LmCht5-1 and LmCht5-2 Promote the Degradation of Serosal and Pro-Nymphal Cuticles during Locust Embryonic Development
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. LmCht5 Expression Pattern during Embryogenesis
2.3. dsRNA Synthesis and Microinjection
2.4. Paraffin Sections of Locust Eggs
2.5. Immunofluorescence Microscopy
2.6. Transmission Electron Microscopy
2.7. Statistical Analysis
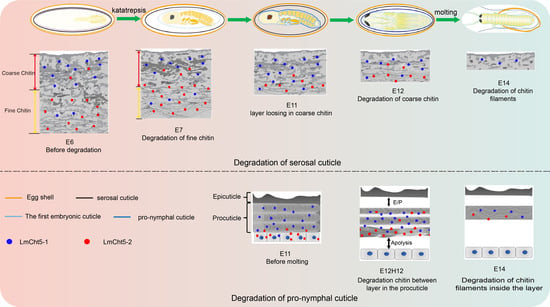
3. Results
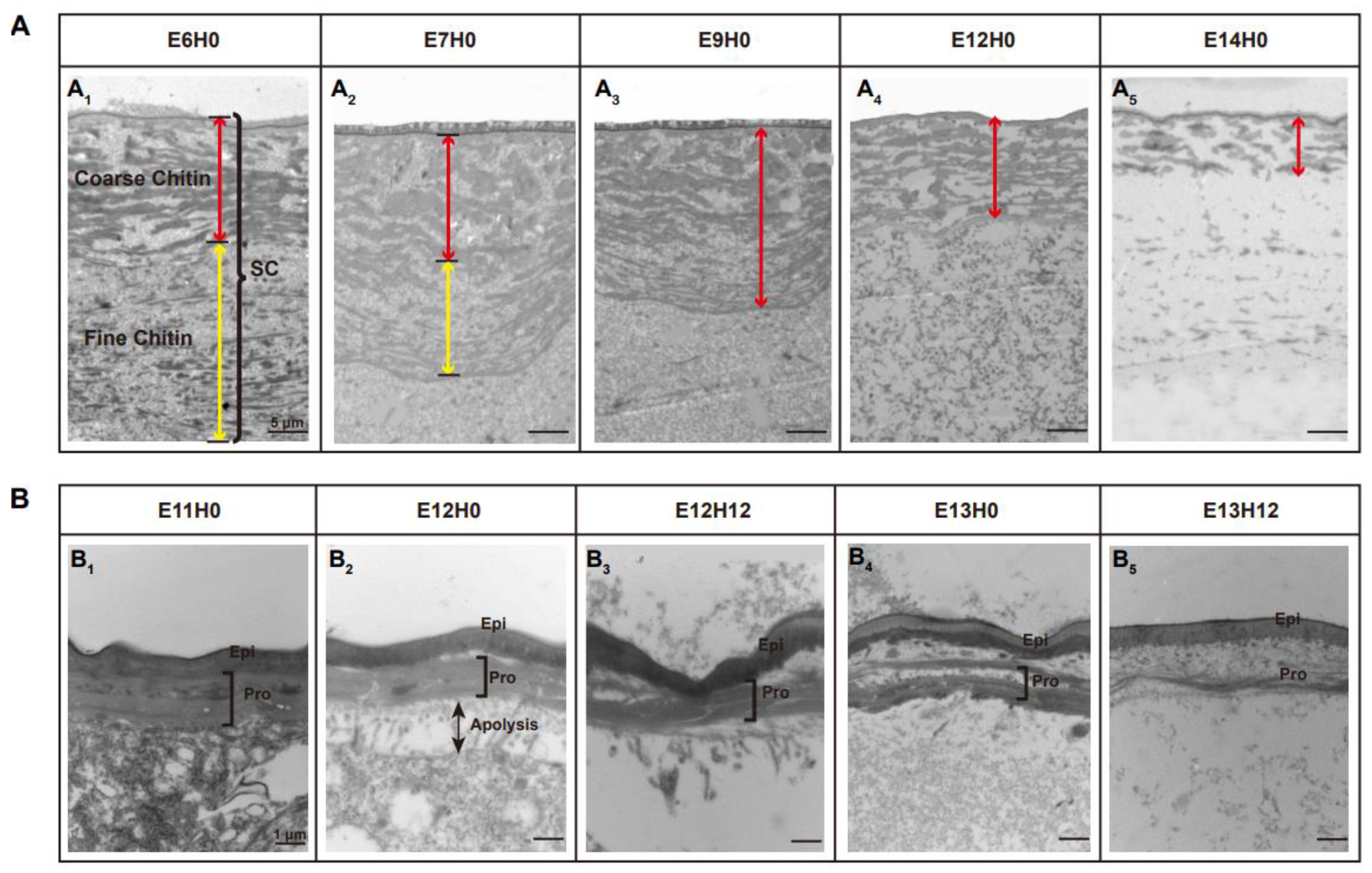
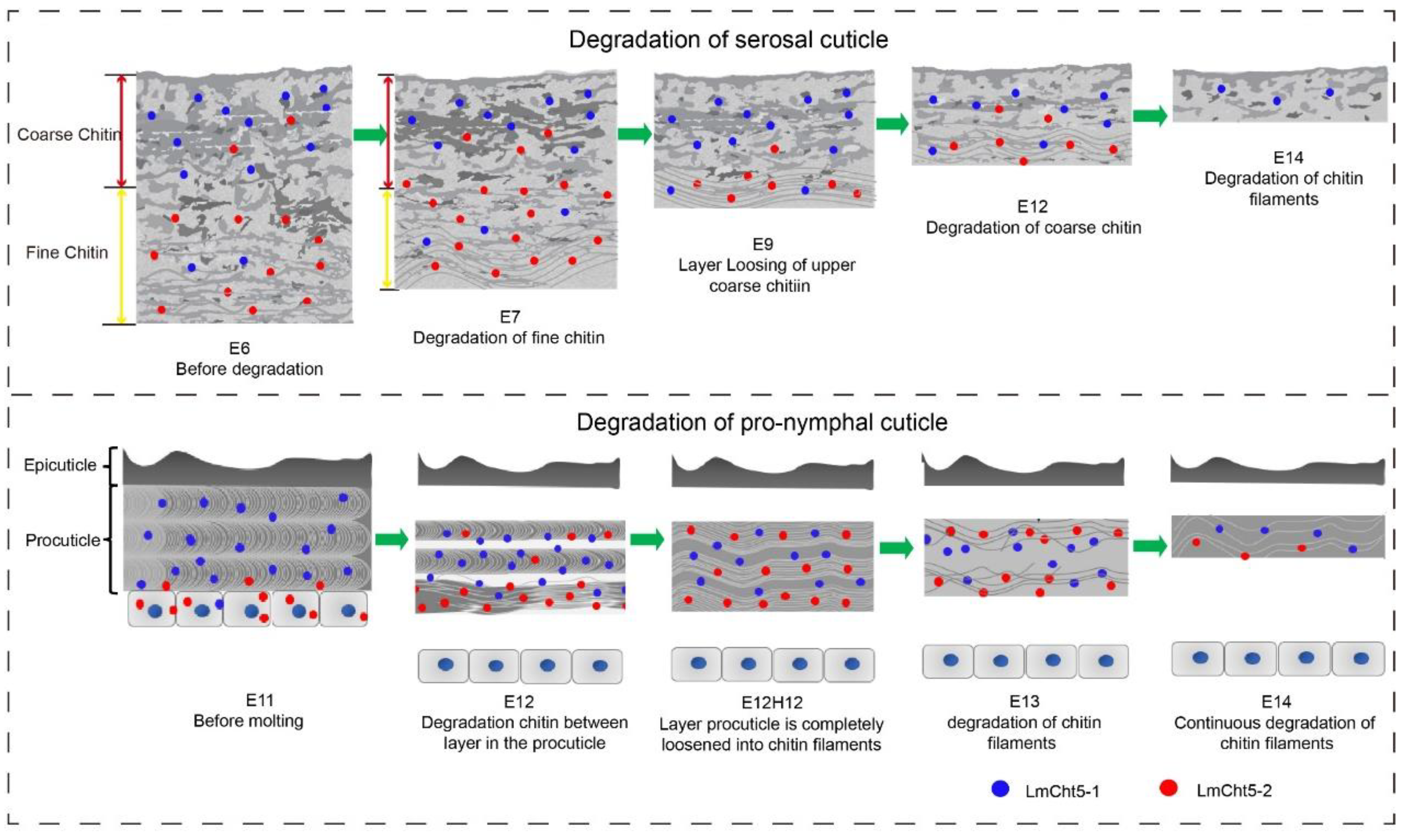
3.1. Ultra-Structure Showed Significant Differences in Cuticle Degradation between the Serosal Cuticle and the Pro-Nymphal Cuticle
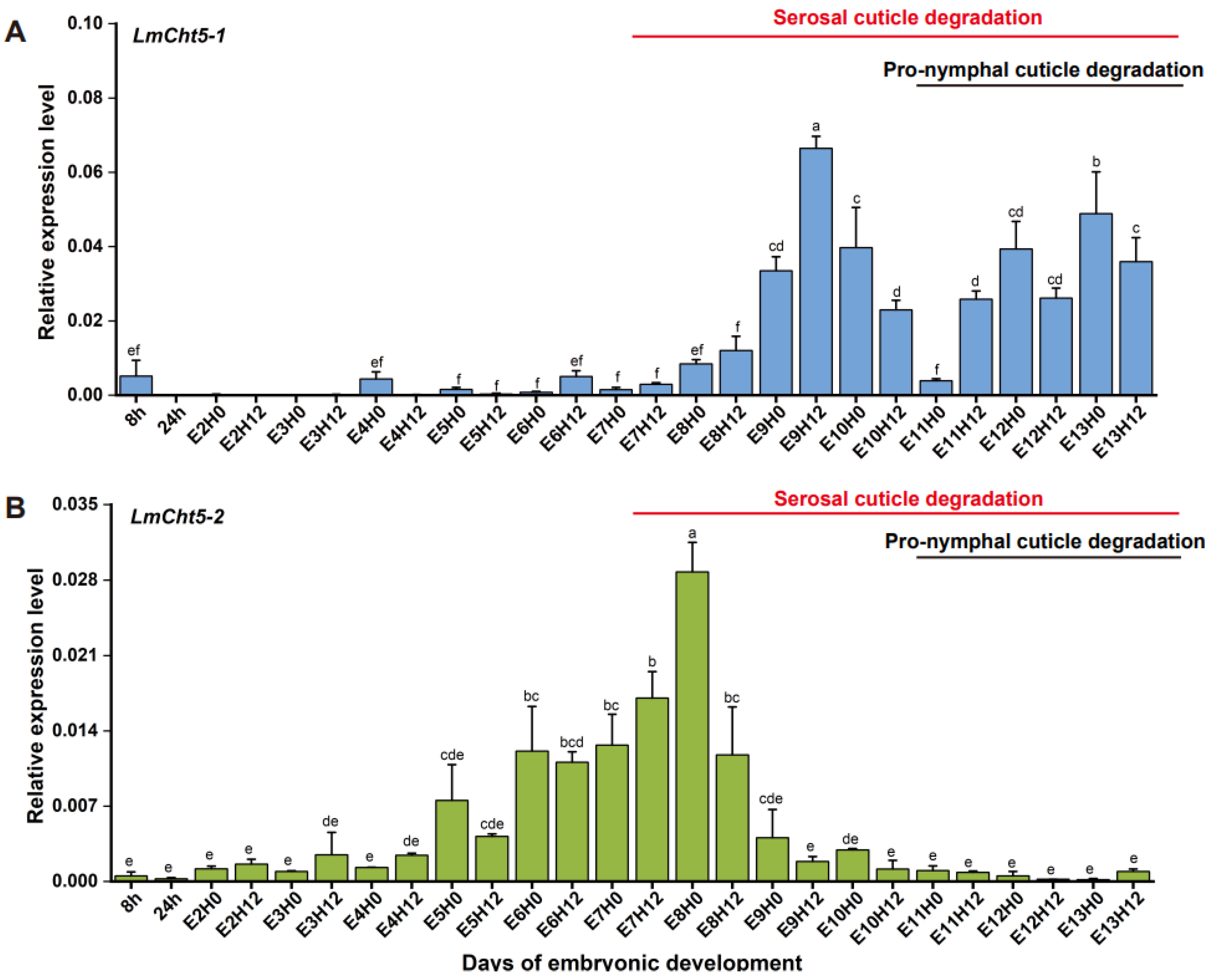
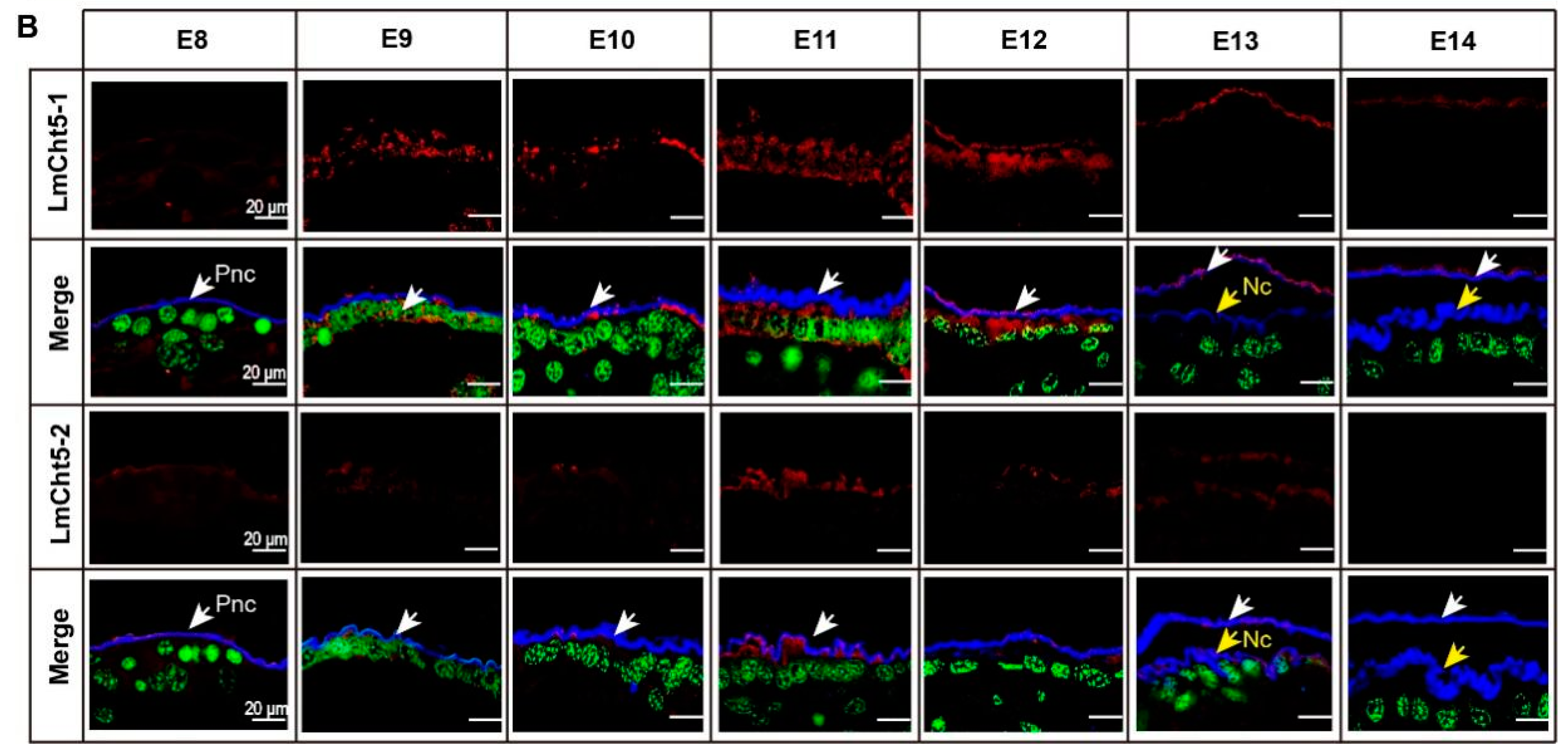
3.2. LmCht5-1 and LmCht5-2 Development Expression Patterns during Embryogenesis
3.3. Both dsLmCht5-1 and dsLmCht5-2 Cause Lethality during Early and Late Embryogenesis of the Locust
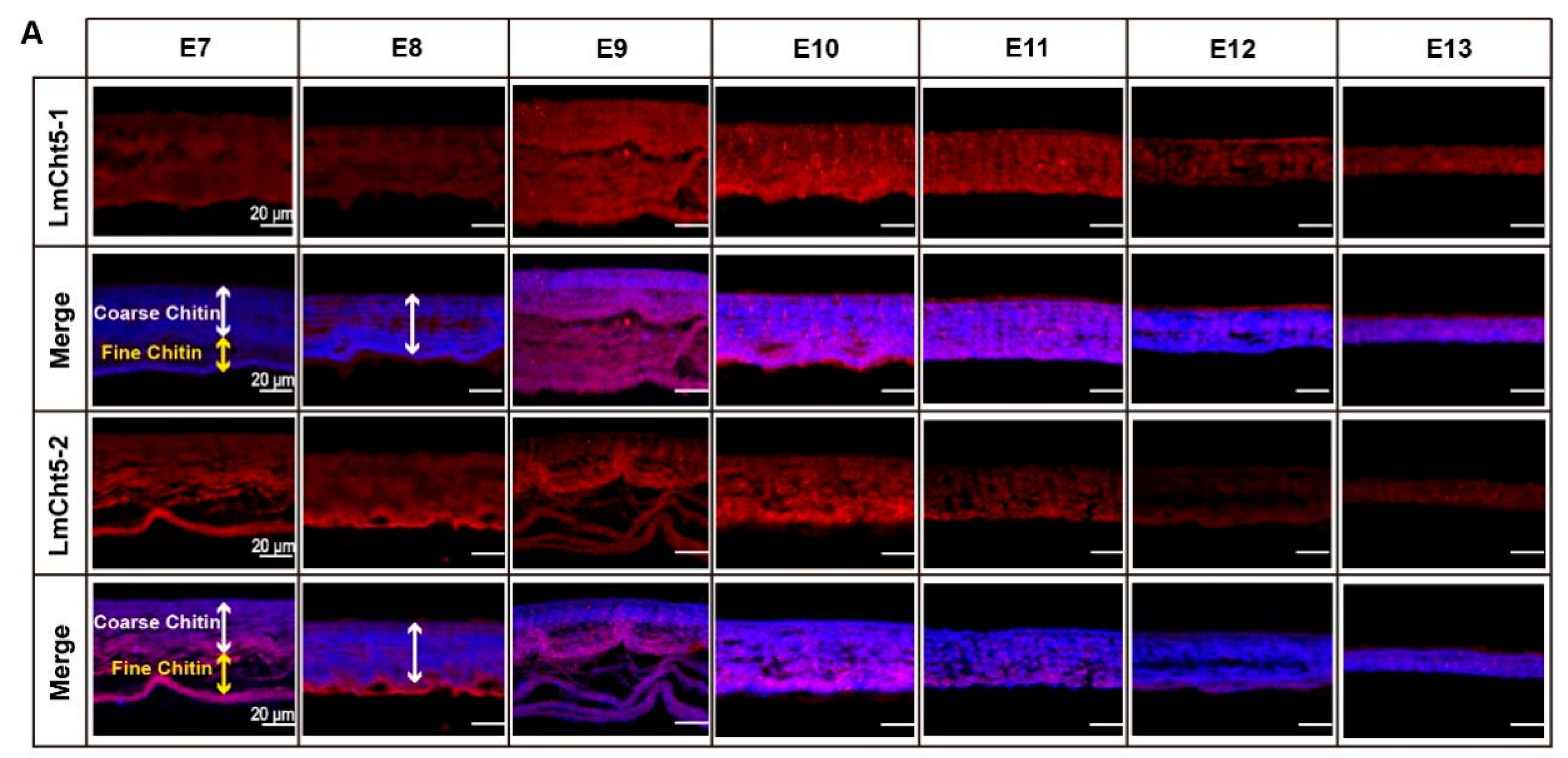
3.4. LmCht5-1 and LmCht5-2 Proteins Were Expressed during Serosal Cuticle and Pro-Nymphal Cuticle Degradation
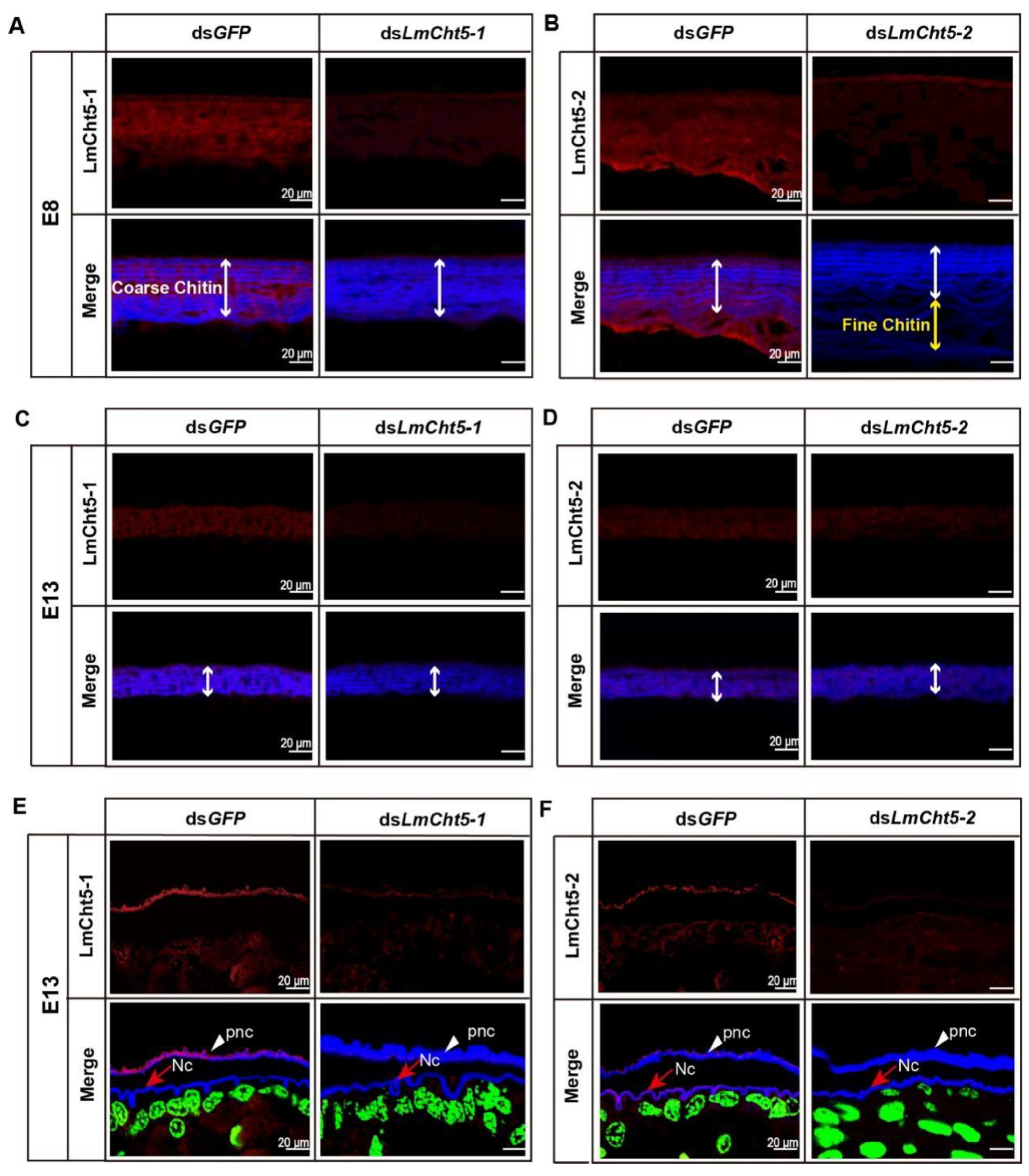
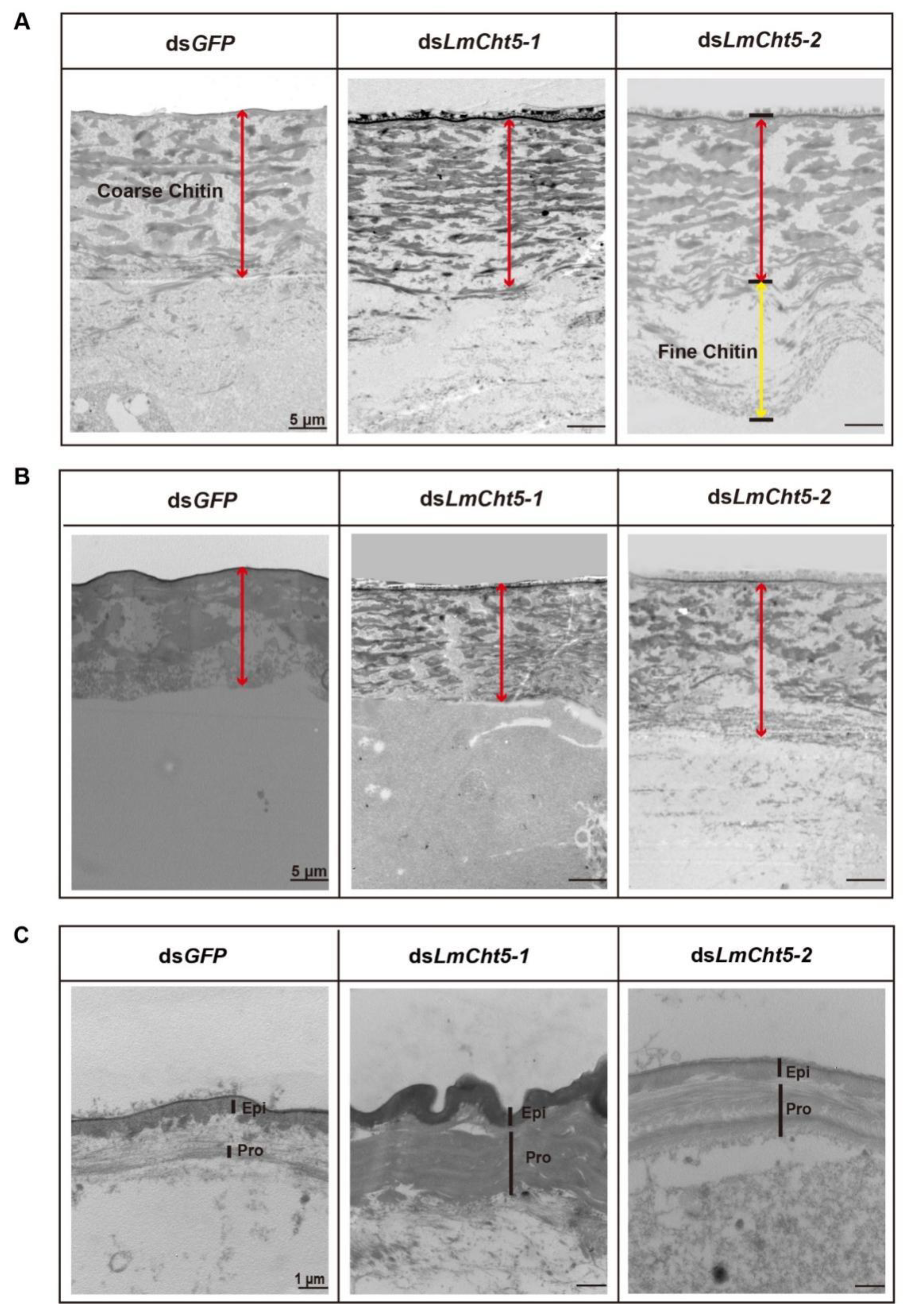
3.5. dsLmCht5-1 and dsLmCht5-2 Inhibited Cuticle Degradation of the Serosal Cuticle in Both Early and Late Embryogenesis of the Locust
