Serial Increases in Human Leukocyte Antigen-DR Expression and Decreases in Interleukin-10 Expression in Alveolar Monocytes of Survivors of Pneumonia-Related Acute Respiratory Distress Syndrome
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Definitions
2.2. BAL Mononuclear Cell Preparation
2.3. Flow Cytometric Analysis of BAL Mononuclear Cells
2.4. Statistical Analyses
3. Results
3.1. Analysis of BAL Mononuclear Cells between Survivors and Non-Survivors
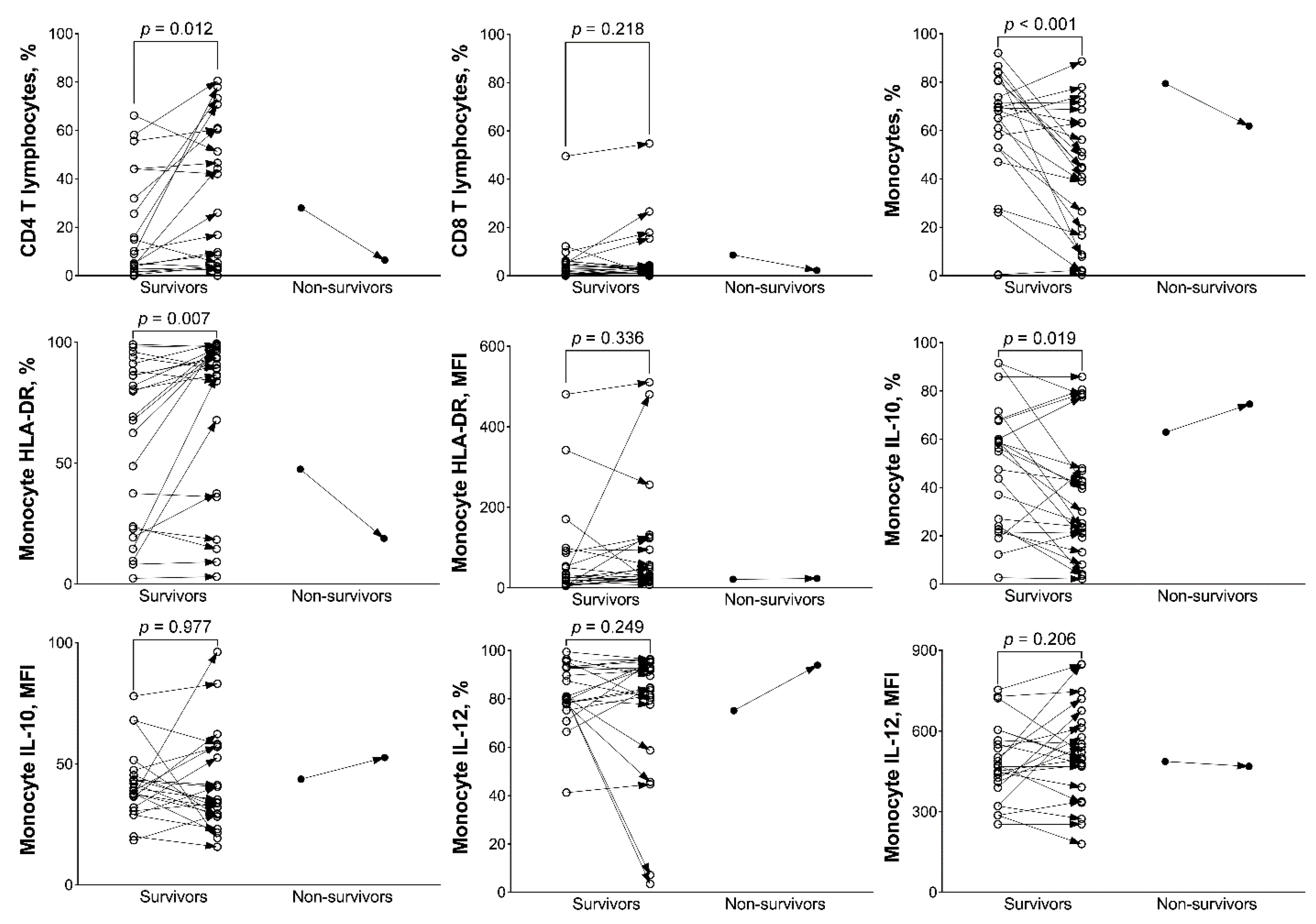
3.2. Analysis of BAL Mononuclear Cells between Day 1 and 8 in Survivors and Non-Survivors
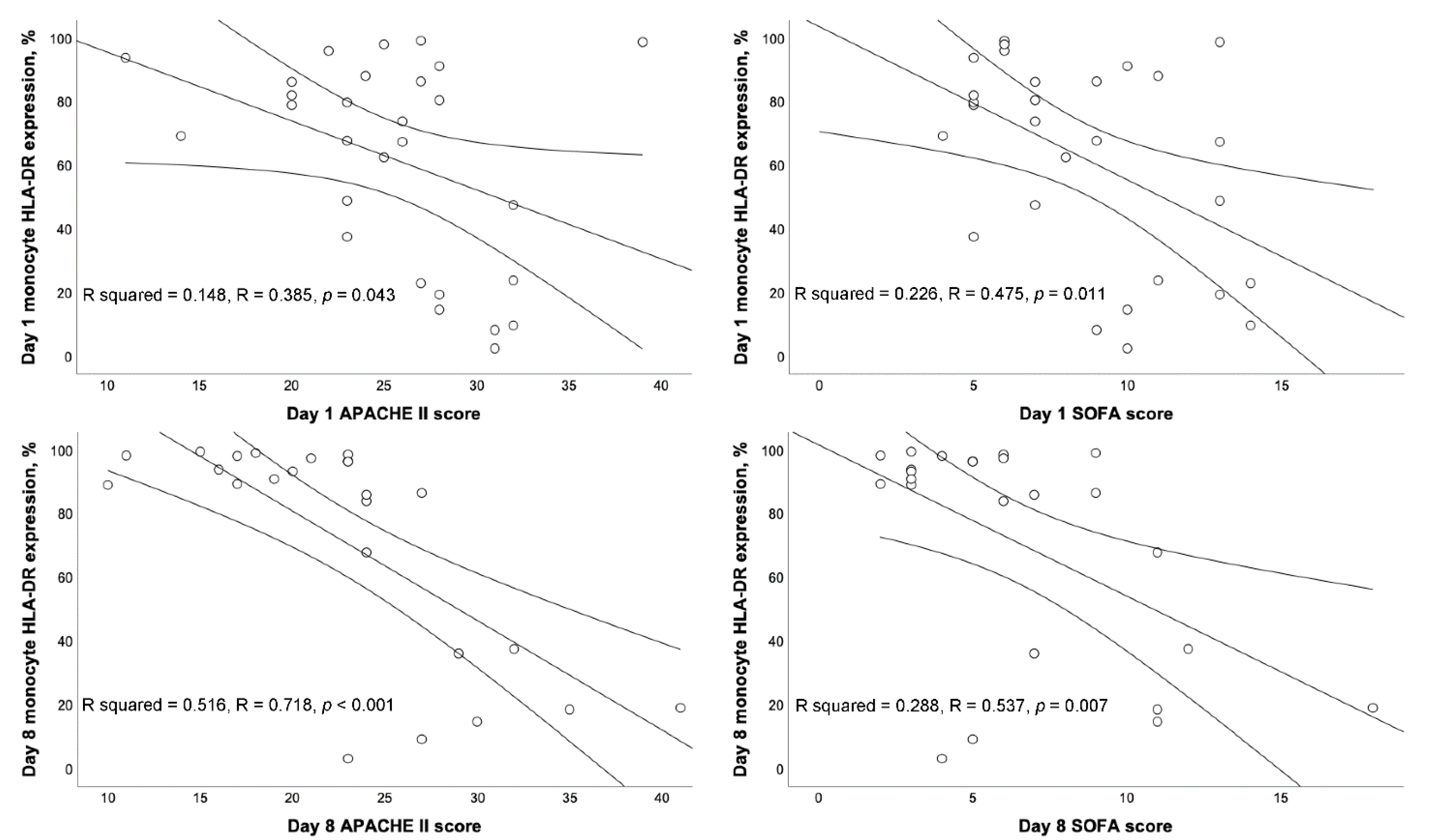
3.3. Correlation between BAL mHLA-DR and Severity Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Pfortmueller, C.A.; Meisel, C.; Fux, M.; Schefold, J.C. Assessment of immune organ dysfunction in critical illness: Utility of innate immune response markers. Intensive Care Med. Exp. 2017, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.P.; Shih, C.C.; Lin, C.Y.; Hua, C.C.; Chuang, D.Y. Serial increase of IL-12 response and human leukocyte antigen-DR expression in severe sepsis survivors. Crit. Care 2011, 15, R224. [Google Scholar] [CrossRef]
- Wu, J.F.; Ma, J.; Chen, J.; Ou-Yang, B.; Chen, M.Y.; Li, L.F.; Liu, Y.J.; Lin, A.H.; Guan, X.D. Changes of monocyte human leukocyte antigen-DR expression as a reliable predictor of mortality in severe sepsis. Crit. Care 2011, 15, R220. [Google Scholar] [CrossRef]
- Wu, H.P.; Chen, C.K.; Chung, K.; Tseng, J.C.; Hua, C.C.; Liu, Y.C.; Chuang, D.Y.; Yang, C.H. Serial cytokine levels in patients with severe sepsis. Inflamm. Res. 2009, 57, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, Y.; Xiong, T.; Chen, C.; Su, X.X.; Huang, Y.; Zhang, L. IL-10 promotes development of acute respiratory distress syndrome via inhibiting differentiation of bone marrow stem cells to alveolar type 2 epithelial cells. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6085–6092. [Google Scholar] [PubMed]
