Term and Preterm Birth Initiation Is Associated with the Macrophages Shifting to M1 Polarization in Gestational Tissues in Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Protocols
2.2. Cell Isolation
2.3. Monoclonal Antibodies (mAbs) and Reagents
2.4. Flow Cytometry
2.5. Total RNA Extraction and Quantitative Real-Time PCR (Q-PCR)
2.6. Statistical Analysis
3. Results
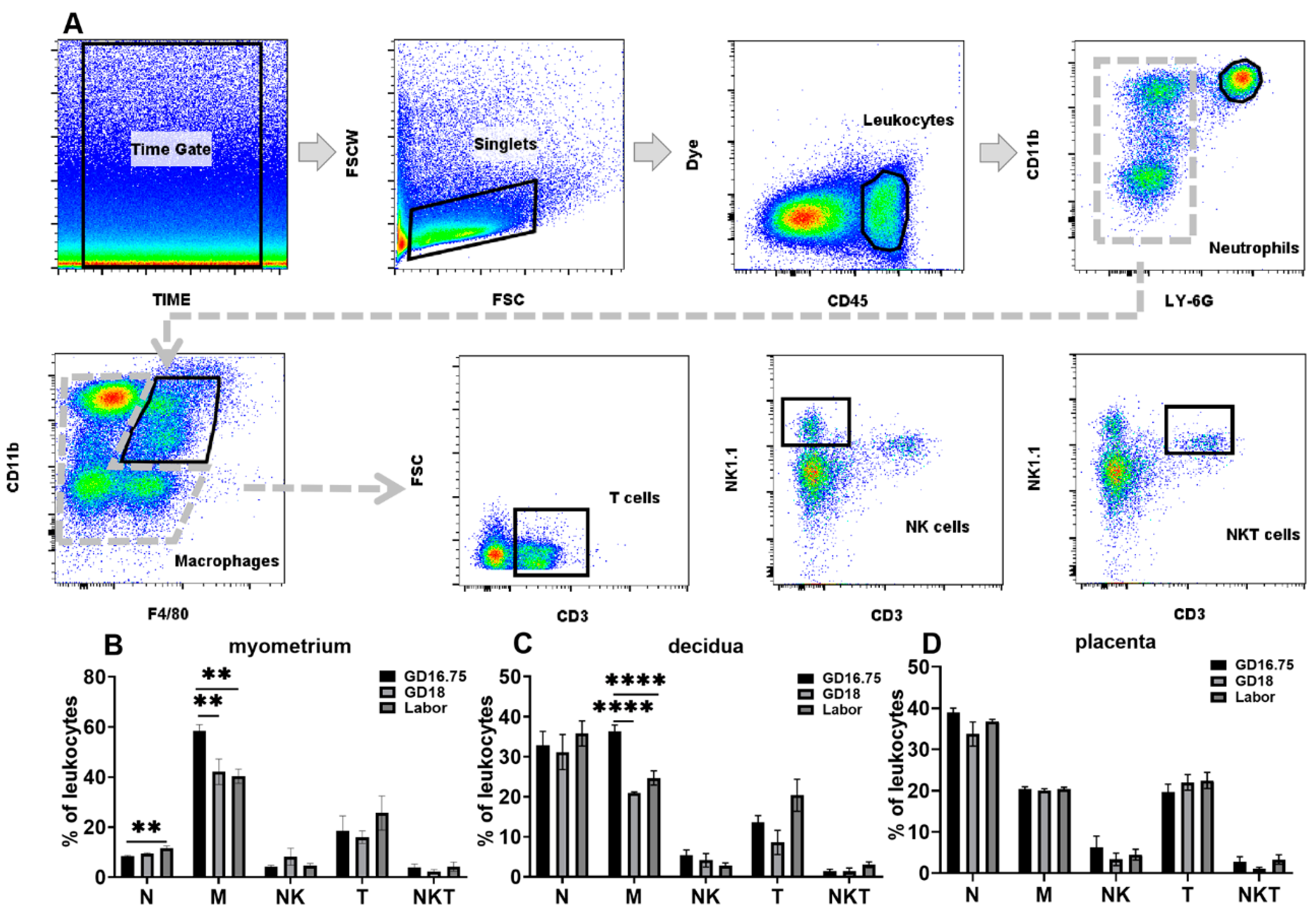
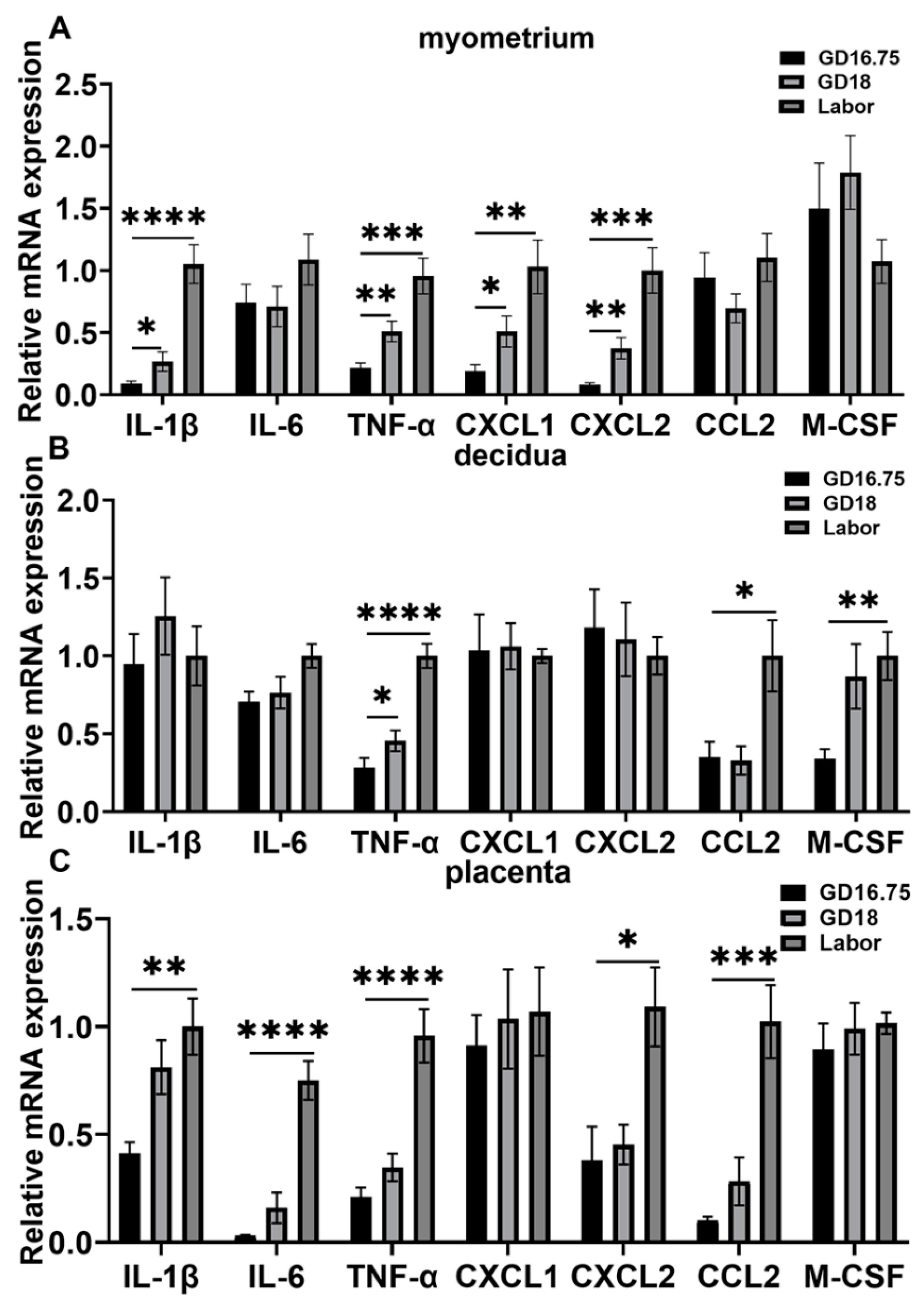
3.1. Dynamic Changes in Leukocyte Infiltration and Cytokine and Chemokine Expression in Myometrium, Decidua, and Placenta at Late Gestation and Labor
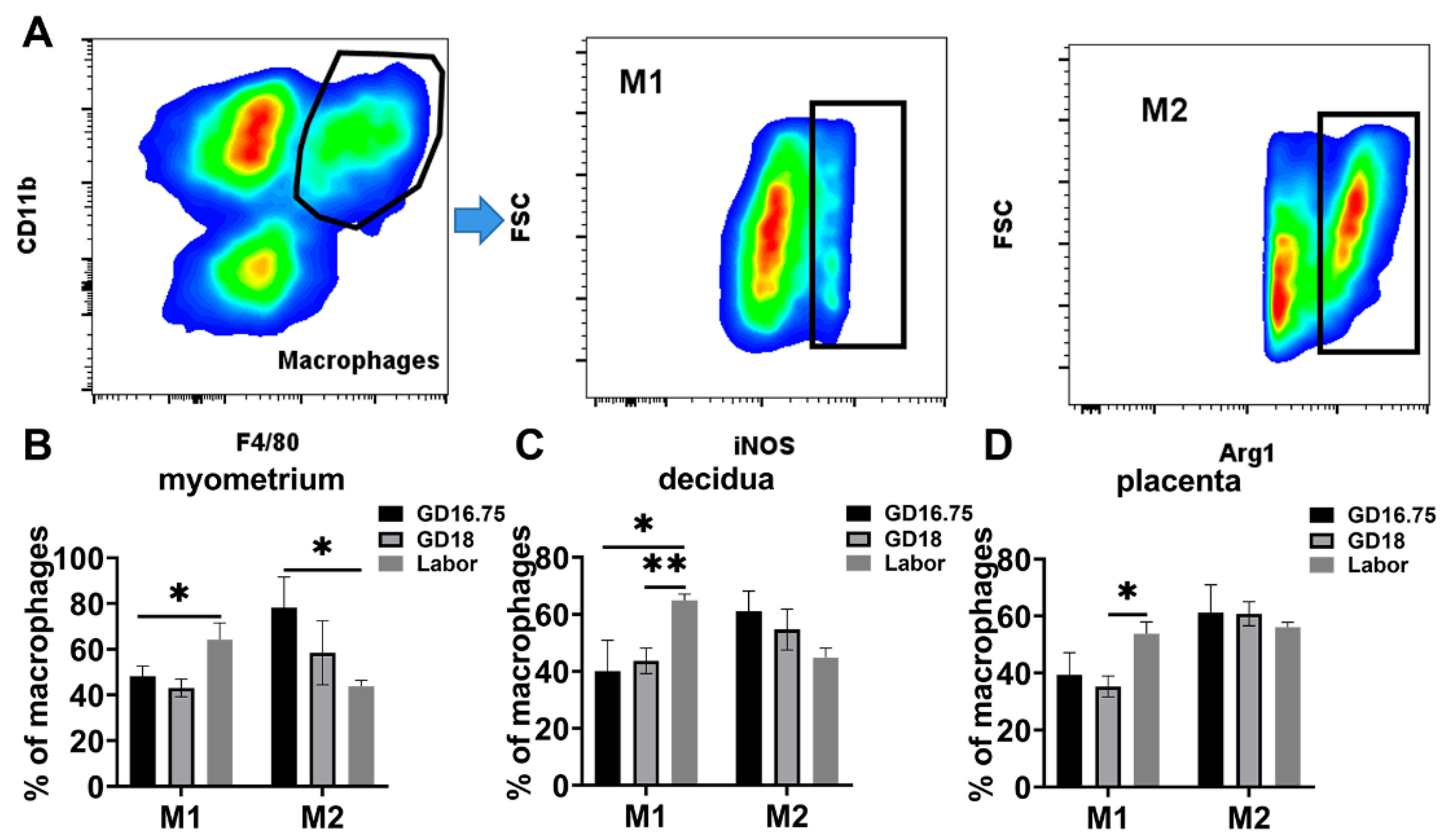
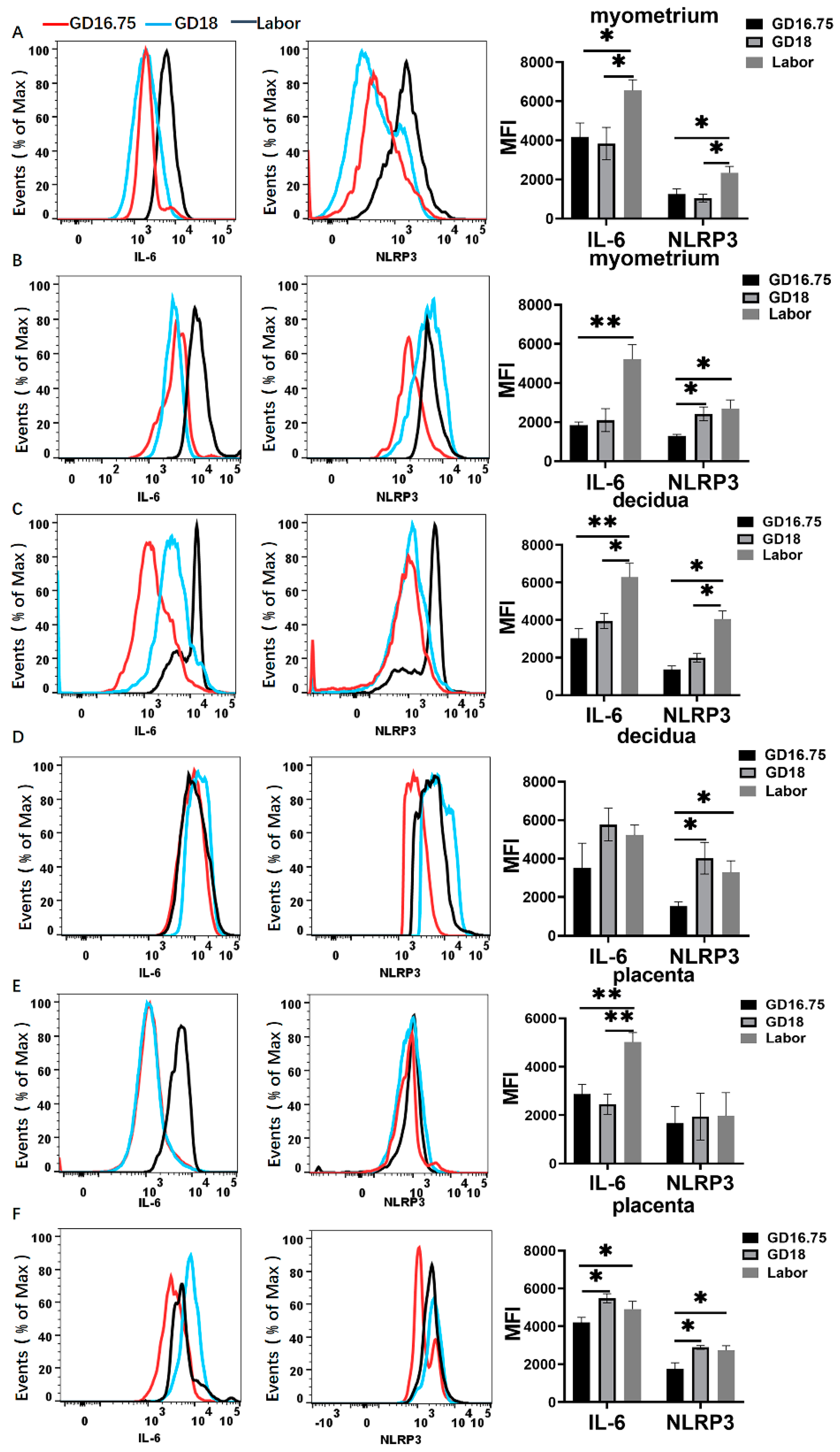
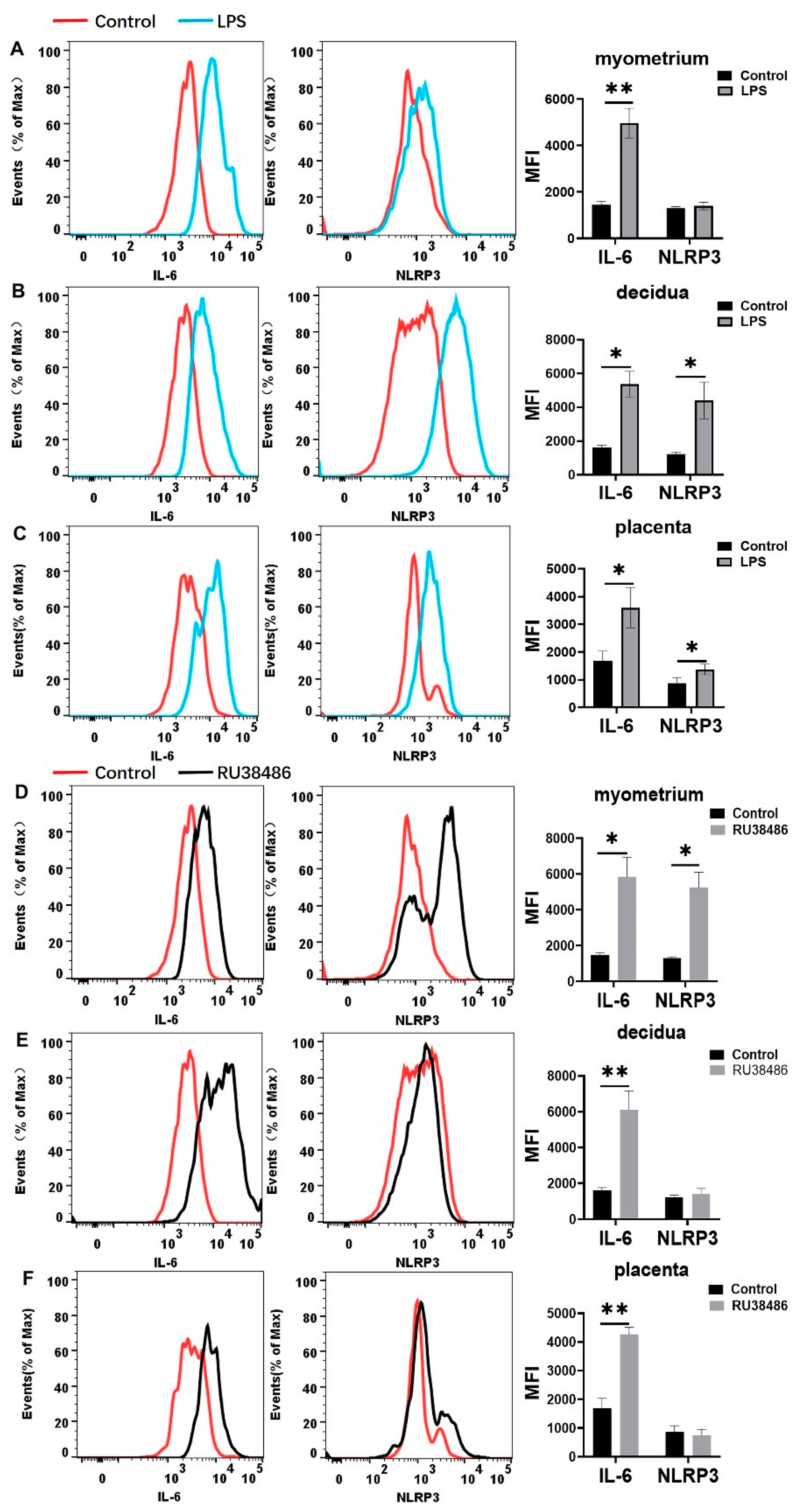
3.2. Macrophages Exhibit M1 Polarization and IL-6 and NLRP3 Expression Is Increased in Myometrium, Decidua, and Placenta at Labor
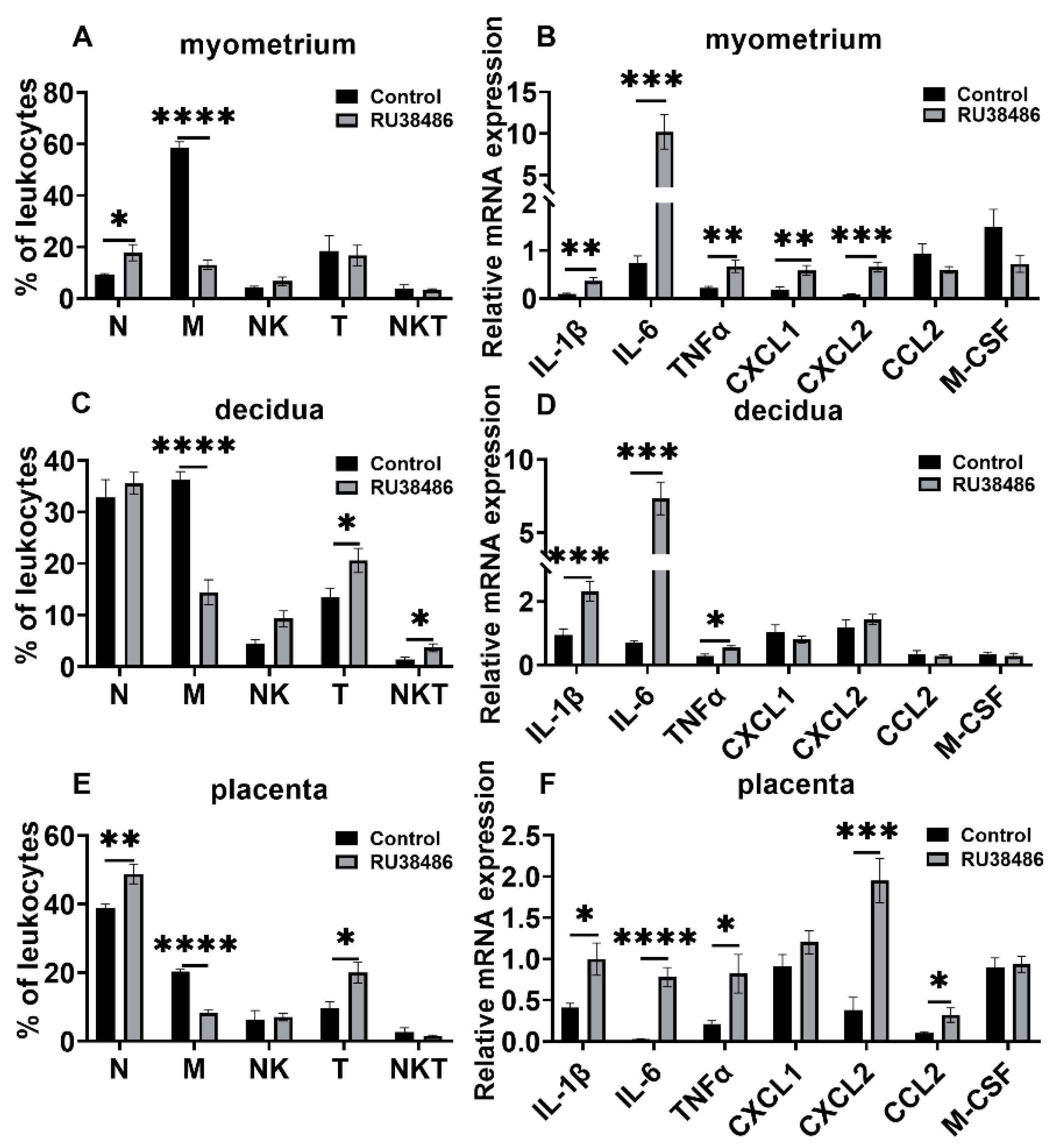
3.3. Changes in Leukocyte Subsets and Cytokine and Chemokine Expression in Myometrium, Decidua, and Placenta in LPS and RU38486 Preterm Birth
3.4. Characteristics in M1 and M2 Populations and IL-6 and NLRP3 Expressions in Myometrium, Decidua, and Placenta at Preterm Labor Induced by LPS and RU38486
3.5. Effects of MCC950 Treatment on Leukocyte Subsets in Gestational Tissues at Term and Preterm Labor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, G.C.; Tosto, V.; Giardina, I. The biological basis and prevention of preterm birth. Best Pract Res. Clin. Obstet. Gynaecol. 2018, 52, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Green, E.S.; Arck, P.C. Pathogenesis of preterm birth: Bidirectional inflammation in mother and fetus. Semin. Immunopathol. 2020, 42, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Gilman-Sachs, A.; Dambaeva, S.; Salazar, G.M.; Hussein, Y.; Kwak-Kim, J.; Beaman, K. Inflammation induced preterm labor and birth. J. Reprod. Immunol. 2018, 129, 53–58. [Google Scholar] [CrossRef]
- Shynlova, O.; Nadeem, L.; Zhang, J.; Dunk, C.; Lye, S. Myometrial activation: Novel concepts underlying labor. Placenta 2020, 92, 28–36. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; StLouis, D.; Lehr, M.A.; Sanchez-Rodriguez, E.N.; Arenas-Hernandez, M. Immune cells in term and preterm labor. Cell. Mol. Immunol. 2014, 11, 571–581. [Google Scholar] [CrossRef]