3.6. dsLmCht5-1 and dsLmCht5-2 Inhibited Chitin Degradation of the Pro-Nymphal Cuticle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, K.; Dhiman, S.; Acharya, B.; Ghorpade, R.; Sukumaran, D. Pyriproxyfen treated surface exposure exhibits reproductive disruption in dengue vector Aedes aegypti. PLoS Negl. Trop. Dis. 2019, 13, e0007842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Zhang, J.; Li, D.; Sun, Y.; Guo, Y.; Ma, E.; Zhu, K. Silencing of two alternative splicing-derived mRNA variants of chitin synthase 1 gene by RNAi is lethal to the oriental migratory locust, Locusta migratoria manilensis (Meyen). Insect Biochem. Mol. Biol. 2010, 40, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, W.; Li, D.; Gao, L.; Ma, E.; Zhu, K.Y.; Moussian, B.; Li, S.; Zhang, J. LmCht5-1 promotes pro-nymphal molting during locust embryonic development. Insect Biochem. Mol. Biol. 2018, 101, 124–130. [Google Scholar] [CrossRef]
- Laguecx, M.; Hetru, C.; Goltzene, F.; Kappler, C.; Hoffmann, J.A. Ecdysone titre and metabolism to cuticulogenesis in embryos of Locusta migratoria. J. Insect Physiol. 1979, 25, 709–723. [Google Scholar] [CrossRef]
- Micciarelli, A.S.; Sbrenna, G. The embryonic apolyses of Schistocerca gregaria (Orthoptera). J. Insect. Physiol. 1972, 18, 1027–1037. [Google Scholar] [CrossRef]
- Liu, W.; Li, R.; Fu, S.; Dong, Q.; Zhang, M.; Zhang, X.; Zhang, J.; Zhang, T. Optimization of paraffin section for embryo to research the development pattern of serosal cuticle in Locusta migratoria (Orthoptera: Acrididae). Acta Entomol. Sin. 2018, 61, 733–740. [Google Scholar]
- Truman, J.W.; Roddford, M.L. The origins of insect metamorphosis. Nature 1999, 401, 447–452. [Google Scholar] [CrossRef]
