Nitrogen and Carbon Mineralization from Green and Senesced Leaf Litter Differ between Cycad and Angiosperm Trees
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
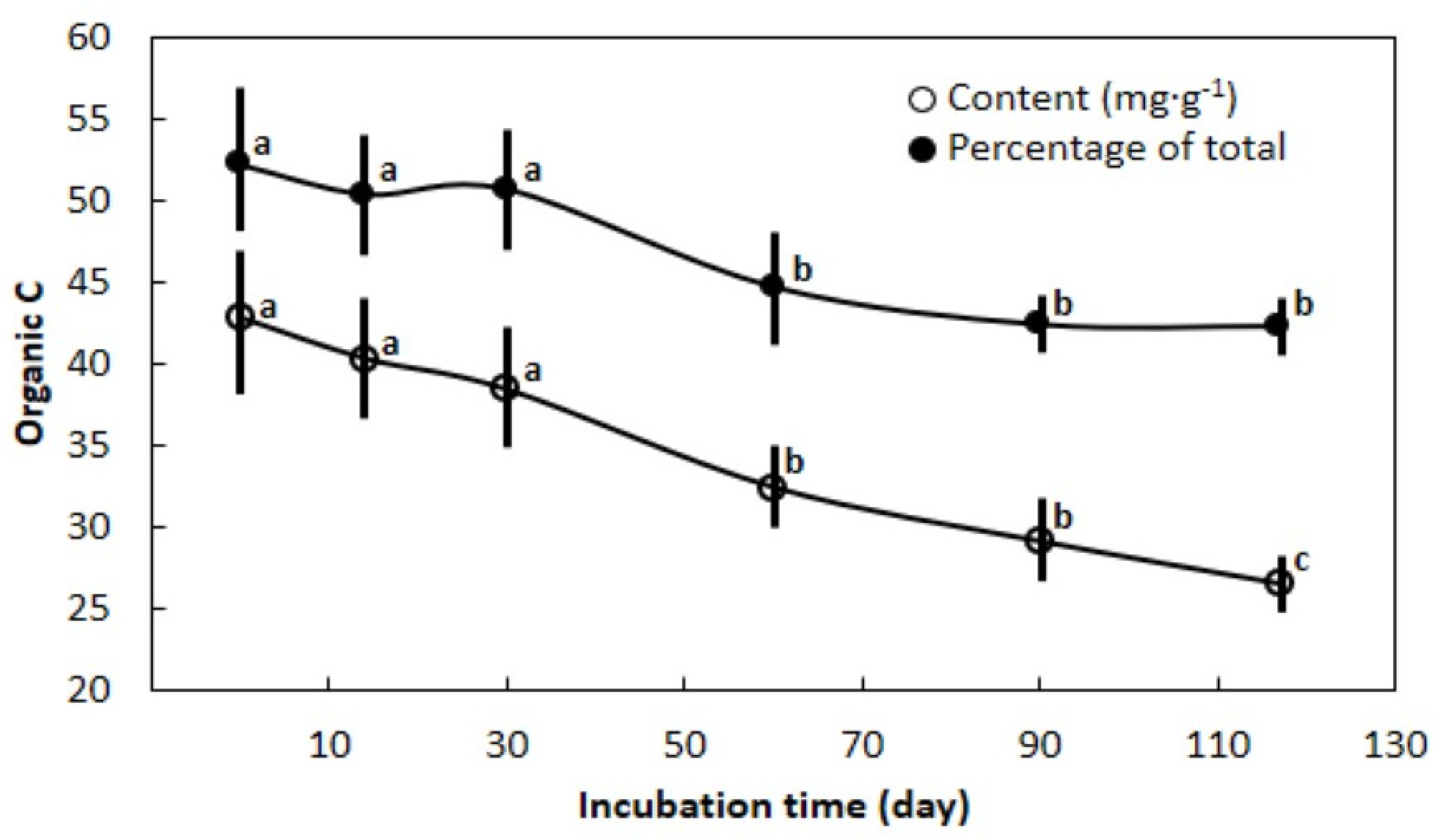
3.1. Carbon Changes with Duration of Incubation
3.2. Nitrogen Changes with Duration of Incubation
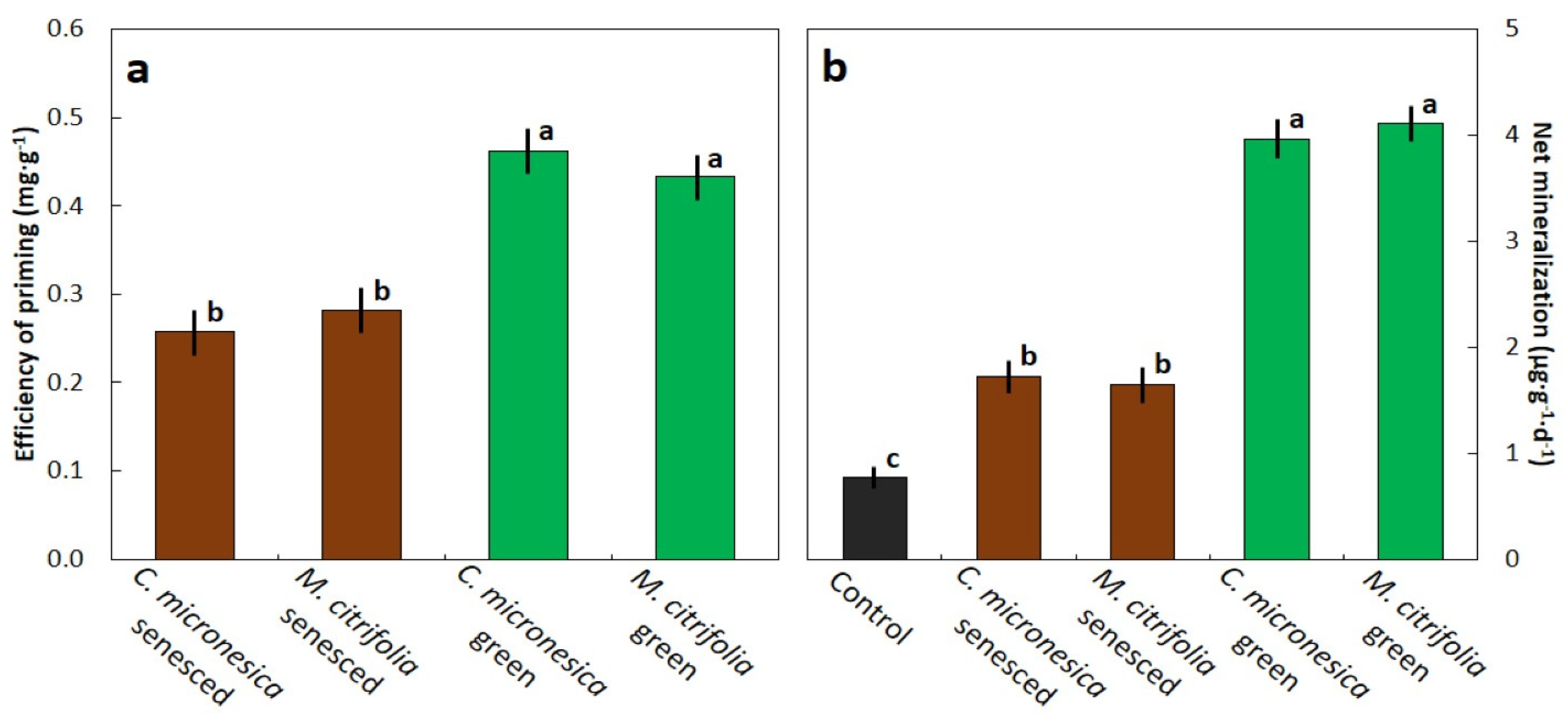
3.3. Soil Priming Effect and Net N Mineralization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aerts, R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: A triangular relationship. Oikos 1997, 79, 439–449. [Google Scholar] [CrossRef]
- Peng, Y.; Holmstrup, M.; Schmidt, I.K.; Bachega, L.R.; Schelfhout, S.; Zheng, H.; Heděnec, P.; Yue, K.; Vesterdal, L. Tree species identity is the predominant modulator of the effects of soil fauna on leaf litter decomposition. Forest Ecol. Manag. 2022, 520, 120396. [Google Scholar] [CrossRef]
- Marler, T.E. Pacific island tropical cyclones are more frequent and globally relevant, yet less studied. Front. Environ. Sci. 2014, 2, 42. [Google Scholar] [CrossRef]
- Wang, H.-C.; Wang, S.-F.; Lin, K.-C.; Lee Shaner, P.-J.; Lin, T.-C. Litterfall and element fluxes in a natural hardwood forest and a Chinese-fir plantation experiencing frequent typhoon disturbance in central Taiwan. Biotropica 2013, 45, 541–548. [Google Scholar] [CrossRef]
- Jaramillo, V.J.; Martínez-Yrízar, A.; Machado, L.I. Hurricane-induced massive nutrient return via tropical dry forest litterfall: Has forest biogeochemistry resilience changed? Ecosystems 2022, 25. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Bernard, L.; Basile-Doelsch, I.; Derrien, D.; Fanin, N.; Fontaine, S.; Guenet, B.; Karimi, B.; Marsden, C.; Maron, P.-A. Advancing the mechanistic understanding of the priming effect on soil organic matter mineralisation. Funct. Ecol. 2022, 36, 1355–1377. [Google Scholar] [CrossRef]
- Mo, F.; Ren, C.; Yu, K.; Zhou, Z.; Phillips, R.P.; Luo, Z.; Zhang, Y.; Dang, Y.; Han, J.; Ye, J.-S.; et al. Global pattern of soil priming effect intensity and its environmental drivers. Ecology 2022, 103, e3790. [Google Scholar] [CrossRef]
