Effects of Caulerpa taxifolia on Physiological Processes and Gene Expression of Acropora hyacinthus during Thermal Stress
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
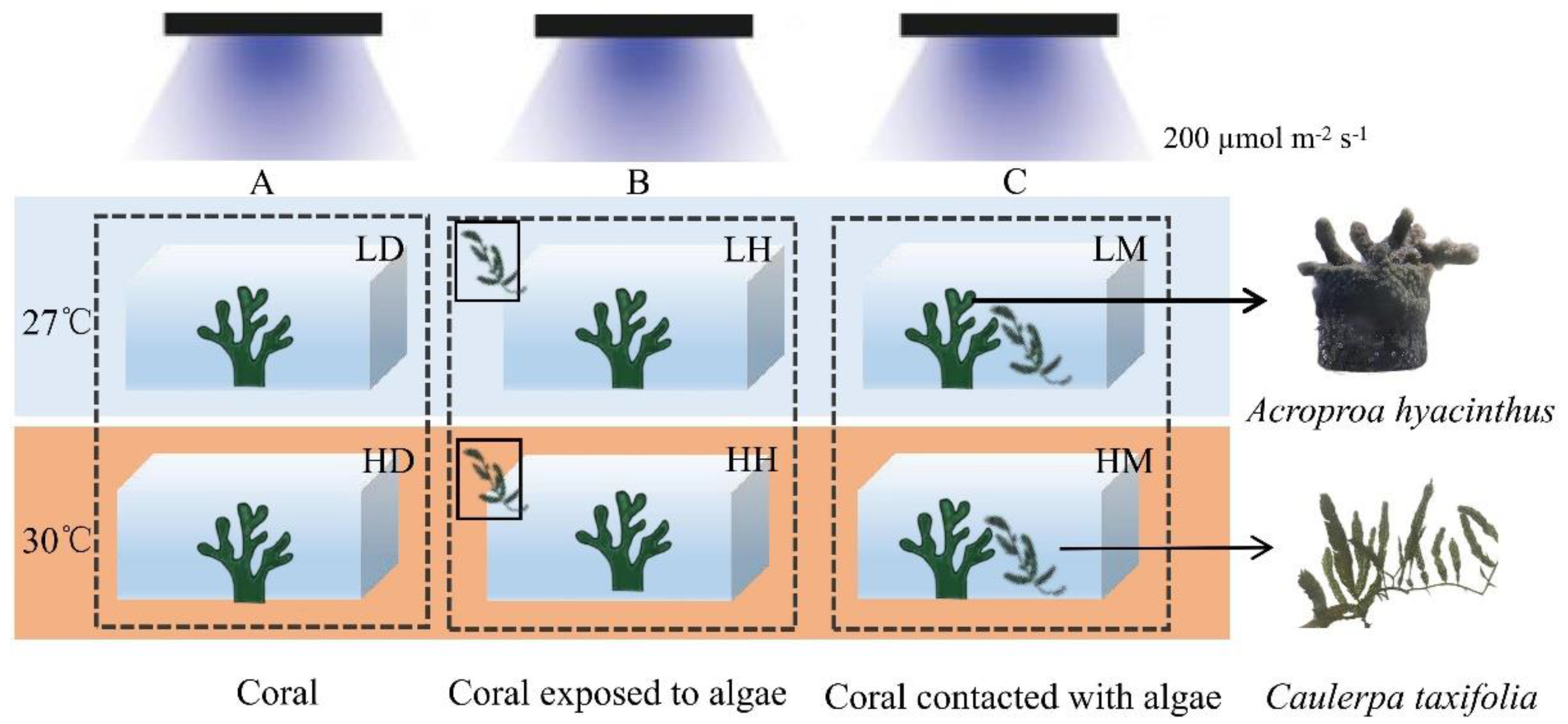
2.1. Experimental Samples
2.2. Experimental Design
2.3. Determination of Physiological and Biochemical Indexes
2.3.1. Sample Collection
2.3.2. Zooxanthellae Density and Chl a Content
2.3.3. Growth Rate
2.3.4. SOD and CAT
2.4. Transcriptome Sequencing and Analysis of A. hyacinthus
2.4.1. RNA-Seq Data Analysis
2.4.2. Quantitative PCR for mRNA Expression
2.5. Data Analysis
3. Results
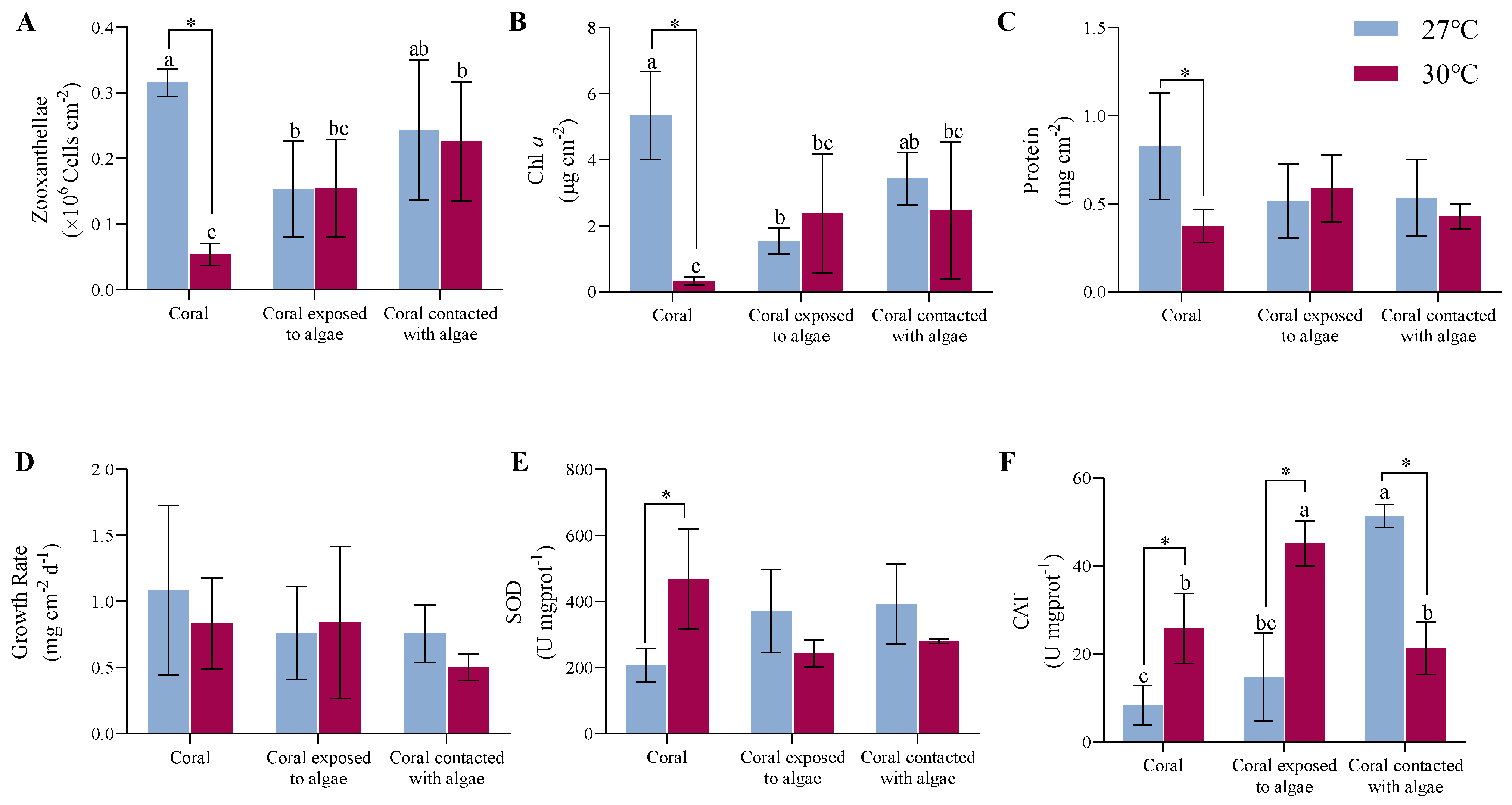
3.1. Results of Macroalgae on Physiological Processes
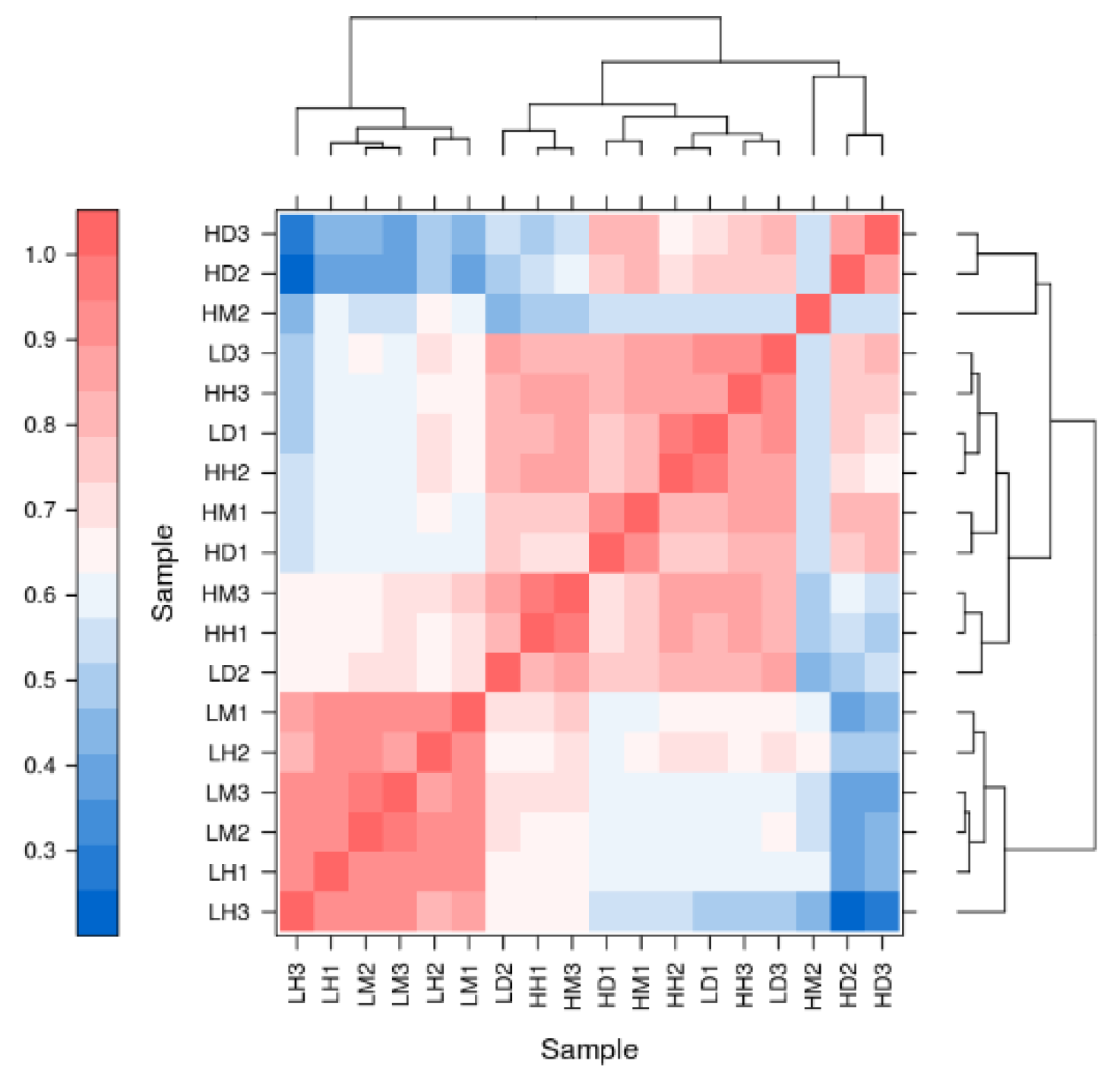
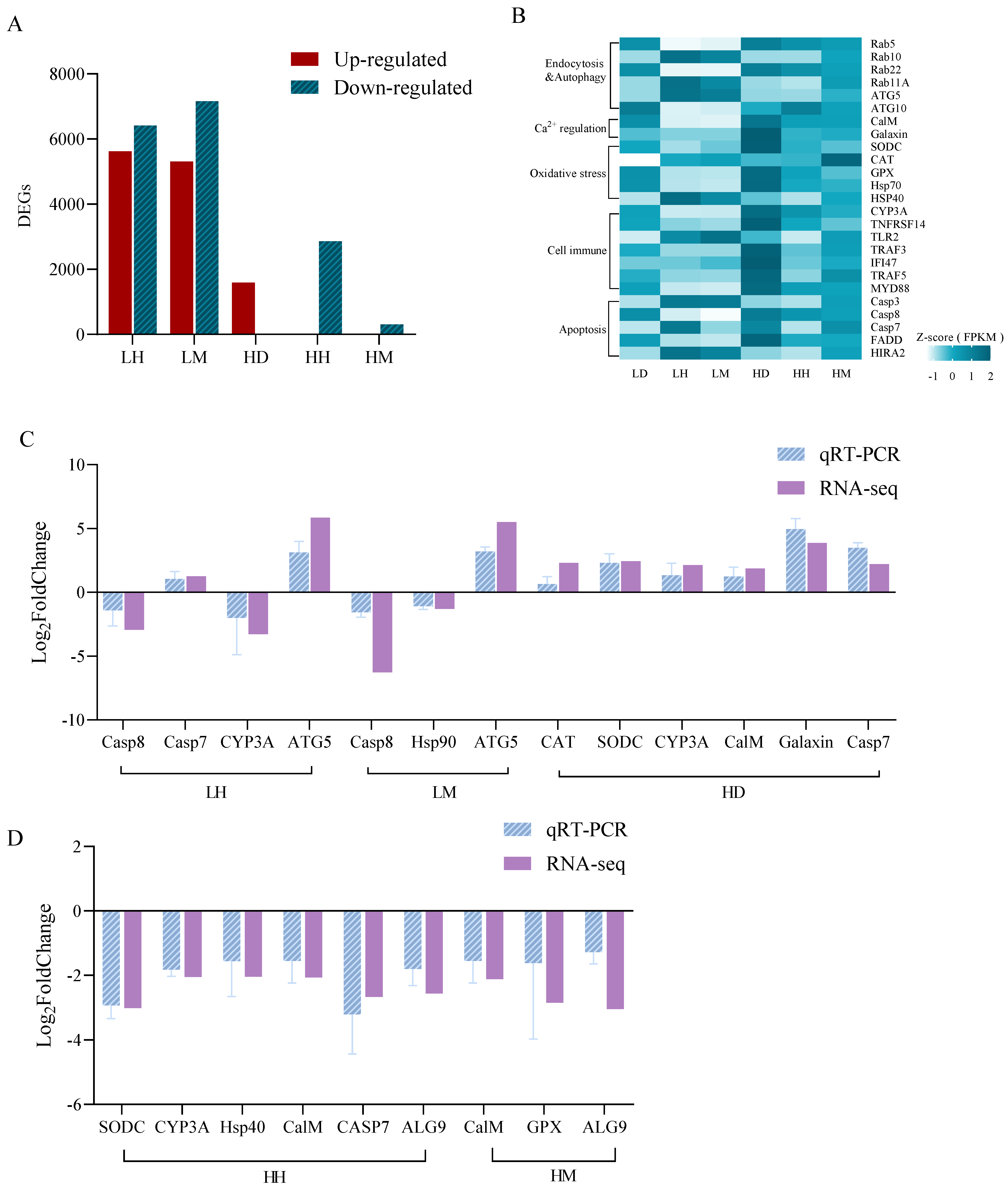
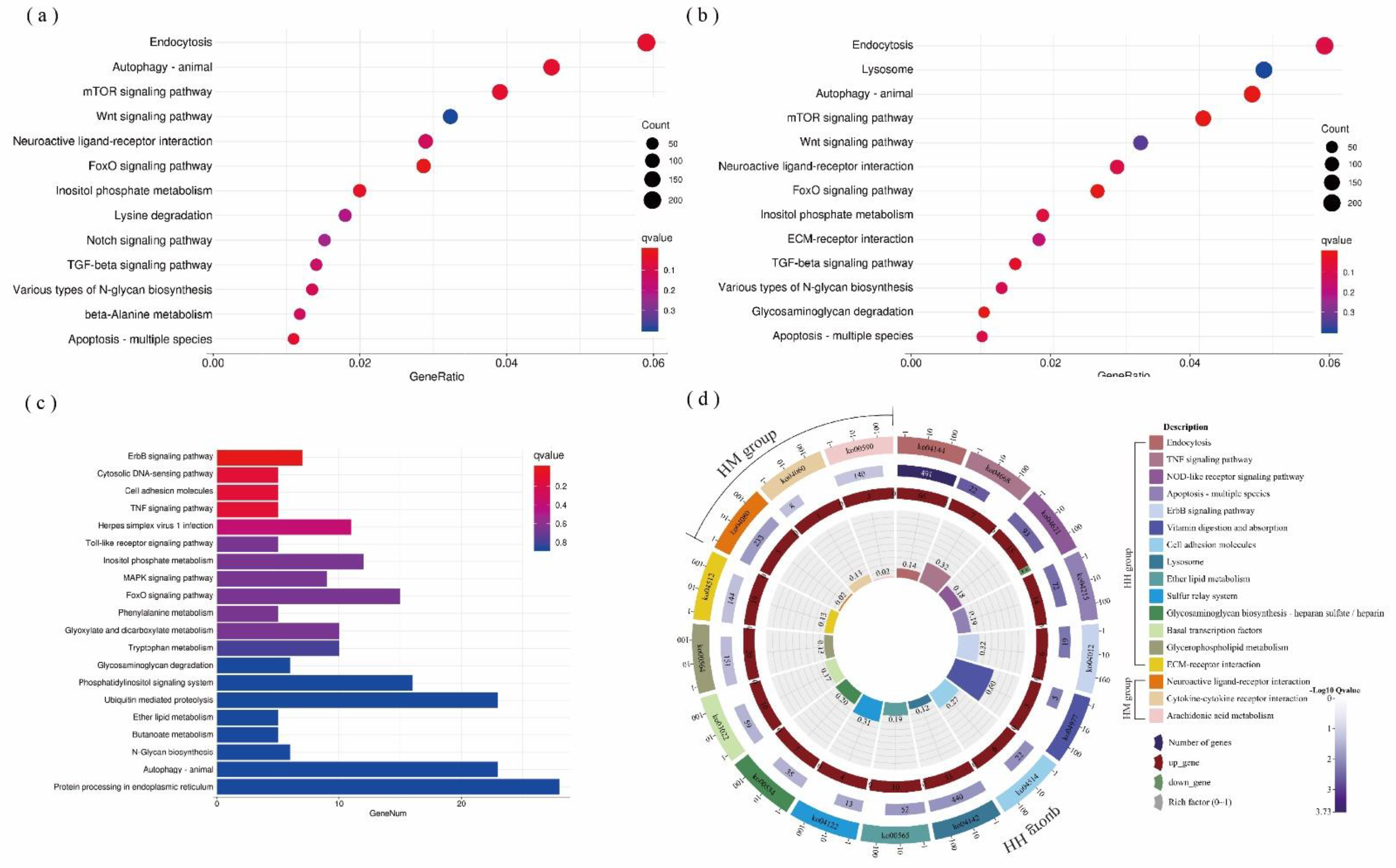
3.2. Results of Transcriptome Analysis of A. hyacinthus
4. Discussion
4.1. Effects of C. taxifolia on the Physiology and Ecology of A. hyacinthus
4.2. Effects of Thermal Stress on the Physiology and Ecology of A. hyacinthus
4.3. Combined Effects of C. taxifolia and Thermal Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Pac, S. Coral-reef area and the contributions of reefs to processes and resources of the world’s oceans. Nature 1978, 273, 18. [Google Scholar]
- Leggat, W.; Heron, S.F.; Fordyce, A.; Suggett, D.J.; Ainsworth, T.D. Experiment Degree Heating Week (eDHW) as a novel metric to reconcile and validate past and future global coral bleaching studies. J. Environ. Manag. 2022, 301, 113919. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P. Catastrophes, Phase Shifts, and Large-Scale Degradation of a Caribbean Coral Reef. Science 1994, 265, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky, Z. Coral Reefs: An Ecosystem in Transition; Stambler, N., Ed.; Springer Science & Business Media: Berlin, Germany, 2010. [Google Scholar]
- Coyer, J.A.; Ambrose, R.F.; Engle, J.M.; Carroll, J.C. Interactions between corals and algae on a temperate zone rocky reef: Mediation by sea urchins. J. Exp. Mar. Biol. Ecol. 1993, 167, 21–37. [Google Scholar] [CrossRef]
