Carbon and Iron Uptake by Phytoplankton in the Amundsen Sea, Antarctica
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
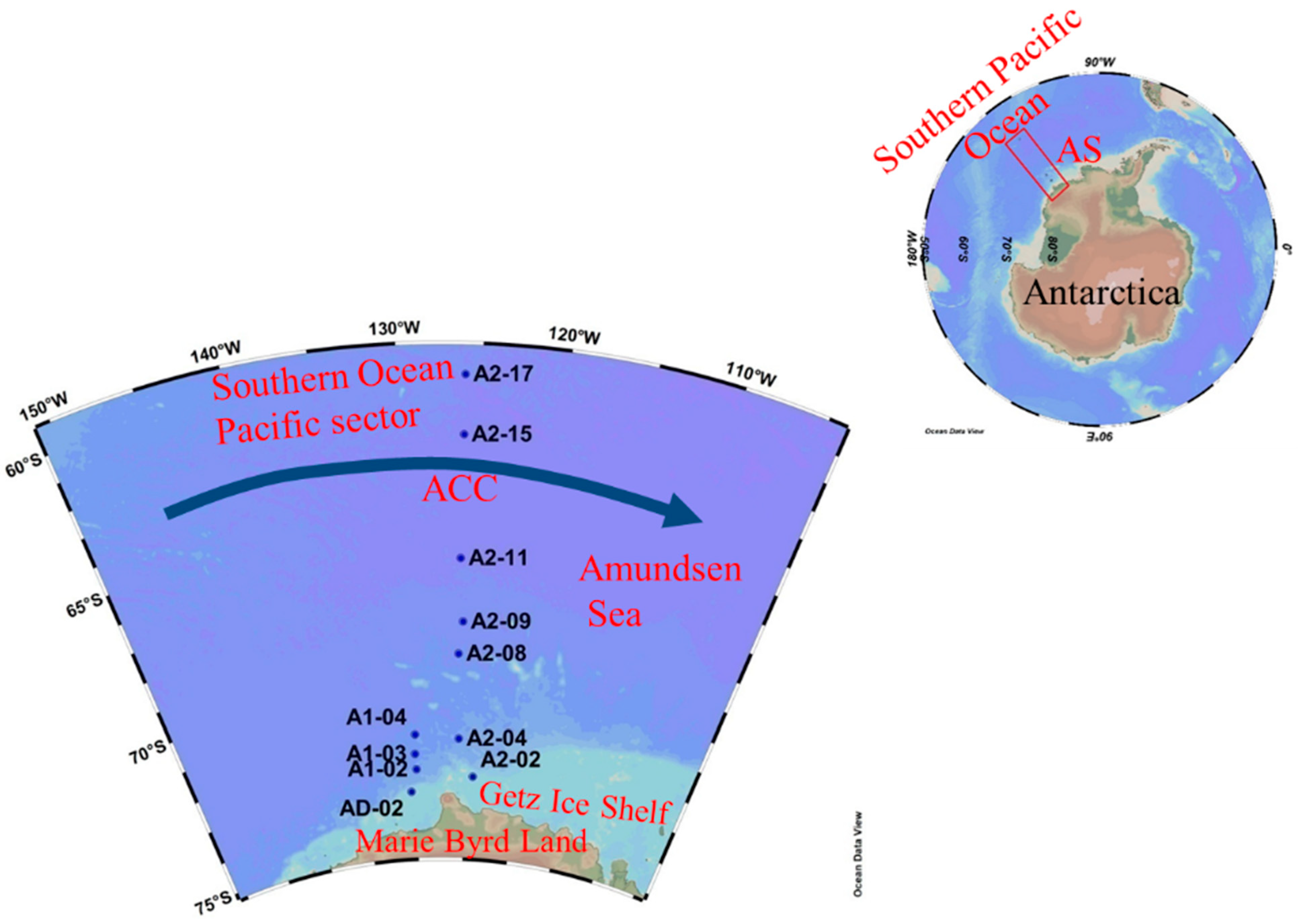
2.1. Study Area
2.2. Sampling
2.3. Determination of Major Nutrients
2.4. Measurement of Carbon Fixation Rate (CFR)
2.5. Measurement of Iron Uptake Rate (FeUR)
2.6. Determination of Mixed Layer Depth (MLD)
2.7. Quantification of Sea Ice Meltwater (SIM) and Meteoric Water (MW)
2.7.1. Measurement of Stable Oxygen Isotopic Composition
2.7.2. Calculation of Freshwater Components (SIM and MW)
2.8. Statistical Analyses
3. Results
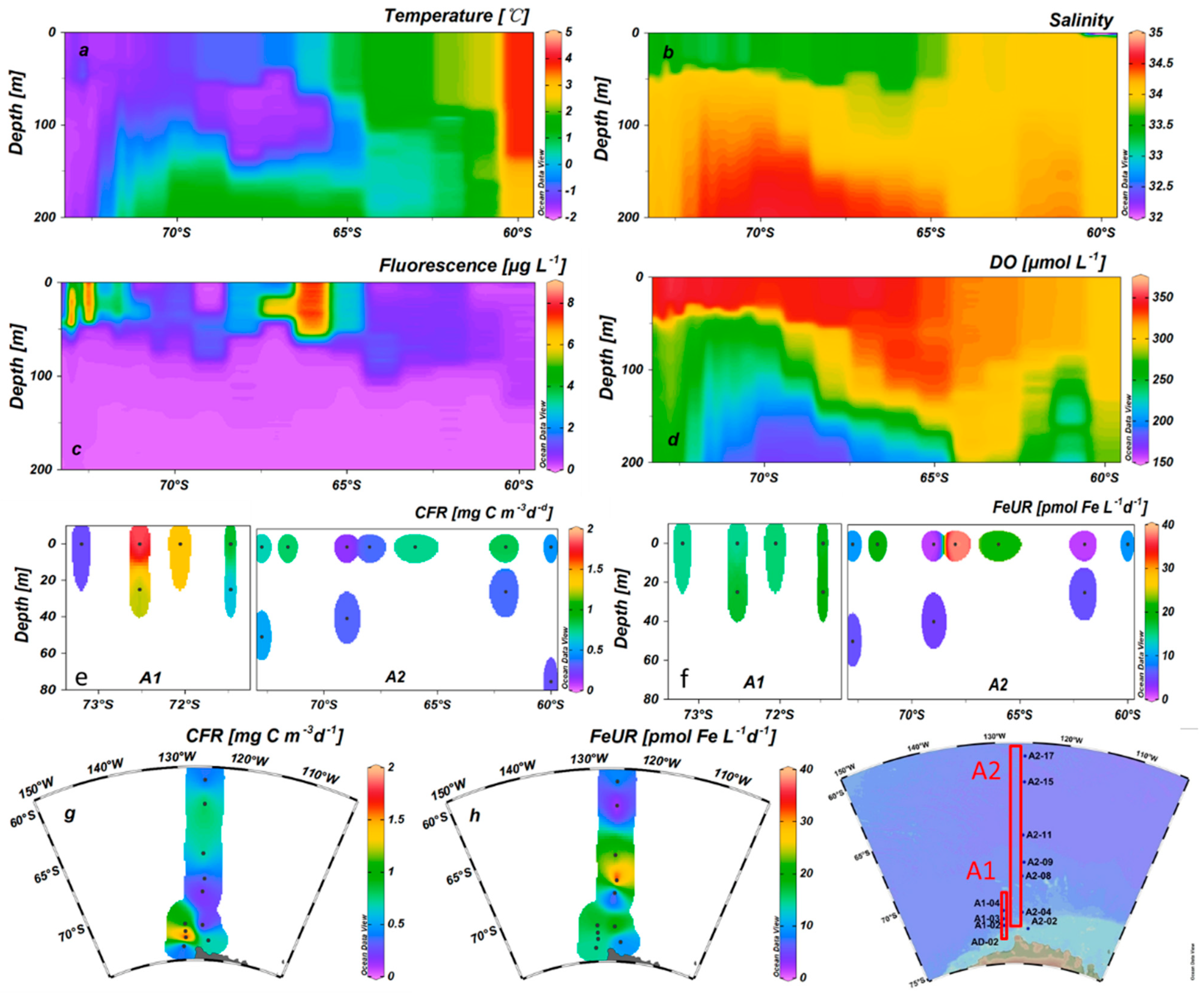
3.1. Physical and Hydrochemical Properties
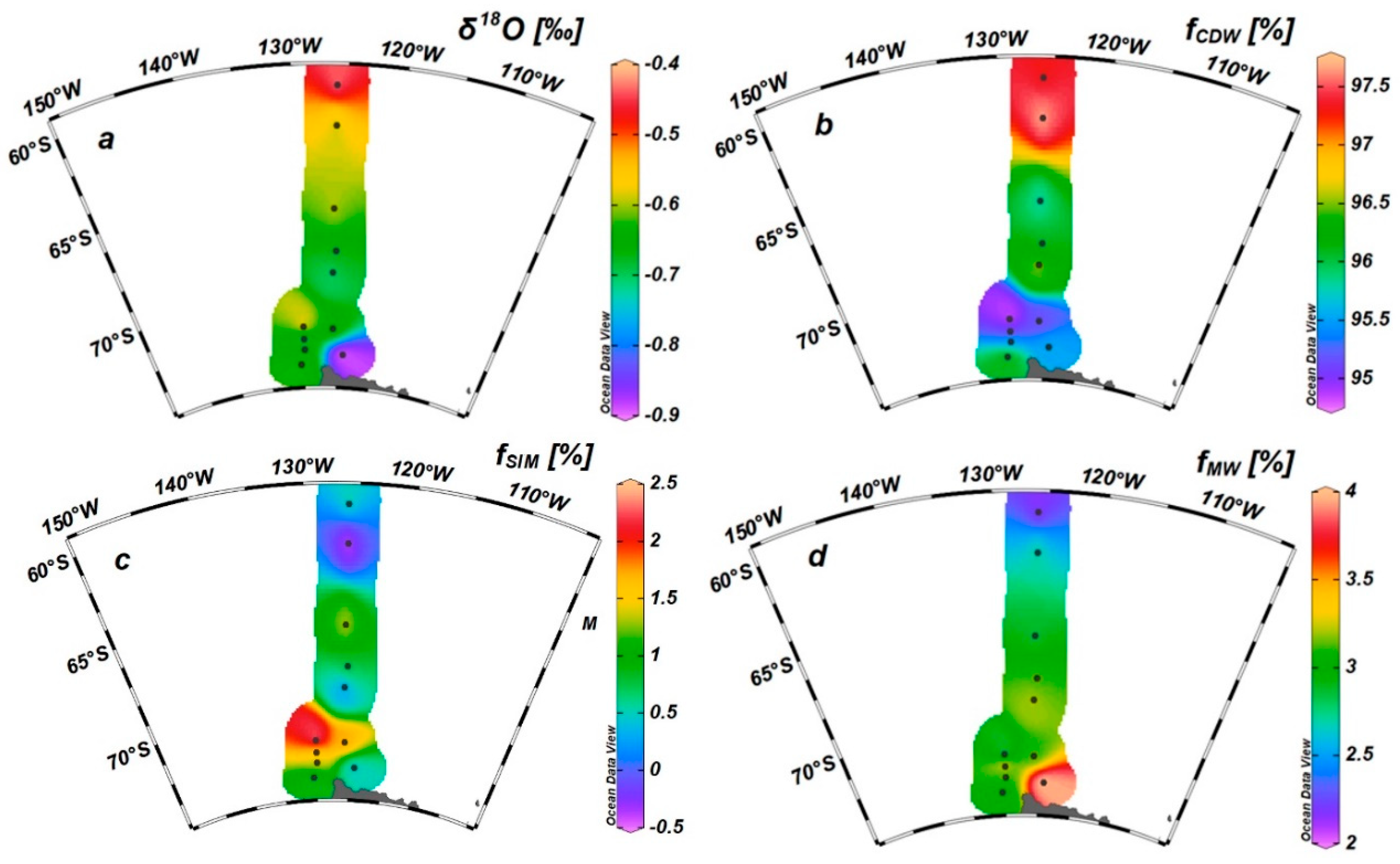
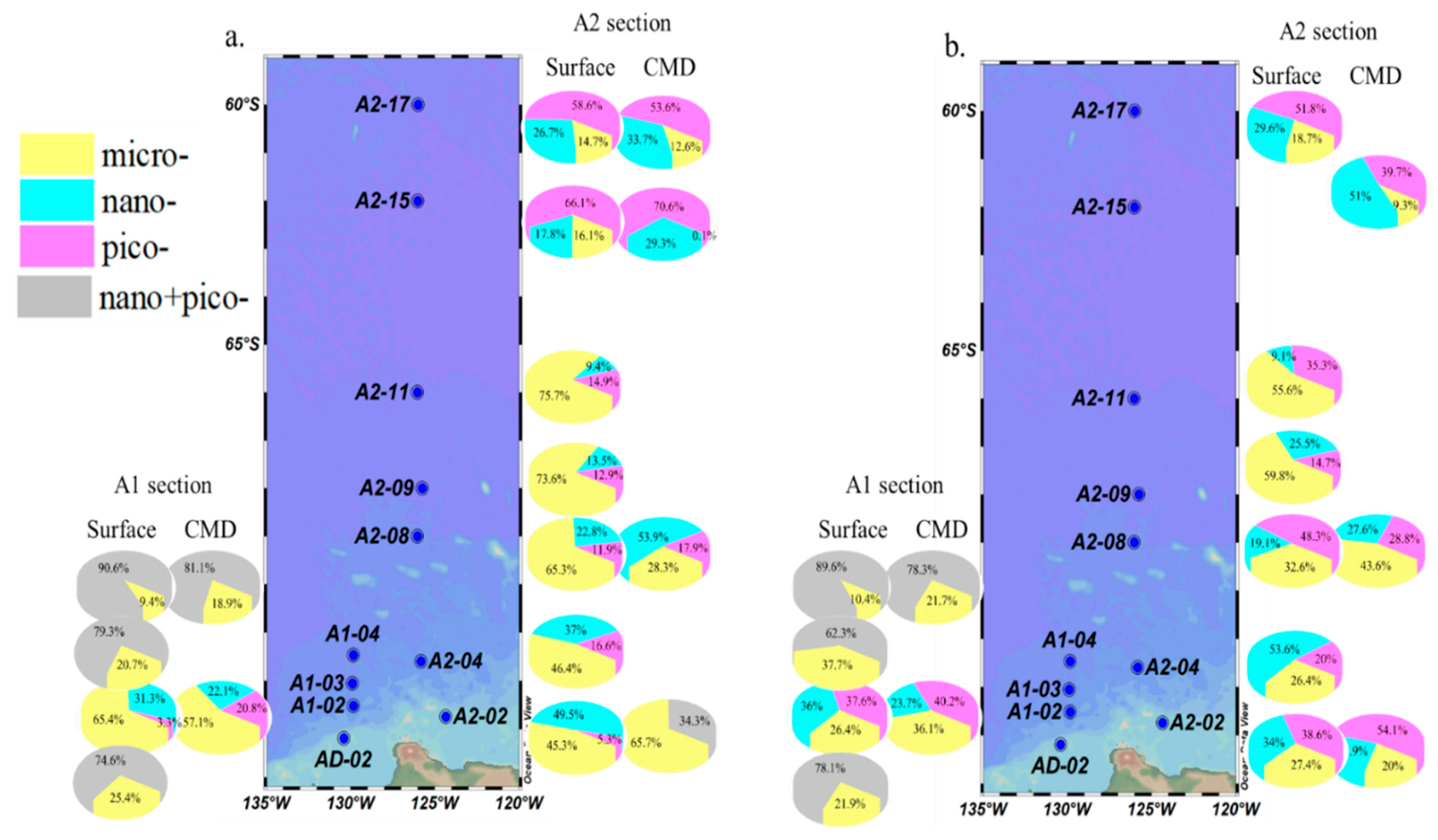
3.2. δ18O in Seawater and Fraction of Freshwater Components
3.3. CFR and FeUR
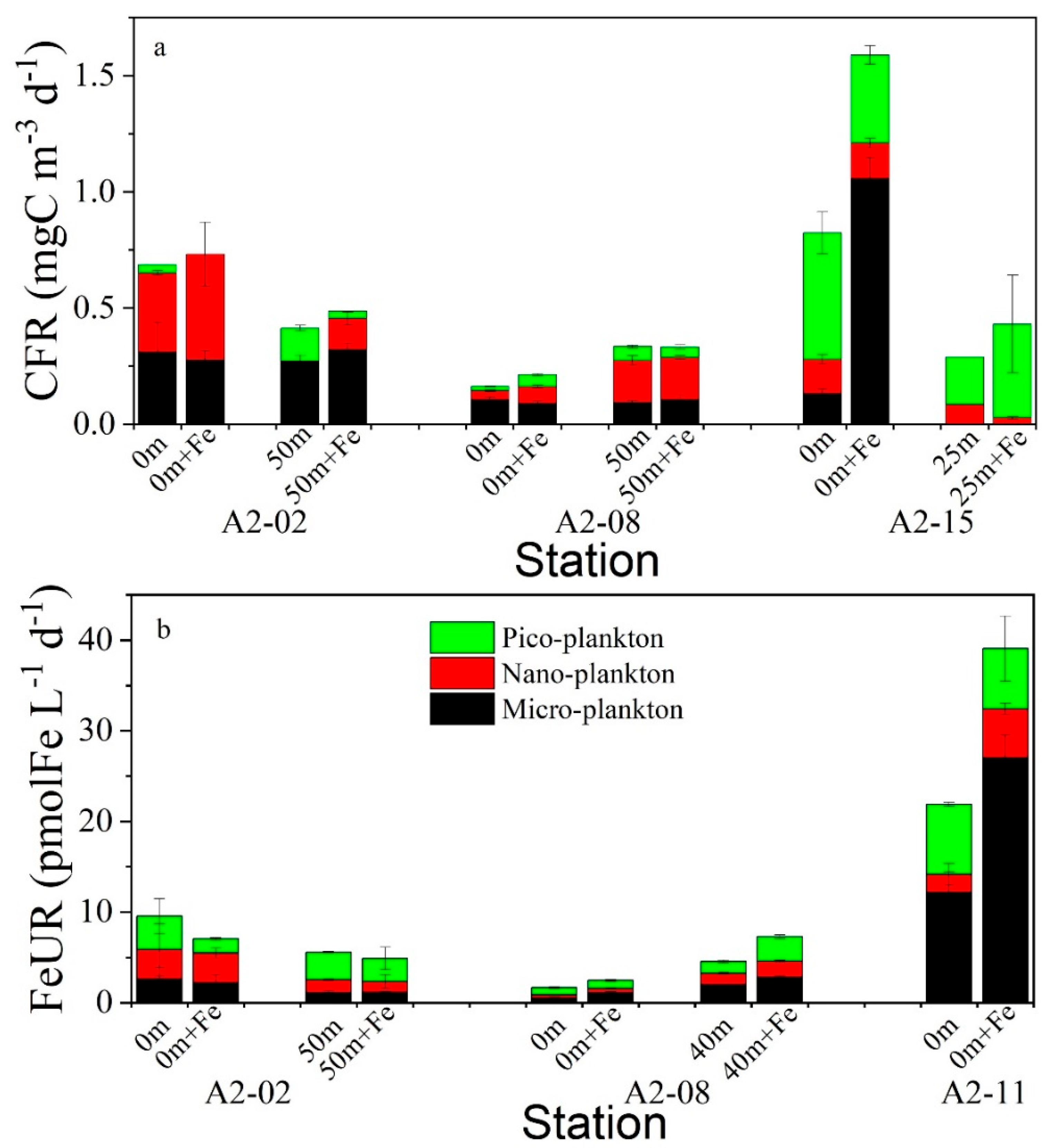
3.4. Size-Fractionated CFR and FeUR
3.5. Effects of Fe Enrichment on CFR and FeUR
4. Discussion
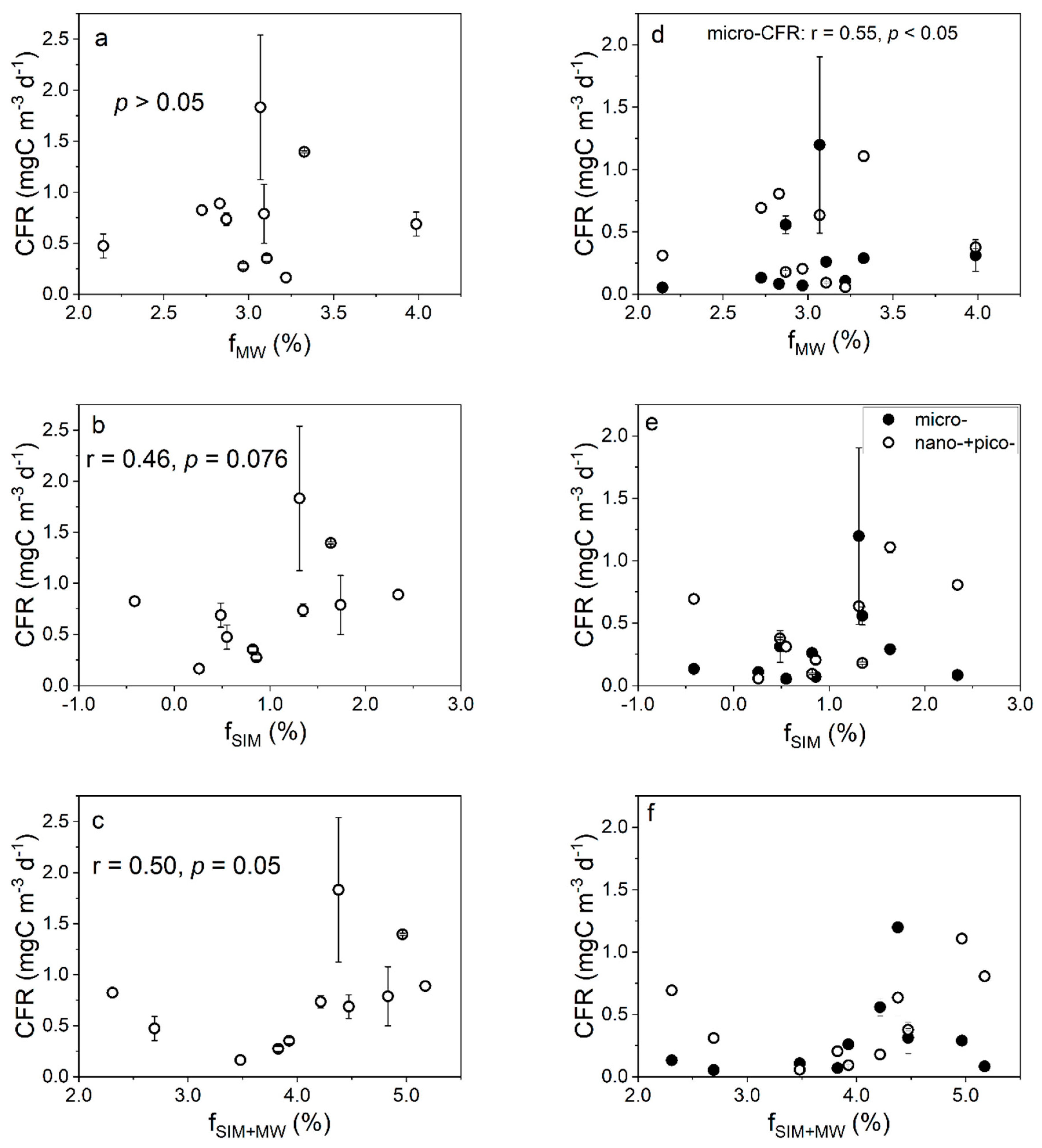
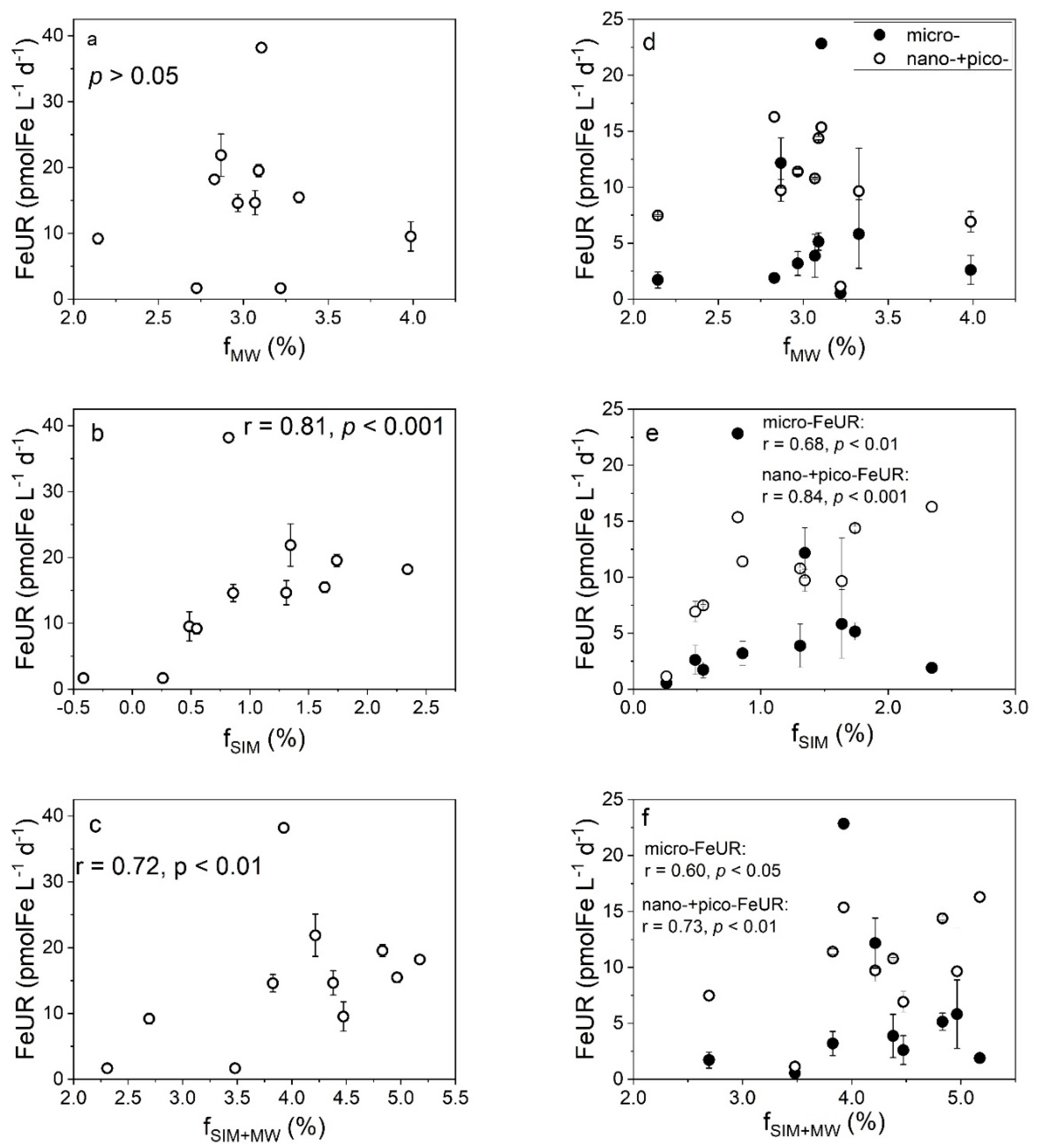
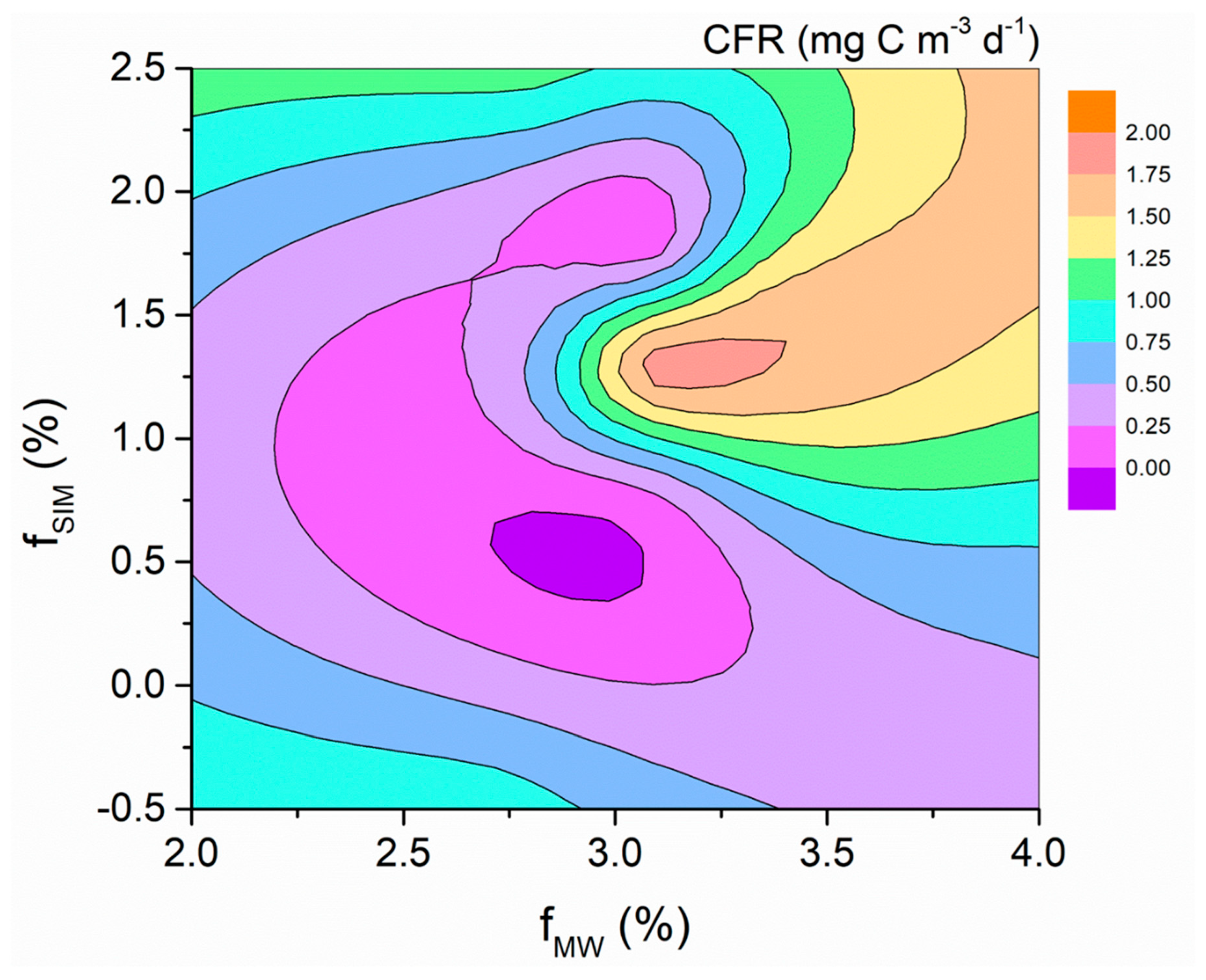
4.1. Effects of Freshwater Components on the CFR
4.2. Effects of Freshwater Components on the FeUR
4.3. Phytoplankton Demands for Fe and C
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arrigo, K.R.; Weiss, A.M.; Smith, W.O. Physical forcing of phytoplankton dynamics in the southwestern Ross Sea. J. Geophys. Res.-Oceans 1998, 103, 1007–1021. [Google Scholar] [CrossRef]
- Arrigo, K.R.; Robinson, D.H.; Worthen, D.L.; Dunbar, R.B.; DiTullio, G.R.; VanWoert, M.; Lizotte, M.P. Phytoplankton community structure and the drawdown of nutrients and CO2 in the Southern Ocean. Science 1999, 283, 365–367. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Interrelated influence of iron, light and cell size on marine phytoplankton growth. Nature 1997, 390, 389–392. [Google Scholar] [CrossRef]
- Timmermans, K.R.; Davey, M.S.; van der Wagt, B.; Snoek, J.; Geider, R.J.; Veldhuis, M.J.; Gerringa, L.J.; de Baar, H.J. Co-limitation by iron and light of Chaetoceros brevis, C. dichaeta and C. calcitrans (Bacillariophyceae). Mar. Ecol. Prog. Ser. 2001, 217, 287–297. [Google Scholar] [CrossRef]
- de Baar, H.J.W.; Boyd, P.W.; Coale, K.H.; Landry, M.R.; Tsuda, A.; Assmy, P.; Bakker, D.C.E.; Bozec, Y.; Barber, R.T.; Brzezinski, M.A.; et al. Synthesis of iron fertilization experiments: From the Iron Age in the Age of Enlightenment. J. Geophys. Res.-Oceans 2005, 110, C09S16. [Google Scholar] [CrossRef]
- Alderkamp, A.-C.; Mills, M.M.; van Dijken, G.L.; Laan, P.; Thuróczy, C.-E.; Gerringa, L.J.A.; de Baar, H.J.W.; Payne, C.D.; Visser, R.J.W.; Buma, A.G.J.; et al. Iron from melting glaciers fuels phytoplankton blooms in the Amundsen Sea (Southern Ocean): Phytoplankton characteristics and productivity. Deep Sea Res. Part II 2012, 71–76, 32–48. [Google Scholar] [CrossRef]
