The Feeding Behaviour of Gall Midge Larvae and Its Implications for Biocontrol of the Giant Reed: Insights from Stable Isotope Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Lasioptera donacis Life Cycle
2.2. Sampling and Stable Isotope Analysis
2.3. Data Analysis
3. Results
3.1. Isotopic Signatures of Reeds, Fungus and Gall Midge Larvae
3.2. Diet of Gall Midge Larvae: Influence of Reed Quality and Parasitism by T. gyraloura
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simberloff, D. Biological Invasions—How Are They Affecting Us, and What Can We Do about Them? West. N. Am. Nat. 2001, 61, 308–315. [Google Scholar]
- Masters, G.; Norgrove, L. Climate Change and Invasive Alien Species. UK CABI Work. Pap. 2010, 1, 30. [Google Scholar]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the Environmental and Economic Costs Associated with Alien-Invasive Species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Pimentel, D. Biological Invasions: Economic and Environmental Costs of Alien Plant, Animal, and Microbe Species, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-1-4398-2990-5. [Google Scholar]
- Sakai, A.K.; Allendorf, F.W.; Holt, J.S.; Lodge, D.M.; Molofsky, J.; With, K.A.; Baughman, S.; Cabin, R.J.; Cohen, J.E.; Ellstrand, N.C.; et al. The Population Biology of Invasive Species. Annu. Rev. Ecol. Syst. 2001, 32, 305–332. [Google Scholar] [CrossRef]
- Brancatelli, G.I.E.; Zalba, S.M. Vector Analysis: A Tool for Preventing the Introduction of Invasive Alien Species into Protected Areas. Nat. Conserv. 2018, 24, 43–63. [Google Scholar] [CrossRef]
- Keane, R. Exotic Plant Invasions and the Enemy Release Hypothesis. Trends Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A Global Assessment of Invasive Plant Impacts on Resident Species, Communities and Ecosystems: The Interaction of Impact Measures, Invading Species’ Traits and Environment. Glob. Chang. Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- David, P.; Thébault, E.; Anneville, O.; Duyck, P.-F.; Chapuis, E.; Loeuille, N. Impacts of Invasive Species on Food Webs. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 56, pp. 1–60. ISBN 978-0-12-804338-7. [Google Scholar]