- Osman, I.; Young, A.; Ledingham, M.A.; Thomson, A.J.; Jordan, F.; Greer, I.A.; Norman, J.E. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol. Hum. Reprod. 2003, 9, 41–45. [Google Scholar] [CrossRef]
- Helmo, F.R.; Alves, E.; Moreira, R.; Severino, V.O.; Rocha, L.P.; Monteiro, M.; Reis, M.; Etchebehere, R.M.; Machado, J.R.; Correa, R. Intrauterine infection, immune system and premature birth. J. Matern Fetal Neonatal. Med. 2018, 31, 1227–1233. [Google Scholar] [CrossRef]
- You, X.; Liu, J.; Xu, C.; Liu, W.; Zhu, X.; Li, Y.; Sun, Q.; Gu, H.; Ni, X. Corticotropin-releasing hormone (CRH) promotes inflammation in human pregnant myometrium: The evidence of CRH initiating parturition? J. Clin. Endocrinol. Metab. 2014, 99, E199–E208. [Google Scholar] [CrossRef]
- Yeh, C.C.; Chen, C.Y.; Wang, P.H. Infection and preterm birth. J. Chin. Med. Assoc. 2017, 80, 530–531. [Google Scholar] [CrossRef] [PubMed]
- Higgins, R.D.; Saade, G.; Polin, R.A.; Grobman, W.A.; Buhimschi, I.A.; Watterberg, K.; Silver, R.M.; Raju, T. Evaluation and Management of Women and Newborns With a Maternal Diagnosis of Chorioamnionitis: Summary of a Workshop. Obstet. Gynecol. 2016, 127, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, M.; Doll, J.R.; Stankiewicz, T.E.; Lawson, M.J.; Sauer, V.; Wen, B.; Kalinichenko, V.V.; Sun, X.; Tilburgs, T.; Divanovic, S. Maternal regulation of inflammatory cues is required for induction of preterm birth. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Yellon, S.M.; Dobyns, A.E.; Beck, H.L.; Kurtzman, J.T.; Garfield, R.E.; Kirby, M.A. Loss of progesterone receptor-mediated actions induce preterm cellular and structural remodeling of the cervix and premature birth. PLoS ONE 2013, 8, e81340. [Google Scholar] [CrossRef] [PubMed]
- Shynlova, O.; Dorogin, A.; Li, Y.; Lye, S. Inhibition of infection-mediated preterm birth by administration of broad spectrum chemokine inhibitor in mice. J. Cell. Mol. Med. 2014, 18, 1816–1829. [Google Scholar] [CrossRef]