- Planquette, H.; Sherrell, R.M.; Stammerjohn, S.; Field, M.P. Particulate iron delivery to the water column of the Amundsen Sea, Antarctica. Mar. Chem. 2013, 153, 15–30. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, K.; Fu, F.; Spackeen, J.L.; Bronk, D.A.; Hutchins, D.A. A comparative study of iron and temperature interactive effects on diatoms and Phaeocystis antarctica from the Ross Sea, Antarctica. Mar. Ecol. Prog. Ser. 2016, 550, 39–51. [Google Scholar] [CrossRef]
- Arrigo, K.R.; van Dijken, G.L. Phytoplankton dynamics within 37 Antarctic coastal polynya systems. J. Geophys. Res.-Oceans 2003, 108, 3271. [Google Scholar] [CrossRef]
- Alderkamp, A.C.; Kulk, G.; Buma, A.G.; Visser, R.J.; Van Dijken, G.L.; Mills, M.M.; Arrigo, K.R. The Effect of Iron Limitation on the Photophysiology of Phaeocystis antarctica (Prymnesiophyceae) and Fragilariopsis cylindrus (Bacillariophyceae) under Dynamic Irradiance 1. J. Phycol. 2012, 48, 45–59. [Google Scholar] [CrossRef]
- Thuróczy, C.-E.; Alderkamp, A.-C.; Laan, P.; Gerringa, L.J.A.; Mills, M.M.; Van Dijken, G.L.; De Baar, H.J.W.; Arrigo, K.R. Key role of organic complexation of iron in sustaining phytoplankton blooms in the Pine Island and Amundsen Polynyas (Southern Ocean). Deep Sea Res. Part II 2012, 71–76, 49–60. [Google Scholar] [CrossRef]
- Boyd, P.W.; Doney, S.C. The impact of climate change and feedback processes on the ocean carbon cycle. In Ocean Biogeochemistry; Fasham, M.J.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 157–193. [Google Scholar]
- Gerringa, L.J.A.; Alderkamp, A.-C.; Laan, P.; Thuróczy, C.-E.; De Baar, H.J.W.; Mills, M.M.; van Dijken, G.L.; Haren, H.v.; Arrigo, K.R. Iron from melting glaciers fuels the phytoplankton blooms in Amundsen Sea (Southern Ocean): Iron biogeochemistry. Deep Sea Res. Part II 2012, 71–76, 16–31. [Google Scholar] [CrossRef]
- de Baar, H.J.W.; de Jong, J.T.M.; Bakker, D.C.E.; Löscher, B.M.; Veth, C.; Bathmann, U.; Smetacek, V. Importance of iron for plankton blooms and carbon dioxide drawdown in the Southern Ocean. Nature 1995, 373, 412. [Google Scholar] [CrossRef]
- Jacobs, S.S.; Comiso, J.C. Climate Variability in the Amundsen and Bellingshausen Seas. J. Clim. 1997, 10, 697–709. [Google Scholar] [CrossRef]
- Rignot, E.; Velicogna, I.; van den Broeke, M.R.; Monaghan, A.; Lenaerts, J.T.M. Acceleration of the contribution of the Greenland and Antarctic ice sheets to sea level rise. Geophys. Res. Lett. 2011, 38, L05503. [Google Scholar] [CrossRef]
- Biddle, L.C.; Heywood, K.J.; Kaiser, J.; Jenkins, A. Glacial Meltwater Identification in the Amundsen Sea. J. Phys. Oceanogr. 2017, 47, 933–954. [Google Scholar] [CrossRef]