- Chadwick, N.E.; Morrow, K.M. Competition among sessile organisms on coral reefs. In Coral Reefs: An Ecosystem in Transition; Springer: Dordrecht, The Netherlands, 2011; pp. 347–371. [Google Scholar]
- Longo, G.O.; Hay, M.E. Seaweed allelopathy to corals: Are active compounds on, or in, seaweeds? Coral Reefs 2017, 36, 247–253. [Google Scholar] [CrossRef]
- Jason, E.; Tanner. Competition between scleractinian corals and macroalgae: An experimental investigation of coral growth, survival and reproduction. J. Exp. Mar. Biol. Ecol. 1995, 190, 151–168. [Google Scholar]
- Rasher, D.B.; Hay, M.E. Chemically rich seaweeds poison corals when not controlled by herbivores. Proc. Natl. Acad. Sci. USA 2010, 107, 9683–9688. [Google Scholar] [CrossRef]
- Rasher, D.B.; Hay, M.E. Seaweed allelopathy degrades the resilience and function of coral reefs. Commun. Integr. Biol. 2010, 3, 564. [Google Scholar] [CrossRef]
- Rasher, D.B.; Stout, E.P.; Engel, S.; Kubanek, J.; Hay, M.E. Macroalgal terpenes function as allelopathic agents against reef corals. Proc. Natl. Acad. Sci. USA 2011, 108, 17726–17731. [Google Scholar] [CrossRef]
- Bonaldo, R.M.; Hay, M.E. Seaweed-coral interactions: Variance in seaweed allelopathy, coral susceptibility, and potential effects on coral resilience. PLoS ONE 2014, 9, e85786. [Google Scholar] [CrossRef]
- Fong, J.; Deignan, L.K.; Bauman, A.G.; Steinberg, P.D.; McDougald, D.; Todd, P.A. Contact-and Water-Mediated Effects of Macroalgae on the Physiology and Microbiome of Three Indo-Pacific Coral Species. Front. Mar. Sci. 2020, 6, 831. [Google Scholar] [CrossRef]
- Diaz-Pulido, G.; Gouezo, M.; Tilbrook, B.; Dove, S.; Anthony, K.R. High CO2 enhances the competitive strength of seaweeds over corals. Ecol. Lett. 2010, 14, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Rölfer, L.; Reuter, H.; Ferse, S.C.A.; Kubicek, A.; Dove, S.; Hoegh-Guldberg, O.; Bender-Champ, D. Coral-macroalgal competition under ocean warming and acidification. J. Exp. Mar. Biol. Ecol. 2021, 534, 151477. [Google Scholar] [CrossRef]