- Ren, C.; Mo, F.; Zhou, Z.; Bastida, F.; Delgado-Baquerizo, M.; Wang, J.; Zhang, X.; Luo, Y.; Griffis, T.J.; Han, X.; et al. The global biogeography of soil priming effect intensity. Glob. Ecol. Biogeogr. 2022, 31, 1679–1687. [Google Scholar] [CrossRef]
- Huys, R.; Poirier, V.; Bourget, M.Y.; Roumet, C.; Hättenschwiler, S.; Fromin, N.; Munson, A.D.; Freschet, G.T. Plant litter chemistry controls coarse-textured soil carbon dynamics. J. Ecol. 2022, in press. [Google Scholar] [CrossRef]
- Heimann, M.; Reichstein, M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 2008, 451, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E. Leaf elemental concentrations, stoichiometry, and resorption in Guam’s coastal karst forests. Diversity 2021, 13, 545. [Google Scholar] [CrossRef]
- Young, F.J. Soil Survey of Territory of Guam; U. S. Dept. of Agric. Soil Conservation Service: Washington, DC, USA, 1988. [Google Scholar]
- Eno, C.F. Nitrate production in the field by incubating the soil in polyethylene bags. Proc. Soil Sci. Soc. Am. 1960, 24, 277–279. [Google Scholar] [CrossRef]
- Palozzi, J.E.; Lindo, Z. Are leaf litter and microbes team players? Interpreting home-field advantage decomposition dynamics. Soil Biol. Biochem. 2018, 124, 189–198. [Google Scholar] [CrossRef]
- Marler, T.E. Perennial trees associating with nitrogen-fixing symbionts differ in leaf after-life nitrogen and carbon release. Nitrogen 2020, 1, 111–124. [Google Scholar] [CrossRef]
- Dumas, J.B.A. Procedes de L’analyse Organique. Ann. Chim. Phys. 1831, 47, 198–205. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 3. Chemical Methods; SSSA Book Series No. 5; Sparks, D.L., Ed.; SSSA and ASA: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Cataldo, D.A.; Haroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Berghage, R.D.; Krauskopf, D.M.; Warncke, D.D.; Widders, I. Micronutrient testing of plant growth media extractant, identification and evaluation. Commun. Soil Sci. Plant Anal. 1987, 18, 1089–1109. [Google Scholar] [CrossRef]