- Romanuk, T.N.; Zhou, Y.; Valdovinos, F.S.; Martinez, N.D. Robustness Trade-Offs in Model Food Webs. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 56, pp. 263–291. ISBN 978-0-12-804338-7. [Google Scholar]
- López-Núñez, F.A.; Heleno, R.H.; Ribeiro, S.; Marchante, H.; Marchante, E. Four-Trophic Level Food Webs Reveal the Cascading Impacts of an Invasive Plant Targeted for Biocontrol. Ecology 2017, 98, 782–793. [Google Scholar] [CrossRef]
- Culliney, T.W. Benefits of Classical Biological Control for Managing Invasive Plants. Crit. Rev. Plant Sci. 2005, 24, 131–150. [Google Scholar] [CrossRef]
- Weber, E. Invasive Plant Species of the World: A Reference Guide to Environmental Weeds; CABI: Wallingford, UK, 2017; ISBN 1-78064-386-1. [Google Scholar]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; Invasive Species Specialist Group Auckland: Auckland, New Zealand, 2000; Volume 12. [Google Scholar]
- Bell, G.P. Ecology and Management of Arundo donax, and Approaches to Riparian Habitat Restoration in Southern California. In Plant Invasions. Studies from North America and Europe; Backhuys Publishers: Leiden, The Netherlands, 1998. [Google Scholar]
- Dudley, T.L. Arundo donax L. In Invasive Plants California’s Wildlands; University of California Press: Berkeley, CA, USA, 2000; pp. 53–58. [Google Scholar]
- Goolsby, J.A.; Racelis, A.E.; Goolsby, J.B.; Kirk, A.A.; Cristofaro, M.; Grusak, M.A.; de Leon, A.P. Evaluation of Biogeographical Factors in the Native Range to Improve the Success of Biological Control Agents in the Introduced Range. Biocontrol Sci. Technol. 2013, 23, 1213–1230. [Google Scholar] [CrossRef]
