Mycorrhizal Colonization Modulates the Essential Oil Profile and Enzymatic and Non-Enzymatic Antioxidants to Mitigate the Adverse Effects of Water Deficit in Salvia subg. Perovskia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design and Treatments
2.3. Measured Parameters
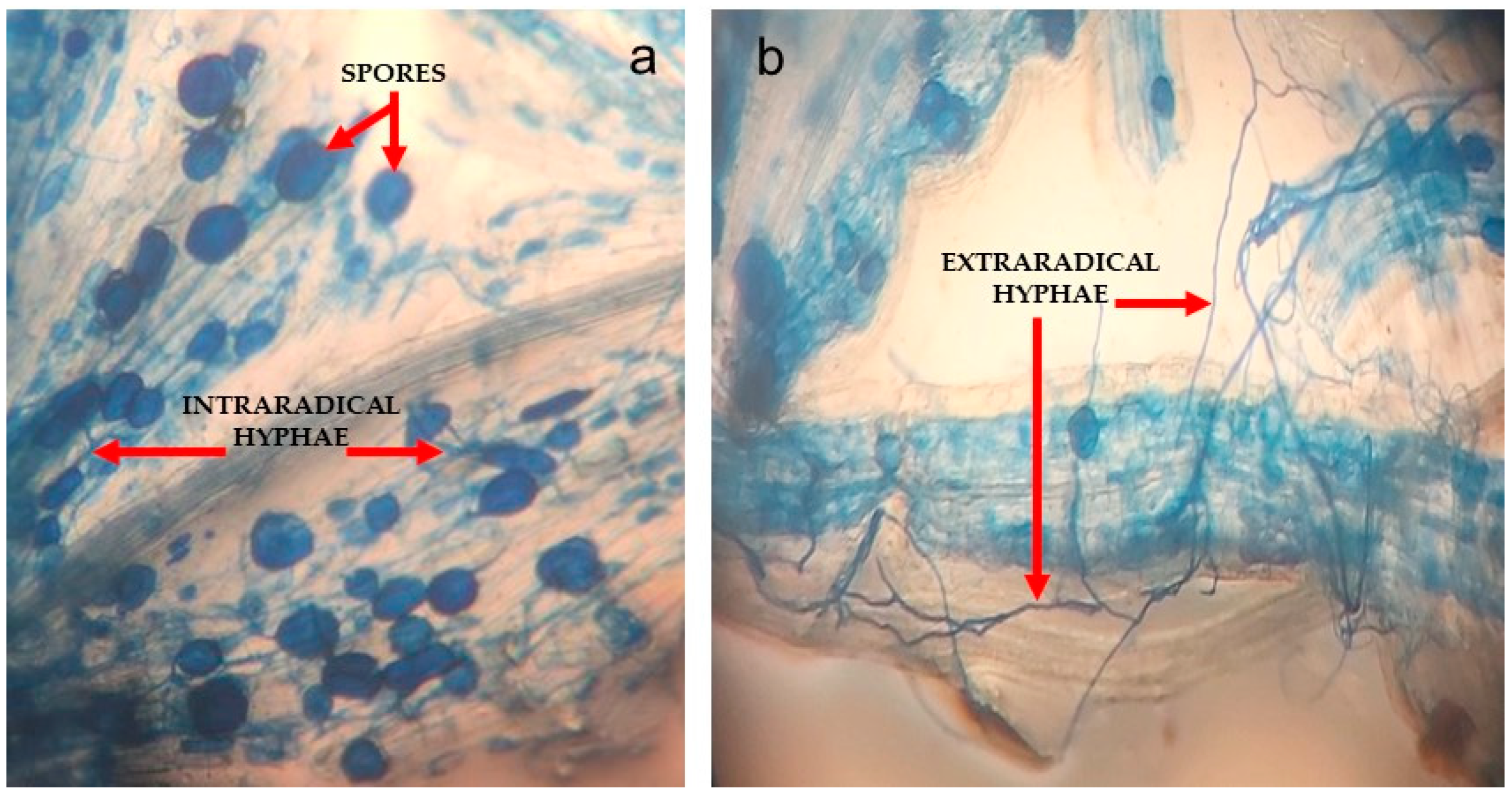
2.3.1. Percentage of Root Colonization by AMF
2.3.2. Determination of Total Phenolics and Flavonoids
2.3.3. Antioxidant Enzyme Activities
2.3.4. Leaf Phosphorus Concentration and Relative Water Content
2.3.5. Chlorophyll and Carotenoid Contents
2.3.6. Essential Oil Content
2.3.7. GC–FID and GC/MS Analyses
2.3.8. Essential Oil Components Identification
2.4. Data Analysis
3. Results
3.1. Analysis of Variance
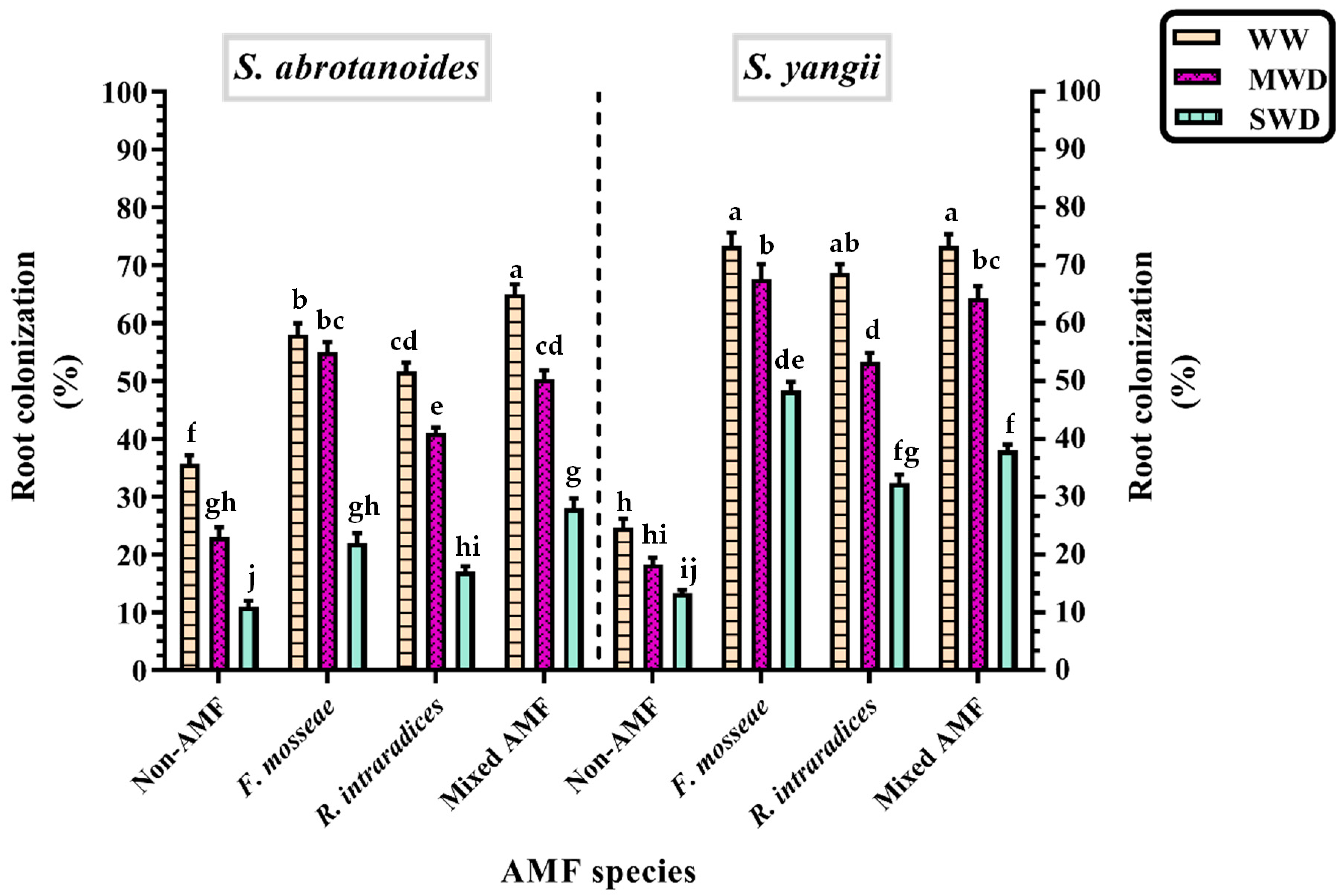
3.2. Root Colonization Percentage
3.3. Total Phenolics and Flavonoids
3.4. Enzymatic Antioxidant Defense Systems
3.5. Leaf Phosphorus Concentration and Relative Water Content
3.6. Photosynthetic Pigments
3.7. Essential Oil Content
3.8. Essential Oil Composition
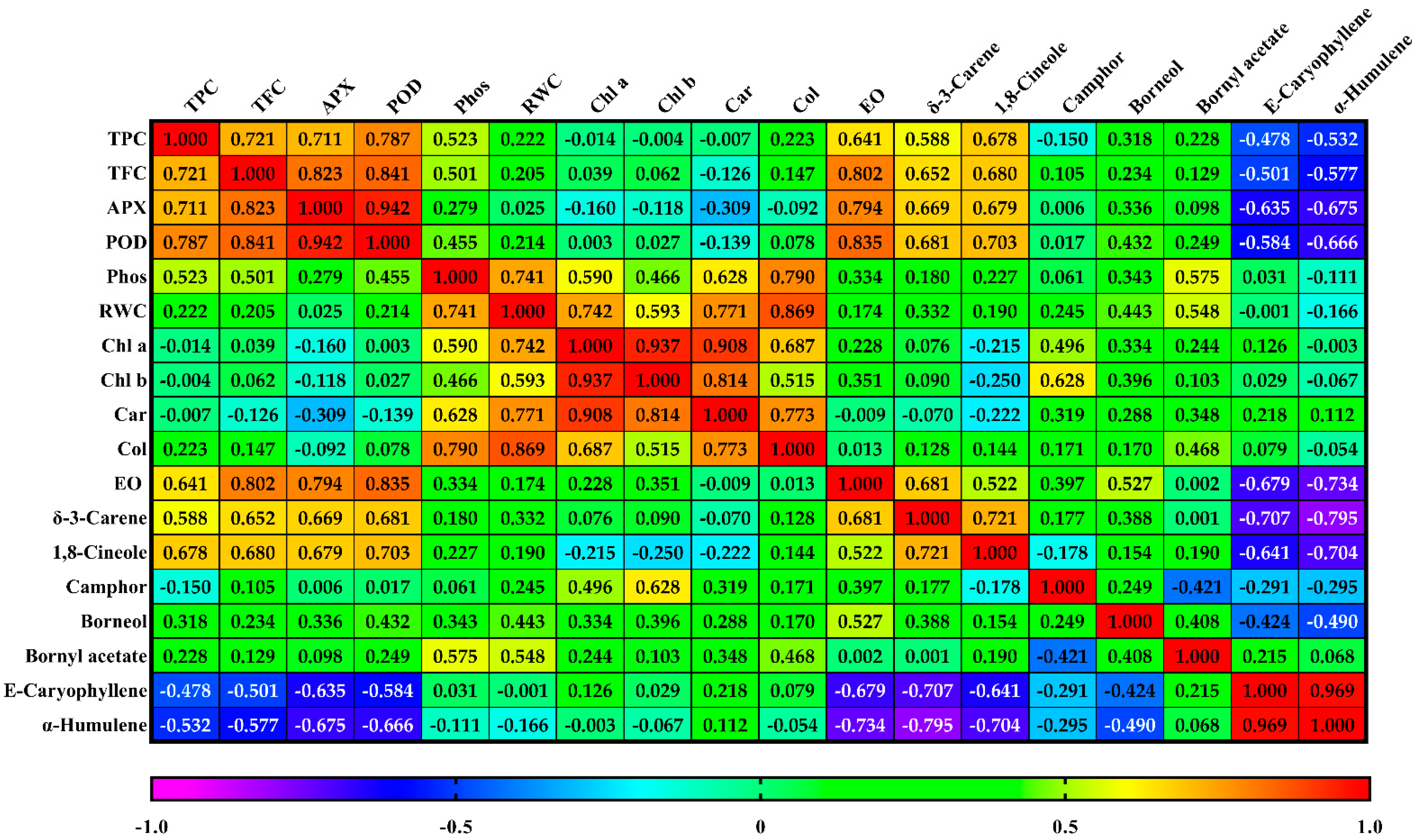
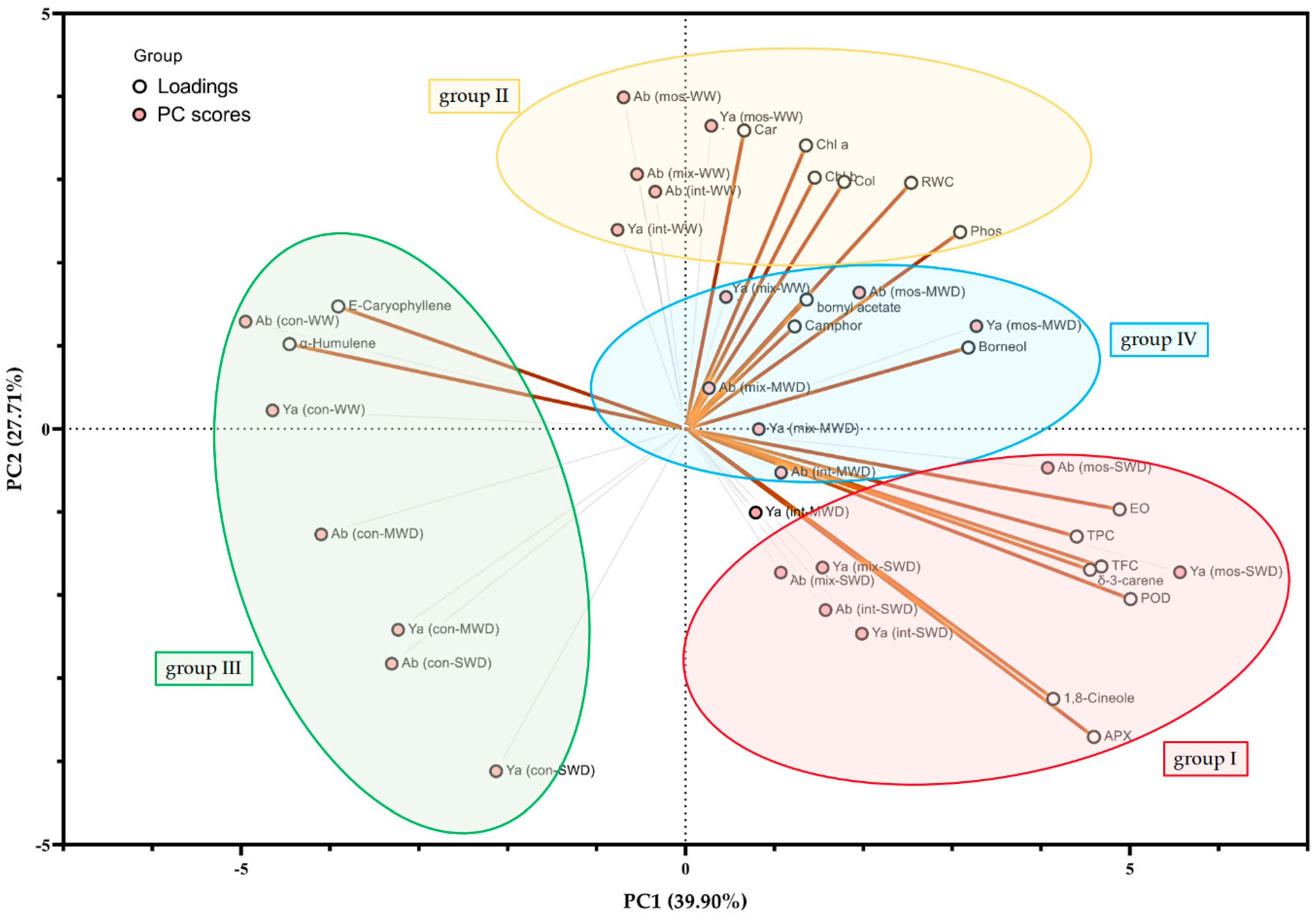
3.9. Correlation and Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMF | arbuscular mycorrhizal fungi |
| TPC | total phenolic content |
| TFC | total flavonoid content |
| APX | ascorbate peroxidase |
| POD | guaiacol peroxidase |
| Phos | phosphorus concentration |
| RWC | relative water content |
| Chl a | chlorophyll a |
| Chl b | chlorophyll b |
| Car | carotenoid |
| EO | essential oil content |
| WW | well-watered |
| MWD | moderate drought stress |
| SWD | severe drought stress |
References
- Rahimmalek, M.; Afshari, M.; Sarfaraz, D.; Miroliaei, M. Using HPLC and multivariate analyses to investigate variations in the polyphenolic compounds as well as antioxidant and antiglycative activities of some Lamiaceae species native to Iran. Ind. Crops Prod. 2020, 154, e112640. [Google Scholar] [CrossRef]
- Drew, B.T.; González-Gallegos, J.G.; Xiang, C.L.; Kriebel, R.; Drummond, C.P.; Walked, J.B.; Sytsma, K.J. Salvia united: The greatest good for the greatest number. Taxon 2017, 66, 133–145. [Google Scholar] [CrossRef]
- Bielecka, M.; Pencakowski, B.; Stafiniak, M.; Jakubowski, K.; Rahimmalek, M.; Gharibi, S.; Matkowski, A.; Ślusarczyk, S. Metabolomics and DNA-based authentication of two traditional Asian medicinal and aromatic species of Salvia subg. Perovskia. Cells 2021, 10, 112–137. [Google Scholar] [CrossRef] [PubMed]
- Mohammadhosseini, M.; Venditti, A.; Akbarzadeh, A. The genus Perovskia Kar.: Ethnobotany, chemotaxonomy and phytochemistry: A review. Toxin Rev. 2021, 40, 484–505. [Google Scholar] [CrossRef]
- Ghaffari, Z.; Rahimmalek, M.; Sabzalian, M.R. Variations in essential oil composition and antioxidant activity in Perovskia abrotanoides Kar. collected from different regions in Iran. Chem. Biodivers. 2018, 15, e1700565. [Google Scholar] [CrossRef] [PubMed]
- Afshari, M.; Rahimmalek, M. Variation in essential oil composition, anatomical, and antioxidant characteristics of Achillea filipendulina Lam. as affected by different phenological stages. J. Essent. Oil Res. 2021, 33, 283–298. [Google Scholar] [CrossRef]
- Khaliq, S.; Volk, F.J.; Frahm, A.W. Phytochemical investigation of Perovskia abrotanoides. Planta Med. 2006, 73, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Sairafianpour, M.; Christensen, J.; Stærk, D.; Budnik, B.A.; Kharazmi, A.; Bagherzadeh, K.; Jaroszewski, J.W. Leishmanicidal, antiplasmodial, and cytotoxic activity of novel diterpenoid 1,2-quinones from Perovskia abrotanoides: New source of tanshinones. J. Nat. Prod. 2001, 64, 1398–1403. [Google Scholar] [CrossRef]
- Arabi, F.; Moharramipour, S.; Sefidkon, F. Chemical composition and insecticidal activity of essential oil from Perovskia abrotanoides (Lamiaceae) against Sitophilus oryzae (Coleoptera: Curculionidae) and TriboChun-Yanm castaneum (Coleoptera: Tenebrionidae). Int. J. Trop. Insect Sci. 2008, 28, 144–150. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Masoudi, S.; Ameri, N.; Samiee, K.; Monfared, A. Volatile constituents of Ballota aucheri Boiss.; Stachys benthamiana Boiss. and Perovskia abrotanoides Karel. growing wild in Iran. J. Essent. Oil Res. 2006, 18, 218–221. [Google Scholar] [CrossRef]