- Olsen, S.; Cole, C.; Watanabe, F.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA Circular Nr 939, US Gov. Print Office: Washington DC, USA, 1954. [Google Scholar]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Hengl, T.; de Jesus, J.M.; Heuvelink, G.B.M.; Gonzalez, M.R.; Kilibarda, M.; Blagotić, A.; Shangguan, W.; Wright, M.N.; Geng, X.; Bauer-Marschallinger, B.; et al. SoilGrids250m: Global gridded soil information based on machine learning. PLoS ONE 2017, 12, e0169748. [Google Scholar] [CrossRef]
- Chapin, I.F.S.; Matson, P.A.; Vitousek, P. Principles of Terrestrial Ecosystem Ecology; Springer Science & Business Media: Berlin, Germany, 2011. [Google Scholar]
- Rumpel, C.; Amiraslani, F.; Koutika, L.-S.; Smith, P.; Whitehead, D.; Wollenberg, E. Put more carbon in soils to meet Paris climate pledges. Nature 2018, 564, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Cassart, B.; Basia, A.A.; Jonard, M.; Ponette, Q. Average leaf litter quality drives the decomposition of single-species, mixed-species and transplanted leaf litters for two contrasting tropical forest types in the Congo Basin (DRC). Ann. For. Sci. 2020, 77, 33. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Song, Q.; Compson, Z.G.; LeRoy, C.J.; Luan, F.; Wang, H.; Hu, Y.; Yang, Q. Synergistic effects: A common theme in mixed-species litter decomposition. New Phytol. 2020, 227, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Butenschoen, O.; Barantal, S.; Handa, I.T.; Makkonen, M.; Vos, V.; Aerts, R.; Berg, M.P.; McKie, B.; Van Ruijven, J.; et al. Decomposition of leaf litter mixtures across biomes: The role of litter identity, diversity and soil fauna. J. Ecol. 2020, 108, 2283–2297. [Google Scholar] [CrossRef]
- Hill, P.W.; Quilliam, R.S.; DeLuca, T.H.; Farrar, J.; Farrell, M.; Roberts, P.; Newsham, K.K.; Hopkins, D.W.; Bardgett, R.D.; Jones, D.L. Acquisition and assimilation of nitrogen as peptide-bound and D-enantiomers of amino acids by wheat. PLoS ONE 2011, 6, e19220. [Google Scholar] [CrossRef]
- Vranova, V.; Zahradnickova, H.; Janous, D.; Skene, K.R.; Matharu, A.S.; Rejsek, K.; Formanek, P. The significance of D-amino acids in soil, fate and utilization by microbes and plants: Review and identification of knowledge gaps. Plant Soil 2012, 354, 21–39. [Google Scholar] [CrossRef]