- Motomura, K.; Romero, R.; Galaz, J.; Tao, L.; Garcia-Flores, V.; Xu, Y.; Done, B.; Arenas-Hernandez, M.; Miller, D.; Gutierrez-Contreras, P.; et al. Fetal and maternal NLRP3 signaling is required for preterm labor and birth. JCI Insight 2022, 7. [Google Scholar] [CrossRef]
- Chen, Z.; Shan, Y.; You, X.; Gu, H.; Xu, C.; Long, J.; Ni, X. NLRP3 inflammasome is involved in uterine activation for labor at term and preterm. Reproduction 2021, 162, 449–460. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, Q.; Jin, L. Dynamic Function and Composition Changes of Immune Cells During Normal and Pathological Pregnancy at the Maternal-Fetal Interface. Front. Immunol. 2019, 10, 2317. [Google Scholar] [CrossRef]
- Pique-Regi, R.; Romero, R.; Tarca, A.L.; Sendler, E.D.; Xu, Y.; Garcia-Flores, V.; Leng, Y.; Luca, F.; Hassan, S.S.; Gomez-Lopez, N. Single cell transcriptional signatures of the human placenta in term and preterm parturition. eLife 2019, 8, e52004. [Google Scholar] [CrossRef]
- Li, L.P.; Fang, Y.C.; Dong, G.F.; Lin, Y.; Saito, S. Depletion of invariant NKT cells reduces inflammation-induced preterm delivery in mice. J. Immunol. 2012, 188, 4681–4689. [Google Scholar] [CrossRef]
- Arenas-Hernandez, M.; Romero, R.; Xu, Y.; Panaitescu, B.; Garcia-Flores, V.; Miller, D.; Ahn, H.; Done, B.; Hassan, S.S.; Hsu, C.D.; et al. Effector and Activated T Cells Induce Preterm Labor and Birth That Is Prevented by Treatment with Progesterone. J. Immunol. 2019, 202, 2585–2608. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Abrahams, V.M. Neutrophils in preterm birth: Friend or foe? Placenta 2020, 102, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Shynlova, O.; Nedd-Roderique, T.; Li, Y.; Dorogin, A.; Nguyen, T.; Lye, S.J. Infiltration of myeloid cells into decidua is a critical early event in the labour cascade and post-partum uterine remodelling. J. Cell. Mol. Med. 2013, 17, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Garcia-Flores, V.; Chin, P.Y.; Groome, H.M.; Bijland, M.T.; Diener, K.R.; Romero, R.; Robertson, S.A. Macrophages exert homeostatic actions in pregnancy to protect against preterm birth and fetal inflammatory injury. JCI Insight 2021, 6. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Xu, Y.; Romero, R.; Miller, D.; Kadam, L.; Mial, T.N.; Plazyo, O.; Garcia-Flores, V.; Hassan, S.S.; Xu, Z.; Tarca, A.L.; et al. An M1-like Macrophage Polarization in Decidual Tissue during Spontaneous Preterm Labor That Is Attenuated by Rosiglitazone Treatment. J. Immunol. 2016, 196, 2476–2491. [Google Scholar] [CrossRef]
