left Ventricular Dysfunction and Brain Structure and Function: Findings from the SABRE (Southall and Brent Revisited) Study. J. Am. Heart Assoc. 2017, 6, e004898. [Google Scholar] [CrossRef]
- Nagata, T.; Ohara, T.; Hata, J.; Sakata, S.; Furuta, Y.; Yoshida, D.; Honda, T.; Hirakawa, Y.; Ide, T.; Kanba, S.; et al. NT-proBNP and Risk of Dementia in a General Japanese Elderly Population: The Hisayama Study. J. Am. Heart Assoc. 2019, 8, e011652. [Google Scholar] [CrossRef]
- Zuccalà, G.; Cattel, C.; Manes-Gravina, E.; Di Niro, M.G.; Cocchi, A.; Bernabei, R. Left ventricular dysfunction: A clue to cog-nitive impairment in older patients with heart failure. J. Neurol. Neurosurg. Psychiatry 1997, 63, 509–512. [Google Scholar] [CrossRef]
- Shin, M.-S.; Lan, S.J.; Kim, S.; Shim, J.L.; Park, J.-K.; Kim, J. Concomitant diastolic dysfunction further interferes with cognitive performance in moderate to severe systolic heart failure. PLoS ONE 2017, 12, e0184981. [Google Scholar] [CrossRef]
- Festa, J.R.; Jia, X.; Cheung, K.; Marchidann, A.; Schmidt, M.; Shapiro, P.A.; Mancini, D.M.; Naka, Y.; Deng, M.; Lantz, E.R.; et al. Association of Low Ejection Fraction with Impaired Verbal Memory in Older Patients With Heart Failure. Arch. Neurol. 2011, 68, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Carey, B.J.; Eames, P.J.; Blake, M.J.; Panerai, R.B.; Potter, J.F. Dynamic Cerebral Autoregulation Is Unaffected by Aging. Stroke 2000, 31, 2895–2900. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C. Brain hypoperfusion: A critical factor in vascular dementia. Neurol. Res. 2004, 26, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Athilingam, P.; D’Aoust, R.; Miller, L.; Chen, L. Cognitive Profile in Persons with Systolic and Diastolic Heart Failure. Congest. Hear. Fail. 2012, 19, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Bratzke-Bauer, L.C.; Pozehl, B.J.; Paul, S.M.; Johnson, J.K. Neuropsychological Patterns Differ by Type of Left Ventricle Dys-function in Heart Failure. Arch. Clin. Neuropsychol. 2012, 28, 114–124. [Google Scholar] [CrossRef]
- Almeida, O.P.; Beer, C.; Lautenschlager, N.T.; Arnolda, L.; Alfonso, H.; Flicker, L. Two-year course of cognitive function and mood in adults with congestive heart failure and coronary artery disease: The Heart-Mind Study. Int. Psychogeriatrics 2011, 24, 38–47. [Google Scholar] [CrossRef]
- Farokhian, F.; Yang, C.; Beheshti, I.; Matsuda, H.; Wu, S. Age-Related Gray and White Matter Changes in Normal Adult Brains. Aging Dis. 2017, 8, 899–909. [Google Scholar] [CrossRef]
- Almeida, O.P.; Garrido, G.J.; Etherton-Beer, C.; Lautenschlager, N.T.; Arnolda, L.; Alfonso, H.; Flicker, L. Brain and mood changes over 2 years in healthy controls and adults with heart failure and ischaemic heart disease. Eur. J. Hear. Fail. 2013, 15, 850–858. [Google Scholar] [CrossRef]
- Simons, J.S.; Spiers, H. Prefrontal and medial temporal lobe interactions in long-term memory. Nat. Rev. Neurosci. 2003, 4, 637–648. [Google Scholar] [CrossRef]
- Frey, A.; Homola, G.A.; Henneges, C.; Muhlbauer, L.; Sell, R.; Kraft, P.; Franke, M.; Morbach, C.; Vogt, M.; Mullges, W.; et al. Temporal changes in total and hippocampal brain volume and cognitive function in patients with chronic heart failure-the COGNITION.MATTERS-HF cohort study. Eur. Heart J. 2021, 42, 1569–1578. [Google Scholar] [CrossRef]