- Edge, S.E.; Morgan, M.B.; Gleason, D.F.; Snell, T.W. Development of a coral cDNA array to examine gene expression profiles in Montastraea faveolata exposed to environmental stress. Mar. Pollut. Bull. 2005, 51, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Desalvo, M.K.; Sunagawa, S.; Fisher, P.L.; Voolstra, C.R.; Iglesias-Prieto, R.; Medina, M. Coral host transcriptomic states are correlated with Symbiodinium genotypes. Mol. Ecol. 2010, 19, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.E.; Shearer, T.L.; Morgan, M.B.; Snell, T.W. Sub-lethal coral stress: Detecting molecular responses of coral populations to environmental conditions over space and time. Aquat. Toxicol. 2013, 128, 135–146. [Google Scholar] [CrossRef]
- Traylor-Knowles, N.; Rose, N.H.; Sheets, E.A.; Palumbi, S.R. Early transcriptional responses during heat stress in the coral. Acropora hyacinthus. Biol. Bull. 2017, 232, 91–100. [Google Scholar] [CrossRef]
- Todd, P.A. Morphological plasticity in scleractinian corals. Biol. Rev. 2008, 83, 315–337. [Google Scholar] [CrossRef]
- Drury, C.; Manzello, D.; Lirman, D. Genotype and local environment dynamically influence growth, disturbance response and survivorship in the threatened coral, Acropora cervicornis. PLoS ONE 2017, 12, e0174000. [Google Scholar] [CrossRef]
- Drury, C.; Lirman, D. Genotype by environment interactions in coral bleaching. Proc. R. Soc. B 2021, 288, 20210177. [Google Scholar] [CrossRef]
- Drury, C.; Dilworth, J.; Majerová, E.; Caruso, C.; Greer, J.B. Expression plasticity regulates intraspecific variation in the acclimatization potential of a reef-building coral. Nat. Commun. 2022, 13, 4790. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.W.; Adrian, M.; Ries, J.B.; Castillo, K.D. Thermal and pCO2 Stress Elicit Divergent Transcriptomic Responses in a Resilient Coral. Front. Mar. Sci. 2016, 3, 112. [Google Scholar] [CrossRef]
- Zhongming, Z.; Linong, L.; Xiaona, Y.; Wangqiang, Z.; Wei, L. AR6 Climate Change 2021: The Physical Science Basis. 2021. Available online: https://www.ipcc.ch/report/sixth-assessment-report-working-group-i/ (accessed on 7 November 2022).