- Hamilton, S.; Oomomian, Y.; Stephen, G.; Shynlova, O.; Tower, C.L.; Garrod, A.; Lye, S.J.; Jones, R.L. Macrophages infiltrate the human and rat decidua during term and preterm labor: Evidence that decidual inflammation precedes labor. Biol. Reprod. 2012, 86, 39. [Google Scholar] [CrossRef]
- Zhang, Y.H.; He, M.; Wang, Y.; Liao, A.H. Modulators of the Balance between M1 and M2 Macrophages during Pregnancy. Front. Immunol. 2017, 8, 120. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef]
- Shynlova, O.; Nedd-Roderique, T.; Li, Y.; Dorogin, A.; Lye, S.J. Myometrial immune cells contribute to term parturition, preterm labour and post-partum involution in mice. J. Cell. Mol. Med. 2013, 17, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Shynlova, O.; Boros-Rausch, A.; Farine, T.; Adams, W.K.; Dunk, C.; Lye, S.J. Decidual Inflammation Drives Chemokine-Mediated Immune Infiltration Contributing to Term Labor. J. Immunol. 2021, 207, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shynlova, O.; Sabra, S.; Bang, A.; Briollais, L.; Lye, S.J. Immunophenotyping and activation status of maternal peripheral blood leukocytes during pregnancy and labour, both term and preterm. J. Cell. Mol. Med. 2017, 21, 2386–2402. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hara, H.; Nunez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
- Fang, X.; Wang, Y.; Zhang, Y.; Li, Y.; Kwak-Kim, J.; Wu, L. NLRP3 Inflammasome and Its Critical Role in Gynecological Disorders and Obstetrical Complications. Front. Immunol. 2020, 11, 555826. [Google Scholar] [CrossRef]
- Arenas-Hernandez, M.; Sanchez-Rodriguez, E.N.; Mial, T.N.; Robertson, S.A.; Gomez-Lopez, N. Isolation of Leukocytes from the Murine Tissues at the Maternal-Fetal Interface. J. Vis. Exp. 2015, e52866. [Google Scholar] [CrossRef]