- Moore, G.W.; Watts, D.A.; Goolsby, J.A. Ecophysiological Responses of Giant Reed (Arundo donax) to Herbivory. Invasive Plant Sci. Manag. 2010, 3, 521–529. [Google Scholar] [CrossRef]
- Oakins, A.J. An Assessment and Management Protocol for Arundo donax in the Salinas Valley Watershed. Bachelor’s Thesis, California State University, Long Beach, CA, USA, 2001. [Google Scholar]
- Goolsby, J.A.; Vacek, A.T.; Salinas, C.; Racelis, A.; Moran, P.J.; Kirk, A.A. Host Range of the European Leaf Sheath Mining Midge, Lasioptera donacis Coutin (Diptera: Cecidomyiidae), a Biological Control of Giant Reed, Arundo donax L. Biocontrol Sci. Technol. 2017, 27, 781–795. [Google Scholar] [CrossRef]
- Thomas, D.B.; Goolsby, J.A. Morphology of the Preimaginal Stages of Lasioptera donacis Coutin (Diptera: Cecidomyiidae), a Candidate Biocontrol Agent for Giant Arundo Cane. Psyche 2015, 2015, 262678. [Google Scholar]
- Marshall, M.; Goolsby, J.A.; Vacek, A.T.; Racelis, A. Biotic and Abiotic Factors Influencing Infestation Levels of the Arundo Leafminer, Lasioptera donacis, in Its Native Range in Mediterranean Europe. Subtrop. Agric. Environ. 2018, 69, 8–18. [Google Scholar]
- Bon, M.-C.; Guermache, F.; de Simone, D.; Cristofaro, M.; Vacek, A.; Goolsby, J. Insights into the Microbes and Nematodes Hosted by Pupae of the Arundo Leaf Miner, Lasioptera donacis (Diptera: Cecidomyiidae). Fla. Entomol. 2018, 101, 505–507. [Google Scholar] [CrossRef]
- Coutin, R. Une Nouvelle Cécidomyie Des Gaines Foliaires de La Canne de Provence, Lasioptera donacis n. sp.(Diptera, Cecidomyiidae). Bull. Société Entomol. Fr. 2001, 106, 105–108. [Google Scholar] [CrossRef]
- Poinar, G.; Thomas, D.B. Tripius gyraloura n. Sp. (Aphelenchoidea: Sphaerulariidae) Parasitic in the Gall Midge Lasioptera donacis Coutin (Diptera: Cecidomyiidae). Syst. Parasitol. 2014, 89, 247–252. [Google Scholar] [CrossRef]