- Marsh, J.A. Primary Productivity of Reef-Building Calcareous Red Algae. Ecology 1970, 51, 255–263. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent Sets of Spectrophotometric Chlorophyll Equations for Acetone, Methanol and Ethanol Solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.S. Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar. Biol. 1989, 101, 389–395. [Google Scholar] [CrossRef]
- Stefanik, D.J.; Wolenski, F.S.; Friedman, L.E.; Gilmore, T.D.; Finnerty, J.R. Isolation of DNA, RNA and protein from the starlet sea anemone Nematostella vectensis. Nat. Protoc. 2013, 8, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Blackall, L.L.; Wilson, B.; Van Oppen, M.J.H. Coral—The world’s most diverse symbiotic ecosystem. Mol. Ecol. 2015, 24, 5330–5347. [Google Scholar] [CrossRef]
- Van Oppen, M.J.H.; Blackall, L.L. Coral microbiome dynamics, functions and design in a changing world. Nat. Rev. Microbiol. 2019, 17, 557–567. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Mizerek, T.L.; Baird, A.H.; Madin, J.S. Species traits as indicators of coral bleaching. Coral Reefs 2018, 37, 791–800. [Google Scholar] [CrossRef]
- Lough, J.M.; Van Oppen, M.J.H. Coral Bleaching Introduction: Coral Bleaching—Patterns, Processes, Causes and Consequences; Springe: Berlin/Heidelberg, Germany, 2009; pp. 1–5. [Google Scholar]
- Shearer, T.L.; Snell, T.W.; Hay, M.E. Gene Expression of Corals in Response to Macroalgal Competitors. PLoS ONE 2014, 9, e114525. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.R.; Schnitzler, C.E.; Weis, V.M. Apoptosis and autophagy as mechanisms of dinoflagellate symbiont release during cnidarian bleaching: Every which way you lose. Proc. R. Soc. B Biol. Sci. 2007, 274, 3079–3085. [Google Scholar] [CrossRef] [PubMed]
- Staiger, K.; Schatz, U.; Staiger, H.; Weyrich, P.; Haas, C.; Guirguis, A.; Machicao, F.; Häring, H.U.; Kellerer, M. Protein kinase C iota mediates lipid-induced apoptosis of human coronary artery endothelial cells. Microvasc. Res. 2009, 78, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Hutagalung, A.H.; Novick, P.J. Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 2011, 91, 119–149. [Google Scholar] [CrossRef]