- Sherrell, R.; Lagerström, M.; Forsch, K.; Stammerjohn, S.; Yager, P. Dynamics of dissolved iron and other bioactive trace metals (Mn, Ni, Cu, Zn) in the Amundsen Sea Polynya, Antarctica. Elem. Sci. Anth. 2015, 3, 000071. [Google Scholar] [CrossRef]
- Kim, B.K.; Joo, H.; Song, H.J.; Yang, E.J.; Lee, S.H.; Hahm, D.; Rhee, T.S.; Lee, S.H. Large seasonal variation in phytoplankton production in the Amundsen Sea. Polar Biol. 2015, 38, 319–331. [Google Scholar] [CrossRef]
- Schofield, O.; Miles, T.; Alderkamp, A.-C.; Lee, S.; Haskins, C.; Rogalsky, E.; Sipler, R.; Sherrell, R.M.; Yager, P.L. In situ phytoplankton distributions in the Amundsen Sea Polynya measured by autonomous gliders. Elementa-Sci. Anthrop. 2015, 3, 1. [Google Scholar] [CrossRef]
- Wang, B.; Chen, M.; Chen, F.; Jia, R.; Li, X.; Zheng, M.; Qiu, Y. Meteoric water promotes phytoplankton carbon fixation and iron uptake off the eastern tip of the Antarctic Peninsula (eAP). Prog. Oceanogr. 2020, 185, 102347. [Google Scholar] [CrossRef]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis; Verlag Chemie, GmbH: Weinheim, Germany, 1983. [Google Scholar]
- Strickland, J.D.; Parsons, T.R. A Practical Handbook of Seawater Analysis; Fisheries Reasearch Board of Canada Bulletin, Queen’s Printer: Ottawa, ON, Canada, 1972. [Google Scholar]
- Gall, M.P.; Strzepek, R.; Maldonado, M.; Boyd, P.W. Phytoplankton processes. Part 2: Rates of primary production and factors controlling algal growth during the Southern Ocean Iron RElease Experiment (SOIREE). Deep Sea Res. Part II 2001, 48, 2571–2590. [Google Scholar] [CrossRef]
- Vernet, M.; Martinson, D.; Iannuzzi, R.; Stammerjohn, S.; Kozlowski, W.; Sines, K.; Smith, R.; Garibotti, I. Primary production within the sea-ice zone west of the Antarctic Peninsula: I—Sea ice, summer mixed layer, and irradiance. Deep Sea Res. Part II 2008, 55, 2068–2085. [Google Scholar] [CrossRef]
- Wang, B.; Chen, M.; Zheng, M.; Qiu, Y. The biological uptake of dissolved iron in the changing Daya Bay, South China Sea: Effect of pH and DO. Mar. Pollut. Bull. 2022, 178, 113635. [Google Scholar] [CrossRef] [PubMed]
- Welschmeyer, N.A.; Lorenzen, C.J. Carbon-14 labeling of phytoplankton carbon and chlorophyll a carbon: Determination of specific growth rates1. Limnol. Oceanogr. 1984, 29, 135–145. [Google Scholar] [CrossRef]
- Price, N.M.; Harrisona, G.I.; Heringa, J.G.; Hudsona, R.J.; Nirela, P.M.V.; Palenika, B.; Morela, F.M.M. Preparation and Chemistry of the Artificial Algal Culture Medium Aquil. Biol. Oceanogr. 1989, 6, 443–461. [Google Scholar] [CrossRef]
