The Effect of Salinity Stress on Enzyme Activities, Histology, and Transcriptome of Silver Carp (Hypophthalmichthys molitrix)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Preparation and Maintenance
2.2. Experimental Design and Sample Collection
2.3. Assay of Enzyme Activities
2.4. Histological Examination Structure
2.5. RNA-Seq
2.5.1. RNA Extraction, Library Preparation, and Sequencing
2.5.2. Quality Control and Sequence Alignment
2.5.3. Transcriptome Assembly and Annotation
2.5.4. Differential Expression Analysis of Genes and Enrichment Analysis
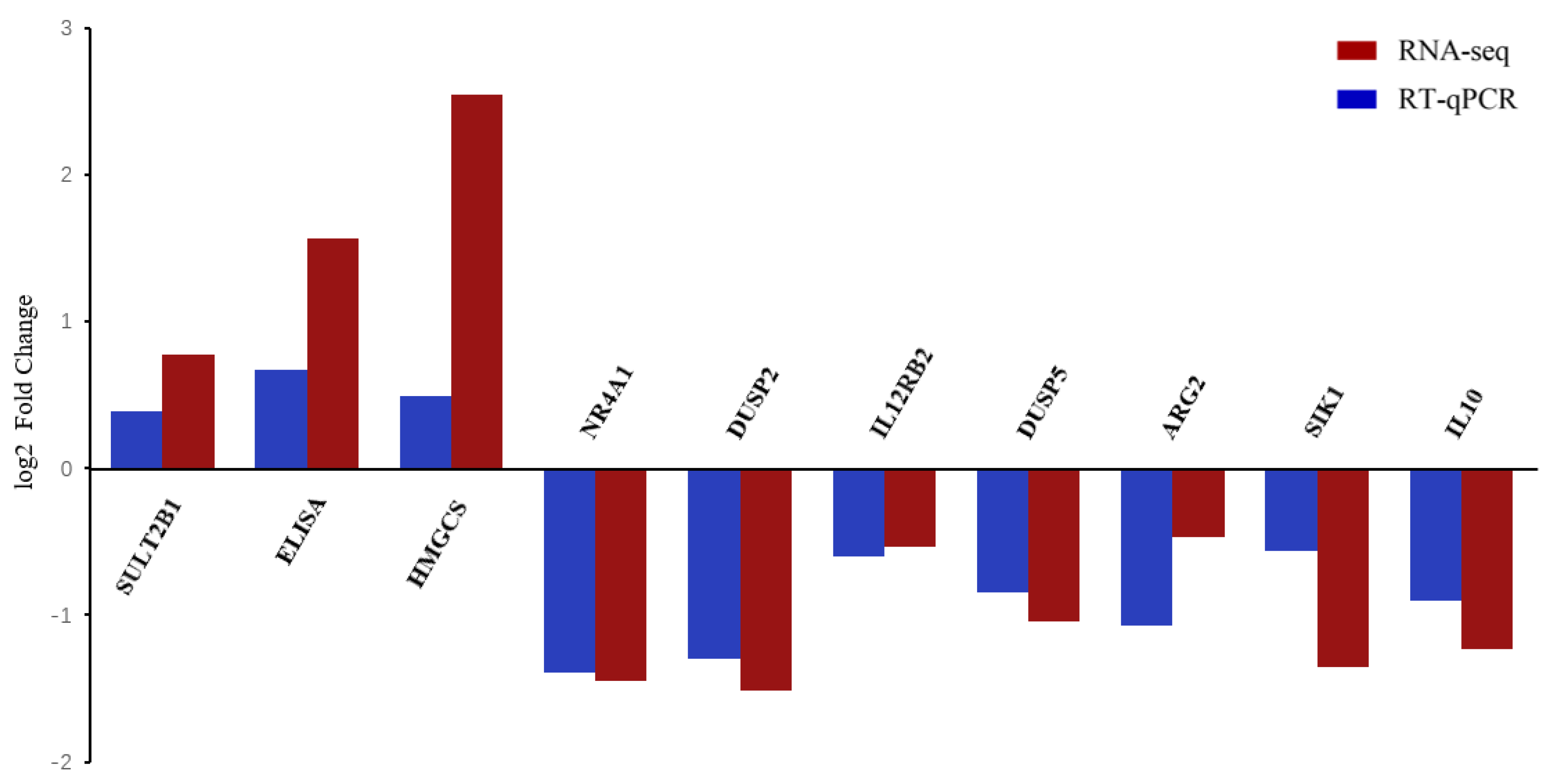
2.6. Real-Time Quantitative PCR Validation
2.7. Statistical Analysis
3. Results
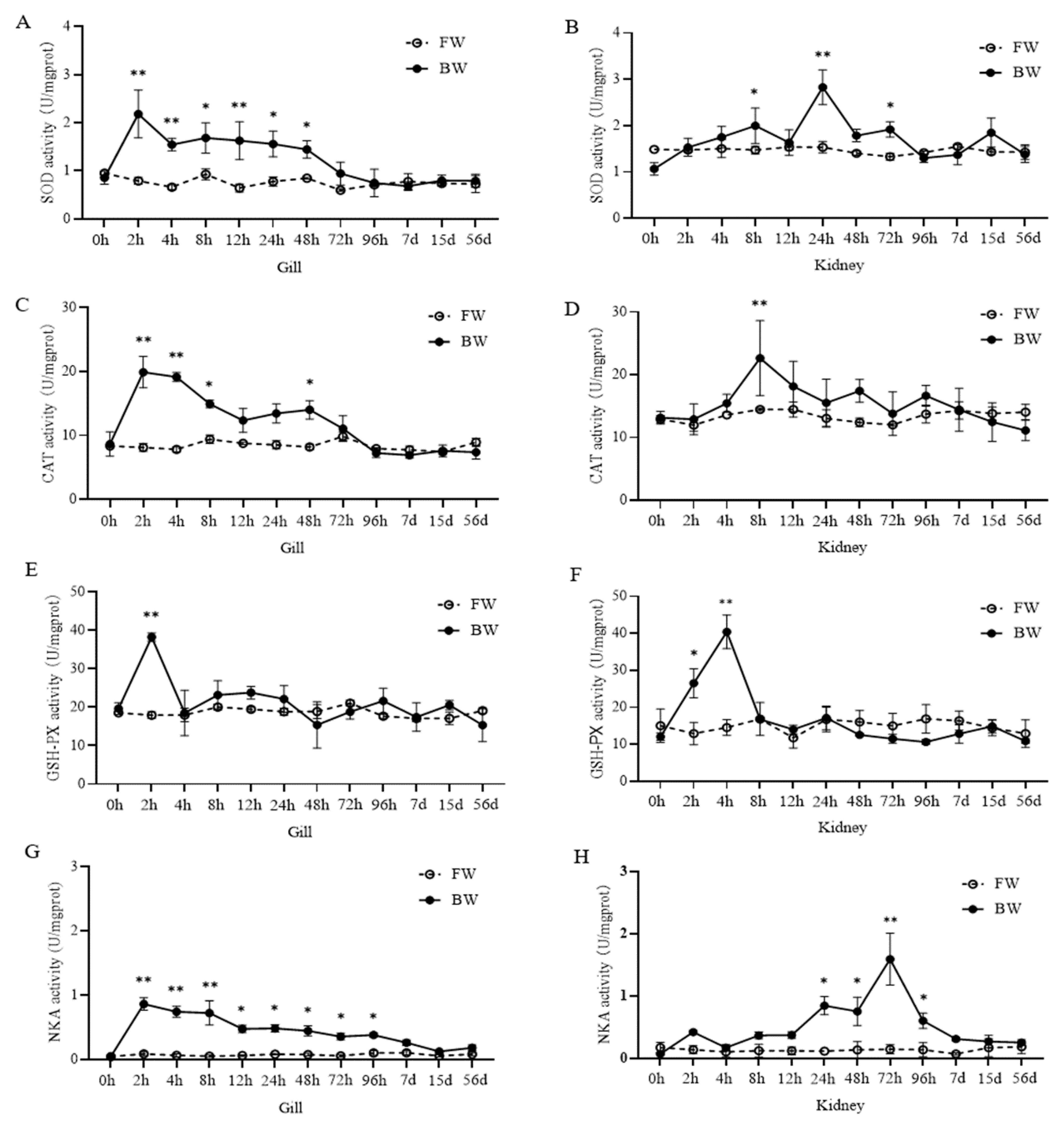
3.1. Antioxidant Enzyme Activities
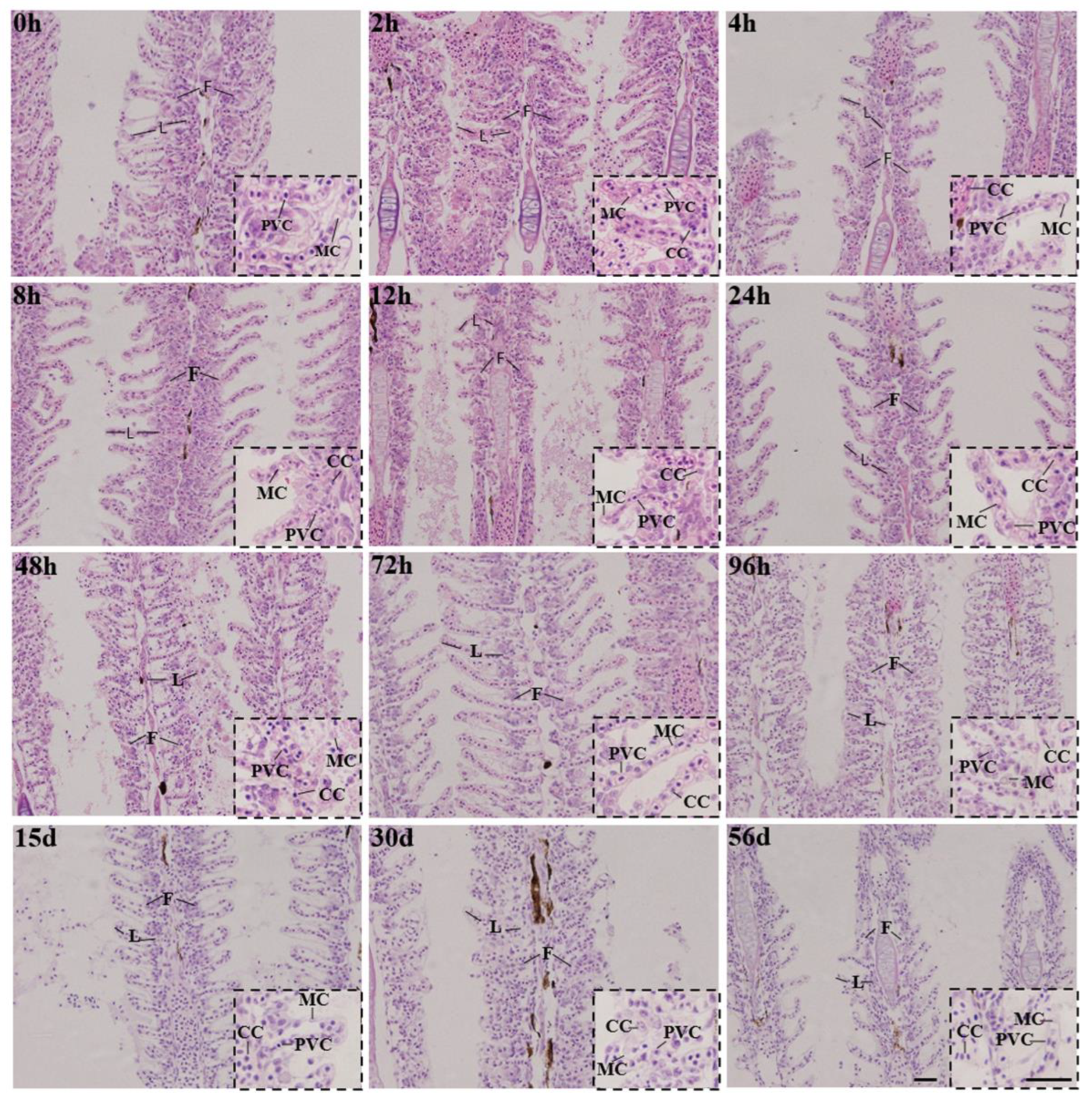
3.2. Histology Structure
3.3. Transcriptome Sequencing
3.3.1. Quality Assessment of Transcriptome Sequencing Data
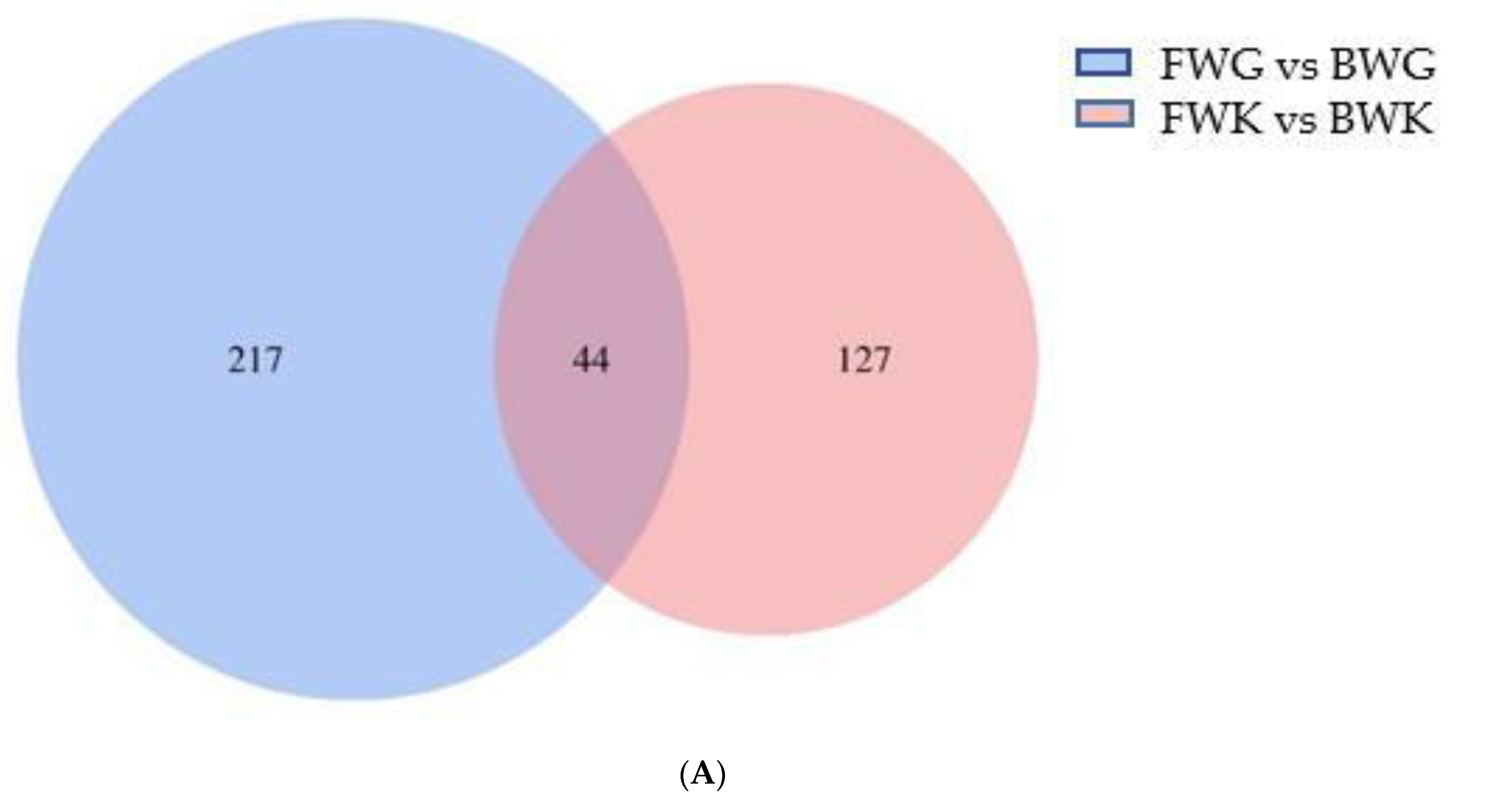
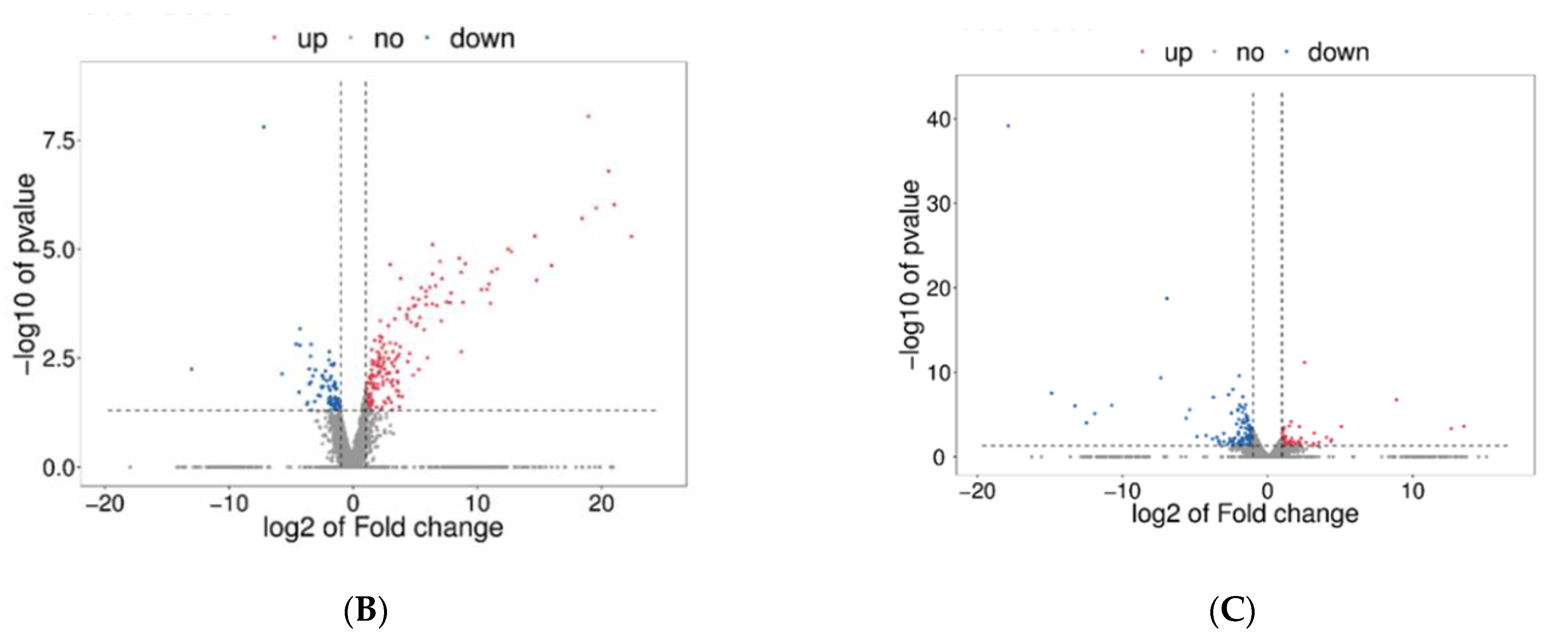
3.3.2. Functional Annotation of Differentially Expressed Genes (DEGs)
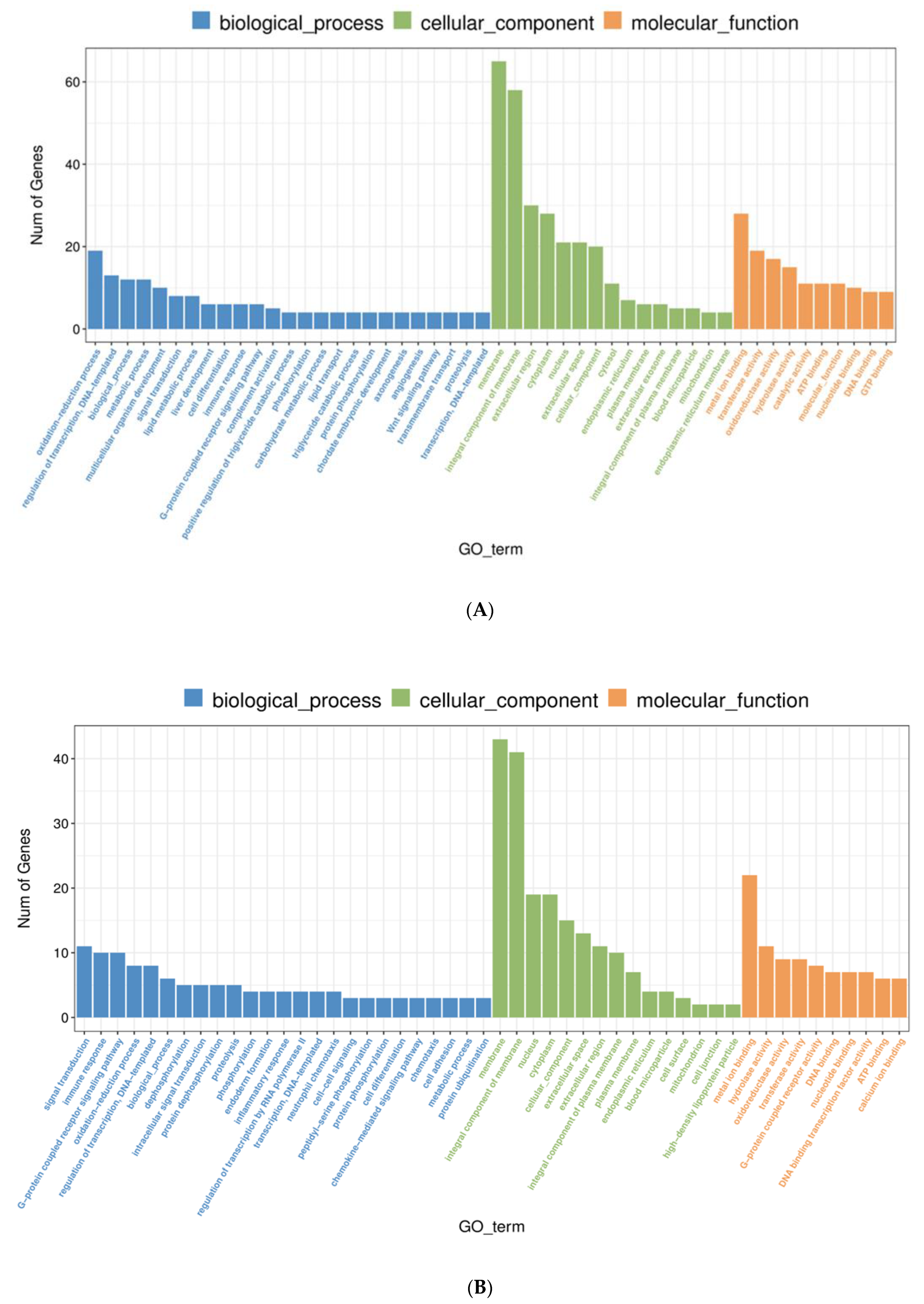
3.3.3. GO Function Enrichment Analysis of DEGs
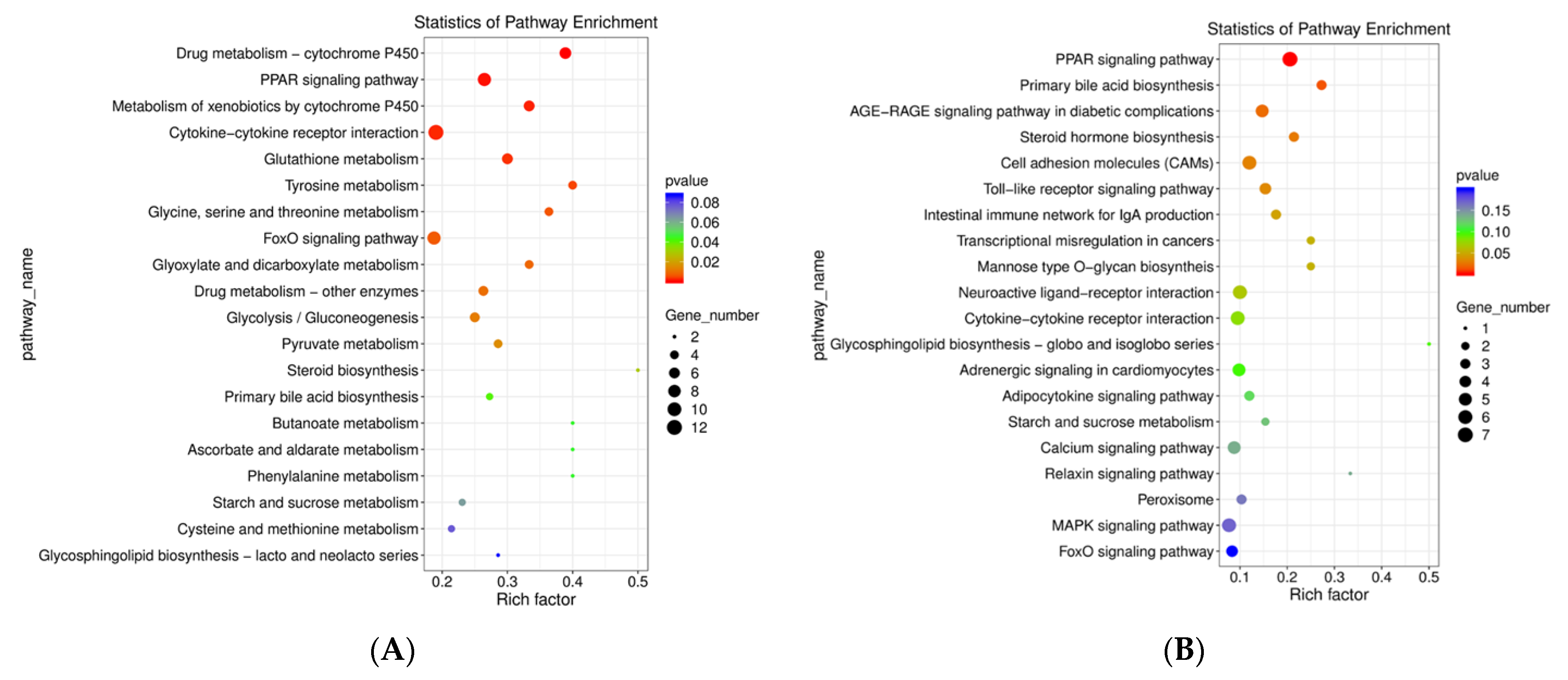
3.3.4. KEGG Pathway Enrichment Analysis of DEGs
4. Discussion
4.1. Antioxidant Enzyme Activity
4.2. Histological Pathology
4.3. Transcriptome Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Overton, J.L.; Bayley, M.; Paulsen, H.; Wang, T.J.A. Salinity tolerance of cultured Eurasian perch, Perca fluviatilis L.: Effects on growth and on survival as a function of temperature. Aquaculture 2008, 277, 282–286. [Google Scholar] [CrossRef]
- Fridman, S.; Bron, J.; Rana, K.J.A. Influence of salinity on embryogenesis, survival, growth and oxygen consumption in embryos and yolk-sac larvae of the Nile tilapia. Aquaculture 2012, 334, 182–190. [Google Scholar] [CrossRef]
- Chen, Y.J.; Liu, H.J.; Yang, H.J.; Yuan, Y.; Liu, F.J.; Tian, L.X.; Liang, G.Y.; Yuan, R.M. Effect of dietary oxidized fish oil on growth performance, body composition, antioxidant defense mechanism and liver histology of juvenile largemouth bass Micropterus salmonids. Aquac. Nutr. 2011, 18, 321–331. [Google Scholar] [CrossRef]
- Long, S.S.; Dong, X.H.; Yan, X.B.; Liu, H.; Tan, B.P.; Zhang, S.; Chi, S.Y.; Yang, Q.H.; Liu, H.Y.; Yang, Y.Z. The effect of oxidized fish oil on antioxidant ability, histology, and transcriptome in intestine of the juvenile hybrid grouper (female Epimetheus fuscoguttatus x male Epimetheus lanceolatus). Aquac. Rep. 2022, 22, 100921. [Google Scholar] [CrossRef]
- Monaghan, P.; Metcalfe, N.B.; Torres, R. Oxidative stress as a mediator of life history trade-offs: Mechanisms, measurements and interpretation. Ecol. Lett. 2009, 12, 75–92. [Google Scholar] [CrossRef]
- Mourente, G.; Diaz-Salvago, E.; Bell, J.G.; Tocher, D.R. Increased activities of hepatic antioxidant defence enzymes in juvenile gilthead sea bream (Sparus aurata L.) fed dietary oxidised oil: Attenuation by dietary vitamin E. Aquaculture 2002, 214, 343–361. [Google Scholar] [CrossRef]