- Spencer, D.; Sher, A.; Thornby, D.; Liow, P.-S.; Ksander, G.; Tan, W. Non-Destructive Assessment of Arundo donax (Poaceae) Leaf Quality. J. Freshw. Ecol. 2007, 22, 277–285. [Google Scholar] [CrossRef]
- Layman, C.A.; Araujo, M.S.; Boucek, R.; Hammerschlag-Peyer, C.M.; Harrison, E.; Jud, Z.R.; Matich, P.; Rosenblatt, A.E.; Vaudo, J.J.; Yeager, L.A.; et al. Applying Stable Isotopes to Examine Food-Web Structure: An Overview of Analytical Tools. Biol. Rev. 2012, 87, 545–562. [Google Scholar] [CrossRef]
- Michener, R.H.; Lajtha, K. Stable Isotopes in Ecology and Environmental Science, 2nd ed.; Ecological Methods and Concepts Series; Blackwell Pub: Malden, MA, USA, 2007; ISBN 978-1-4051-2680-9. [Google Scholar]
- Peterson, B.J.; Fry, B. Stable Isotopes in Ecosystem Studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Post, D.M. Using Stable Isotopes to Estimate Trophic Position: Models, Methods, and Assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Bearhop, S.; Adams, C.E.; Waldron, S.; Fuller, R.A.; Macleod, H. Determining Trophic Niche Width: A Novel Approach Using Stable Isotope Analysis: Stable Isotopes as Measures of Niche Width. J. Anim. Ecol. 2004, 73, 1007–1012. [Google Scholar] [CrossRef]
- Newsome, S.D.; Martinez del Rio, C.; Bearhop, S.; Phillips, D.L. A Niche for Isotopic Ecology. Front. Ecol. Environ. 2007, 5, 429–436. [Google Scholar] [CrossRef]
- Madeira, F.; di Lascio, A.; Costantini, M.L.; Rossi, L.; Rösch, V.; Pons, X. Intercrop Movement of Heteropteran Predators between Alfalfa and Maize Examined by Stable Isotope Analysis. J. Pest Sci. 2019, 92, 757–767. [Google Scholar] [CrossRef]
- di Lascio, A.; Madeira, F.; Costantini, M.L.; Rossi, L.; Pons, X. Movement of Three Aphidophagous Ladybird Species between Alfalfa and Maize Revealed by Carbon and Nitrogen Stable Isotope Analysis. BioControl 2016, 61, 35–46. [Google Scholar] [CrossRef]