- Chaika, S.Y. On the Pronymphal Stage in the Migratory Locust (Locusta migratoria, Orthoptera, Acrididae). Entomol. Rev. 2013, 92, 559–571. [Google Scholar] [CrossRef]
- McFarlane, J.E. Structure and function of the egg shell as related to water absorption by the eggs of Acheta domesticus (L.). Can. J. Zool. 1960, 38, 231–241. [Google Scholar] [CrossRef]
- Jacobs, C.G.C.; Rezende, G.L.; Lamers, G.E.; van der Zee, M. The extraembryonic serosa protects the insect egg against desiccation. Proc. Biol. Sci. 2013, 280, 20131082. [Google Scholar] [CrossRef]
- Rezende, G.L.; Martins, A.J.; Gentile, C.; Farnesi, L.C.; Pelajo-Machado, M.; Peixoto, A.A.; Valle, D. Embryonic desiccation resistance in Aedes aegypti: Presumptive role of the chitinized serosal cuticle. BMC Biol. 2008, 8, 182. [Google Scholar] [CrossRef]
- Jacobs, C.G.C.; Braak, N.; Lamers, G.E.; van der Zee, M. Elucidation of the serosal cuticle machinery in the beetle Tribolium by RNA sequencing and functional analysis of Knickkopf1, Retroactive and Laccase2. Insect Biochem. Mol. Biol. 2015, 60, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Farnesi, L.C.; Menna-Barreto, R.F.; Martins, A.J.; Valle, D.; Rezende, G.L. Physical features and chitin content of eggs from the mosquito vectors Aedes aegypti, Anopheles aquasalis and Culex quinquefasciatus: Connection with distinct levels of resistance to desiccation. J. Insect Physiol. 2015, 83, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Beckel, E.W. Investigations of permeability, diapause, and hatching in the eggs of the mosquito Aedes hexodontus Dyar. Can. J. Zool. 1958, 36, 541–554. [Google Scholar] [CrossRef]