- Modesto, K.A.; Martinez, C.B. Roundup causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere 2010, 78, 294–299. [Google Scholar] [CrossRef]
- Gülçin, I.; Beydemir, S.; Hisar, O. Effect of alpha-tocopherol on antioxidant enzyme activities and lipid peroxidation in rainbow trout (Oncorhynchus mykiss). Acta Vet. Hung. 2005, 53, 425–433. [Google Scholar] [CrossRef]
- Pipe, R.K.; Porte, C.; Livingstone, D.R. Antioxidant Enzymes Associated with the Blood Cells and Hemolymph of the Mussel Mytilus edulis. Fish Shellfish Immunol. 1993, 3, 221–233. [Google Scholar] [CrossRef]
- Aliko, V.; Qirjo, M.; Sula, E.; Morina, V.; Faggio, C. Antioxidant defense system, immune response and erythron profile modulation in goldfish, Carassius auratus, after acute manganese treatment. Fish Shellfish Immunol. 2018, 76, 101–109. [Google Scholar] [CrossRef]
- Yada, T.; Azuma, T.; Takagi, Y. Stimulation of non-specific immune functions in seawater-acclimated rainbow trout, Oncorhynchus mykiss, with reference to the role of growth hormone. Comp. Biochem. Physiol. Biochem. Mol. Biol. 2001, 129, 695–701. [Google Scholar] [CrossRef]
- Joanne, S.M.; Stephen, D.; Bern, H.A. Effects of salinity on chloride cells and Na+ k+-ATPase activity in the teleost gillchthys mirabilis. Comp. Biochem. Physiol. Part A Physiol. 1993, 105, 311–317. [Google Scholar] [CrossRef]
- Martínez-Alvarez, R.M.; San, Z.A.; García-Gallego, M.; Domezain, A.; Domezain, J.; Carmona, R.; del Valle Ostos-Garrido, M.; Morales, A.E. Adaptive branchial mechanisms in the sturgeon Acipenser naccarii during acclimation to saltwater. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 41, 183–190. [Google Scholar] [CrossRef]
- Guh, Y.J.; Lin, C.H.; Wang, P.P. Osmoregulation in zebrafish: Ion transport mechanisms and functional regulation. EXCLI J. 2015, 14, 627–659. [Google Scholar] [CrossRef]
- Blair, S.D.; Matheson, D.; He, Y.; Goss, G.G. Reduced salinity tolerance in the Arctic grayling (Thymallus arcticus) is associated with rapid development of a gill interlamellar cell mass: Implications of high-saline spills on native freshwater salmonids. Conserv. Physiol. 2016, 4, cow010. [Google Scholar] [CrossRef] [Green Version]
- Blair, S.D.; Matheson, D.; Goss, G.G. Physiological and morphological investigation of Arctic grayling (Thymallus arcticus) gill filaments with high salinity exposure and recovery. Conserv. Physiol. 2017, 5, cox040. [Google Scholar] [CrossRef] [Green Version]
- Niu, C.J.; Rummer, J.L.; Brauner, C.J.; Schulte, P.M. Heat shock protein (hsp 70) induced by mild heat shock inhibits sharp plasma osmolarity increases upon seawater transfer in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. 2008, 148, 437–444. [Google Scholar] [CrossRef]
- Cui, Q.; Qiu, L.; Yang, X.; Shang, S.; Yang, B.; Chen, M.; Liu, X.; Chen, B.; Fu, X.; Wang, W.; et al. Transcriptome profiling of the low-salinity stress responses in the gills of the juvenile Pseudopleuronectes yokohamae. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 32, 100612. [Google Scholar] [CrossRef]