- Stock, B.C.; Jackson, A.L.; Ward, E.J.; Parnell, A.C.; Phillips, D.L.; Semmens, B.X. Analyzing Mixing Systems Using a New Generation of Bayesian Tracer Mixing Models. PeerJ 2018, 6, e5096. [Google Scholar] [CrossRef]
- Sporta Caputi, S.; Rossi, L.; Pons, X.; Careddu, G.; Calizza, E.; Costantini, M.L. Trophic Attractiveness for Soil Fauna of Residues of Bt and Near-Isogenic Maize: A C and N Stable Isotope-Based Study. Agric. Ecosyst. Environ. 2022, 329, 107868. [Google Scholar] [CrossRef]
- Carneiro, M.A.A.; Branco, C.S.; Braga, C.E.; Almada, E.D.; Costa, M.; Maia, V.C.; Fernandes, G.W. Are Gall Midge Species (Diptera, Cecidomyiidae) Host-Plant Specialists? Rev. Bras. Entomol. 2009, 53, 365–378. [Google Scholar] [CrossRef]
- Gagné, R.; Jaschhof, M. A Catalog of the Cecidomyiidae (Diptera) of the World. 813p. Available online: https://www.ars.usda.gov/ARSUserFiles/12754100/gagne_2010_world_catalog_cecidomyiidae.pdf (accessed on 18 June 2021).
- Borkent, A.; Bissett, J. Gall Midges (Diptera: Cecidomyiidae) Are Vectors for Their Fungal Symbionts. Symbiosis 1985, 1, 185–194. [Google Scholar]
- Rohfritsch, O. A fungus associated gall midge, Lasioptera arundinis (Schiner), on Phragmites australis (Cav.) Trin. Bull. Société Bot. Fr. Lett. Bot. 1992, 139, 45–59. [Google Scholar] [CrossRef]
- Costantini, M.L.; Calizza, E.; Rossi, L. Stable Isotope Variation during Fungal Colonisation of Leaf Detritus in Aquatic Environments. Fungal Ecol. 2014, 11, 154–163. [Google Scholar] [CrossRef][Green Version]
- Ponsard, S.; Arditi, R. Detecting Omnivory with Δ15N. Trends Ecol. Evol. 2001, 16, 20–21. [Google Scholar] [CrossRef]
- McCutchan, J.H., Jr.; Lewis, W.M., Jr.; Kendall, C.; McGrath, C.C. Variation in Trophic Shift for Stable Isotope Ratios of Carbon, Nitrogen, and Sulfur. Oikos 2003, 102, 378–390. [Google Scholar] [CrossRef]