- Alosco, M.L.; Garcia, S.; Spitznagel, M.B.; van Dulmen, M.; Cohen, R.; Sweet, L.H.; Josephson, R.; Hughes, J.; Rosneck, J.; Gunstad, J. Cognitive performance in older adults with stable heart failure: Longitudinal evidence for stability and improve-ment. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2014, 21, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Vogels, R.L.; Oosterman, J.M.; Van Harten, B.; Gouw, A.A.; Schroeder-Tanka, J.M.; Scheltens, P.; van der Flier, W.; Weinstein, H.C. Neuroimaging and Correlates of Cognitive Function among Patients with Heart Failure. Dement. Geriatr. Cogn. Disord. 2007, 24, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Nguyen, H.D.; Ogren, J.A.; Macey, P.; Thompson, P.; Fonarow, G.; Hamilton, M.A.; Harper, R.M.; Woo, M.A. Global and regional putamen volume loss in patients with heart failure. Eur. J. Hear. Fail. 2011, 13, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Yadav, S.K.; Palomares, J.A.; Park, B.; Joshi, S.H.; Ogren, J.A.; Macey, P.M.; Fonarow, G.C.; Harper, R.M.; Woo, M.A. Reduced Regional Brain Cortical Thickness in Patients with Heart Failure. PLoS ONE 2015, 10, e0126595. [Google Scholar] [CrossRef]
- Vogels, R.L.; van der Flier, W.; Van Harten, B.; Gouw, A.A.; Scheltens, P.; Schroeder-Tanka, J.M.; Weinstein, H.C. Brain magnetic resonance imaging abnormalities in patients with heart failure. Eur. J. Hear. Fail. 2007, 9, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Markus, H.S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ 2010, 341, c3666. [Google Scholar] [CrossRef] [PubMed]
- Stegmann, T.; Chu, M.L.; Witte, V.A.; Villringer, A.; Kumral, D.; Riedel-Heller, S.G.; Roehr, S.; Hagendorff, A.; Laufs, U.; Loeffler, M.; et al. Heart failure is independently associated with white matter lesions: Insights from the population-based LIFE-Adult Study. ESC Hear. Fail. 2020, 8, 697–704. [Google Scholar] [CrossRef]
- Jefferson, A.L.; Himali, J.J.; Beiser, A.S.; Au, R.; Massaro, J.M.; Seshadri, S.; Gona, P.; Salton, C.J.; DeCarli, C.; O’Donnell, C.J.; et al. Cardiac index is associated with brain aging: The framingham heart study. Circulation 2010, 122, 690–697. [Google Scholar] [CrossRef]
- Alosco, M.L.; Brickman, A.M.; Spitznagel, M.B.; Garcia, S.L.; Narkhede, A.; Griffith, E.Y.; Raz, N.; Cohen, R.; Sweet, L.H.; Colbert, L.H.; et al. Cerebral perfusion is associated with white matter hyperintensities in older adults with heart failure. Congest. Hear. Fail. 2013, 19, E29–E34. [Google Scholar] [CrossRef]
- Choi, B.-R.; Kim, J.S.; Yang, Y.J.; Park, K.-M.; Lee, C.W.; Kim, Y.-H.; Hong, M.-K.; Song, J.-K.; Park, S.-W.; Park, S.-J. Factors Associated with Decreased Cerebral Blood Flow in Congestive Heart Failure Secondary to Idiopathic Dilated Cardiomyopathy. Am. J. Cardiol. 2006, 97, 1365–1369. [Google Scholar] [CrossRef]
- Babayiğit, E.; Murat, S.; Mert, K.U.; Çavuşoğlu, Y. Assesment of Cerebral Blood Flow Velocities with Transcranial Doppler Ultrasonography in Heart Failure Patients with Reduced Ejection Fraction. J. Stroke Cerebrovasc. Dis. 2021, 30, 105706. [Google Scholar] [CrossRef] [PubMed]