- Rajarathnam, K.; Schnoor, M.; Richardson, R.M.; Rajagopal, S. How do chemokines navigate neutrophils to the target site: Dissecting the structural mechanisms and signaling pathways. Cell. Signal. 2019, 54, 69–80. [Google Scholar] [CrossRef]
- Tarique, A.A.; Logan, J.; Thomas, E.; Holt, P.G.; Sly, P.D.; Fantino, E. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am. J. Respir. Cell Mol. Biol. 2015, 53, 676–688. [Google Scholar] [CrossRef]
- Sierra-Filardi, E.; Nieto, C.; Dominguez-Soto, A.; Barroso, R.; Sanchez-Mateos, P.; Puig-Kroger, A.; Lopez-Bravo, M.; Joven, J.; Ardavin, C.; Rodriguez-Fernandez, J.L.; et al. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: Identification of CCL2/CCR2-dependent gene expression profile. J. Immunol. 2014, 192, 3858–3867. [Google Scholar] [CrossRef]
- Prairie, E.; Cote, F.; Tsakpinoglou, M.; Mina, M.; Quiniou, C.; Leimert, K.; Olson, D.; Chemtob, S. The determinant role of IL-6 in the establishment of inflammation leading to spontaneous preterm birth. Cytokine Growth Factor Rev. 2021, 59, 118–130. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Presicce, P.; Park, C.W.; Senthamaraikannan, P.; Bhattacharyya, S.; Jackson, C.; Kong, F.; Rueda, C.M.; DeFranco, E.; Miller, L.A.; Hildeman, D.A.; et al. IL-1 signaling mediates intrauterine inflammation and chorio-decidua neutrophil recruitment and activation. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.F.; Catalano, R.D.; Wade, J.; Rossi, A.G.; Norman, J.E. Decidual neutrophil infiltration is not required for preterm birth in a mouse model of infection-induced preterm labor. J. Immunol. 2014, 192, 2315–2325. [Google Scholar] [CrossRef] [PubMed]
- Hudalla, H.; Karenberg, K.; Kuon, R.J.; Poschl, J.; Tschada, R.; Frommhold, D. LPS-induced maternal inflammation promotes fetal leukocyte recruitment and prenatal organ infiltration in mice. Pediatr. Res. 2018, 84, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Tamassia, N.; Bianchetto-Aguilera, F.; Arruda-Silva, F.; Gardiman, E.; Gasperini, S.; Calzetti, F.; Cassatella, M.A. Cytokine production by human neutrophils: Revisiting the “dark side of the moon”. Eur. J. Clin. Investig. 2018, 48 (Suppl. 2), e12952. [Google Scholar] [CrossRef]