- Felton, G.W. Nutritive Quality of Plant Protein: Sources of Variation and Insect Herbivore Responses. Arch. Insect Biochem. Physiol. Publ. Collab. Entomol. Soc. Am. 1996, 32, 107–130. [Google Scholar] [CrossRef]
- Simpson, S.J.; Clissold, F.J.; Lihoreau, M.; Ponton, F.; Wilder, S.M.; Raubenheimer, D. Recent Advances in the Integrative Nutrition of Arthropods. Annu. Rev. Entomol. 2015, 60, 293–311. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. The Nature of Nutrition: A Unifying Framework. Aust. J. Zool. 2011, 59, 350. [Google Scholar] [CrossRef]
- Neto, C.P.; Seca, A.; Nunes, A.M.; Coimbra, M.A.; Domingues, F.; Evtuguin, D.; Silvestre, A.; Cavaleiro, J.A.S. Variations in Chemical Composition and Structure of Macromolecular Components in Different Morphological Regions and Maturity Stages of Arundo donax. Ind. Crops Prod. 1997, 6, 51–58. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. The Constituents and Biological Effects of Arundo donax-A Review. Int. J. Phytopharm. Res. 2015, 6, 34–40. [Google Scholar]
- Moran, P.J.; Goolsby, J.A. Effect of Nitrogen Fertilization on Growth of Arundo donax and on Rearing of a Biological Control Agent, the Shoot Gall-Forming Wasp Tetramesa romana. Biocontrol Sci. Technol. 2014, 24, 503–517. [Google Scholar] [CrossRef]
- Spencer, D.F.; Ksander, G.G.; Whitehand, L.C. Spatial and Temporal Variation in RGR and Leaf Quality of a Clonal Riparian Plant: Arundo donax. Aquat. Bot. 2005, 81, 27–36. [Google Scholar] [CrossRef]
- Calizza, E.; Favero, F.; Rossi, D.; Careddu, G.; Fiorentino, F.; Sporta Caputi, S.; Rossi, L.; Costantini, M.L. Isotopic Biomonitoring of N Pollution in Rivers Embedded in Complex Human Landscapes. Sci. Total Environ. 2020, 706, 136081. [Google Scholar] [CrossRef] [PubMed]
- Paredes, I.; Ramírez, F.; Forero, M.G.; Green, A.J. Stable Isotopes in Helophytes Reflect Anthropogenic Nitrogen Pollution in Entry Streams at the Doñana World Heritage Site. Ecol. Indic. 2019, 97, 130–140. [Google Scholar] [CrossRef]