- Sajjadi, S.E.; Mehregan, I.; Khatamsaz, M.; Asgari, G. Chemical composition of the essential oil of Perovskia abrotanoides karel. growing wild in Iran. Flavour Fragr. J. 2005, 20, 445–446. [Google Scholar] [CrossRef]
- Arpanahi, A.A.; Feizian, M.; Mehdipourian, G.; Khojasteh, D.N. Arbuscular mycorrhizal fungi inoculation improves essential oil and physiological parameters and nutritional values of Thymus daenensis Celak and Thymus vulgaris L. under normal and drought stress conditions. Eur. J. Soil Biol. 2020, 100, e103217. [Google Scholar] [CrossRef]
- Nouraei, S.; Rahimmalek, M.; Saeidi, G. Variation in polyphenolic composition, antioxidants and physiological characteristics of globe artichoke (Cynara cardunculus var. scolymus Hayek L.) as affected by drought stress. Sci. Hortic. 2018, 233, 378–385. [Google Scholar] [CrossRef]
- Gholinezhad, E.; Darvishzadeh, R. Influence of arbuscular mycorrhiza fungi and drought stress on fatty acids profile of sesame (Sesamum indicum L.). Field Crops Res. 2021, 262, e108035. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N.; Xia, R.X. Effects of water stress and arbuscular mycorrhizal fungi on reactive oxygen metabolism and antioxidant production by citrus (Citrus tangerine) roots. Eur. J. Soil Biol. 2006, 42, 166–172. [Google Scholar] [CrossRef]
- Chen, S.; Jin, W.; Chun-Yan, A.; Zhang, S.; Chun-Yan, D.; Wang, F.; Lin, X.; He, C. Arbuscular mycorrhizal fungi (AMF) increase growth and secondary metabolism in cucumber subjected to low temperature stress. Sci. Hortic. 2013, 160, 222–229. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef]
- Amiri, R.; Nikbakht, A.; Etemadi, N. Alleviation of drought stress on rose geranium [Pelargonium graveolens (L.) Herit.] in terms of antioxidant activity and secondary metabolites by mycorrhizal inoculation. Sci. Hortic. 2015, 197, 373–380. [Google Scholar] [CrossRef]
- Rebey, I.B.; Jabri-Karoui, I.; Hamrouni-Sellami, I.; Bourgou, S.; Limam, F.; Marzouk, B. Effect of drought on the biochemical composition and antioxidant activities of cumin (Cuminum cyminum L.) seeds. Ind. Crops Prod. 2012, 36, 238–245. [Google Scholar] [CrossRef]
- Amiri, R.; Nikbakht, A.; Rahimmalek, M.; Hosseini, H. Variation in the essential oil composition, antioxidant capacity, and physiological characteristics of Pelargonium graveolens L. inoculated with two species of mycorrhizal fungi under water deficit conditions. J. Plant Growth Regul. 2017, 36, 502–515. [Google Scholar] [CrossRef]
- Hazzoumi, Z.; Moustakime, Y.; Joutei, K.A. Effect of arbuscular mycorrhizal fungi and water stress on ultrastructural change of glandular hairs and essential oil compositions in Ocimum gratissimum. Chem. Biol. Technol. Agric. 2017, 4, 20. [Google Scholar] [CrossRef]
- Paravar, A.; Farahani, S.M.; Rezazadeh, A. Lallemantia species response to drought stress and Arbuscular mycorrhizal fungi application. Ind. Crops Prod. 2021, 172, e114002. [Google Scholar] [CrossRef]
- Rechinger, K.H. Flora Iranica; Akademische Druck-u, Verlagsanstalt: Graz, Austria, 1982; pp. 354–396. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper 56; Food and Agricultural Organization of the United Nations: Rome, Italy, 1998; p. 300. [Google Scholar]
- Alipour, A.; Rahimi, M.M.; Hosseini, S.M.A.; Bahrani, A. Mycorrhizal fungi and growth-promoting bacteria improves fennel essential oil content under water stress. Ind. Crops Prod. 2021, 170, e113792. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Toor, R.K.; Savage, G.P. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 2005, 38, 487–494. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gericke, S.; Kurmies, B. The colorimetric determination of phosphoric acid ammonium vanadate molybdate and its application in plant analysis. J. Plant Nutr. Soil Sci. 1952, 159, 11–21. [Google Scholar]
- He, J.D.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhizas enhance drought tolerance of trifoliate orange by enhancing activities and gene expression of antioxidant enzymes. Sci. Hortic. 2020, 262, e108745. [Google Scholar] [CrossRef]
- Arnon, A.N. Method of extraction of chlorophyll in the plants. Agron. J. 1967, 23, 112–121. [Google Scholar]