- Zhou, Z.; Hu, F.W.; Li, W.J.; Yang, X.H.; Hallerman, E.; Huang, Z.T. Effects of salinity on growth, hematological parameters, gill microstructure and transcriptome of fat greenling Hexagrammos otakii. Aquaculture 2021, 531, 735945. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, H.J.; Kim, Y.K. Comparative transcriptome profiling of selected osmotic regulatory proteins in the gill during seawater acclimation of chum salmon (Oncorhynchus keta) fry. Sci. Rep. 2020, 10, 1987. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Qi, D.; Liu, S.; Qi, H.; Wang, Y.; Zhao, K.; Tian, F. Physiological, morphological and transcriptomic responses of Tibetan naked carps (Gymnocypris przewalskii) to salinity variations. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 42, 100982. [Google Scholar] [CrossRef] [PubMed]
- Domaizon, I.; Devaux, J. Experimental study of the impacts of silver carp on plankton communities of eutrophic Villerest reservoir. Aquat. Ecol. 1999, 33, 193–204. [Google Scholar] [CrossRef]
- Chen, J.; Xie, P.; Zhang, D.; Ke, Z.; Yang, H.J.A. In situ studies on the bioaccumulation of microcystins in the phytoplanktivorous silver carp (Hypophthalmichthys molitrix) stocked in Lake Taihu with dense toxic Microcystis blooms. Aquaculture 2006, 261, 1026–1038. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, X.; Feng, W.; Liu, S.; Zhuang, Z. Analyses of the molecular mechanisms associated with salinity adaption of Trachidermus fasciatus through combined iTRAQ-based proteomics and RNA sequencing-based transcriptomics. Prog. Biophys. Mol. Biol. 2018, 136, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Salati, A.P.; Baghbanzadeh, A.; Soltani, M.; Peyghan, R.; Riazi, G. Effect of different levels of salinity on gill and kidney function in common carp Cyprinus carpio (Pisces: Cyprinidae). Ital. J. Zool. 2011, 78, 298–303. [Google Scholar] [CrossRef]
- Fang, H.; Yang, Y.Y.; Wu, X.M.; Zheng, S.Y.; Song, Y.J.; Zhang, J.; Chang, M.X. Effects and Molecular Regulation Mechanisms of Salinity Stress on the Health and Disease Resistance of Grass Carp. Front. Immunol. 2022, 13, 917497. [Google Scholar] [CrossRef]
- Wang, H.; Lai, Q.F.; Me, Z.L. Water Quality for Aquaculture in Saline-Alkaline Land; Aquatic Industry Standard of the People’s Republic of China: Beijing, China, 2012; pp. 1–6. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hirsch, C.N.; Foerster, J.M.; Johnson, J.M.; Sekhon, R.S.; Muttoni, G.; Vaillancourt, B.; Peñagaricano, F.; Lindquist, E.; Pedraza, M.A.; Barry, K.; et al. Insights into the maize pan-genome and pan-transcriptome. Plant Cell 2014, 26, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klopfenstein, D.V.; Zhang, L.; Pedersen, B.S.; Ramírez, F.; Warwick Vesztrocy, A.; Naldi, A.; Mungall, C.J.; Yunes, J.M.; Botvinnik, O.; Weigel, M.; et al. Goatools: A Python library for Gene Ontology analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- Issac, P.K.; Guru, A.; Velayutham, M.; Pachaiappan, R.; Arasu, M.V.; Al-Dhabi, N.A.; Choi, K.C.; Harikrishnan, R.; Arockiaraj, J. Oxidative stress induced antioxidant and neurotoxicity demonstrated in vivo zebrafish embryo or larval model and their normalization due to morin showing therapeutic implications. Life Sci. 2021, 283, 119864. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Wang, G.; Chen, Y.; Guo, J.; Pan, C.; Liu, E.; Ling, Q. Physicochemical changes in liver and Hsc70 expression in pikeperch Sander lucioperca under heat stress. Ecotoxicol. Environ. Saf. 2019, 181, 130–137. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Mukherjee, M.; Kumar, S.; Chakraborty, S.B. Effects of salinity stress on antioxidant status and inflammatory responses in females of a "Near Threatened" economically important fish species Notopterus chitala: A mechanistic approach. Environ. Sci. Pollut. Res. Int. 2022, 29, 75031–75042. [Google Scholar] [CrossRef]
- Hossain, M.A.; Aktar, S.; Qin, J.G. Salinity Stress Response in Estuarine Fishes from the Murray Estuary and Coorong, South Australia. Fish Physiol. Biochem. 2016, 42, 1571–1580. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, M.; Li, S.; Wei, X.; Ding, L.; Han, S. Integrated application of multi-omics approach and biochemical assays provides insights into physiological responses to Saline-Alkaline Stress in the Gills of Crucian Carp (Carassius Auratus). Sci. Total Environ. 2022, 822, 153622. [Google Scholar] [CrossRef]
- Telahigue, K.; Rabeh, I.; Mhadhbi, L.; Nechi, S.; Chelbi, E.; Ben Ali, M.; Hedfi, A.; Al-Harbi, M.S.; Hajji, T. Glyphosate exposure modulates lipid composition, histo-architecture and oxidative stress status and induces neurotoxicity in the smooth scallop Flexopecten glaber. Pestic. Biochem. Physiol. 2022, 184, 105099. [Google Scholar] [CrossRef]
- Nordberg, J.; Arnér, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radiv. Biol. Med. 2001, 2017, 1287–1312. [Google Scholar] [CrossRef]
- Elarabany, N.; Bahnasawy, M.; Edrees, G.; Alkazagli, R. Effects of salinity on some haematological and biochemical parameters in Nile tilapia Oreochromus niloticus. Agric. For. Fish. 2017, 6, 200–205. [Google Scholar] [CrossRef]
- Tietze, S.M.; Gerald, G.W. Trade-offs between salinity preference and antipredator behaviour in the euryhaline sailfin molly Poecilia latipinna. J. Fish Biol. 2016, 88, 1918–1931. [Google Scholar] [CrossRef] [Green Version]
- Nepal, V.; Fabrizio, M.C. High salinity tolerance of invasive blue catfish suggests potential for further range expansion in the Chesapeake Bay region. PLoS ONE 2019, 14, e0224770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leite, T.; Branco, P.; Ferreira, M.T.; Santos, J.M. Activity, boldness and schooling in freshwater fish are affected by river salinization. Sci. Total Environ. 2022, 819, 153046. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Liu, C.H.; Chang, Y.P.; Hsieh, S.L. Effects of hot-water extract of Toona sinensis on immune response and resistance to Aeromonas hydrophila in Oreochromis mossambicus. Fish Shellfish Immunol. 2010, 29, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Oruc, E. Oxidative stress responses and recovery patterns in the liver of Oreochromis niloticus exposed to chlorpyrifos-ethyl. Bull. Environ. Contam. Toxicol. 2012, 88, 678–684. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Yin, S. Effects of salinity change on two superoxide dismutases (SODs) in juvenile marbled eel Anguilla marmorata. PeerJ 2016, 4, e2149. [Google Scholar] [CrossRef] [Green Version]
- Saoud, I.P.; Kreydiyyeh, S.; Chalfoun, A.; Fakih, M. Influence of salinity on survival, growth, plasma osmolality and gill Na+-K+-ATPase activity in the rabbitfish Siganus rivulatus. J. Exp. Mar. Biol. Ecol. 2007, 348, 183–190. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Yang, L.; Chen, Y.; Yang, Z. Changes in plasma osmolality and Na+/K+ ATPase activity of juvenile obscure puffer Takifugu obscurus following salinity challenge. Biochem. Syst. Ecol. 2014, 56, 111–117. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The Multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar]
- Shen, A.C.; Leatherland, J.F. Histogenesis ofthe pituitary in rainbow trout (Salmo gairdner) in different am bient salinities particular reference to the rostral pars distalis. Cell Tiss. Res. 1978, 189, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Xu, W. Tolerance of 5 Species of Fish and Changes of Immune Related Indicators Under Salinity and Immunological Stress Reaction; Shanghai Ocean University: Shanghai, China, 2014. [Google Scholar]
- Zhuang, Q.Q.; Zhao, J.L.; Zhao, L.H. Effects of salinity stress on the adjustment of branchial chloride cells in Oreochromis niloticus. Chin. J. Ecol. 2012. [Google Scholar]
- Hou, J.L.; Chen, L.Q.; Zhuang, P. Structural changes of chloride cells in gills epithelia of juvenile Acipenser schrenckii acclimated to various salinities. J. Fish. China 2006. [Google Scholar]
- Hwang, P.P.; Lee, T.H. New insights into fish ion regulation and mitochondrion-rich cells. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.C.; Wang, P.P. Some insights into energy metabolism for osmoregulation in fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 148, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Jung, H.; Nguyen, T.M.; Hurwood, D.; Mather, P. Evaluation of potential candidate genes involved in salinity tolerance in striped catfish (Pangasianodon hypophthalmus) using an RNA-Seq approach. Mar. Genom. 2016, 25, 75–88. [Google Scholar] [CrossRef]
- Zhuang, P.; Zhang, L.Z.; Tian, H.J.; Zhao, F.; Song, C. Effects of salinity on digestive enzyme activities of juvenile Acipenser schrenckii. J. Fish. Sci. China 2008, 15, 198–203. [Google Scholar]