- Calizza, E.; Costantini, M.L.; Rossi, D.; Pasquali, V.; Careddu, G.; Rossi, L. Stable Isotopes and Digital Elevation Models to Study Nutrient Inputs in High-Arctic Lakes. Rend. Lincei 2016, 27, 191–199. [Google Scholar] [CrossRef]
- Watts, D.A.; Moore, G.W. Water-Use Dynamics of an Invasive Reed, Arundo donax, from Leaf to Stand. Wetlands 2011, 31, 725–734. [Google Scholar] [CrossRef]
- Ebert, D.; Herre, E.A. The Evolution of Parasitic Diseases. Parasitol. Today 1996, 12, 96–101. [Google Scholar] [CrossRef]
- Jensen, K.H.; Little, T.; Skorping, A.; Ebert, D. Empirical Support for Optimal Virulence in a Castrating Parasite. PLoS Biol. 2006, 4, e197. [Google Scholar] [CrossRef]
- Little, T.J.; Shuker, D.M.; Colegrave, N.; Day, T.; Graham, A.L. The Coevolution of Virulence: Tolerance in Perspective. PLoS Pathog. 2010, 6, e1001006. [Google Scholar] [CrossRef]
- Molnár, B.P.; Boddum, T.; Hill, S.R.; Hansson, B.S.; Hillbur, Y.; Birgersson, G. Ecological and Phylogenetic Relationships Shape the Peripheral Olfactory Systems of Highly Specialized Gall Midges (Cecidomiiydae). Front. Physiol. 2018, 9, 323. [Google Scholar] [CrossRef]
- Pyke, G.H.; Pulliam, H.R.; Charnov, E.L. Optimal Foraging: A Selective Review of Theory and Tests. Q. Rev. Biol. 1977, 52, 137–154. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, Q.; Liang, D.; Huang, S.; Liao, J. The Potential Application of Giant Reed (Arundo donax) in Ecological Remediation. Front. Environ. Sci. 2021, 9, 652367. [Google Scholar] [CrossRef]
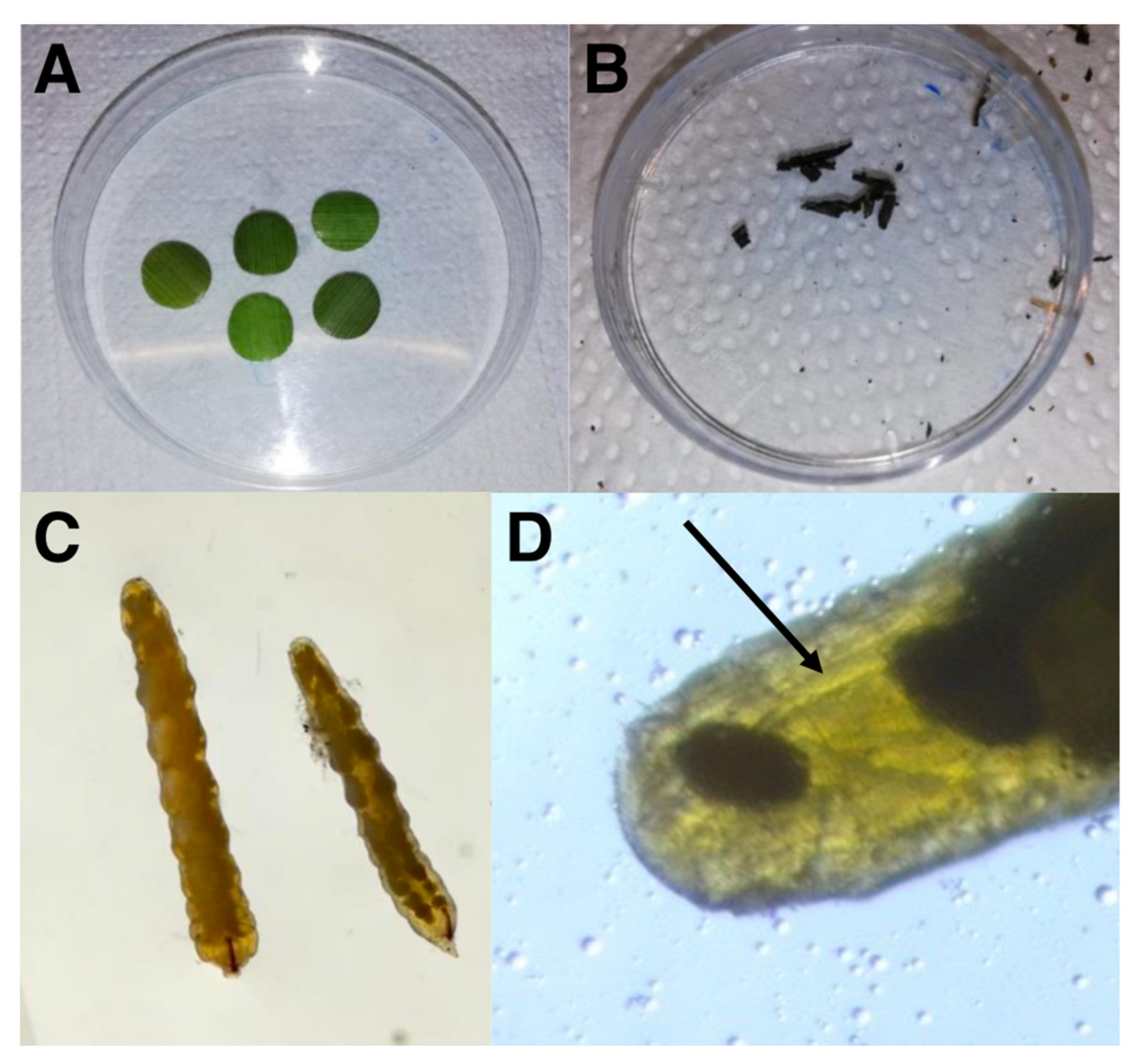
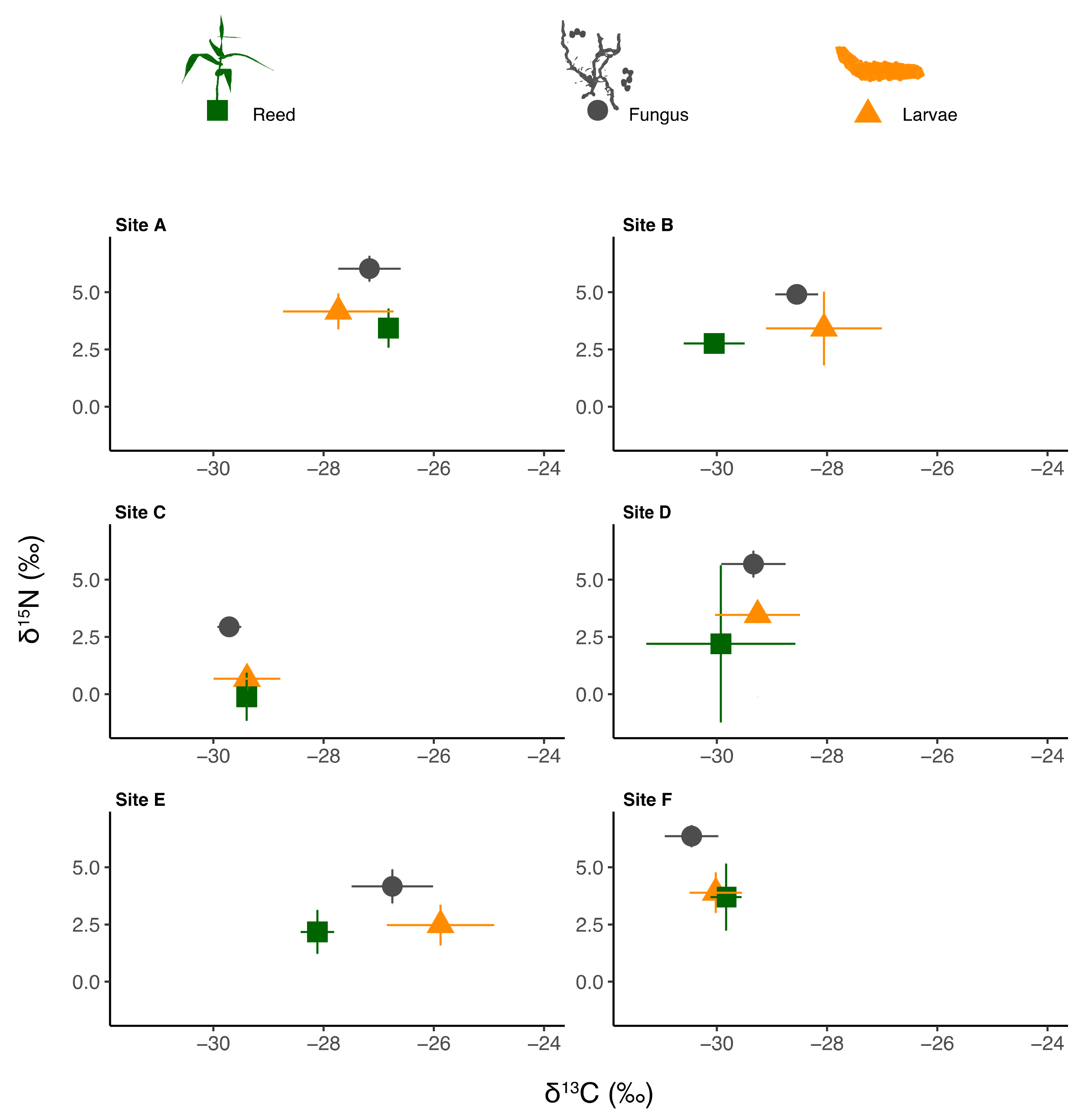
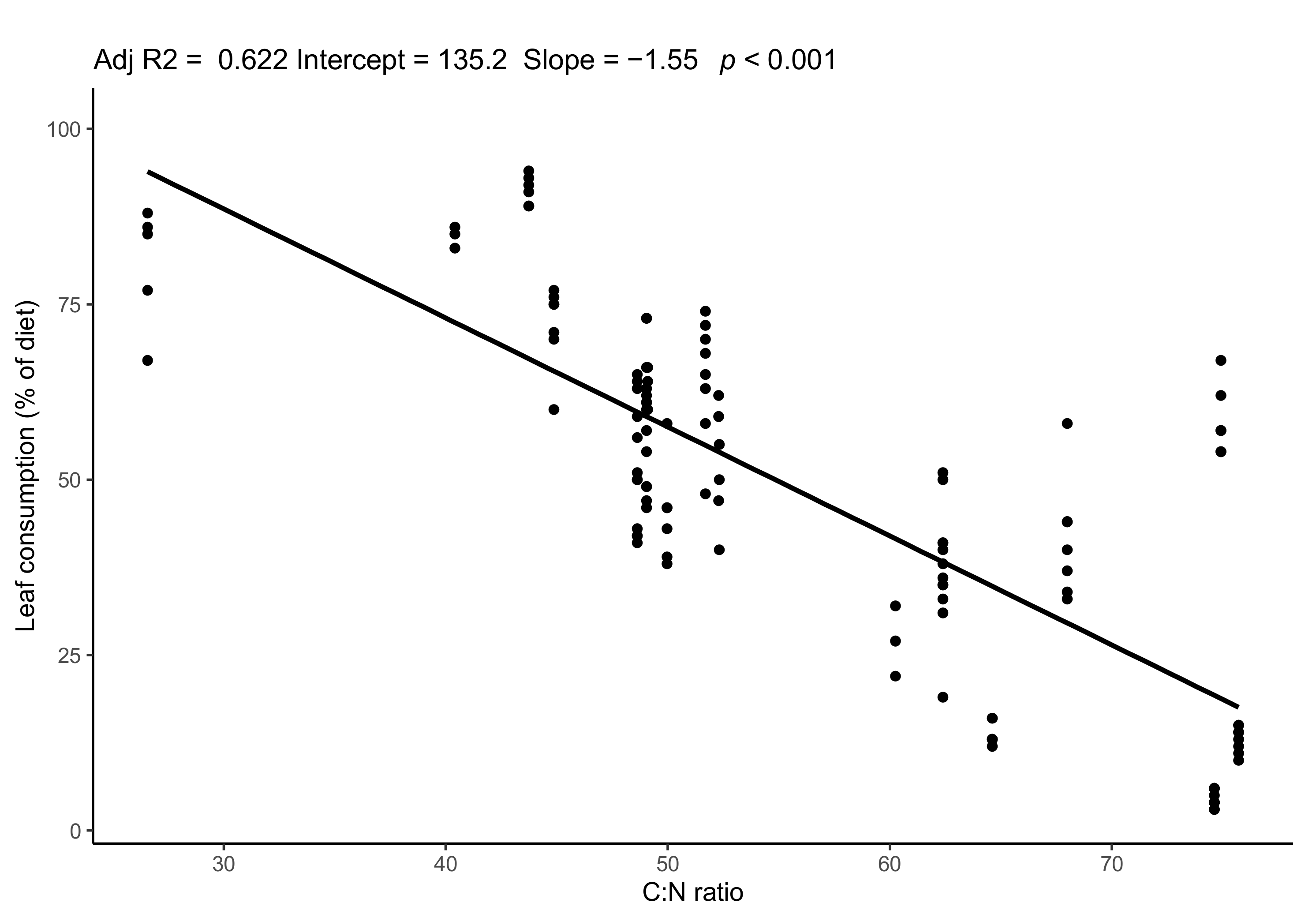
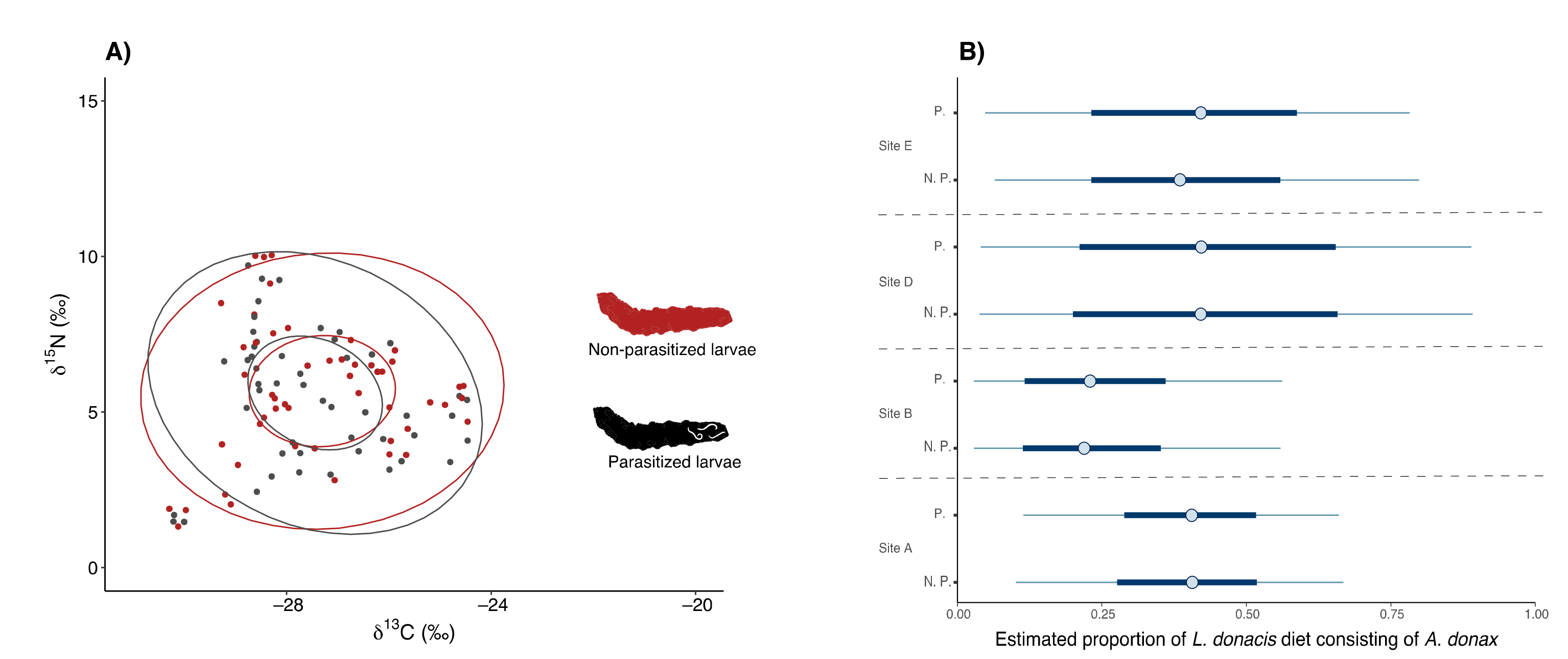
| δ13C (‰) | δ15N (‰) | C:N | Sample Size | |
|---|---|---|---|---|
| Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | N° | |
| A. donax | −29.0 ± 1.3 a | 2.5 ± 1.9 a | 55.0 ± 13.2 a | 18 |
| A. arundinis | −28.7 ± 1.5 a,b | 5.1 ± 1.7 b | 22.1 ± 4.4 b | 18 |