- Jin, X.; Hu, Z.; Kang, Y.; Liu, C.; Zhou, Y.; Wu, X.; Liu, J.; Zhong, M.; Luo, C.; Deng, L.; et al. Association of IL-10-1082 G/G genotype with lower mortality of acute respiratory distress syndrome in a Chinese population. Mol. Biol. Rep. 2012, 39, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lekkou, A.; Karakantza, M.; Mouzaki, A.; Kalfarentzos, F.; Gogos, C.A. Cytokine production and monocyte HLA-DR expression as predictors of outcome for patients with community-acquired severe infections. Clin. Diagn. Lab. Immunol. 2004, 11, 161–167. [Google Scholar] [CrossRef]
- Bendib, I.; Beldi-Ferchiou, A.; Schlemmer, F.; Surenaud, M.; Maitre, B.; Plonquet, A.; Carteaux, G.; Razazi, K.; Godot, V.; Hue, S.; et al. Alveolar compartmentalization of inflammatory and immune cell biomarkers in pneumonia-related ARDS. Crit. Care 2021, 25, 23. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pr. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Karakike, E.; Kyriazopoulou, E.; Tsangaris, I.; Routsi, C.; Vincent, J.L.; Giamarellos-Bourboulis, E.J. The early change of SOFA score as a prognostic marker of 28-day sepsis mortality: Analysis through a derivation and a validation cohort. Crit. Care 2019, 23, 387. [Google Scholar] [CrossRef]
- Drewry, A.M.; Ablordeppey, E.A.; Murray, E.T.; Beiter, E.R.; Walton, A.H.; Hall, M.W.; Hotchkiss, R.S. Comparison of monocyte human leukocyte antigen-DR expression and stimulated tumor necrosis factor alpha production as outcome predictors in severe sepsis: A prospective observational study. Crit. Care 2016, 20, 334. [Google Scholar] [CrossRef]
- Cajander, S.; Rasmussen, G.; Tina, E.; Magnuson, A.; Soderquist, B.; Kallman, J.; Stralin, K. Dynamics of monocytic HLA-DR expression differs between bacterial etiologies during the course of bloodstream infection. PLoS ONE 2018, 13, e0192883. [Google Scholar] [CrossRef]