- Chen, M.; Wang, W.-X.; Guo, L. Phase partitioning and solubility of iron in natural seawater controlled by dissolved organic matter. Glob. Biogeochem. Cycles 2004, 18, GB4013. [Google Scholar] [CrossRef]
- Tovar-Sanchez, A.; Sañudo-Wilhelmy, S.A.; Garcia-Vargas, M.; Weaver, R.S.; Popels, L.C.; Hutchins, D.A. A trace metal clean reagent to remove surface-bound iron from marine phytoplankton. Mar. Chem. 2003, 82, 91–99. [Google Scholar] [CrossRef]
- Tagliabue, A.; Arrigo, K.R. Processes governing the supply of iron to phytoplankton in stratified seas. J. Geophys. Res.-Oceans 2006, 111, C06019. [Google Scholar] [CrossRef]
- Hewes, C.D.; Reiss, C.S.; Kahru, M.; Mitchell, B.G.; Holm-Hansen, O. Control of phytoplankton biomass by dilution and mixed layer depth in the western Weddell-Scotia Confluence. Mar. Ecol. Prog. Ser. 2008, 366, 15–29. [Google Scholar] [CrossRef]
- Carvalho, F.; Kohut, J.; Oliver, M.J.; Schofield, O. Defining the ecologically relevant mixed-layer depth for Antarctica’s coastal seas. Geophys. Res. Lett. 2017, 44, 338–345. [Google Scholar] [CrossRef]
- Höfer, J.; Giesecke, R.; Hopwood, M.J.; Carrera, V.; Alarcón, E.; González, H.E. The role of water column stability and wind mixing in the production/export dynamics of two bays in the Western Antarctic Peninsula. Prog. Oceanogr. 2019, 174, 105–116. [Google Scholar] [CrossRef]
- de Boyer Montégut, C.; Madec, G.; Fischer, A.S.; Lazar, A.; Iudicone, D. Mixed layer depth over the global ocean: An examination of profile data and a profile-based climatology. J. Geophys. Res.-Oceans 2004, 109, C12003. [Google Scholar] [CrossRef]
- Toyoda, T.; Fujii, Y.; Kuragano, T.; Kamachi, M.; Ishikawa, Y.; Masuda, S.; Sato, K.; Awaji, T.; Hernandez, F.; Ferry, N.; et al. Intercomparison and validation of the mixed layer depth fields of global ocean syntheses. Clim. Dynam. 2017, 49, 753–773. [Google Scholar] [CrossRef]
- Li, Q.; Chen, M.; Jia, R.; Zeng, J.; Lin, H.; Zheng, M.; Qiu, Y. Transit time of river water in the Bering and Chukchi Seas estimated from δ18O and radium isotopes. Prog. Oceanogr. 2017, 159, 115–129. [Google Scholar] [CrossRef]
- Meredith, M.P.; Wallace, M.I.; Stammerjohn, S.E.; Renfrew, I.A.; Clarke, A.; Venables, H.J.; Shoosmith, D.R.; Souster, T.; Leng, M.J. Changes in the freshwater composition of the upper ocean west of the Antarctic Peninsula during the first decade of the 21st century. Prog. Oceanogr. 2010, 87, 127–143. [Google Scholar] [CrossRef]
- Meredith, M.P.; Stammerjohn, S.E.; Venables, H.J.; Ducklow, H.W.; Martinson, D.G.; Iannuzzi, R.A.; Leng, M.J.; van Wessem, J.M.; Reijmer, C.H.; Barrand, N.E. Changing distributions of sea ice melt and meteoric water west of the Antarctic Peninsula. Deep Sea Res. Part II 2017, 139, 40–57. [Google Scholar] [CrossRef]