- Alosco, M.L.; Spitznagel, M.B.; Cohen, R.; Raz, N.; Sweet, L.H.; Josephson, R.; Hughes, J.; Rosneck, J.; Gunstad, J. Reduced cerebral perfusion predicts greater depressive symptoms and cognitive dysfunction at a 1-year follow-up in patients with heart failure. Int. J. Geriatr. Psychiatry 2013, 29, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Kure, C.E.; Rosenfeldt, F.L.; Scholey, A.; Pipingas, A.; Kaye, D.M.; Bergin, P.J.; Croft, K.; Wesnes, K.; Myers, S.P.; Stough, C. Relationships Among Cognitive Function and Cerebral Blood Flow, Oxidative Stress, and Inflammation in Older Heart Failure Patients. J. Card. Fail. 2016, 22, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Matsumoto, Y.; Ota, H.; Sugimura, K.; Takahashi, J.; Ito, K.; Miyata, S.; Furukawa, K.; Arai, H.; Fukumoto, Y.; et al. Hippocampal Blood Flow Abnormality Associated with Depressive Symptoms and Cognitive Impairment in Patients With Chronic Heart Failure. Circ. J. 2016, 80, 1773–1780. [Google Scholar] [CrossRef]
- van Hout, M.J.P.; Dekkers, I.A.; Westenberg, J.J.M.; Schalij, M.J.; Scholte, A.J.H.A.; Lamb, H.J. Associations between left ventricular function, vascular function and measures of cerebral small vessel disease: A cross-sectional magnetic resonance imaging study of the UK Biobank. Eur. Radiol. 2021, 31, 5068–5076. [Google Scholar] [CrossRef]
- Triantafyllidi, H.; Arvaniti, C.; Lekakis, J.; Ikonomidis, I.; Siafakas, N.; Tzortzis, S.; Trivilou, P.; Zerva, L.; Stamboulis, E.; Kremastinos, D.T. Cognitive Impairment Is Related to Increased Arterial Stiffness and Microvascular Damage in Patients with Never-Treated Essential Hypertension. Am. J. Hypertens. 2009, 22, 525–530. [Google Scholar] [CrossRef]
- van Exel, E.; de Craen, A.J.; Remarque, E.J.; Gussekloo, J.; Houx, P.; der Wiel, A.B.-V.; Frolich, M.; Macfarlane, P.W.; Blauw, G.J.; Westendorp, R.G. Interaction of atherosclerosis and inflammation in elderly subjects with poor cognitive function. Neurology 2003, 61, 1695–1701. [Google Scholar] [CrossRef]
- Sabayan, B.; van Buchem, M.A.; Sigurdsson, S.; Zhang, Q.; Harris, T.B.; Gudnason, V.; Arai, A.E.; Launer, L.J. Cardiac he-modynamics are linked with structural and functional features of brain aging: The age, gene/environment susceptibility (AG-ES)-Reykjavik Study. J. Am. Heart Assoc. 2015, 4, e001294. [Google Scholar] [CrossRef]
- Yun, M.; Nie, B.; Wen, W.; Zhu, Z.; Liu, H.; Nie, S.; Lanzenberger, R.; Wei, Y.; Hacker, M.; Shan, B.; et al. Assessment of cerebral glucose metabolism in patients with heart failure by 18F-FDG PET/CT imaging. J. Nucl. Cardiol. 2020, 1–13. [Google Scholar] [CrossRef]
- Moody, D.M.; Bell, M.A.; Challa, V.R. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxy-genation deficiency: An anatomic study. Am. J. Neuroradiol. 1990, 11, 431–439. [Google Scholar]
- Leeuwis, A.E.; Hooghiemstra, A.M.; Bron, E.E.; Kuipers, S.; Oudeman, E.A.; Kalay, T.; Rocca, H.B.; Kappelle, L.J.; Van Oostenbrugge, R.J.; Greving, J.P.; et al. Cerebral blood flow and cognitive functioning in patients with disorders along the heart–brain axis. Alzheimer’s Dementia: Transl. Res. Clin. Interv. 2020, 6. [Google Scholar] [CrossRef]