- Manzoli, T.F.; Troster, E.J.; Ferranti, J.F.; Sales, M.M. Prolonged suppression of monocytic human leukocyte antigen-DR expression correlates with mortality in pediatric septic patients in a pediatric tertiary Intensive Care Unit. J. Crit. Care 2016, 33, 84–89. [Google Scholar] [CrossRef]
- Wu, H.P.; Chuang, L.P.; Liu, P.H.; Chu, C.M.; Yu, C.C.; Lin, S.W.; Kao, K.C.; Li, L.F.; Chuang, D.Y. Decreased Monocyte HLA-DR Expression in Patients with Sepsis and Acute Kidney Injury. Medicina 2022, 58, 1198. [Google Scholar] [CrossRef]
- Trimmel, H.; Luschin, U.; Kohrer, K.; Anzur, C.; Vevera, D.; Spittler, A. Clinical outcome of critically ill patients cannot be defined by cutoff values of monocyte human leukocyte antigen-DR expression. Shock 2012, 37, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.K.; Li, W.Q.; Li, N.; Li, J.S. Mononuclear histocompatibility leukocyte antigen-DR expression in the early phase of acute pancreatitis. Pancreatology 2004, 4, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Martignoni, A.; Tschop, J.; Goetzman, H.S.; Choi, L.G.; Reid, M.D.; Johannigman, J.A.; Lentsch, A.B.; Caldwell, C.C. CD4-expressing cells are early mediators of the innate immune system during sepsis. Shock 2008, 29, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Adamzik, M.; Broll, J.; Steinmann, J.; Westendorf, A.M.; Rehfeld, I.; Kreissig, C.; Peters, J. An increased alveolar CD4 + CD25 + Foxp3 + T-regulatory cell ratio in acute respiratory distress syndrome is associated with increased 30-day mortality. Intensive Care Med. 2013, 39, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.P.; Chung, K.; Lin, C.Y.; Jiang, B.Y.; Chuang, D.Y.; Liu, Y.C. Associations of T helper 1, 2, 17 and regulatory T lymphocytes with mortality in severe sepsis. Inflamm. Res. 2013, 62, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Fabri, A.; Kandara, K.; Coudereau, R.; Gossez, M.; Abraham, P.; Monard, C.; Cour, M.; Rimmele, T.; Argaud, L.; Monneret, G.; et al. Characterization of Circulating IL-10-Producing Cells in Septic Shock Patients: A Proof of Concept Study. Front. Immunol. 2021, 11, 615009. [Google Scholar] [CrossRef]