- Biddle, L.C.; Loose, B.; Heywood, K.J. Upper ocean distribution of glacial meltwater in the Amundsen Sea, Antarctica. J. Geophys. Res.-Oceans 2019, 124, 6854–6870. [Google Scholar] [CrossRef]
- Randall-Goodwin, E.; Meredith, M.P.; Jenkins, A.; Yager, P.L.; Sherrell, R.M.; Abrahamsen, E.P.; Guerrero, R.; Yuan, X.; Mortlock, R.A.; Gavahan, K.; et al. Freshwater distributions and water mass structure in the Amundsen Sea Polynya region, Antarctica. Elementa-Sci. Anthrop. 2015, 3, 000065. [Google Scholar] [CrossRef]
- Price, M.R.; Heywood, K.J.; Nicholls, K.W. Ice-shelf—Ocean interactions at Fimbul Ice Shelf, Antarctica from oxygen isotope ratio measurements. Ocean Sci. 2008, 4, 89–98. [Google Scholar] [CrossRef]
- Potter, J.R.; Paren, J.G. Interaction between Ice Shelf and Ocean in George VI Sound, Antarctica. In Oceanology of the Antarctic Continental Shelf; American Geophysical Union: Washington, DC, USA, 1985; pp. 35–58. [Google Scholar]
- Hassler, C.S.; Schoemann, V.; Nichols, C.M.; Butler, E.C.V.; Boyd, P.W. Saccharides enhance iron bioavailability to Southern Ocean phytoplankton. Proc. Natl. Acad. Sci. USA 2011, 108, 1076–1081. [Google Scholar] [CrossRef]
- Maldonado, M.T.; Strzepek, R.F.; Sander, S.; Boyd, P.W. Acquisition of iron bound to strong organic complexes, with different Fe binding groups and photochemical reactivities, by plankton communities in Fe-limited subantarctic waters. Glob. Biogeochem. Cycles 2005, 19, GB4S23. [Google Scholar] [CrossRef]
- Francois, R.; Altabet, M.A.; Yu, E.-F.; Sigman, D.M.; Bacon, M.P.; Frank, M.; Bohrmann, G.; Bareille, G.; Labeyrie, L.D. Contribution of Southern Ocean surface-water stratification to low atmospheric CO2 concentrations during the last glacial period. Nature 1997, 389, 929. [Google Scholar] [CrossRef]
- de Jong, J.; Schoemann, V.; Maricq, N.; Mattielli, N.; Langhorne, P.; Haskell, T.; Tison, J.-L. Iron in land-fast sea ice of McMurdo Sound derived from sediment resuspension and wind-blown dust attributes to primary productivity in the Ross Sea, Antarctica. Mar. Chem. 2013, 157, 24–40. [Google Scholar] [CrossRef]
- Venables, H.J.; Clarke, A.; Meredith, M.P. Wintertime controls on summer stratification and productivity at the western Antarctic Peninsula. Limnol. Oceanogr. 2013, 58, 1035–1047. [Google Scholar] [CrossRef]
- Rozema, P.D.; Venables, H.J.; van de Poll, W.H.; Clarke, A.; Meredith, M.P.; Buma, A.G.J. Interannual variability in phytoplankton biomass and species composition in northern Marguerite Bay (West Antarctic Peninsula) is governed by both winter sea ice cover and summer stratification. Limnol. Oceanogr. 2017, 62, 235–252. [Google Scholar] [CrossRef]