- Chen, M.C.; Cheng, Y.; Sung, P.J.; Kuo, C.E.; Fang, L.S. Molecular identification of Rab7 (ApRab7) in Aiptasia pulchella and its exclusion from phagosomes harboring zooxanthellae. Biochem. Biophys. Res. Commun. 2003, 308, 586–595. [Google Scholar] [CrossRef]
- Chen, M.C.; Cheng, Y.M.; Hong, M.C.; Fang, L.S. Molecular cloning of Rab5 (ApRab5) in Aiptasia pulchella and its retention in phagosomes harboring live zooxanthellae. Biochem. Biophys. Res. Commun. 2004, 324, 1024–1033. [Google Scholar] [CrossRef]
- Chen, M.C.; Hong, M.C.; Huang, Y.S.; Liu, M.C.; Cheng, Y.M.; Fang, L.S. ApRab11, a cnidarian homologue of the recycling regulatory protein Rab11, is involved in the establishment and maintenance of the Aiptasia-Symbiodinium endosymbiosis. Biochem. Biophys. Res. Commun. 2005, 338, 1607–1616. [Google Scholar] [CrossRef]
- Kim, M.; Sandford, E.; Gatica, D.; Qiu, Y.; Liu, X.; Zheng, Y.; Schulman, B.A.; Xu, J.; Semple, I.; Ro, S.H.; et al. Mutation in ATG5 reduces autophagy and leads to ataxia with developmental delay. Elife 2016, 5, e12245. [Google Scholar] [CrossRef]
- Smith, J.E.; Shaw, M.; Edwards, R.A.; Obura, D.; Pantos, O.; Sala, E.; Sandin, S.A.; Smriga, S.; Hatay, M.; Rohwer, F.L. Indirect effects of algae on coral: Algae-mediated, microbe-induced coral mortality. Ecol. Lett. 2006, 9, 835–845. [Google Scholar] [CrossRef]
- Del Monaco, C.; Hay, M.E.; Gartrell, P.; Mumby, P.J.; Diaz-Pulido, G. Effects of ocean acidification on the potency of macroalgal allelopathy to a common coral. Sci. Rep. 2017, 7, 41053. [Google Scholar] [CrossRef] [PubMed]
- Ulido, G.D.; Barrón, C. CO2 Enrichment Stimulates Dissolved Organic Carbon Release in Coral Reef Macroalgae. J. Phycol. 2020, 56, 8. [Google Scholar]
- Garren, M. Microbial ecology: Algae feed a shift on coral reefs. Nat. Microbiol. 2016, 1, 1–2. [Google Scholar] [CrossRef]
- Krueger, T.; Hawkins, T.D.; Becker, S.; Pontasch, S.; Dove, S.; Hoegh-Guldberg, O.; Leggat, W.; Fisher, P.L.; Davy, S.K. Differential coral bleaching—Contrasting the activity and response of enzymatic antioxidants in symbiotic partners under thermal stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 190, 15–25. [Google Scholar] [CrossRef]