- Liu, C.H.; Kuo, S.W.; Ko, W.J.; Tsai, P.R.; Wu, S.W.; Lai, C.H.; Wang, C.H.; Chen, Y.S.; Chen, P.L.; Liu, T.T.; et al. Early measurement of IL-10 predicts the outcomes of patients with acute respiratory distress syndrome receiving extracorporeal membrane oxygenation. Sci. Rep. 2017, 7, 1021. [Google Scholar] [CrossRef]
- Volk, C.F.; Burgdorf, S.; Edwardson, G.; Nizet, V.; Sakoulas, G.; Rose, W.E. Interleukin (IL)-1beta and IL-10 Host Responses in Patients With Staphylococcus aureus Bacteremia Determined by Antimicrobial Therapy. Clin. Infect. Dis. 2020, 70, 2634–2640. [Google Scholar] [CrossRef]
- Rose, W.E.; Shukla, S.K.; Berti, A.D.; Hayney, M.S.; Henriquez, K.M.; Ranzoni, A.; Cooper, M.A.; Proctor, R.A.; Nizet, V.; Sakoulas, G. Increased Endovascular Staphylococcus aureus Inoculum Is the Link Between Elevated Serum Interleukin 10 Concentrations and Mortality in Patients With Bacteremia. Clin. Infect. Dis. 2017, 64, 1406–1412. [Google Scholar] [CrossRef]
- Udomsinprasert, W.; Jittikoon, J.; Sangroongruangsri, S.; Chaikledkaew, U. Circulating Levels of Interleukin-6 and Interleukin-10, But Not Tumor Necrosis Factor-Alpha, as Potential Biomarkers of Severity and Mortality for COVID-19: Systematic Review with Meta-analysis. J. Clin. Immunol. 2021, 41, 11–22. [Google Scholar] [CrossRef]

| Survivors (n = 25) | Non-Survivors (n = 3) | All Patients (n = 28) | |
|---|---|---|---|
| Age (years old) | 71.8 ± 16.0 | 77.7 ± 18.0 | 72.5 ± 16.0 |
| Male (%) | 16 (64.0) | 2 (66.7) | 18 (64.3) |
| APACHE II score | 24.7 ± 5.1 | 32.3 ± 6.5 * | 25.4 ± 5.7 |
| SOFA score | 8.4 ± 3.1 | 11.0 ± 3.5 | 8.6 ± 3.2 |
| History (%) | |||
| COPD | 4 (16.0) | 1 (33.3) | 5 (17.9) |
| Heart failure | 1 (4.0) | 0 (0.0) | 1 (3.6) |
| Hypertension | 12 (48.0) | 3 (100.0) | 15 (53.6) |