- De Jong, J.T.M.; Stammerjohn, S.E.; Ackley, S.F.; Tison, J.L.; Mattielli, N.; Schoemann, V. Sources and fluxes of dissolved iron in the Bellingshausen Sea (West Antarctica): The importance of sea ice, icebergs and the continental margin. Mar. Chem. 2015, 177, 518–535. [Google Scholar] [CrossRef]
- Sedwick, P.N.; DiTullio, G.R. Regulation of algal blooms in Antarctic Shelf Waters by the release of iron from melting sea ice. Geophys. Res. Lett. 1997, 24, 2515–2518. [Google Scholar] [CrossRef]
- Eveleth, R.; Cassar, N.; Sherrell, R.M.; Ducklowd, H.; Meredith, M.P.; Venables, H.J.; Lin, Y.; Li, Z. Ice melt influence on summertime net community production along the Western Antarctic Peninsula. Deep Sea Res. Part II 2017, 139, 89–102. [Google Scholar] [CrossRef]
- Wang, S.; Bailey, D.; Lindsay, K.; Moore, J.K.; Holland, M. Impact of sea ice on the marine iron cycle and phytoplankton productivity. Biogeosciences 2014, 11, 4713–4731. [Google Scholar] [CrossRef]
- Lannuzel, D.; Grotti, M.; Abelmoschi, M.L.; van der Merwe, P. Organic ligands control the concentrations of dissolved iron in Antarctic sea ice. Mar. Chem. 2015, 174, 120–130. [Google Scholar] [CrossRef]
- Lizotte, M.P. The Contributions of Sea Ice Algae to Antarctic Marine Primary Production. BioOne Am. Zool. 2001, 41, 57–73. [Google Scholar] [CrossRef]
- Gradinger, R. Sea-ice algae: Major contributors to primary production and algal biomass in the Chukchi and Beaufort Seas during May/June 2002. Deep Sea Res. Part II 2009, 56, 1201–1212. [Google Scholar] [CrossRef]
- Annett, A.L.; Fitzsimmons, J.N.; Séguret, M.J.M.; Lagerström, M.; Meredith, M.P.; Schofield, O.; Sherrell, R.M. Controls on dissolved and particulate iron distributions in surface waters of the Western Antarctic Peninsula shelf. Mar. Chem. 2017, 196, 81–97. [Google Scholar] [CrossRef]
- St-Laurent, P.; Yager, P.L.; Sherrell, R.M.; Stammerjohn, S.E.; Dinniman, M.S. Pathways and supply of dissolved iron in the Amundsen Sea (Antarctica). J. Geophys. Res.-Oceans 2017, 122, 7135–7162. [Google Scholar] [CrossRef]
- Wang, B.; Chen, M.; Zheng, M.; Qiu, Y. Responses of two coastal algae (Skeletonema costatum and Chlorella vulgaris) to changes in light and iron levels. J. Phycol. 2020, 56, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Ardelan, M.V.; Holm-Hansen, O.; Hewes, C.D.; Reiss, C.S.; Silva, N.S.; Dulaiova, H.; Steinnes, E.; Sakshaug, E. Natural iron enrichment around the Antarctic Peninsula in the Southern Ocean. Biogeosciences 2010, 7, 11–25. [Google Scholar] [CrossRef]
- Annett, A.L.; Skiba, M.; Henley, S.F.; Venables, H.J.; Meredith, M.P.; Statham, P.J.; Ganeshram, R.S. Comparative roles of upwelling and glacial iron sources in Ryder Bay, coastal western Antarctic Peninsula. Mar. Chem. 2015, 176, 21–33. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Hutchins, D.A. Size-fractionated biological iron and carbon uptake along a coastal to offshore transect in the NE Pacific. Deep Sea Res. Part II 1999, 46, 2487–2503. [Google Scholar] [CrossRef]