- Flores-Ramírez, L.A.; Liñán-Cabello, M.A. Relationships among thermal stress, bleaching and oxidative damage in the hermatypic coral, Pocillopora capitata. Comp. Biochem. Physiol. C Toxicol. Pharm. 2007, 146, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Sun, Y.F.; Zhou, G.W.; Tong, H.Y.; Huang, L.T.; Yu, X.L.; Liu, C.Y.; Zhang, Y.Y.; Yuan, X.C.; Qian, P.Y.; et al. Ocean acidification elicits differential bleaching and gene expression patterns in larval reef coral Pocillopora damicornis under heat stress. Sci. Total Environ. 2022, 842, 156851. [Google Scholar] [CrossRef]
- Diaz, J.; Hansel, C.; Apprill, A.; Brighi, C.; Zhang, T.; Weber, L.; McNally, S.; Xun, L. Species-specific control of external superoxide levels by the coral holobiont during a natural bleaching event. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Weis, V.M. Cellular mechanisms of Cnidarian bleaching: Stress causes the collapse of symbiosis. J. Exp. Biol. 2008, 211, 3059–3066. [Google Scholar] [CrossRef]
- Cai, J.; Jones, D.P. Superoxide in apoptosis: Mitochondrial generation triggered by cytochromec loss. J. Biol. Chem. 1998, 273, 11401–11404. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Varrella, S.; Tangherlini, M.; Dell’Anno, A.; Canensi, S.; Cerrano, C.; Danovaro, R. Changes in coral forest microbiomes predict the impact of marine heatwaves on habitat-forming species down to mesophotic depths. Sci. Total Environ. 2022, 823, 153701. [Google Scholar] [CrossRef]
- Williams, A.; Pathmanathan, J.S.; Stephens, T.G.; Su, X.; Chiles, E.N.; Conetta, D.; Putnam, H.M.; Bhattacharya, D. Multi-omic characterization of the thermal stress phenome in the stony coral Montipora capitata. PeerJ 2021, 9, e12335. [Google Scholar] [CrossRef] [PubMed]