- Lavado, R.; Aparicio-Fabre, R.; Schlenk, D. Effects of salinity acclimation on the expression and activity of Phase I enzymes (CYP450 and FMOs) in coho salmon (Oncorhynchus kisutch). Fish Physiol. Biochem. 2014, 40, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Tine, M.; McKenzie, D.J.; Bonhomme, F.; Durand, J.D. Salinity-related variation in gene expression in wild populations of the black-chinned tilapia from various West African coastal marine, estuarine and freshwater habitats. Estuar. Coast. Shelf Sci. 2011, 91, 102–109. [Google Scholar] [CrossRef]
- Israelsen, W.J.; Vander Heiden, M.G. Pyruvate kinase: Function, regulation, and role in cancer. Semin. Cell Dev. Biol. 2015, 43, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Lustig, B.; Jerchow, B.; Sachs, M.; Weiler, S.; Pietsch, T.; Karsten, U.; Wetering, M.; Clevers, H.; Schlag, P.M.; Birchmeier, W.; et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 2002, 22, 1184–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starai, V.J.; Celic, I.; Cole, R.N.; Boeke, J.D.; Escalante-Semerena, J.C. Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science 2002, 298, 2390–2392. [Google Scholar] [CrossRef] [PubMed]
- Schwer, B.; Bunkenborg, J.; Verdin, R.O.; Andersen, J.S.; Verdin, E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc. Natl. Acad. Sci. USA 2006, 103, 10224–10229. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Shang, Y.P.; Guo, R.; Chang, Y.Q.; Jiang, Y.A. Salinity stress-induced differentially expressed miRNAs and target genes in sea cucumbers Apostichopus japonicus. Cell Stress Chaperones 2019, 24, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Guo, J.T.; Zhao, L.H.; Zhao, J.L. MiR-30c: A novel regulator of salt tolerance in tilapia. Biochem. Biophys. Res. Commun. 2012, 425, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, E.; Li, T.; Xu, C.; Wang, X.; Lin, H.; Qin, J.G.; Chen, L. Transcriptome and Molecular Pathway Analysis of the Hepatopancreas in the Pacific White Shrimp Litopenaeus vannamei under Chronic Low-Salinity Stress. PLoS ONE 2015, 10, e0131503. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, W.N.; Wang, A.L.; Wang, J.M.; Sun, R.Y. Effects of dietary vitamin e supplementation on antioxidant enzyme activities in litopenaeus vannamei (boone, 1931) exposed to acute salinity changes. Aquaculture 2007, 265, 351–358. [Google Scholar] [CrossRef]
- Choi, C.Y.; An, K.W.; An, M.I. Molecular characterization and mRNA expression of glutathione peroxidase and glutathione S-transferase during osmotic stress in olive flounder (Paralichthys olivaceus). Comp. Biochem. Physiol. Part A-Mol. Integr. Physiol. 2008, 149, 330–337. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, Z.C.; Gao, T.X.; Song, N. Transcriptome Profiling Reveals a Divergent Adaptive Response to Hyper- and Hypo-Salinity in the Yellow Drum, Nibea albiflora. Animals 2021, 11, 2201. [Google Scholar] [CrossRef]
- Koskinen, H.; Krasnov, A.; Rexroad, C.; Gorodilov, Y.; Afanasyev, S.; Molsa, H. The 14-3-3 proteins in the teleost fish rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 2004, 207, 3361–3368. [Google Scholar] [CrossRef] [Green Version]