- Merzendorfer, H. Chitin synthesis inhibitors: Old molecules and new developments. Insect Sci. 2013, 20, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Tetreau, G.; Cao, X.; Chen, Y.R.; Muthukrishnan, S.; Jiang, H.; Blissard, G.W.; Kanost, M.R.; Wang, P. Overview of chitin metabolism enzymes in Manduca sexta: Identification, domain organization, phylogenetic analysis and gene expression. Insect Biochem. Mol. Biol. 2015, 62, 114–126. [Google Scholar] [CrossRef]
- Lu, Y.; Zen, K.C.; Muthukrishnan, S.; Kramer, K.J. Site-directed mutagenesis and functional analysis of active site acidic amino acid residues D142, D144 and E146 in Manduca sexta (tobacco hornworm) chitinase. Insect Biochem. Mol. Biol. 2002, 32, 1369–1382. [Google Scholar] [CrossRef]
- Zhu, Q.; Arakane, Y.; Banerjee, D.; Beeman, R.W.; Kramer, K.J.; Muthukrishnan, S. Domain organization and phylogenetic analysis of the chitinase-like family of proteins in three species of insects. Insect Biochem. Mol. Biol. 2008, 38, 452–466. [Google Scholar] [CrossRef]
- Zhu, Q.; Arakane, Y.; Beeman, R.W.; Kramer, K.J.; Muthukrishnan, S. Characterization of recombinant chitinase-like proteins of Drosophila melanogaster and Tribolium castaneum. Insect Biochem. Mol. Biol. 2008, 38, 467–477. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, J.; Yao, Q.; Pan, Z.; Chen, J.; Zhang, W. Functional analysis of two chitinase genes during the pupation and eclosion stages of the beet armyworm Spodoptera exigua by RNA interference. Arch. Insect Biochem. Physiol. 2012, 79, 220–234. [Google Scholar] [CrossRef]
- Arakane, Y.; Muthukrishnan, S. Insect chitinase and chitinase-like proteins. Cell. Mol. Life Sci. 2009, 67, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, J.; Wang, Y.; Liu, X.; Ma, E.; Sun, Y.; Li, S.; Zhu, K.Y.; Zhang, J. Two chitinase 5 genes from Locusta migratoria: Molecular characteristics and functional differentiation. Insect Biochem. Mol. Biol. 2015, 58, 46–54. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Gao, L.; Li, R.; Fu, H.; Li, D.; Liu, W.; Zhang, J. The Antibody Preparation and Expression Analysis of Chitinase 5-1 in Locusta migratoria. J. Integr. Agric. 2018, 51, 2418–2428. [Google Scholar]
- Yu, R.; Liu, W.; Li, D.; Zhao, X.; Ding, G.; Zhang, M.; Ma, E.; Zhu, K.; Li, S.; Moussian, B.; et al. Helicoidal organization of chitin in the cuticle of the migratory locust requires the function of the chitin deacetylase2 enzyme (LmCDA2). J. Biol. Chem. 2016, 291, 24352–24363. [Google Scholar] [CrossRef] [PubMed]
- Moussian, B.; Letizia, A.; Martinez-Corrales, G.; Rotstein, B.; Casali, A.; Llimargas, M. Deciphering the genetic programme triggering timely and spatially-regulated chitin deposition. PLoS Genet. 2015, 11, e1004939. [Google Scholar] [CrossRef][Green Version]