- Parsell, D.A.; Lindquist, S. The Function of Heat-Shock Proteins in Stress Tolerance: Degradation and Reactivation of Damaged Proteins. Annu. Rev. Genet. 1993, 27, 437. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, W.C.; Starcevic, A.; Baranasic, D.; Diminic, J.; Zucko, J.; Gacesa, R.; van Oppen, M.J.; Hranueli, D.; Cullum, J.; Long, P.F. KEGG orthology-based annotation of the predicted proteome of Acropora digitifera: ZoophyteBase—An open access and searchable database of a coral genome. BMC Genom. 2013, 14, 509. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, S.; Akira, S. Toll-like receptors and Type I interferons. J. Biol. Chem. 2007, 282, 15319–15323. [Google Scholar] [CrossRef]
- Velez, D.R.; Wejse, C.; Stryjewski, M.E.; Abbate, E.; Hulme, W.F.; Myers, J.L.; Estevan, R.; Patillo, S.G.; Olesen, R.; Tacconelli, A.; et al. Variants in toll-like receptors 2 and 9 influence susceptibility to pulmonary tuberculosis in Caucasians, African-Americans, and West Africans. Hum. Genet. 2010, 127, 65–73. [Google Scholar] [CrossRef]
- Bista, P.; Zeng, W.; Ryan, S.; Bailly, V.; Browning, J.L.; Lukashev, M.E. TRAF3 controls activation of the canonical and alternative NFκB by the lymphotoxin beta receptor. J. Biol. Chem. 2010, 285, 12971–12978. [Google Scholar] [CrossRef]
- Khanal, S.K.; Xie, B.; Thompson, M.L.; Sung, S.; Ong, S.K.; Van Leeuwen, J. Fate, transport, and biodegradation of natural estrogens in the environment and engineered systems. Environ. Sci. Technol. 2007, 38, 6537–6546. [Google Scholar] [CrossRef]
- Rieger, J.K.; Klein, K.; Winter, S.; Zanger, U.M. Expression variability of absorption, distribution, metabolism, excretion–Related microRNAs in human liver: Influence of nongenetic factors and association with gene expression. Drug Metab. Dispos. 2013, 41, 1752–1762. [Google Scholar] [CrossRef]
- Brown, K.T.; Bender-Champ, D.; Kenyon, T.M.; Rémond, C.; Hoegh-Guldberg, O.; Dove, S. Temporal effects of ocean warming and acidification on coral–algal competition. Coral Reefs 2019, 38, 297–309. [Google Scholar] [CrossRef]
- Hauri, C.; Fabricius, K.E.; Schaffelke, B.; Humphrey, C. Chemical and Physical Environmental Conditions Underneath Mat- and Canopy-Forming Macroalgae, and Their Effects on Understorey Corals. PLoS ONE 2010, 5, e12685. [Google Scholar] [CrossRef]