- Tuo, X.Q.; He, L.; Zou, Y.N. Alleviation of drought stress in white clover after inoculation with arbuscular mycorrhizal fungi. Not. Bot. Horti Agrobot. Cluj Napoca 2017, 45, 220–224. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, A.; Jha, A.; Salunkhe, O.; Vyas, D. Soil moisture levels affect mycorrhization during early stages of development of agroforestry plants. Biol. Fertil. Soils 2013, 49, 545–554. [Google Scholar] [CrossRef]
- Hazzoumi, Z.; Moustakime, Y.; Elharchli, E.H.; Joutei, K.A. Effect of arbuscular mycorrhizal fungi (AMF) and water stress on growth, phenolic compounds, glandular hairs, and yield of essential oil in basil (Ocimum gratissimum L.). Chem. Biol. Technol. Agric. 2015, 2, 10–21. [Google Scholar] [CrossRef]
- Giovannetti, M.; Avio, L.; Sbrana, C. Fungal spore germination and pre-symbiotic mycelial growth-physiological and genetic aspects. In Arbuscular Mycorrhizas: Physiology and Function; Koltai, H., Kapulnik, Y., Eds.; Springer: New York, NY, USA, 2010; pp. 3–32. [Google Scholar] [CrossRef]
- Afshari, M.; Rahimmalek, M.; Sabzalian, M.R.; Bielecka, M.; Matkowski, A.; Talebi, M. Changes in physiological, phytochemical traits and gene expression of two Perovskia species in response to water deficit. Sci. Hortic. 2022, 293, e110747. [Google Scholar] [CrossRef]
- Manukyan, A. Effect of growing factors on productivity and quality of lemon catmint, lemon balm and sage under soilless greenhouse production: I. drought stress. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 119–125. [Google Scholar]
- Pirbalouti, A.G.; Malekpoor, F.; Salimi, A.; Golparvar, A. Exogenous application of chitosan on biochemical and physiological characteristics, phenolic content and antioxidant activity of two species of basil (Ocimum ciliatum and Ocimum basilicum) under reduced irrigation. Sci. Hortic. 2017, 217, 114–122. [Google Scholar] [CrossRef]
- Amanifar, S.; Toghranegar, Z. The efficiency of arbuscular mycorrhiza for improving tolerance of Valeriana officinalis L. and enhancing valerenic acid accumulation under salinity stress. Ind. Crops Prod. 2020, 147, e112234. [Google Scholar] [CrossRef]
- Begum, N.; Akhtar, K.; Ahanger, M.A.; Iqbal, M.; Wang, P.; Mustafa, N.S.; Zhang, L. Arbuscular mycorrhizal fungi improve growth, essential oil, secondary metabolism, and yield of tobacco (Nicotiana tabacum L.) under drought stress conditions. Environ. Sci. Pollut. Res. 2021, 28, 45276–45295. [Google Scholar] [CrossRef]
- Seifi, E.; Teymoor, Y.S.; Alizadeh, M.; Fereydooni, H. Olive mycorrhization: Influences of genotype, mycorrhiza, and growing periods. Sci. Hortic. 2014, 180, 214–219. [Google Scholar] [CrossRef]
- Bahari, A.A.; Sokhtesaraei, R.; Chaghazardi, H.R.; Masoudi, F.; Nazarli, H. Effect of water deficit stress and foliar application of salicylic acid on antioxidants enzymes activity in leaves of Thymus daenensis subsp. LancifoChun-Yans. Cercet. Agron. Mold. 2015, 48, 57–67. [Google Scholar] [CrossRef]
- Prathyusha, I.V.S.N.; Chaitanya, K.V. Effect of water stress on the physiological and biochemical responses of two different Coleus (Plectranthus) species. Biol. Futur. 2019, 70, 312–322. [Google Scholar] [CrossRef]
- Chun-Yan, L.; Yu-Juan, W.; Qiang-Sheng, W.; Tian-Yuan, Y.; Kamil, K. Arbuscular mycorrhizal fungi improve the antioxidant capacity of tea (Camellia sinensis) seedlings under drought stress. Not. Bot. Horti Agrobot. Cluj Napoca 2020, 48, 1993–2005. [Google Scholar] [CrossRef]
- Haghighi, T.M.; Saharkhiz, M.J. Mycorrhizal colonization and silicon nutrition mitigates drought stress in Licorice (Glycyrrhiza glabra L.) with morphophysiological and biochemical perspectives. Ind. Crops Prod. 2022, 178, e114650. [Google Scholar] [CrossRef]
- Rezaei-Chiyaneh, E.; Mahdavikia, H.; Subramanian, S.; Alipour, H.; Siddique, K.H.; Smith, D.L. Co-inoculation of phosphate-solubilizing bacteria and mycorrhizal fungi: Effect on seed yield, physiological variables, and fixed oil and essential oil productivity of Ajowan (Carum copticum L.) under water deficit. J. Soil Sci. Plant Nutr. 2021, 21, 3159–3179. [Google Scholar] [CrossRef]
- Ghanbarzadeh, Z.; Mohsenzadeh, S.; Rowshan, V.; Moradshahi, A. Evaluation of the growth, essential oil composition and antioxidant activity of Dracocephalum moldavica L. under water deficit stress and symbiosis with Claroideoglomus etunicatum and Micrococcus yunnanensis. Sci. Hortic. 2019, 256, e108652. [Google Scholar] [CrossRef]