- Mackintosh, C. Dynamic interactions between 14-3-3 proteins and phosphoproteins regulate diverse cellular processes. Biochem. J. 2004, 381, 329–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hemert, M.J.; Niemantsverdriet, M.; Schmidt, T.; Backendorf, C.; Spaink, H.P. Isoform-specific differences in rapid nucleocytoplasmic shuttling cause distinct subcellular distributions of 14-3-3 sigma and 14-3-3 zeta. J. Cell Sci. 2004, 117, 1411–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, G.R.; Schulte, P.M.; Wood, C.M. Plasticity of osmoregulatory function in the killifish intestine: Drinking rates, salt and water transport, and gene expression after freshwater transfer. J. Exp. Biol. 2006, 209, 4040–4050. [Google Scholar] [CrossRef] [Green Version]
- Kültz, D.; Chakravarty, D.; Adilakshmi, T. A novel 14-3-3 gene is osmoregulated in gill epithelium of the euryhaline teleost Fundulus heteroclitus. J. Exp. Biol. 2001, 204, 2975–2985. [Google Scholar] [CrossRef] [PubMed]
- Kyosseva, S.V. Mitogen-activated protein kinase signaling. Int. Rev. Neurobiol. 2004, 59, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Itakura, M.; Kashiwagi, M.; Nakamura, N.; Matsuki, T.; Sakuta, H.; Naito, N.; Takano, K.; Fujita, T.; Hirose, S. Identification by differential display of a hypertonicity-inducible inward rectifier potassium channel highly expressed in chloride cells. J. Biol. Chem. 1999, 274, 11376–11382. [Google Scholar] [CrossRef] [Green Version]
- Vojtek, A.B.; Cooper, J.A. Rho family members: Activators of MAP kinase cascades. Cell 1995, 82, 527–529. [Google Scholar] [CrossRef] [Green Version]
- Ciano-Oliveira, C.D.; Thirone, A.C.; Szászi, K.; Kapus, A. Osmotic stress and the cytoskeleton: The R(h)ole of Rho GTPases. Acta Physiol. 2006, 187, 257–272. [Google Scholar] [CrossRef]
- Brocker, C.; Thompson, D.C.; Vasiliou, V. The role of hyperosmotic stress in inflammation and disease. Biomol. Concepts 2012, 3, 345–364. [Google Scholar] [CrossRef]
- Lim, H.K.; Min, B.H.; Kwon, M.G.; Byun, G.; Park, M.S.; Jeong, M.H.; Kim, Y.S.; Chang, Y.J. Blood physiological responses and growth of juvenile starry flounder, Platichthys stellatus exposed to different salinities. J. Environ. Biol. 2013, 34, 885–890. [Google Scholar]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A.; Roach, J.L.; Zhang, S.J.; Galvez, F. Genomic mechanisms of evolved physiological plasticity in killifish distributed along an environmental salinity gradient. Proc. Natl. Acad. Sci. USA 2011, 108, 6193–6198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.Y.; Hou, J.L.; Liu, H.J.; Zhu, H.Y.; Xu, G.C.; Xu, J. Adaptive evolution of low-salinity tolerance and hypoosmotic regulation in a euryhaline teleost, Takifugu obscurus. Mar. Biol. 2020, 167, 90. [Google Scholar] [CrossRef]
- Jiang, J.L.; Xu, J.; Ye, L.; Sun, M.L.; Jiang, Z.Q.; Mao, M.G. Identification of differentially expressed genes in gills of tiger puffer (Takifugu rubripes) in response to low-salinity stress. Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 2020, 243, 110437. [Google Scholar] [CrossRef]