- Hawkins, H.J.; Johansen, A.; George, E. Uptake and transport of organic and inorganic nitrogen by arbuscular mycorrhizal fungi. Plant Soil 2000, 226, 275–285. [Google Scholar] [CrossRef]
- Leigh, J.; Hodge, A.; Fitter, A.H. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol. 2009, 181, 199–207. [Google Scholar] [CrossRef]
- Talbot, J.M.; Treseder, K.K. Controls over mycorrhizal uptake of organic nitrogen. Pedobiologia 2010, 53, 169–179. [Google Scholar] [CrossRef]
- Miransari, M. Arbuscular mycorrhizal fungi and nitrogen uptake. Arch. Microbiol. 2011, 193, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.W.; Broughton, R.; Bougoure, J.; Havelange, W.; Newsham, K.K.; Grant, H.; Murphy, D.V.; Clode, P.; Ramayah, S.; Marsden, K.A.; et al. Angiosperm symbioses with non-mycorrhizal fungal partners enhance N acquisition from ancient organic matter in a warming maritime Antarctic. Ecol. Lett. 2019, 22, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.O.; Kim, H.J.; Gil Nam, H. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef]
- Diaz, C.; Lemaitre, T.; Christ, A.; Azzopardi, M.; Kato, Y.; Sato, F.; Morot-Gaudry, J.F.; Le Dily, F.; Masclaux-Daubresse, C. Nitrogen recycling and remobilization are differentially controlled by leaf senescence and development stage in Arabidopsis under low nitrogen nutrition. Plant Physiol. 2008, 147, 1437–1449. [Google Scholar] [CrossRef]
- Marler, T.E.; Krishnapillai, M.V. Longitude, forest fragmentation, and plant size influence Cycas micronesica mortality following island insect invasions. Diversity 2020, 12, 194. [Google Scholar] [CrossRef]
- Deloso, B.E.; Terry, L.I.; Yudin, L.S.; Marler, T.E. Biotic threats to Cycas micronesica continue to expand to complicate conservation decisions. Insects 2020, 11, 888. [Google Scholar] [CrossRef] [PubMed]
- Gawel, A.M.; Rogers, H.S.; Miller, R.H.; Kerr, A.M. Contrasting ecological roles of non-native ungulates in a novel ecosystem. R. Soc. Open Sci. 2018, 5, 170151. [Google Scholar] [CrossRef]