- Zardak, S.G.; Dehnavi, M.M.; Salehi, A.; Gholamhoseini, M. Responses of field grown fennel (Foeniculum vulgare Mill.) to different mycorrhiza species under varying intensities of drought stress. J. Appl. Res. Med. Aromat. Plants 2017, 5, 16–25. [Google Scholar] [CrossRef]
- Davies, F.T.; Olalde-Portugal, V.; Aguilera-Gomez, L.; Alvarado, M.J.; Ferrera-Cerrato, R.C.; Boutton, T.W. Alleviation of drought stress of Chile ancho pepper (Capsicum annuum L. cv. San Luis) with arbuscular mycorhhiza indigenous to Mexico. Sci. Hortic. 2002, 92, 347–359. [Google Scholar] [CrossRef]
- Mahdi Abadi, B.H.; Ganjali, H.R.; Mobasser, H.R. Effect of mycorrhiza and phosphorus fertilizer on some characteristics of black cumin. Biol. Forum Int. J. 2015, 7, 1115–1120. [Google Scholar]
- Setayeshmehr, Z.; Ganjali, A. Effects of drought stress on growth and physiological characteristics of dill (Anethum graveolens L.). J. Hortic. Sci. 2013, 27, 27–35. [Google Scholar]
- Zhu, X.Q.; Tang, M.; Zhan, H.Q. Arbuscular mycorrhizal fungi enhanced the growth, photosynthesis, and calorific value of black locust under salt stress. Photosynthetica 2017, 55, 378–385. [Google Scholar] [CrossRef]
- Mahdavikia, H.; Rezaei-Chiyaneh, E.; Rahimi, A.; Mohammadkhani, N. Effects of fertilizer treatments on antioxidant activities and physiological traits of basil (Ocimum basilicum L.) under water limitation conditions. J. Med. Plants By-Prod. 2019, 8, 143–151. [Google Scholar] [CrossRef]
- Baslam, M.; Esteban, R.; García-Plazaola, J.I.; Goicoechea, N. Effectiveness of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of major carotenoids, chlorophylls and tocopherol in green and red leaf lettuces. Appl. Microbiol. Biotechnol. 2013, 97, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Asrar, A.A.; Abdel-Fattah, G.M.; Elhindi, K.M. Improving growth, flower yield, and water relations of snapdragon (Antirrhinum majus L.) plants grown under well-watered and water-stress conditions using arbuscular mycorrhizal fungi. Photosynthetica 2012, 50, 305–316. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Morshedloo, M.R.; Aghaee, A.; Maggi, F. Funneliformis mosseae inoculation under water deficit stress improves the yield and phytochemical characteristics of thyme in intercropping with soybean. Sci. Rep. 2021, 11, 15279. [Google Scholar] [CrossRef]
- De Abreu, I.N.; Mazzafera, P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 2005, 43, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, S.; Pirzad, A. Pseudomonas and mycorrhizal fungi co-inoculation alter seed quality of fax under various water supply conditions. Ind. Crops Prod. 2019, 129, 518–524. [Google Scholar] [CrossRef]
- Rydlová, J.; Püschel, D.; Sudová, R.; Gryndler, M.; Mikanová, O.; Vosátka, M. Interaction of arbuscular mycorrhizal fungi and rhizobia: Effects on flax yield in spoil-bank clay. J. Plant Nutr. Soil Sci. 2011, 174, 128–134. [Google Scholar] [CrossRef]
- Habibzadeh, Y.; Jalilian, J.; Zardashti, M.R.; Pirzad, A.; Eini, O. Some morphophysiological characteristics of mung bean mycorrhizal plants under different irrigation regimes in field condition. J. Plant Nutr. 2015, 38, 1754–1767. [Google Scholar] [CrossRef]
- Pirzad, A.; Mohammadzadeh, S. Water use efficiency of three mycorrhizal Lamiaceae species (Lavandula officinalis, Rosmarinus officinalis and Thymus vulgaris). Agric. Water Manag. 2018, 204, 1–10. [Google Scholar] [CrossRef]
- Prasad, A.; Kumar, S.; Pandey, A.; Chand, S. Microbial and chemical sources of phosphorus supply modulate the yield and chemical composition of essential oil of rose-scented geranium (Pelargonium species) in sodic soils. Biol. Fertil. Soils 2012, 48, 117–122. [Google Scholar] [CrossRef]
- Tarraf, W.; Ruta, C.; De Cillis, F.; Tagarelli, A.; Tedone, L.; De Mastro, G. Effects of mycorrhiza on growth and essential oil production in selected aromatic plants. Ital. J. Agron. 2015, 10, 160–162. [Google Scholar] [CrossRef]
- Mirzaie, M.; Ladanmoghadam, A.R.; Hakimi, L.; Danaee, E. Water stress modifies essential oil content and composition, glandular trichomes and stomatal features of lemongrass (Cymbopogon citratus) inoculated with arbuscular mycorrhizal fungi. J. Agric. Sci. Technol. 2020, 22, 1575–1585. [Google Scholar]