| Sample | Raw Data | Valid Data | Valid Ratio (Reads) | Q20% | Q30% | GC Content% | ||
|---|---|---|---|---|---|---|---|---|
| Read | Base | Read | Base | |||||
| FW_1K | 45,347,198 | 6.80 G | 43,643,336 | 6.55 G | 96.24 | 99.98 | 97.53 | 45 |
| FW_2K | 47,957,634 | 7.19 G | 46,287,490 | 6.94 G | 96.52 | 99.98 | 97.64 | 48 |
| FW_3K | 44,558,076 | 6.68 G | 42,830,398 | 6.42 G | 96.12 | 99.99 | 97.55 | 45 |
| FW_1G | 46,015,408 | 6.90 G | 44,324,996 | 6.65 G | 96.33 | 99.98 | 98.10 | 46 |
| FW_2G | 51,657,304 | 7.75 G | 50,051,380 | 7.51 G | 96.89 | 99.99 | 97.83 | 47 |
| FW_3G | 50,027,992 | 7.50 G | 48,022,254 | 7.20 G | 95.99 | 99.99 | 97.85 | 45.50 |
| BW_1K | 46,640,790 | 7.00 G | 45,595,748 | 6.84 G | 97.76 | 99.99 | 97.94 | 47 |
| BW_2K | 36,694,278 | 5.50 G | 35,067,796 | 5.26 G | 95.57 | 99.98 | 97.60 | 45 |
| BW_3K | 51,008,526 | 7.65 G | 49,212,978 | 7.38 G | 96.48 | 99.98 | 97.77 | 46 |
| BW_1G | 42,950,828 | 6.44 G | 41,499,428 | 6.22 G | 96.62 | 99.98 | 97.37 | 46 |
| BW_2G | 51,213,734 | 7.68 G | 49,349,280 | 7.40 G | 96.36 | 99.98 | 97.45 | 46 |
| BW_3G | 46,942,238 | 7.04 G | 45,502,674 | 6.83 G | 96.93 | 99.98 | 97.50 | 47 |
| Primer | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| (Gene Name) | ||
| SULT2B1 | CAAATATACGGCTCCCTGTTG | GTGAATGAGCGAGCGATAG |
| ELISA | TCCGAACGGATTCCTTCTCT | ACCACTCGGTCCACTAAC |
| HMGCS | AGGAGGATTAGGTCATCCG | CTTCCAGACAGGCTTGTAG |
| NR4A1 | TGTTCGTCTGACGTTCTTAATG | AGTTCTGTGAAGTGATTTCGG |
| DUSP2 | TCATCACTGCCCTTCAGTTAGA | TGCAAAGAACCCGTCAAATC |
| IL12RB2 | TCGTGTCACTCAATGTCTCTAA | TGAGCAGTTCACATGACTGAT |
| DUSP5 | TACCTCCTTCCTAACACCAATG | TTTAAGGCAGCGCAGTTAT |
| ARG2 | TGTTGCCTTCCCGAAGAT | TTGGAATGCAAAGTGGTTCA |
| SIK1 | TCAGAGTGCCCTTCTTCAT | GAGCCACACTGAATCTCTTG |
| IL10 | CATGATGACGTGAGTCAAGTT | GATGAAGTCCATTTGTGCCT |
| β-Actin | CTTTTCCAGCCATCCTTCCT | GGTCAGCAATGCCAGGGTA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Yuan, C.; Qi, M.; Liu, Q.; Hu, Z. The Effect of Salinity Stress on Enzyme Activities, Histology, and Transcriptome of Silver Carp (Hypophthalmichthys molitrix). Biology 2022, 11, 1580. https://doi.org/10.3390/biology11111580
Jiang Y, Yuan C, Qi M, Liu Q, Hu Z. The Effect of Salinity Stress on Enzyme Activities, Histology, and Transcriptome of Silver Carp (Hypophthalmichthys molitrix). Biology. 2022; 11(11):1580. https://doi.org/10.3390/biology11111580
Chicago/Turabian StyleJiang, Yuhan, Chen Yuan, Ming Qi, Qigen Liu, and Zhongjun Hu. 2022. "The Effect of Salinity Stress on Enzyme Activities, Histology, and Transcriptome of Silver Carp (Hypophthalmichthys molitrix)" Biology 11, no. 11: 1580. https://doi.org/10.3390/biology11111580
APA StyleJiang, Y., Yuan, C., Qi, M., Liu, Q., & Hu, Z. (2022). The Effect of Salinity Stress on Enzyme Activities, Histology, and Transcriptome of Silver Carp (Hypophthalmichthys molitrix). Biology, 11(11), 1580. https://doi.org/10.3390/biology11111580