- Gruhn, N.; Larsen, F.S.; Boesgaard, S.; Knudsen, G.M.; Mortensen, S.A.; Thomsen, G.; Aldershvile, J. Cerebral Blood Flow in Patients with Chronic Heart Failure Before and After Heart Transplantation. Stroke 2001, 32, 2530–2533. [Google Scholar] [CrossRef]
- Roman, D.D.; Kubo, S.H.; Ormaza, S.; Francis, G.S.; Bank, A.J.; Shumway, S.J. Memory improvement following cardiac transplantation. J. Clin. Exp. Neuropsychol. 1997, 19, 692–697. [Google Scholar] [CrossRef]
- Vorovich, E.; Andrei, A.-C.; Xu, Y.; Kao, A.; Hsich, E.M.; Dew, M.A.; Kormos, R.L.; Pham, D.T.; Yancy, C.W.; LaRue, S.; et al. Improvement in cognitive function after heart transplant and mechanical circulatory support: Findings from the sustaining quality of life of the aged (sustain-it) study. Circulation 2019, 140. [Google Scholar]
- Bhat, G.; Yost, G.; Mahoney, E. Cognitive function and left ventricular assist device implantation. J. Hear. Lung Transplant. 2015, 34, 1398–1405. [Google Scholar] [CrossRef]
- McIlvennan, C.; Bryce, K.; Lindenfeld, J.; Allen, L.; Lanfear, D. Assessment of Cognitive Function Prior to and After Implantation of Left Ventricular Assist Device. J. Hear. Lung Transplant. 2016, 35, S165–S166. [Google Scholar] [CrossRef][Green Version]
- Schall, R.R.; Petrucci, R.J.; Brozena, S.C.; Cavarocchi, N.C.; Jessup, M. Cognitive function in patients with symptomatic dilated cardiomyopathy before and after cardiac transplantation. J. Am. Coll. Cardiol. 1989, 14, 1666–1672. [Google Scholar] [CrossRef]
- Georgiadis, D.; Sievert, M.; Cencetti, S.; Uhlmann, F.; Krivokuca, M.; Zierz, S.; Werdan, K. Cerebrovascular reactivity is im-paired in patients with cardiac failure. Eur. Heart J. 2000, 21, 407–413. [Google Scholar] [CrossRef]
- Erkelens, C.D.; van der Wal, H.H.; de Jong, B.M.; Elting, J.-W.; Renken, R.; Gerritsen, M.; van Laar, P.J.; van Deursen, V.M.; van der Meer, P.; van Veldhuisen, D.J.; et al. Dynamics of cerebral blood flow in patients with mild non-ischaemic heart failure. Eur. J. Heart Fail. 2017, 19, 261–268. [Google Scholar] [CrossRef]
- Fraser, K.S.; Heckman, G.A.; McKelvie, R.S.; Harkness, K.; Middleton, L.E.; Hughson, R.L. Cerebral hypoperfusion is exag-gerated with an upright posture in heart failure: Impact of depressed cardiac output. JACC Heart Fail. 2015, 3, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Bronzwaer, A.G.; Bogert, L.W.; Westerhof, B.E.; Piek, J.; Daemen, M.J.; Van Lieshout, J.J. Abnormal haemodynamic postural response in patients with chronic heart failure. ESC Hear. Fail. 2017, 4, 146–153. [Google Scholar] [CrossRef]
- Kharraziha, I.; Holm, H.; Magnusson, M.; Wollmer, P.; Molvin, J.; Jujic, A.; Fedorowski, A.; Bachus, E.; Hamrefors, V. Impaired cerebral oxygenation in heart failure patients at rest and during head-up tilt testing. ESC Hear. Fail. 2020, 8, 586–594. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Reichenberg, A.; Yirmiya, R.; Schuld, A.; Kraus, T.; Haack, M.; Morag, A.; Pollmächer, T. Cytokine-Associated Emotional and Cognitive Disturbances in Humans. Arch. Gen. Psychiatry 2001, 58, 445–452. [Google Scholar] [CrossRef]