| Herbarium Voucher Specimen No. | Species | Location | Longitude | Latitude | Altitude m a.s.l. | Annual Precipitation (mm) |
|---|---|---|---|---|---|---|
| 13,368 | S. yangii | Khash- Sistan and Baluchestan- Iran | 28°22′ E | 61°18′ N | 1410 | 141.22 |
| 13,363 | S. abrotanoides | Kalat- Laein- Khorasan Razavi- Iran | 37°11′ E | 59°25′ N | 748.8 | 76.50 |
| Source of Variation | df | TPC | TFC | APX | POD | Phos | RWC | Chl a | Chl b | Car | EO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Irrigation regime | 2 | 28585.88 ** | 58.44 ** | 740680.43 ** | 4776.20 ** | 0.04 ** | 1808.63 ** | 284.31 ** | 8.84 ** | 3.44 ** | 6.14 ** |
| AMF | 3 | 42646.31 ** | 65.38 ** | 319437.26 ** | 4789.57 ** | 0.17 ** | 2074.50 ** | 254.38 ** | 14.50 ** | 1.31 ** | 6.88 ** |
| Irrigation regime × AMF | 6 | 3111.68 ** | 0.63 ** | 41997.87 ** | 303.01 ** | 0.01 ** | 117.45 ** | 3.73 ** | 0.44 ** | 0.05 ** | 0.28 ** |
| Plant species | 1 | 36802.74 ** | 26.43 ** | 137172.74 ** | 1584.84 ** | 0.08 ** | 175.71 ** | 178.10 ** | 25.13 ** | 0.41 ** | 1.21 ** |
| Irrigation regime × Plant species | 2 | 9015.89 ** | 7.75 ** | 25602.60 ** | 0.64 ns | 0.01 ** | 30.33 ** | 2.64 ** | 0.21 ** | 0.03 ** | 0.10 ** |
| AMF × Plant species | 3 | 11632.79 ** | 10.88 ** | 1682.23 ** | 93.15 ** | 0.01 ** | 14.01 ** | 10.89 ** | 0.48 ** | 0.09 ** | 0.25 ** |
| Irrigation regime × AMF × Plant species | 6 | 3497.07 ** | 0.91 ** | 18158.74 ** | 62.64 ** | 0.01 ** | 2.58 ** | 4.12 ** | 0.17 ** | 0.01 ** | 0.06 ** |
| Error | 48 | 1.74 | 0.07 | 1.18 | 6.35 | 0.01 | 0.46 | 0.08 | 0.01 | 0.01 | 0.01 |
| Plant Species | AMF | Irrigation Regime | TPC | TFC | APX | POD | Phos | RWC | Chl a | Chl b | Car |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg TAE g−1 DW) | (mg QUE g−1 DW) | (μmol min−1 mg−1 protein) | (mg g−1) | (%) | (mg g−1 FW) | ||||||
| S. abrotanoides | Non-AMF | WW | 94.65 ± 3.62 i | 3.35 ± 0.52 g | 181.79 ± 7.22 j | 8.44 ± 0.91 h | 0.37 ± 0.04 e | 64.02 ± 1.92 c | 10.45 ± 1.17 fg | 3.86 ± 0.72 d | 1.31 ± 0.14 b |
| MWD | 111.82 ± 6.11 h | 7.71 ± 0.81 e | 359.16 ± 10.04 g | 14.63 ± 1.28 g | 0.35 ± 0.05 ef | 53.06 ± 1.05 de | 5.57 ± 0.55 hi | 3.15 ± 0.39 e | 0.83 ± 0.18 df | ||
| SWD | 138.25 ± 5.73 g | 8.43 ± 0.66 d | 486.22 ± 7.51 f | 21.41 ± 0.89 e | 0.34 ± 0.09 ef | 34.90 ± 1.66 f | 4.50 ± 0.64 i | 2.78 ± 0.11 f | 0.76 ± 0.09 f | ||
| Funneliformis mosseae | WW | 172.17 ± 8.08 bc | 9.58 ± 0.75 c | 233.26 ± 8.85 i | 25.55 ± 1.72 d | 0.58 ± 0.11 a | 79.24 ± 2.17 a | 20.48 ± 1.82 a | 6.05 ± 0.96 a | 1.98 ± 0.27 a | |
| MWD | 178.90 ± 6.43 b | 11.54 ± 0.18 b | 775.15 ± 9.27 b | 51.39 ± 1.26 b | 0.52 ± 0.03 b | 75.12 ± 1.33 ab | 18.56 ± 1.12 b | 5.88 ± 0.93 ab | 1.46 ± 0.43 b | ||
| SWD | 201.95 ± 7.21 a | 12.59 ± 0.95 a | 862.67 ± 10.66 a | 72.38 ± 1.80 a | 0.49 ± 0.10 bc | 67.42 ± 2.04 bc | 13.34 ± 0.86 e | 5.35 ± 0.47 b | 1.03 ± 0.07 c | ||
| Rhizophagus intraradices | WW | 150.47 ± 9.10 e | 6.36 ± 0.27 f | 381.27 ± 5.13 g | 21.35 ± 0.97 e | 0.50 ± 0.05 b | 77.19 ± 1.70 a | 15.48 ± 0.98 cd | 4.98 ± 0.39 c | 1.68 ± 0.18 ab | |
| MWD | 163.78 ± 5.57 de | 9.37 ± 0.81 b | 525.82 ± 8.82 e | 38.47 ± 1.34 c | 0.43 ± 0.03 cd | 69.51 ± 1.69 b | 9.46 ± 0.33 g | 3.67 ± 0.58 de | 1.01 ± 0.08 c | ||
| SWD | 166.93 ± 6.75 cd | 11.20 ± 0.53 b | 738.68 ± 11.92 bc | 49.39 ± 1.66 b | 0.41 ± 0.04 d | 63.89 ± 2.28 c | 6.69 ± 1.14 h | 3.40 ± 0.19 de | 0.88 ± 0.08 d | ||
| Mixed AMF | WW | 144.79 ± 8.32 f | 8.70 ± 0.26 d | 325.26 ± 5.07 gh | 14.60 ± 0.32 g | 0.48 ± 0.04 bc | 79.61 ± 1.91 a | 16.63 ± 1.76 c | 5.40 ± 0.55 b | 1.79 ± 0.41 a | |
| MWD | 165.90 ± 9.16 cd | 11.27 ± 0.74 b | 374.31 ± 8.29 g | 19.39 ± 0.28 ef | 0.44 ± 0.03 c | 71.79 ± 1.88 b | 11.75 ± 0.89 f | 4.44 ± 0.60 c | 1.07 ± 0.07 c | ||
| SWD | 180.15 ± 7.37 b | 11.80 ± 0.19 b | 608.28 ± 7.46 d | 40.54 ± 1.58 c | 0.37 ± 0.08 e | 57.11 ± 0.97 d | 9.13 ± 0.27 g | 4.25 ± 0.37 cd | 0.87 ± 0.12 d | ||
| S. yangii | Non-AMF | WW | 111.22 ± 6.28 hi | 7.45 ± 0.27 g | 279.28 ± 7.30 f | 10.42 ± 0.35 g | 0.38 ± 0.08 f | 66.60 ± 1.22 c | 7.69 ± 0.32 de | 2.91 ± 0.18 c | 1.01 ± 0.08 bc |
| MWD | 124.23 ± 4.91 h | 8.07 ± 0.96 f | 401.01 ± 9.92 e | 17.29 ± 0.92 f | 0.36 ± 0.05 f | 51.62 ± 1.68 d | 3.49 ± 0.65 g | 2.61 ± 0.29 cd | 0.65 ± 0.08 e | ||