- Strzepek, R.F.; Hunter, K.A.; Frew, R.D.; Harrison, P.J.; Boyd, P.W. Iron-light interactions differ in Southern Ocean phytoplankton. Limnol. Oceanogr. 2012, 57, 1182–1200. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Iron uptake and growth limitation in oceanic and coastal phytoplankton. Mar. Chem. 1995, 50, 189–206. [Google Scholar] [CrossRef]
- Middlemiss, J.K.; Anderson, A.M.; Stratilo, C.W.; Weger, H.G. Oxygen Consumption Associated with Ferric Reductase Activity and Iron Uptake by Iron-Limited Cells of Chlorella kessleri (Chlorophyceae). J. Phycol. 2001, 37, 393–399. [Google Scholar] [CrossRef]
- Kustka, A.B.; Allen, A.E.; Morel, F.M.M. Sequence Analysis and Transcriptional Regulation of Iron Acquisition Genes in Two Marine Diatoms1. J. Phycol. 2007, 43, 715–729. [Google Scholar] [CrossRef]
- Boyd, P.W.; Watson, A.J.; Law, C.S.; Abraham, E.R.; Trull, T.; Murdoch, R.; Bakker, D.C.E.; Bowie, A.R.; Buesseler, K.O.; Chang, H.; et al. A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization. Nature 2000, 407, 695–702. [Google Scholar] [CrossRef] [PubMed]
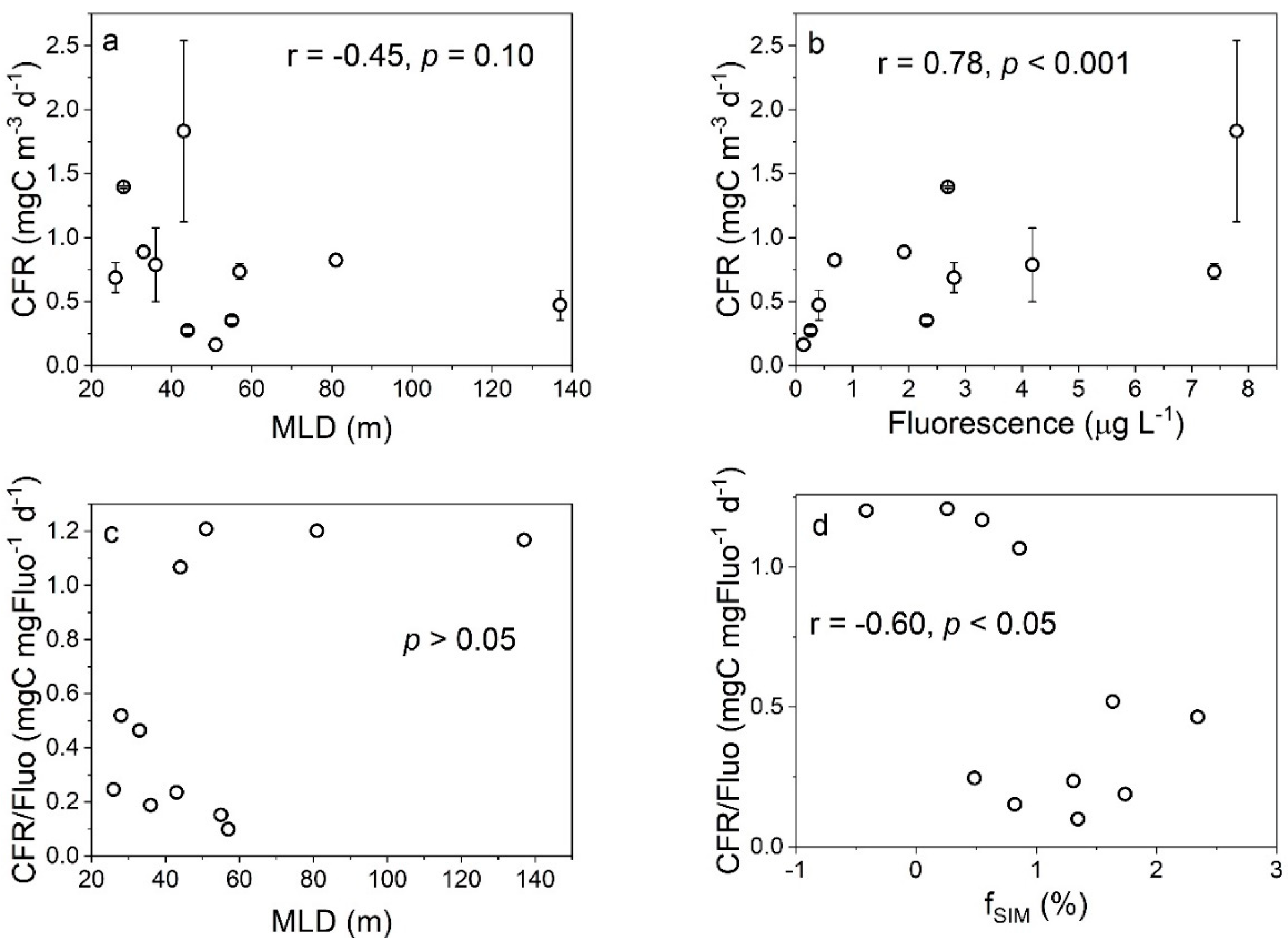
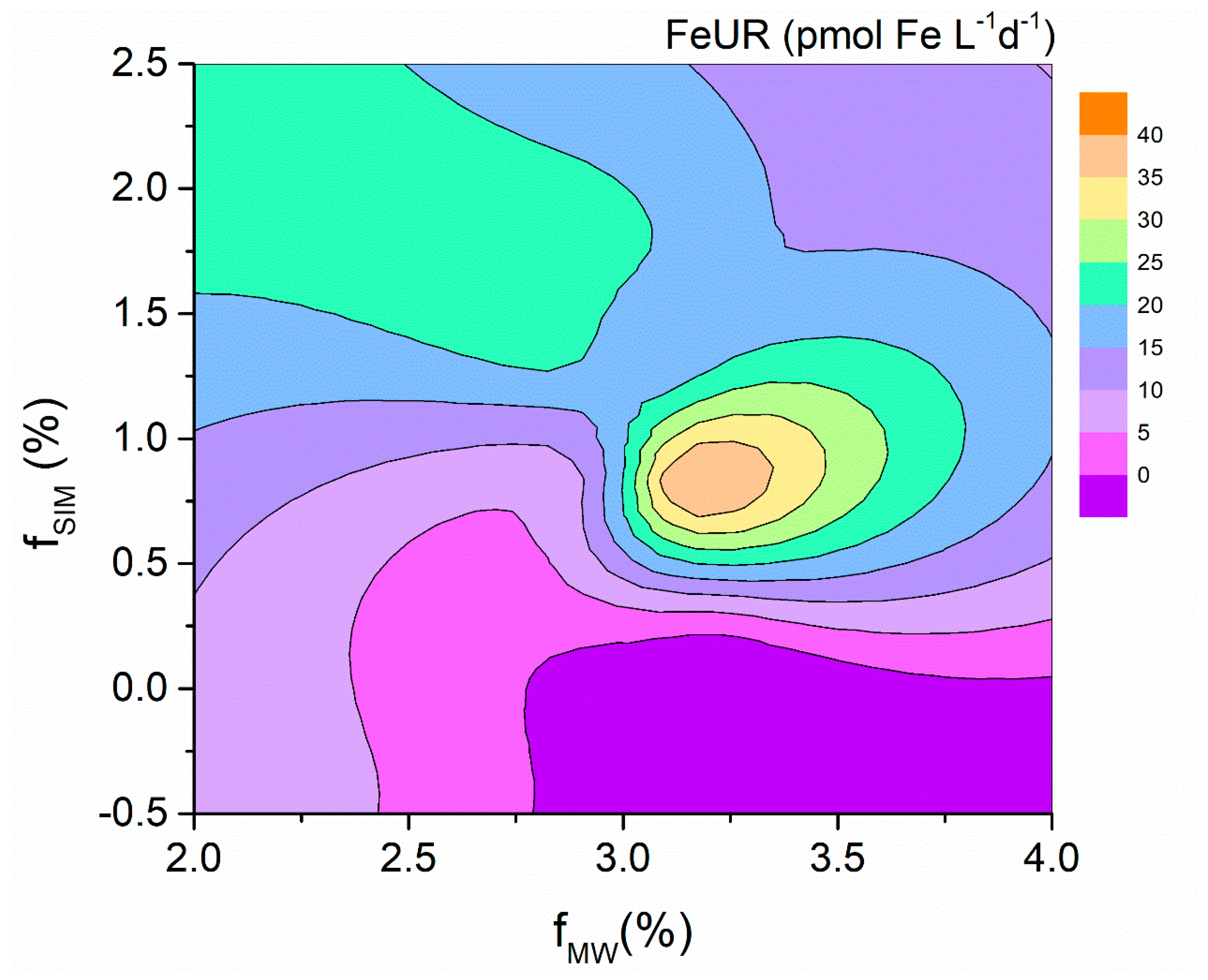
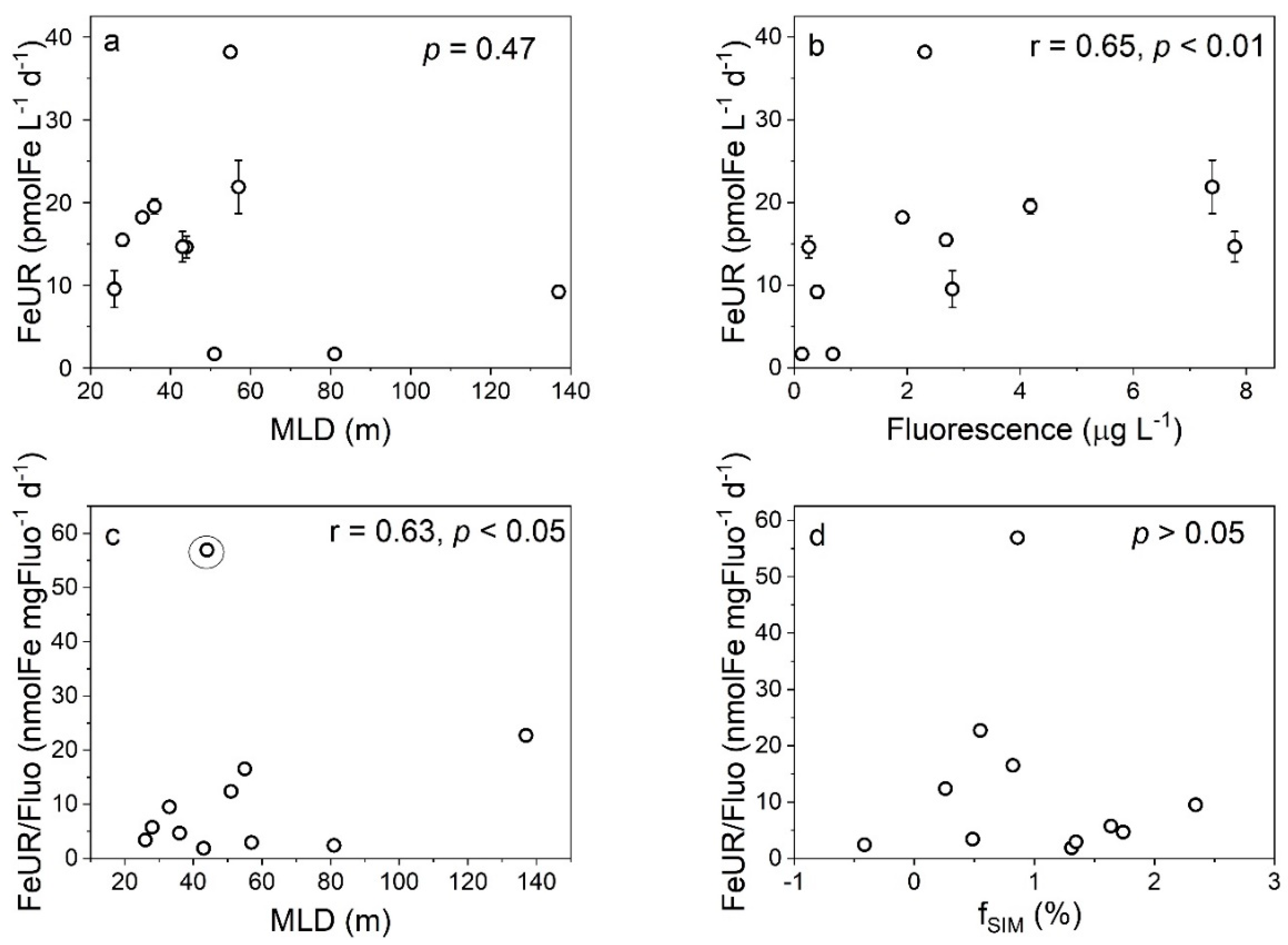
| Station | Depth (m) | CFR (mgC m−3 d−1) | Micro-CFR (mgC m−3 d−1) | Nano-CFR (mgC m−3 d−1) | Pico-CFR (mgC m−3 d−1) | FeUR (pmolFe L−1 d−1) | Micro-FeUR (pmolFe L−1 d−1) | Nano-FeUR (pmolFe L−1 d−1) | Pico-FeUR (pmolFe L−1 d−1) |
|---|---|---|---|---|---|---|---|---|---|
| AD-02 | 0 | 0.27 ± 0.03 | 0.07 ± 0.00 | 0.20 ± 0.03 * | 14.59 ± 1.32 | 3.20 ± 1.08 | 11.40 ± 0.24 * | ||
| A1-02 | 0 | 1.83 ± 0.71 | 1.20 ± 0.71 | 0.57 ± 0.00 | 0.06 ± 0.00 | 14.65 ± 1.84 | 3.87 ± 1.93 | 5.27 ± 0.09 | 5.51 ± 0.00 |
| 25 | 1.34 ± 0.36 | 0.77 ± 0.20 | 0.30 ± 0.30 | 0.28 ± 0.16 | 17.58 ± 1.50 | 6.35 ± 1.39 | 4.17 ± 0.26 | 7.06 ± 0.37 | |
| A1-03 | 0 | 1.40 ± 0.01 | 0.29 ± 0.03 | 1.11 ± 0.04 * | 15.47 ± 0.78 | 5.83 ± 3.07 | 9.64 ± 3.85 * | ||
| A1-04 | 0 | 0.89 ± 0.00 | 0.08 ± 0.00 | 0.81 ± 0.00 * | 18.19 ± 0.00 | 1.90 ± 0.00 | 16.29 ± 0.00 * | ||
| 25 | 0.60 ± 0.00 | 0.11 ± 0.00 | 0.49 ± 0.00 * | 20.29 ± 0.00 | 4.41 ± 0.00 | 15.88 ± 0.00 * | |||
| A2-02 | 0 | 0.69 ± 0.12 | 0.31 ± 0.13 | 0.34 ± 0.01 | 0.04 ± 0.00 | 9.53 ± 2.21 | 2.61 ± 1.29 | 3.24 ± 2.86 | 3.68 ± 1.94 |
| 50 | 0.41 ± 0.18 | 0.27 ±0.03 | 0.14 ± 0.01 * | 5.55 ± 0.18 | 1.11 ± 0.18 | 1.44 ± 0.08 | 3.00 ± 0.08 | ||
| A2-04 | 0 | 0.20 ± 0.29 | nd | nd | nd | 19.53 ± 0.92 | 5.15 ± 0.78 | 10.47 ± 0.61 | 3.91 ± 0.46 |