- Poh, X.Y.; Loh, F.K.; Friedland, J.S.; Ong, C. Neutrophil-Mediated Immunopathology and Matrix Metalloproteinases in Central Nervous System—Tuberculosis. Front. Immunol. 2021, 12, 788976. [Google Scholar] [CrossRef]
- Zhang, L.; Mamillapalli, R.; Habata, S.; McAdow, M.; Taylor, H.S. Myometrial-derived CXCL12 promotes lipopolysaccharide induced preterm labour by regulating macrophage migration, polarization and function in mice. J. Cell. Mol. Med. 2022, 26, 2566–2578. [Google Scholar] [CrossRef]
- Renaud, S.J.; Graham, C.H. The role of macrophages in utero-placental interactions during normal and pathological pregnancy. Immunol. Investig. 2008, 37, 535–564. [Google Scholar] [CrossRef]
- Mridha, A.R.; Wree, A.; Robertson, A.; Yeh, M.M.; Johnson, C.D.; Van Rooyen, D.M.; Haczeyni, F.; Teoh, N.C.; Savard, C.; Ioannou, G.N.; et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J. Hepatol. 2017, 66, 1037–1046. [Google Scholar] [CrossRef]
- Sano, M.; Shimazaki, S.; Kaneko, Y.; Karasawa, T.; Takahashi, M.; Ohkuchi, A.; Takahashi, H.; Kurosawa, A.; Torii, Y.; Iwata, H.; et al. Palmitic acid activates NLRP3 inflammasome and induces placental inflammation during pregnancy in mice. J. Reprod. Dev. 2020, 66, 241–248. [Google Scholar] [CrossRef]
- Romero, R.; Espinoza, J.; Goncalves, L.F.; Kusanovic, J.P.; Friel, L.A.; Nien, J.K. Inflammation in preterm and term labour and delivery. Semin. Fetal. Neonatal. Med. 2006, 11, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Wan, C.; Liu, Y.; Wang, Y.; Meng, C.; Zhang, Y.; Jiang, C. NLRP3 inflammasome mediates M1 macrophage polarization and IL-1beta production in inflammatory root resorption. J. Clin. Periodontol. 2020, 47, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Arenas-Hernandez, M.; Romero, R.; Miller, D.; Garcia-Flores, V.; Leng, Y.; Xu, Y.; Galaz, J.; Hassan, S.S.; Hsu, C.D.; et al. Regulatory T Cells Play a Role in a Subset of Idiopathic Preterm Labor/Birth and Adverse Neonatal Outcomes. Cell Rep. 2020, 32, 107874. [Google Scholar] [CrossRef]
- Busse, M.; Campe, K.J.; Redlich, A.; Oettel, A.; Hartig, R.; Costa, S.D.; Zenclussen, A.C. Regulatory B Cells Are Decreased and Impaired in Their Function in Peripheral Maternal Blood in Pre-term Birth. Front. Immunol. 2020, 11, 386. [Google Scholar] [CrossRef]