| SWD | 187.13 ± 8.47 d | 9.35 ± 0.25 e | 529.80 ± 5.69 d | 25.52 ± 1.08 e | 0.35 ± 0.05 fg | 40.07 ± 1.91 e | 2.85 ± 0.14 gh | 1.86 ± 0.44 f | 0.38 ± 0.04 f | ||
| Funneliformis mosseae | WW | 171.60 ± 8.12 df | 11.77 ± 0.61 c | 505.61 ± 7.17 d | 38.30 ± 1.01 d | 0.72 ± 0.09 a | 79.43 ± 1.96 ab | 17.01 ± 0.45 a | 4.85 ± 0.42 a | 1.78 ± 0.39 a | |
| MWD | 351.03 ± 10.64 b | 12.30 ± 0.11 b | 638.93 ± 8.85 c | 55.38 ± 0.79 b | 0.68 ± 0.04 a | 76.77 ± 2.08 b | 10.52 ± 1.16 c | 4.52 ± 0.85 a | 1.45 ± 0.11 ab | ||
| SWD | 393.97 ± 9.73 a | 14.32 ± 0.58 a | 1044.66 ± 11.39 a | 86.38 ± 1.86 a | 0.59 ± 0.11 b | 72.77 ± 1.77 bc | 8.47 ± 0.70 d | 3.11 ± 0.17 bc | 1.04 ± 0.08 bc | ||
| Rhizophagus intraradices | WW | 126.09 ± 6.68 h | 10.36 ± 0.82 d | 536.58 ± 4.22 d | 37.73 ± 1.55 d | 0.61 ± 0.08 b | 77.90 ± 2.14 b | 11.83 ± 0.85 bc | 3.95 ± 0.63 b | 1.29 ± 0.09 b | |
| MWD | 198.69 ± 9.45 cd | 11.66 ± 0.37 c | 610.64 ± 8.39 c | 47.40 ± 1.87 c | 0.55 ± 0.09 c | 71.07 ± 1.55 bc | 6.48 ± 0.19 e | 2.62 ± 0.10 cd | 0.81 ± 0.12 d | ||
| SWD | 211.24 ± 5.07 c | 12.51 ± 0.44 b | 786.60 ± 7.09 b | 56.44 ± 1.45 b | 0.48 ± 0.05 d | 68.31 ± 1.69 c | 5.50 ± 0.62 f | 2.14 ± 0.08 e | 0.70 ± 0.08 de | ||
| Mixed AMF | WW | 158.99 ± 7.82 g | 8.47 ± 0.18 f | 433.80 ± 7.61 e | 21.45 ± 0.79 e | 0.51 ± 0.06 c | 84.17 ± 1.31 a | 12.46 ± 0.48 b | 3.81 ± 0.97 b | 1.71 ± 0.24 a | |
| MWD | 182.24 ± 5.33 d | 9.64 ± 0.71 e | 503.81 ± 8.55 d | 42.37 ± 1.33 c | 0.47 ± 0.08 d | 76.62 ± 1.08 b | 10.68 ± 0.75 c | 3.45 ± 0.46 b | 1.05 ± 0.09 bc | ||
| SWD | 195.93 ± 8.49 cd | 10.54 ± 0.59 d | 628.72 ± 9.12 c | 51.44 ± 1.68 b | 0.42 ± 0.04 de | 65.01 ± 1.28 c | 7.32 ± 0.66 de | 3.21 ± 0.15 bc | 0.99 ± 0.05 bc | ||
| Compounds | RI a | Non-AMF | Funneliformis mosseae | Rhizophagus intraradices | Mixed AMF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WW | MWD | SWD | WW | MWD | SWD | WW | MWD | SWD | WW | MWD | SWD | ||
| α-Pinene | 937 | nd b | 2.90 | 3.85 | 1.09 | 4.89 | 3.99 | 1.12 | 5.88 | 5.51 | 2.18 | 2.60 | 5.67 |
| Camphene | 951 | nd | 1.11 | 1.40 | 1.05 | 3.99 | 3.28 | 1.06 | 4.66 | 4.66 | 2.03 | 2.37 | 4.55 |
| β-Pinene | 973 | nd | 0.18 | 0.22 | nd | 0.49 | 0.61 | nd | 0.47 | 0.48 | nd | nd | 0.59 |
| Myrcene | 991 | 0.79 | 1.15 | 1.11 | 1.01 | 1.52 | 0.94 | 1.12 | 2.00 | 2.44 | 1.27 | 1.35 | 2.40 |
| δ-3-Carene | 1011 | 1.91 | 0.15 | 0.70 | 4.27 | 7.40 | 6.90 | 3.60 | 7.93 | 8.16 | 5.19 | 6.95 | 7.34 |
| p-Cymene | 1025 | nd | 0.38 | 0.95 | nd | 0.85 | 0.76 | nd | 0.95 | 1.07 | 0.61 | 0.77 | 1.02 |
| Limonene | 1030 | 0.75 | 1.05 | 1.85 | 0.91 | 2.01 | 1.24 | 1.01 | 1.43 | 1.60 | 1.15 | 1.20 | 1.58 |
| 1,8-Cineole | 1032 | 5.20 | 9.41 | 12.77 | 11.15 | 17.34 | 19.33 | 11.50 | 19.11 | 20.09 | 12.63 | 17.13 | 20.34 |
| Linalool | 1099 | nd | 0.82 | 0.40 | nd | 0.57 | nd | nd | nd | nd | nd | nd | nd |
| Camphor | 1142 | 10.13 | 9.90 | 15.88 | 18.82 | 23.16 | 19.34 | 17.62 | 15.48 | 17.66 | 21.66 | 16.69 | 23.53 |
| cis-Chrysanthemol | 1160 | nd | 1.45 | 0.65 | nd | 0.51 | nd | nd | nd | 0.52 | nd | nd | 0.50 |
| Borneol | 1167 | 5.34 | 4.77 | 3.20 | 5.46 | 4.88 | 11.60 | 11.92 | 11.03 | 8.78 | 7.55 | 6.71 | 5.05 |
| Terpinen-4-ol | 1177 | nd | nd | 1.69 | nd | 0.49 | nd | nd | nd | nd | nd | nd | nd |
| Myrtenol | 1195 | nd | 0.71 | 0.13 | 0.78 | 0.81 | 1.09 | 0.78 | 0.98 | 1.04 | 0.74 | 1.11 | 0.79 |
| Bornyl acetate | 1285 | 7.65 | 4.18 | 2.10 | 5.05 | 3.08 | 8.43 | 10.23 | 5.93 | 4.25 | 4.82 | 7.17 | 3.89 |
| α-Terpinyl acetate | 1350 | 3.66 | 1.60 | 1.10 | 2.67 | 1.73 | 2.04 | 2.87 | 1.47 | 1.43 | 2.25 | 1.99 | 1.67 |
| Geranyl acetate | 1382 | nd | 1.65 | 2.12 | nd | nd | nd | 0.55 | nd | nd | nd | nd | nd |
| α-Gurjunene | 1409 | 0.82 | 2.92 | 1.10 | 0.70 | nd | nd | 0.72 | nd | 0.62 | nd | nd | 0.45 |
| E-β-Caryophyllene | 1421 | 8.97 | 7.19 | 6.50 | 7.98 | 6.41 | 3.93 | 5.71 | 4.69 | 4.52 | 6.51 | 5.79 | 4.64 |
| α-Humulene | 1454 | 8.02 | 7.44 | 6.91 | 6.80 | 5.19 | 3.26 | 4.83 | 4.10 | 3.65 | 5.84 | 4.88 | 3.82 |
| allo-aromadendrene | 1461 | 0.60 | 2.31 | 1.44 | 1.86 | nd | nd | 0.77 | nd | nd | 2.41 | nd | nd |
| γ-Cadinene | 1513 | 2.24 | 2.18 | 1.55 | 1.74 | 0.68 | 0.91 | 1.27 | 0.73 | 1.27 | 1.47 | 1.31 | 0.96 |