- Aukrust, P.; Ueland, T.; Lien, E.; Bendtzen, K.; Müller, F.; Andreassen, A.K.; Nordøy, I.; Aass, H.; Espevik, T.; Simonsen, S.; et al. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 1999, 83, 376–382. [Google Scholar] [CrossRef]
- Torre-Amione, G.; Kapadia, S.; Benedict, C.; Oral, H.; Young, J.B.; Mann, D. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: A report from the studies of left ventricular dysfunction (SOLVD). J. Am. Coll. Cardiol. 1996, 27, 1201–1206. [Google Scholar] [CrossRef]
- Willis, M.; Patterson, C. Proteotoxicity and Cardiac Dysfunction—Alzheimer’s Disease of the Heart? N. Engl. J. Med. 2013, 368, 455–464. [Google Scholar] [CrossRef]
- Sweeney, P.; Park, H.; Baumann, M.; Dunlop, J.; Frydman, J.; Kopito, R.; McCampbell, A.; Leblanc, G.; Venkateswaran, A.; Nurmi, A.; et al. Protein misfolding in neurodegenerative diseases: Implications and strategies. Transl. Neurodegener. 2017, 6, 6. [Google Scholar] [CrossRef]
- Kittleson, M.M.; Maurer, M.S.; Ambardekar, A.V.; Bullock-Palmer, R.P.; Chang, P.P.; Eisen, H.J.; Nair, A.P.; Nativi-Nicolau, J.; Ruberg, F.L.; and on behalf of the American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement from the American Heart Association. Circulation 2020, 142, e7–e22. [Google Scholar] [CrossRef]
- Heling, A.; Zimmermann, R.; Kostin, S.; Maeno, Y.; Hein, S.; Devaux, B.; Bauer, E.; Klovekorn, W.P.; Schlepper, M.; Schaper, W.; et al. Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ. Res. 2000, 86, 846–853. [Google Scholar] [CrossRef]
- Cannon, J.A.; McMurray, J.J.; Quinn, T.J. ‘Hearts and minds’: Association, causation and implication of cognitive impairment in heart failure. Alzheimer’s Res. Ther. 2015, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Kalantarian, S.; Stern, T.A.; Mansour, M.; Ruskin, J.N. Cognitive impairment associated with atrial fibrillation: A meta-analysis. Ann. Intern. Med. 2013, 158, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Alosco, M.L.; Spitznagel, M.B.; Sweet, L.H.; Josephson, R.; Hughes, J.; Gunstad, J. Atrial Fibrillation Exacerbates Cognitive Dysfunction and Cerebral Perfusion in Heart Failure. Pacing Clin. Electrophysiol. 2014, 38, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, V.G.; Passannante, M.R.; Shah, T.; Modi, K.; Weisse, A.B. Effect of mitral regurgitation on left ventricular thrombus formation in dilated cardiomyopathy. Am. Hear. J. 1998, 135, 215–220. [Google Scholar] [CrossRef]
- Freudenberger, R.S.; Hellkamp, A.S.; Halperin, J.L.; Poole, J.; Anderson, J.; Johnson, G.; Mark, D.B.; Lee, K.L.; Bardy, G.H. Risk of thromboembolism in heart failure: An analysis from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Circulation 2007, 115, 2637–2641. [Google Scholar] [CrossRef]
- Alosco, M.L.; Spitznagel, M.B.; Cohen, R.; Sweet, L.H.; Colbert, L.H.; Josephson, R.; Hughes, J.; Rosneck, J.; Gunstad, J. Reduced cognitive function predicts functional decline in patients with heart failure over 12 months. Eur. J. Cardiovasc. Nurs. 2013, 13, 304–310. [Google Scholar] [CrossRef]
- Lovell, J.; Pham, T.; Noaman, S.Q.; Davis, M.-C.; Johnson, M.; Ibrahim, J.E. Self-management of heart failure in dementia and cognitive impairment: A systematic review. BMC Cardiovasc. Disord. 2019, 19, 1–18. [Google Scholar] [CrossRef]