- Busse, M.; Redlich, A.; Hartig, R.; Costa, S.D.; Rathert, H.; Fest, S.; Zenclussen, A.C. Imbalance between inflammatory and regulatory cord blood B cells following pre-term birth. J. Reprod. Immunol. 2021, 145, 103319. [Google Scholar] [CrossRef] [PubMed]
- Sheller-Miller, S.; Radnaa, E.; Yoo, J.K.; Kim, E.; Choi, K.; Kim, Y.; Kim, Y.N.; Richardson, L.; Choi, C.; Menon, R. Exosomal delivery of NF-kappaB inhibitor delays LPS-induced preterm birth and modulates fetal immune cell profile in mouse models. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, W.; You, X.; Leimert, K.; Popowycz, K.; Fang, X.; Wood, S.L.; Slater, D.M.; Sun, Q.; Gu, H.; et al. PGF2alpha modulates the output of chemokines and pro-inflammatory cytokines in myometrial cells from term pregnant women through divergent signaling pathways. Mol. Hum. Reprod. 2015, 21, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Tartakovsky, B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am. J. Obstet. Gynecol. 1992, 167, 1041–1045. [Google Scholar] [CrossRef]
- Robertson, S.A.; Christiaens, I.; Dorian, C.L.; Zaragoza, D.B.; Care, A.S.; Banks, A.M.; Olson, D.M. Interleukin-6 is an essential determinant of on-time parturition in the mouse. Endocrinology 2010, 151, 3996–4006. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Jin, L.; Batra, S.; Douda, D.N.; Palaniyar, N.; Jeyaseelan, S. CXCL1 contributes to host defense in polymicrobial sepsis via modulating T cell and neutrophil functions. J. Immunol. 2014, 193, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Boro, M.; Balaji, K.N. CXCL1 and CXCL2 Regulate NLRP3 Inflammasome Activation via G-Protein-Coupled Receptor CXCR2. J. Immunol. 2017, 199, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Mazgaeen, L.; Gurung, P. Recent Advances in Lipopolysaccharide Recognition Systems. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, C.R.; Gao, L.; Montalbano, A.P. Multifactorial Regulation of Myometrial Contractility During Pregnancy and Parturition. Front. Endocrinol. 2019, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a1651. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Chavez, F.; Correa, D.; Navarrete-Meneses, P.; Cancino-Diaz, J.C.; Cancino-Diaz, M.E.; Rodriguez-Martinez, S. NF-kappaB and Its Regulators During Pregnancy. Front. Immunol. 2021, 12, 679106. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the Role of NLRP3 Inflammasome in Diseases. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Motomura, K.; Miller, D.; Garcia-Flores, V.; Galaz, J.; Romero, R. Inflammasomes: Their Role in Normal and Complicated Pregnancies. J. Immunol. 2019, 203, 2757–2769. [Google Scholar] [CrossRef]




| Forward Primer (5′ -> 3′) | Reverse Primer (5′ -> 3′) | |
|---|---|---|
| β-actin (Mouse) | CTGTATGCCTCTGGTCGTAC | TGATGTCACGCACGATTTCC |
| IL-1β (Mouse) | TGCCACCTTTTGACAGTGATG | AAGGTCCACGGGAAAGACAC |
| IL-6 (Mouse) | CGGCCTTCCCTACTTCACAA | TTCTGCAAGTGCATCATCGT |
| CXCL1 (Mouse) | CTGGGATTCACCTCAAGAACATC | CAGGGTCAAGGCAAGCCTC |
| CXCL2 (Mouse) | CCAACCACCAGGCTACAGG | GCGTCACACTCAAGCTCTG |
| CCL2 (Mouse) | CACCAGCCAACTCTCACTGAA | CATTCCTTCTTGGGGTCAGC |
| M-CSF (Mouse) | TGATTGGGAATGGACACCTG | AAAGGCAATCTGGCATGAAGT |
| TNF-α (Mouse) | ACCC TCACACTCAGATCATC | GAGTAGACAAGGTACAA CCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, Y.; Shen, S.; Long, J.; Tang, Z.; Wu, C.; Ni, X. Term and Preterm Birth Initiation Is Associated with the Macrophages Shifting to M1 Polarization in Gestational Tissues in Mice. Biology 2022, 11, 1759. https://doi.org/10.3390/biology11121759
Shan Y, Shen S, Long J, Tang Z, Wu C, Ni X. Term and Preterm Birth Initiation Is Associated with the Macrophages Shifting to M1 Polarization in Gestational Tissues in Mice. Biology. 2022; 11(12):1759. https://doi.org/10.3390/biology11121759
Chicago/Turabian StyleShan, Yali, Shiping Shen, Jing Long, Zhengshan Tang, Cichun Wu, and Xin Ni. 2022. "Term and Preterm Birth Initiation Is Associated with the Macrophages Shifting to M1 Polarization in Gestational Tissues in Mice" Biology 11, no. 12: 1759. https://doi.org/10.3390/biology11121759
APA StyleShan, Y., Shen, S., Long, J., Tang, Z., Wu, C., & Ni, X. (2022). Term and Preterm Birth Initiation Is Associated with the Macrophages Shifting to M1 Polarization in Gestational Tissues in Mice. Biology, 11(12), 1759. https://doi.org/10.3390/biology11121759