| δ-Cadinene | 1524 | 1.81 | 1.24 | 0.90 | 1.18 | 0.47 | 0.54 | 0.93 | 0.68 | 0.55 | 0.76 | 0.55 | 0.62 |
| Spathulenol | 1576 | nd | 1.46 | 2.31 | nd | nd | 0.49 | 0.58 | 0.46 | 0.50 | nd | nd | 0.38 |
| Caryophyllene oxide | 1581 | 1.58 | 0.77 | 0.34 | 1.15 | 0.61 | 0.49 | 1.01 | 2.83 | nd | 1.14 | 0.96 | 0.56 |
| Viridiflorol | 1591 | 0.89 | 2.35 | 2.33 | 0.62 | nd | nd | nd | nd | nd | nd | 0.53 | nd |
| β-Oplopenone | 1606 | 1.61 | 2.11 | 1.65 | 0.87 | nd | 0.50 | 0.68 | nd | 0.65 | 0.90 | 0.81 | nd |
| Isomyristicin | 1615 | 0.91 | 3.29 | 3.02 | 0.69 | nd | nd | nd | nd | nd | 1.05 | nd | nd |
| Calarene | 1620 | 1.98 | 3.25 | 2.30 | 1.48 | 0.84 | 0.71 | 1.30 | 0.67 | 0.61 | 1.48 | 1.28 | 0.72 |
| tau-Cadinol | 1640 | 8.89 | 6.11 | 5.20 | 4.54 | 1.75 | 2.30 | 3.45 | 1.73 | 3.22 | 4.68 | 4.34 | 2.29 |
| t-Muurolol | 1642 | 6.11 | 5.29 | 4.12 | 4.49 | 2.79 | 2.41 | 4.11 | 2.41 | 2.39 | 3.47 | 3.89 | 2.34 |
| Fonenol | 1648 | 1.30 | 0.37 | 0.91 | 1.08 | 2.08 | nd | 0.78 | nd | 1.32 | 0.88 | 0.81 | 1.62 |
| α-Eudesmol | 1653 | 2.29 | 0.95 | 1.81 | 1.07 | nd | 0.49 | 0.82 | 0.72 | nd | 0.78 | 0.52 | nd |
| α-Cadinol | 1654 | 5.80 | 4.22 | 2.88 | 3.92 | nd | 1.52 | 3.38 | 1.45 | nd | 2.73 | 3.11 | nd |
| β-Bisabolole | 1673 | 4.16 | 3.74 | 2.50 | 2.95 | 1.79 | 1.22 | 2.34 | 1.42 | 1.26 | 2.32 | 2.33 | 1.38 |
| α-Bisabolol | 1684 | 4.06 | 2.98 | 3.21 | 4.04 | 3.09 | 1.70 | 3.96 | 0.78 | 1.75 | 1.49 | 2.86 | 1.29 |
| Monoterpene hydrocarbons | 3.45 | 6.92 | 10.08 | 8.33 | 21.15 | 17.72 | 7.91 | 23.32 | 23.92 | 12.43 | 15.24 | 23.15 | |
| Oxygenated monoterpenes | 20.67 | 27.06 | 34.72 | 36.21 | 47.76 | 51.36 | 41.82 | 46.60 | 48.09 | 42.58 | 41.64 | 50.21 | |
| Sesquiterpene hydrocarbons | 22.46 | 23.28 | 18.40 | 20.26 | 12.75 | 8.64 | 14.23 | 10.20 | 10.61 | 16.99 | 12.53 | 10.49 | |
| Oxygenated sesquiterpenes | 38.67 | 33.60 | 29.56 | 26.21 | 12.95 | 11.83 | 22.41 | 12.47 | 11.70 | 19.87 | 21.44 | 10.58 | |
| Others | 12.22 | 9.14 | 7.24 | 99.42 | 4.81 | 10.45 | 13.63 | 7.40 | 5.68 | 8.12 | 9.15 | 5.56 | |
| Total identified | 97.47 | 100 | 100 | 99.43 | 99.42 | 100 | 100 | 99.99 | 100 | 99.99 | 100 | 99.99 | |
| Essential oil content (%) | 0.54 | 0.82 | 1.70 | 1.87 | 2.56 | 3.78 | 1.54 | 1.88 | 2.20 | 1.76 | 2.07 | 2.38 | |
| Compounds | RI a | Non-AMF | Funneliformis mosseae | Rhizophagus intraradices | Mixed AMF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WW | MWD | SWD | WW | MWD | SWD | WW | MWD | SWD | WW | MWD | SWD | ||
| α-Pinene | 937 | 1.79 | 2.08 | 3.45 | 1.91 | 3.40 | 7.38 | 1.95 | 3.53 | 4.85 | 4.86 | 5.67 | 5.82 |
| Camphene | 951 | 1.47 | 1.30 | 2.15 | 1.49 | 2.78 | 4.72 | 1.63 | 3.05 | 3.70 | 3.68 | 4.36 | 4.22 |
| β-Pinene | 973 | nd b | 1.89 | 1.93 | 0.60 | 0.90 | 1.04 | nd | 0.57 | 0.56 | 1.08 | 1.01 | 1.27 |
| Myrcene | 991 | 0.76 | 2.95 | 3.26 | 0.83 | 0.79 | 0.98 | 1.03 | 0.97 | 1.69 | 0.71 | 0.70 | 1.18 |
| δ-3-Carene | 1011 | 2.57 | 4.89 | 4.40 | 2.88 | 5.29 | 10.21 | 2.82 | 5.90 | 6.21 | 6.51 | 6.27 | 6.43 |
| p-Cymene | 1025 | nd | 0.88 | 0.37 | 0.70 | 0.70 | 1.30 | nd | 0.51 | 0.93 | 0.98 | 1.16 | 0.98 |
| Limonene | 1030 | 3.22 | 2.19 | 3.24 | 2.47 | 2.62 | 6.00 | 0.99 | 1.52 | 3.97 | 5.59 | 5.32 | 7.07 |
| 1,8-Cineole | 1032 | 14.53 | 14.33 | 17.67 | 15.81 | 24.91 | 26.82 | 11.54 | 21.52 | 23.91 | 24.24 | 25.36 | 30.20 |
| Linalool | 1099 | nd | 1.52 | 2.39 | nd | nd | nd | nd | 1.27 | nd | nd | nd | 0.47 |
| Camphor | 1142 | 4.44 | 8.25 | 2.95 | 6.49 | 14.64 | 2.68 | 15.10 | 13.37 | 13.88 | 11.95 | 15.21 | 3.52 |
| cis-Chrysanthemol | 1160 | nd | 2.34 | 4.21 | nd | 0.65 | nd | nd | 0.78 | 0.53 | 0.63 | 0.74 | 0.76 |
| Borneol | 1167 | 4.64 | 2.87 | 3.63 | 6.61 | 8.35 | 8.48 | 6.55 | 5.46 | 6.45 | 5.19 | 4.58 | 6.76 |
| Terpinen-4-ol | 1177 | nd | 0.29 | 0.24 | nd | 0.57 | nd | nd | nd | 0.50 | 0.53 | 0.66 | 0.52 |
| Myrtenol | 1195 | 1.02 | 1.71 | 1.58 | 0.75 | 0.81 | 0.75 | 0.55 | 1.34 | 0.72 | 0.88 | 0.91 | 0.96 |
| Bornyl acetate | 1285 | 9.92 | 4.52 | 3.66 | 12.93 | 8.50 | 10.52 | 12.02 | 5.84 | 7.13 | 7.71 | 6.96 | 10.96 |
| α-Terpinyl acetate | 1350 | 3.36 | 2.01 | 1.83 | 3.90 | 3.33 | 2.46 | 4.21 | 2.54 | 2.79 | 3.03 | 2.71 | 2.94 |
| α-Gurjunene | 1409 | 0.70 | 0.82 | 0.45 | 0.73 | nd | nd | 0.70 | nd | 0.57 | nd | nd | 0.29 |