| L. donacis | −27.8 ± 1.6 b | 5.4 ± 2.0 b | 10.9 ± 1.9 c | 124 |
| Site | Reed Consumption | Fungus Consumption | N° L. donacis | ||||
|---|---|---|---|---|---|---|---|
| Mean (%) | 2.5% | 97.5% | Mean (%) | 2.5% | 97.5% | ||
| A | 32.1 | 10.7 | 57.1 | 67.9 | 42.9 | 89.3 | 27 |
| B | 6.8 | 0.8 | 20.4 | 93.2 | 79.6 | 99.2 | 26 |
| C | 58.4 | 19.5 | 82.9 | 41.6 | 17.1 | 80.5 | 9 |
| D | 17.8 | 1.5 | 57.4 | 82.2 | 42.6 | 98.5 | 24 |
| E | 8.8 | 0.9 | 25.0 | 91.2 | 75.0 | 99.1 | 25 |
| F | 85.9 | 72.6 | 96.4 | 14.1 | 3.6 | 27.4 | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Careddu, G.; Botti, M.; Cristofaro, M.; Sporta Caputi, S.; Calizza, E.; Rossi, L.; Costantini, M.L. The Feeding Behaviour of Gall Midge Larvae and Its Implications for Biocontrol of the Giant Reed: Insights from Stable Isotope Analysis. Biology 2022, 11, 1805. https://doi.org/10.3390/biology11121805
Careddu G, Botti M, Cristofaro M, Sporta Caputi S, Calizza E, Rossi L, Costantini ML. The Feeding Behaviour of Gall Midge Larvae and Its Implications for Biocontrol of the Giant Reed: Insights from Stable Isotope Analysis. Biology. 2022; 11(12):1805. https://doi.org/10.3390/biology11121805
Chicago/Turabian StyleCareddu, Giulio, Marcovalerio Botti, Massimo Cristofaro, Simona Sporta Caputi, Edoardo Calizza, Loreto Rossi, and Maria Letizia Costantini. 2022. "The Feeding Behaviour of Gall Midge Larvae and Its Implications for Biocontrol of the Giant Reed: Insights from Stable Isotope Analysis" Biology 11, no. 12: 1805. https://doi.org/10.3390/biology11121805
APA StyleCareddu, G., Botti, M., Cristofaro, M., Sporta Caputi, S., Calizza, E., Rossi, L., & Costantini, M. L. (2022). The Feeding Behaviour of Gall Midge Larvae and Its Implications for Biocontrol of the Giant Reed: Insights from Stable Isotope Analysis. Biology, 11(12), 1805. https://doi.org/10.3390/biology11121805