- Konopova, B.; Zrzavy, J. Ultrastructure, development, and homology of insect embryonic cuticles. J. Morphol. 2005, 264, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Rajagopal, R.; Bhatnagar, R.K. Molecular characterization of chitinase from polyphagous pest Helicoverpa armigera. Biochem. Biophys. Res. Commun. 2013, 310, 188–195. [Google Scholar] [CrossRef]
- Rinterknecht, E. A fine structural analysis of serosal cuticulogenesis in the egg of Locusta migratoria migratorioides. Tissue Cell 1993, 25, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Arakane, Y.; Muthukrishnan, S.; Kramer, K.J.; Ma, E.; Zhu, K.Y. Comparative genomic analysis of chitinase and chitinase-like genes in the African malaria mosquito (Anopheles gambiae). PLoS ONE 2011, 6, e19899. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Arakane, Y.; Muthukrishnan, S.; Kramer, K.J.; Ma, E.; Zhu, K.Y. Identification and characterization of a novel chitinase-like gene cluster (AgCht5) possibly derived from tandem duplications in the African malaria mosquito, Anopheles gambiae. Insect Biochem. Mol. 2011, 41, 521–528. [Google Scholar] [CrossRef]
- Li, Y.; Song, H.; Zhang, X.; Li, D.; Zhang, T.; Ma, E.; Zhang, J. Heterologous expression and characterization of two chitinase 5 enzymes from the migratory locust Locusta migratoria. Insect Sci. 2016, 23, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Ma, L.; Chen, P.; Yang, Q. Proteomic analysis of insect molting fluid with a focus on enzymes involved in chitin degradation. J. Proteome Res. 2014, 13, 2931–2940. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qu, M.; Zhou, Y.; Yang, Q. Structural analysis of group II chitinase (ChtII) catalysis completes the puzzle of chitin hydrolysis in insects. J. Biol. Chem. 2018, 293, 2652–2660. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, Y.; Qu, M.; Zhao, Y.; Yang, Q. Fully Deacetylated Chitooligosaccharides Act as Glycoside Hydrolase Family 18 Chitinase Inhibitors. J. Biol. Chem. 2014, 289, 17932–17940. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guo, J.; Chen, X.; Cheng, C.; Luo, X.; Zhang, X.; Mussion, B.; Chen, J.; Li, J.; Zhang, C. Chitin synthase 1 and five cuticle protein genes are involved in serosal cuticle formation during early embryogenesis to enhance eggshells in Nilaparvata lugens. Insect Sci. 2022, 29, 363–378. [Google Scholar] [CrossRef]
| Gene | Application | Primers | Sequence of Primers (5′-3′) |
|---|---|---|---|
| LmCht5-1 | Gene expression | RT-LmCht5-1-F | CATCAAAGCGAAGGGCTACGGC |
| RT-LmCht5-1-R | AGATTAGTGCGTCCTTCGGGCCA | ||
| LmCht5-2 | RT-LmCht5-2-F | ATTTTCAAGGATTATGTGGAGAACC | |
| RT-LmCht5-2-R | TCCACAGTGTTTGTTTTCTTTGATT | ||
| LmRpl32 | RT-LmRpl32-F | ACTGGAAGTCTTGATGATGCAG | |
| RT-LmRpl32-R | CTGAGCCCGTTCTACAATAGC | ||
| dsLmCht5-1 | Double-strand | T7-LmCht5-1-F | taatacgactcactatagggTCGTTGAGTACATGAAGCGG |
| RNA synthesis | T7-LmCht5-1-R | taatacgactcactatagggCCTTGTTGATGTAGGTGCCC | |