| A2-08 | 0 | 0.16 ± 0.00 | 0.11 ± 0.00 | 0.04 ± 0.00 | 0.02 ± 0.00 | 1.68 ± 0.00 | 0.55 ± 0.00 | 0.32 ± 0.00 | 0.81 ± 0.00 |
| 40 | 0.34 ± 0.00 | 0.09 ± 0.00 | 0.18 ± 0.00 | 0.06 ± 0.00 | 4.58 ± 0.00 | 1.99 ± 0.00 | 1.26 ± 0.00 | 1.32 ± 0.00 | |
| A2-09 | 0 | 0.35 ± 0.03 | 0.26 ± 0.02 | 0.05 ± 0.01 | 0.05 ± 0.00 | 38.19 ± 0.00 | 22.83 ± 0.00 | 9.76 ± 0.00 | 5.60 ± 0.00 |
| A2-11 | 0 | 0.74 ± 0.06 | 0.56 ± 0.07 | 0.07 ± 0.00 | 0.11 ± 0.01 | 21.88 ± 3.23 | 12.17 ± 2.25 | 1.99 ± 1.21 | 7.73 ± 0.23 |
| A2-15 | 0 | 0.82 ± 0.00 | 0.13 ± 0.00 | 0.15 ± 0.00 | 0.55 ±0.00 | 1.66 ± 0.63 | 1.66 ± 0.63 | nd | nd |
| 25 | 0.29 ± 0.00 | 0.00 ± 0.00 | 0.08 ± 0.00 | 0.20 ± 0.00 | 5.63 ± 3.25 | 0.52 ± 0.26 | 2.87 ± 1.89 | 2.23 ± 1.10 | |
| A2-17 | 0 | 0.36 ± 0.04 | 0.05 ± 0.00 | 0.10 ± 0.00 | 0.21 ± 0.04 | 9.19 ± 0.79 | 1.72 ± 0.72 | 2.72 ± 1.00 | 4.76 ± 1.08 |
| 75 | 0.29 ± 0.02 | 0.04 ± 0.00 | 0.10 ± 0.04 | 0.15 ± 0.06 | nd | nd | nd | nd | |
| Depth (m) | Fe/C Ratio (μmol/mol) | ||||||
|---|---|---|---|---|---|---|---|
| Station | Ambient | Fe Addition | |||||
| Total | Micro- | Nano-+Pico- | Total | Micro- | Nano-+Pico- | ||
| AD-02 | 0 | 640 | 552 | 670 | 1783 | 784 | 2534 |
| A1-02 | 0 | 96 | 39 | 204 | 1972 | 249 | n.d. |
| 25 | 171 | 99 | 234 | 972 | 2595 | 679 | |
| A1-03 | 0 | 133 | 242 | 105 | 151 | 245 | 126 |
| A1-04 | 0 | 245 | 272 | 243 | 154 | 66 | 172 |
| 25 | 403 | 464 | 389 | 116 | 34 | 195 | |
| A2-02 | 0 | 166 | 101 | 221 | 76 | 96 | 215 |
| 50 | 129 | 49 | 376 | 116 | 43 | 270 | |
| A2-04 | 0 | 297 | n.d. | n.d. | 322 | n.d. | n.d. |
| A2-08 | 0 | 123 | 61 | 239 | 139 | 149 | 131 |
| 40 | 164 | 253 | 129 | 262 | 318 | 236 | |
| A2-09 | 0 | 1301 | 1056 | 1982 | 1602 | 1754 | 1318 |
| A2-11 | 0 | 357 | 262 | 653 | 662 | 437 | 391 |
| A2-15 | 0 | 24 | 151 | nd | 14 | 22 | nd |
| 25 | 195 | 19,387 | 212 | nd | nd | nd | |
| A2-17 | 0 | 233 | 384 | 289 | nd | nd | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Fan, L.; Zheng, M.; Qiu, Y.; Chen, M. Carbon and Iron Uptake by Phytoplankton in the Amundsen Sea, Antarctica. Biology 2022, 11, 1760. https://doi.org/10.3390/biology11121760
Wang B, Fan L, Zheng M, Qiu Y, Chen M. Carbon and Iron Uptake by Phytoplankton in the Amundsen Sea, Antarctica. Biology. 2022; 11(12):1760. https://doi.org/10.3390/biology11121760
Chicago/Turabian StyleWang, Bo, Lingfang Fan, Minfang Zheng, Yusheng Qiu, and Min Chen. 2022. "Carbon and Iron Uptake by Phytoplankton in the Amundsen Sea, Antarctica" Biology 11, no. 12: 1760. https://doi.org/10.3390/biology11121760
APA StyleWang, B., Fan, L., Zheng, M., Qiu, Y., & Chen, M. (2022). Carbon and Iron Uptake by Phytoplankton in the Amundsen Sea, Antarctica. Biology, 11(12), 1760. https://doi.org/10.3390/biology11121760