- Dolansky, M.A.; Hawkins, M.A.; Schaefer, J.T.; Sattar, A.; Gunstad, J.; Redle, J.D.; Josephson, R.; Moore, S.M.; Hughes, J.W. Association Between Poorer Cognitive Function and Reduced Objectively Monitored Medication Adherence in Patients with Heart Failure. Circ. Hear. Fail. 2016, 9. [Google Scholar] [CrossRef]
- Kewcharoen, J.; Trongtorsak, A.; Kanitsoraphan, C.; Prasitlumkum, N.; Mekritthikrai, R.; Techorueangwiwat, C.; Limpruttidham, N.; Rattanawong, P. Cognitive impairment and 30-day rehospitalization rate in patients with acute heart failure: A systematic review and meta-analysis. Indian Hear. J. 2019, 71, 52–59. [Google Scholar] [CrossRef]
- Huynh, Q.; Negishi, K.; Blizzard, L.; Saito, M.; De Pasquale, C.; Hare, J.L.; Leung, D.; Stanton, T.; Sanderson, K.; Venn, A.J.; et al. Mild cognitive impairment predicts death and readmission within 30days of discharge for heart failure. Int. J. Cardiol. 2016, 221, 212–217. [Google Scholar] [CrossRef]
- Lan, H.; Hawkins, L.A.; Kashner, M.; Perez, E.; Firek, C.J.; Silvet, H. Cognitive impairment predicts mortality in outpatient veterans with heart failure. Hear. Lung 2018, 47, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Pressler, S.J.; Kim, J.; Riley, P.; Ronis, D.L.; Gradus-Pizlo, I. Memory dysfunction, psychomotor slowing, and decreased execu-tive function predict mortality in patients with heart failure and low ejection fraction. J. Card. Fail 2010, 16, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Alagiakrishnan, K.; Mah, D.; Dyck, J.R.; Senthilselvan, A.; Ezekowitz, J. Comparison of two commonly used clinical cognitive screening tests to diagnose mild cognitive impairment in heart failure with the golden standard European Consortium Criteria. Int. J. Cardiol. 2017, 228, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.; Kure, C.E.; Pressler, S.J.; Ski, C.F.; Clark, A.M.; Thompson, D.R. Diagnostic Accuracy of Cognitive Screening Instruments in Heart Failure: A Systematic Review. J. Cardiovasc. Nurs. 2016, 31, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.A.W.; Gathright, E.C.; Gunstad, J.; Dolansky, M.A.; Redle, J.D.; Josephson, R.; Moore, S.M.; Hughes, J.W. The MoCA and MMSE as screeners for cognitive impairment in a heart failure population: A study with comprehensive neuro-psychological testing. Heart Lung. 2014, 43, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Borson, S.; Scanlan, J.; Brush, M.; Vitaliano, P.; Dokmak, A. The mini-cog: A cognitive “vital signs” measure for dementia screening in multi-lingual elderly. Int. J. Geriatr. Psychiatry 2000, 15, 1021–1027. [Google Scholar] [CrossRef]
- Zuccalà, G.; Onder, G.; Marzetti, E.; Lo Monaco, M.R.; Cesari, M.; Cocchi, A.; Carbonin, P.; Bernabei, R. Use of angioten-sin-converting enzyme inhibitors and variations in cognitive performance among patients with heart failure. Eur. Heart J. 2005, 26, 226–233. [Google Scholar] [CrossRef]