- Coelho, V.R.; Fenner, D.; Caruso, C.; Bayles, B.R.; Huang, Y.; Birkeland, C. Shading as a mitigation tool for coral bleaching in three common Indo-Pacific species. J. Exp. Mar. Biol. Ecol. 2017, 497, 152–163. [Google Scholar] [CrossRef]
- Strober, W.; Murray, P.J.; Kitani, A.; Watanabe, T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 2006, 6, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Stefels, J. Physiological aspects of the production and conversion of DMSP in marine algae and higher plants. J. Sea Res. 2000, 43, 183–197. [Google Scholar] [CrossRef]
- Raina, J.B.; Tapiolas, D.M.; Forêt, S.; Lutz, A.; Abrego, D.; Ceh, J.; Seneca, F.O.; Clode, P.L.; Bourne, D.G.; Willis, B.L.; et al. DMSP biosynthesis by an animal and its role in coral thermal stress response. Nature 2013, 502, 677. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, F.E.; Bell, T.G.; Yang, M.; Suggett, D.J.; Steinke, M. Air exposure of coral is a significant source of dimethylsulfide (DMS) to the atmosphere. Sci. Rep. 2016, 6, 36031. [Google Scholar] [CrossRef]
- Sunda, W.; Kieber, D.J.; Kiene, R.P.; Huntsman, S. An antioxidant function for DMSP and DMS in marine algae. Nature 2002, 418, 317–320. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.-R.; Zhou, J.; Zhang, Y.-P.; Liu, L. Effects of Caulerpa taxifolia on Physiological Processes and Gene Expression of Acropora hyacinthus during Thermal Stress. Biology 2022, 11, 1792. https://doi.org/10.3390/biology11121792
Fu J-R, Zhou J, Zhang Y-P, Liu L. Effects of Caulerpa taxifolia on Physiological Processes and Gene Expression of Acropora hyacinthus during Thermal Stress. Biology. 2022; 11(12):1792. https://doi.org/10.3390/biology11121792
Chicago/Turabian StyleFu, Jian-Rong, Jie Zhou, Yan-Ping Zhang, and Li Liu. 2022. "Effects of Caulerpa taxifolia on Physiological Processes and Gene Expression of Acropora hyacinthus during Thermal Stress" Biology 11, no. 12: 1792. https://doi.org/10.3390/biology11121792
APA StyleFu, J.-R., Zhou, J., Zhang, Y.-P., & Liu, L. (2022). Effects of Caulerpa taxifolia on Physiological Processes and Gene Expression of Acropora hyacinthus during Thermal Stress. Biology, 11(12), 1792. https://doi.org/10.3390/biology11121792