| Diabetes mellitus | 10 (40.0) | 1 (33.3) | 11 (39.3) |
| Previous CVA | 6 (24.0) | 1 (33.3) | 7 (25.0) |
| End stage renal disease | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Liver cirrhosis | 1 (4.0) | 0 (0.0) | 1 (3.6) |
| Active malignancy | 0 (0.0) | 1 (33.3) | 1 (3.6) |
| Adverse event | |||
| New arrhythmia | 1 (4.0) | 2 (66.7) * | 3 (10.7) |
| Gastrointestinal bleeding | 6 (24.0) | 0 (0.0) | 6 (21.4) |
| Acute kidney injury | 8 (32.0) | 2 (66.7) | 10 (35.7) |
| Shock | 6 (24.0) | 2 (66.7) | 8 (28.6) |
| Thrombocytopenia | 6 (24.0) | 2 (66.7) | 8 (28.6) |
| Jaundice | 3 (12.0) | 0 (0.0) | 3 (10.7) |
| Bacteremia | 4 (16.0) | 1 (33.3) | 5 (17.9) |
| PaO2/FiO2 ratio (mm Hg) | 151.9 ± 64.3 | 177.0 ± 49.8 | 154.6 ± 62.6 |
| Blood leukocytes | |||
| WBC/μL | 11,480.0 ± 8683.7 | 11,466.7 ± 1601.0 | 11,478.6 ± 8198.7 |
| Neutrophils, % | 84.6 ± 9.2 | 89.7 ± 5.2 | 85.2 ± 9.0 |
| Lymphocytes, % | 10.3 ± 8.1 | 7.0 ± 4.3 | 9.9 ± 7.8 |
| Monocytes, % | 4.0 ± 2.0 | 3.0 ± 2.1 | 3.9 ± 2.0 |
| Day 8 | (n = 23) | (n = 1) | (n = 24) |
| APACHE II score | 22.1 ± 6.3 | 41.0 * | 22.9 ± 7.3 |
| SOFA score | 6.0 ± 3.2 | 18.0 * | 6.5 ± 4.0 |
| Survivors | Non-Survivors | p Value | |
|---|---|---|---|
| Day 1 | (n = 25) | (n = 3) | |
| CD4 T lymphocytes, % | 16.2 ± 21.0 | 27.1 ± 8.4 | 0.389 |
| CD8 T lymphocytes, % | 4.8 ± 9.9 | 7.1 ± 1.3 | 0.685 |
| Monocytes, % | 59.3 ± 27.0 | 59.9 ± 18.3 | 0.967 |
| Monocyte HLA-DR expression, % | 60.8 ± 33.0 | 71.2 ± 25.8 | 0.605 |
| Monocyte HLA-DR expression, MFI | 69.1 ± 112.0 | 150.6 ± 228.2 | 0.295 |
| Monocyte IL-10 expression, % | 47.7 ± 24.7 | 42.8 ± 21.3 | 0.742 |
| Monocyte IL-10 expression, MFI | 41.7 ± 13.7 | 43.0 ± 3.2 | 0.870 |
| Monocyte IL-12 expression, % | 82.3 ± 13.0 | 84.7 ± 8.7 | 0.762 |
| Monocyte IL-12 expression, MFI | 479.7 ± 137.7 | 621.8 ± 146.9 | 0.105 |
| Day 8 | (n = 23) | (n = 1) | |
| CD4 T lymphocytes, % | 30.1 ± 29.6 | 6.4 | 0.443 |