- Donnegan, J.A.; Butler, S.L.; Grabowiecki, W.; Hiserote, B.A.; Limtiaco, D. Guam’s Forest Resources, 2002; Resource Bulletin PNW-RB-243; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2004. [Google Scholar]
- Lazaro, M.; Kuegler, O.; Stanton, S.; Lehman, A.; Mafnas, J.; Yatskov, M. Guam’s Forest Resources: Forest Inventory and Analysis, 2013; Resour. Bull. PNW-RB-270; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2020. [Google Scholar]


| Response Variable | Soil Treatment f4,58 | Soil Treatment p | Time f5,58 | Time p | S × T f20,58 | S × T p |
|---|---|---|---|---|---|---|
| Total C | 3.5 | 0.049 | 1.8 | 0.122 | 1.2 | 0.332 |
| Organic C | 13.7 | <0.001 | 12.4 | <0.001 | 1.1 | 0.395 |
| Percent organic C | 7.8 | 0.007 | 5.1 | <0.001 | 1.6 | 0.092 |
| Response Variable | Soil Treatment f4,58 | Soil Treatment p | Time f5,58 | Time p | S × T f20,58 | S × T p |
|---|---|---|---|---|---|---|
| Total N | 3.7 | 0.054 | 1.6 | 0.169 | 2.3 | 0.010 |
| Ammonium N | 109.3 | <0.001 | 56.6 | <0.001 | 51.1 | <0.001 |
| Nitrate N | 151.5 | <0.001 | 203.4 | <0.001 | 22.3 | <0.001 |
| Available N | 269.6 | <0.001 | 202.0 | <0.001 | 105.7 | <0.001 |
| Percent available N | 68.1 | <0.001 | 111.0 | <0.001 | 13.0 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulino, C.A.; Marler, T.E. Nitrogen and Carbon Mineralization from Green and Senesced Leaf Litter Differ between Cycad and Angiosperm Trees. Biology 2022, 11, 1758. https://doi.org/10.3390/biology11121758
Paulino CA, Marler TE. Nitrogen and Carbon Mineralization from Green and Senesced Leaf Litter Differ between Cycad and Angiosperm Trees. Biology. 2022; 11(12):1758. https://doi.org/10.3390/biology11121758
Chicago/Turabian StylePaulino, Charles A., and Thomas E. Marler. 2022. "Nitrogen and Carbon Mineralization from Green and Senesced Leaf Litter Differ between Cycad and Angiosperm Trees" Biology 11, no. 12: 1758. https://doi.org/10.3390/biology11121758
APA StylePaulino, C. A., & Marler, T. E. (2022). Nitrogen and Carbon Mineralization from Green and Senesced Leaf Litter Differ between Cycad and Angiosperm Trees. Biology, 11(12), 1758. https://doi.org/10.3390/biology11121758