| dsLmCht5-2 | T7-LmCht5-2-F | taatacgactcactatagggCAGGAAGACTCCTCCACTCG | |
| T7-LmCht5-2-R | taatacgactcactatagggATTCCCAGTCCACGTCAAAG | ||
| dsGFP | T7-GFP-F | taatacgactcactatagggGTGGAGAGGGTGAAGG | |
| T7-GFP-R | taatacgactcactatagggGGGCAGATTGTGTGGAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Huo, Y.; Dong, Q.; Liu, W.; Gao, L.; Zhou, J.; Li, D.; Zhang, X.; Zhang, J.; Zhang, M. LmCht5-1 and LmCht5-2 Promote the Degradation of Serosal and Pro-Nymphal Cuticles during Locust Embryonic Development. Biology 2022, 11, 1778. https://doi.org/10.3390/biology11121778
Zhang T, Huo Y, Dong Q, Liu W, Gao L, Zhou J, Li D, Zhang X, Zhang J, Zhang M. LmCht5-1 and LmCht5-2 Promote the Degradation of Serosal and Pro-Nymphal Cuticles during Locust Embryonic Development. Biology. 2022; 11(12):1778. https://doi.org/10.3390/biology11121778
Chicago/Turabian StyleZhang, Tingting, Yanjun Huo, Qing Dong, Weiwei Liu, Lu Gao, Jiannan Zhou, Daqi Li, Xueyao Zhang, Jianzhen Zhang, and Min Zhang. 2022. "LmCht5-1 and LmCht5-2 Promote the Degradation of Serosal and Pro-Nymphal Cuticles during Locust Embryonic Development" Biology 11, no. 12: 1778. https://doi.org/10.3390/biology11121778
APA StyleZhang, T., Huo, Y., Dong, Q., Liu, W., Gao, L., Zhou, J., Li, D., Zhang, X., Zhang, J., & Zhang, M. (2022). LmCht5-1 and LmCht5-2 Promote the Degradation of Serosal and Pro-Nymphal Cuticles during Locust Embryonic Development. Biology, 11(12), 1778. https://doi.org/10.3390/biology11121778