| CD8 T lymphocytes, % | 6.7 ± 12.3 | 2.2 | 0.722 |
| Monocytes, % | 41.6 ± 26.9 | 61.9 | 0.469 |
| Monocyte HLA-DR expression, % | 73.2 ± 33.8 | 18.9 | 0.131 |
| Monocyte HLA-DR expression, MFI | 93.2 ± 139.7 | 23.2 | 0.629 |
| Monocyte IL-10 expression, % | 37.3 ± 26.9 | 74.6 | 0.188 |
| Monocyte IL-10 expression, MFI | 41.4 ± 20.2 | 52.7 | 0.590 |
| Monocyte IL-12 expression, % | 76.7 ± 27.0 | 93.9 | 0.540 |
| Monocyte IL-12 expression, MFI | 520.1 ± 177.9 | 469.3 | 0.783 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, C.-M.; Chung, C.-J.; Huang, C.-Y.; Yu, C.-C.; Wang, C.-H.; Li, L.-F.; Wu, H.-P. Serial Increases in Human Leukocyte Antigen-DR Expression and Decreases in Interleukin-10 Expression in Alveolar Monocytes of Survivors of Pneumonia-Related Acute Respiratory Distress Syndrome. Biology 2022, 11, 1793. https://doi.org/10.3390/biology11121793
Chu C-M, Chung C-J, Huang C-Y, Yu C-C, Wang C-H, Li L-F, Wu H-P. Serial Increases in Human Leukocyte Antigen-DR Expression and Decreases in Interleukin-10 Expression in Alveolar Monocytes of Survivors of Pneumonia-Related Acute Respiratory Distress Syndrome. Biology. 2022; 11(12):1793. https://doi.org/10.3390/biology11121793
Chicago/Turabian StyleChu, Chien-Ming, Chia-Jung Chung, Chih-Yu Huang, Chung-Chieh Yu, Chao-Hung Wang, Li-Fu Li, and Huang-Pin Wu. 2022. "Serial Increases in Human Leukocyte Antigen-DR Expression and Decreases in Interleukin-10 Expression in Alveolar Monocytes of Survivors of Pneumonia-Related Acute Respiratory Distress Syndrome" Biology 11, no. 12: 1793. https://doi.org/10.3390/biology11121793
APA StyleChu, C.-M., Chung, C.-J., Huang, C.-Y., Yu, C.-C., Wang, C.-H., Li, L.-F., & Wu, H.-P. (2022). Serial Increases in Human Leukocyte Antigen-DR Expression and Decreases in Interleukin-10 Expression in Alveolar Monocytes of Survivors of Pneumonia-Related Acute Respiratory Distress Syndrome. Biology, 11(12), 1793. https://doi.org/10.3390/biology11121793