- Culman, J.; Blume, A.; Gohlke, P.; Unger, T. The renin-angiotensin system in the brain: Possible therapeutic implications for AT1-receptor blockers. J. Hum. Hypertens. 2002, 16, S64–S70. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabes, J.A.; Boriani, G.; Braun-schweig, F.; Brignole, M.; et al. ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA). Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar]
- van Bommel, R.J.; Marsan, N.A.; Koppen, H.; Delgado, V.; Borleffs, C.J.W.; Ypenburg, C.; Bertini, M.; Schalij, M.J.; Bax, J.J. Effect of Cardiac Resynchronization Therapy on Cerebral Blood Flow. Am. J. Cardiol. 2010, 106, 73–77. [Google Scholar] [CrossRef]
- Fumagalli, S.; Pieragnoli, P.; Ricciardi, G.; Mascia, G.; Mascia, F.; Michelotti, F.; Mascioli, G.; Beltrami, M.; Padeletti, M.; Nesti, M.; et al. Cardiac resynchronization therapy improves functional status and cognition. Int. J. Cardiol. 2016, 219, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Duncker, D.; Friedel, K.; König, T.; Schreyer, H.; Lüsebrink, U.; Duncker, M.; Oswald, H.; Klein, G.; Gardiwal, A. Cardiac resynchronization therapy improves psycho-cognitive performance in patients with heart failure. Europace 2015, 17, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Tanne, D.; Freimark, D.; Poreh, A.; Merzeliak, O.; Bruck, B.; Schwammenthal, Y.; Schwammenthal, E.; Motro, M.; Adler, Y. Cognitive functions in severe congestive heart failure before and after an exercise training program. Int. J. Cardiol. 2005, 103, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.R.; Edner, M.; Henriksson, P.; Mejhert, M.; Persson, H.; Grut, M.; Billing, E. A nurse-based management program in heart failure patients affects females and persons with cognitive dysfunction most. Patient Educ. Couns. 2005, 58, 146–153. [Google Scholar] [CrossRef]
- Stanek, K.M.; Gunstad, J.; Spitznagel, M.B.; Waechter, D.; Hughes, J.W.; Luyster, F.; Josephson, R.; Rosneck, J. Improvements in Cognitive Function Following Cardiac Rehabilitation for Older Adults with Cardiovascular Disease. Int. J. Neurosci. 2010, 121, 86–93. [Google Scholar] [CrossRef]
- Cannon, J.A.; Moffitt, P.; Perez-Moreno, A.C.; Walters, M.R.; Broomfield, N.M.; McMurray, J.J.V.; Quinn, T.J. Cognitive Im-pairment and Heart Failure: Systematic Review and Meta-Analysis. J. Card. Fail 2017, 23, 464–475. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, F.Q.; Kong, W.K.F.; Wong, R.C.C.; Chong, Y.F.; Chew, N.W.S.; Yeo, T.-C.; Sharma, V.K.; Poh, K.K.; Sia, C.-H. Cognitive Impairment in Heart Failure—A Review. Biology 2022, 11, 179. https://doi.org/10.3390/biology11020179
Goh FQ, Kong WKF, Wong RCC, Chong YF, Chew NWS, Yeo T-C, Sharma VK, Poh KK, Sia C-H. Cognitive Impairment in Heart Failure—A Review. Biology. 2022; 11(2):179. https://doi.org/10.3390/biology11020179
Chicago/Turabian StyleGoh, Fang Qin, William K. F. Kong, Raymond C. C. Wong, Yao Feng Chong, Nicholas W. S. Chew, Tiong-Cheng Yeo, Vijay Kumar Sharma, Kian Keong Poh, and Ching-Hui Sia. 2022. "Cognitive Impairment in Heart Failure—A Review" Biology 11, no. 2: 179. https://doi.org/10.3390/biology11020179
APA StyleGoh, F. Q., Kong, W. K. F., Wong, R. C. C., Chong, Y. F., Chew, N. W. S., Yeo, T.-C., Sharma, V. K., Poh, K. K., & Sia, C.-H. (2022). Cognitive Impairment in Heart Failure—A Review. Biology, 11(2), 179. https://doi.org/10.3390/biology11020179