| E-β-Caryophyllene | 1421 | 11.42 | 7.07 | 6.14 | 8.59 | 5.87 | 4.49 | 8.70 | 6.41 | 4.62 | 4.66 | 5.37 | 5.15 |
| endo Bornyl acetate | 1448 | nd | 0.21 | 0.15 | 1.06 | nd | nd | nd | nd | nd | nd | nd | nd |
| α-Humulene | 1454 | 10.39 | 6.91 | 5.45 | 7.24 | 4.94 | 3.66 | 7.25 | 5.59 | 3.81 | 4.09 | 4.45 | 3.82 |
| allo-aromadendrene | 1461 | 4.15 | 1.18 | 1.44 | 10.07 | 2.05 | 2.15 | 2.53 | 3.43 | 3.05 | 1.57 | 2.81 | 3.46 |
| Calamenene | 1497 | 0.65 | 2.37 | 2.45 | 0.80 | nd | nd | nd | nd | nd | nd | nd | nd |
| γ-Cadinene | 1513 | 1.23 | 1.19 | 1.78 | 0.70 | 0.82 | 0.66 | 1.57 | 0.60 | 0.99 | 1.07 | 0.53 | 0.31 |
| δ-Cadinene | 1524 | 2.08 | 1.87 | 1.12 | nd | 0.56 | 0.34 | 1.11 | nd | 0.63 | 0.41 | 0.41 | nd |
| Caryophyllene oxide | 1581 | 1.42 | 0.75 | 0.32 | 1.23 | 0.83 | 0.42 | 0.75 | 1.05 | 0.51 | 0.91 | 0.50 | 0.37 |
| Viridiflorol | 1591 | 0.73 | 1.63 | 1.25 | nd | nd | nd | 0.91 | 0.57 | nd | nd | nd | nd |
| β-Oplopenone | 1606 | nd | 1.82 | 1.25 | nd | nd | 0.32 | 0.97 | nd | nd | 0.62 | nd | nd |
| Isomyristicin | 1615 | 1.00 | 0.28 | 0.19 | 2.17 | 0.48 | 0.42 | 1.20 | 0.84 | 0.74 | 0.49 | 0.45 | 0.49 |
| Calarene | 1620 | 1.70 | 0.40 | 0.18 | 1.55 | 0.95 | 0.54 | 1.15 | 1.36 | 0.66 | 1.08 | 0.64 | 0.31 |
| tau-Cadinol | 1640 | 1.94 | 1.91 | 0.18 | 2.04 | 2.18 | 1.61 | 5.64 | 1.72 | 2.34 | 3.40 | 0.99 | 0.71 |
| t-Muurolol | 1642 | 3.52 | 4.48 | 5.63 | 1.91 | 1.02 | 0.83 | 3.14 | 2.75 | 1.76 | 1.55 | 0.74 | 0.56 |
| Fonenol | 1648 | 1.49 | 3.14 | 2.38 | 1.85 | nd | 0.68 | nd | 2.23 | 1.38 | 1.35 | nd | nd |
| α-Eudesmol | 1653 | 2.93 | 3.27 | 3.01 | nd | 0.51 | nd | 1.07 | nd | nd | nd | 0.44 | nd |
| α-Cadinol | 1654 | 3.62 | 1.89 | 2.28 | nd | 0.91 | nd | 2.29 | nd | nd | nd | 0.62 | nd |
| β-Bisabolole | 1673 | 3.29 | 1.27 | 2.07 | 2.02 | 0.66 | 0.54 | 2.11 | 1.63 | 1.13 | 1.22 | 0.43 | nd |
| α-Bisabolol | 1684 | nd | 1.62 | 0.18 | nd | nd | nd | nd | 3.03 | nd | nd | nd | nd |
| Monoterpene hydrocarbons | - | 9.81 | 24.18 | 26.80 | 10.88 | 16.48 | 30.33 | 8.42 | 16.05 | 21.91 | 23.41 | 24.49 | 26.97 |
| Oxygenated monoterpenes | - | 24.63 | 31.31 | 32.67 | 29.66 | 49.93 | 38.73 | 33.74 | 43.74 | 45.99 | 43.42 | 47.46 | 43.19 |
| Sesquiterpene hydrocarbons | - | 28.54 | 11.54 | 9.71 | 28.13 | 13.68 | 10.96 | 20.75 | 16.03 | 13.04 | 11.39 | 13.16 | 13.03 |
| Oxygenated sesquiterpenes | - | 22.72 | 24.05 | 19.85 | 10.60 | 7.62 | 5.28 | 19.14 | 14.34 | 8.41 | 10.54 | 4.77 | 1.95 |
| Others | - | 14.28 | 7.02 | 5.83 | 20.06 | 12.29 | 13.40 | 17.43 | 9.22 | 10.65 | 11.23 | 10.12 | 14.39 |
| Total identified | - | 99.98 | 98.10 | 94.86 | 99.30 | 100 | 98.70 | 99.48 | 99.38 | 100 | 99.99 | 100 | 99.53 |
| Essential oil content (%) | 0.61 | 0.94 | 1.39 | 1.58 | 2.36 | 2.90 | 1.37 | 1.86 | 2.17 | 1.16 | 1.63 | 2.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afshari, M.; Rahimmalek, M.; Sabzalian, M.R.; Szumny, A.; Matkowski, A.; Jezierska-Domaradzka, A. Mycorrhizal Colonization Modulates the Essential Oil Profile and Enzymatic and Non-Enzymatic Antioxidants to Mitigate the Adverse Effects of Water Deficit in Salvia subg. Perovskia. Biology 2022, 11, 1757. https://doi.org/10.3390/biology11121757
Afshari M, Rahimmalek M, Sabzalian MR, Szumny A, Matkowski A, Jezierska-Domaradzka A. Mycorrhizal Colonization Modulates the Essential Oil Profile and Enzymatic and Non-Enzymatic Antioxidants to Mitigate the Adverse Effects of Water Deficit in Salvia subg. Perovskia. Biology. 2022; 11(12):1757. https://doi.org/10.3390/biology11121757
Chicago/Turabian StyleAfshari, Mahvash, Mehdi Rahimmalek, Mohammad R. Sabzalian, Antoni Szumny, Adam Matkowski, and Anna Jezierska-Domaradzka. 2022. "Mycorrhizal Colonization Modulates the Essential Oil Profile and Enzymatic and Non-Enzymatic Antioxidants to Mitigate the Adverse Effects of Water Deficit in Salvia subg. Perovskia" Biology 11, no. 12: 1757. https://doi.org/10.3390/biology11121757
APA StyleAfshari, M., Rahimmalek, M., Sabzalian, M. R., Szumny, A., Matkowski, A., & Jezierska-Domaradzka, A. (2022). Mycorrhizal Colonization Modulates the Essential Oil Profile and Enzymatic and Non-Enzymatic Antioxidants to Mitigate the Adverse Effects of Water Deficit in Salvia subg. Perovskia. Biology, 11(12), 1757. https://doi.org/10.3390/biology11121757









